Summary
Feeding is dynamically regulated by the palatability of the food source and the physiological needs of the animal. How consumption is controlled by external sensory cues and internal metabolic state remains under intense investigation. Here, we identify four GABAergic interneurons in the Drosophila brain that establish a central feeding threshold which is required to inhibit consumption. Inactivation of these cells results in indiscriminate and excessive intake of all compounds, independent of taste quality or nutritional state. Conversely, acute activation of these neurons suppresses consumption of water and nutrients. The output from these neurons is required to gate activity in motor neurons that control meal initiation and consumption. Thus, our study reveals a new layer of inhibitory control in feeding circuits that is required to suppress a latent state of unrestricted and non-selective consumption.
Introduction
Feeding behavior is critical for restoring metabolic homeostasis and is essential for survival. Animals have evolved sophisticated feedback mechanisms that monitor and rectify imbalances in energy stores by regulating food intake. Plasticity in food intake is achieved by altering feeding thresholds in response to internal needs and food availability (Dethier, 1976; Morton et al., 2006). How the nervous system coordinates internal physiological state with external sensory information to trigger feeding behaviors is insufficiently understood.
The fruit fly Drosophila melanogaster is a promising model system to dissect the neural basis of feeding decisions. Many of the endocrine and neuropeptide systems that control feeding in mammals are conserved in Drosophila (Baker and Thummel, 2007; Leopold and Perrimon, 2007; Nassel and Homberg, 2006). Furthermore, the rapid development of genetic and physiological tools makes it an attractive organism to study molecular and cellular mechanisms underlying behavior (Venken et al., 2011). The fly nervous system contains approximately 100,000 neurons, with many cells uniquely identifiable between animals, which significantly facilitates circuit analysis (Ito et al., 2013; Olsen and Wilson, 2008). The numerical simplicity of this system enables cellular and synaptic examination of feeding regulation and may provide insight into mechanisms of regulation used throughout evolution.
The detection of gustatory cues drives feeding initiation and ingestion in Drosophila. The neural circuits that process gustatory cues and elicit feeding behavior are just beginning to be elucidated. The fruit fly assesses the quality of potential food sources using gustatory neurons that detect sweet and bitter compounds and drive acceptance and rejection, respectively (Thorne et al., 2004; Wang et al., 2004). In addition, motor neurons controlling feeding subprograms for proboscis extension and ingestion have been described (Gordon and Scott, 2009; Manzo and Scott, 2012; Rajashekhar and Singh, 1994; Tissot et al., 1998). Only one taste-responsive interneuron has been characterized to date, a putative feeding command neuron that is activated by sugar and promotes feeding (Flood et al., 2013).
The response to gustatory cues is highly regulated based on internal metabolic state. Significant progress has been made in identifying signaling mechanisms that communicate the physiological state to the nervous system. Circulating hormones from the neuroendocrine system and fat body, Drosophila insulin-like peptides, adipokinetic hormone, and the leptin homolog Unpaired-2, signal the status of available carbohydrate and lipid stores (Geminard et al., 2009; Ikeya et al., 2002; Kim and Rulifson, 2004; Noyes et al., 1995; Rajan and Perrimon, 2012; Wu et al., 2005). It was recently found that circulating fructose also reports the nutritional state and alters feeding behavior by direct activation of a few central neurons that express the fructose receptor, Gr43a (Miyamoto et al., 2012). Furthermore, post-ingestive feedback from the gut likely inhibits feeding, as severing the recurrent nerve or the medial abdominal nerve, which transmit information from the gut to the brain, results in overconsumption in blowflies (Dethier and Gelperin, 1967). How the detection of peripheral signals of metabolic state are translated to alter feeding thresholds is largely unknown.
Several central effector pathways regulate feeding by promoting or inhibiting carbohydrate uptake. Neuropeptide Y, small Neuropeptide F and dopamine promote nutrient intake (Hergarden et al., 2012; Inagaki et al., 2012; Lee et al., 2004; Marella et al., 2012; Wu et al., 2003), whereas allatostatin, hugin, leukokinin, and drosulfakinin inhibit specific aspects of feeding (Hergarden et al., 2012; Melcher and Pankratz, 2005; Söderberg et al., 2012; Wu et al., 2003). For example, leukokinin limits meal size whereas drosulfakinin decreases consumption of nutrients. Although many molecular signaling pathways have been identified, the precise neuronal substrates mediating modulation and their effects on feeding circuits remain unclear. Moreover, the gating mechanisms for behavioral feeding subprograms as well as neural correlates for central feeding thresholds are unknown.
Here, we identify four GABAergic interneurons that impart an inhibitory tone on ingestive behavior that is required for regulation by taste quality or satiety state. Inactivation of these neurons leads to robust and indiscriminate overconsumption regardless of the chemical properties of the ingested substance. We show that these neurons act upstream of motor neurons for multiple feeding subprograms. This study opens the door to analyzing how central inhibition regulates feeding behaviors in Drosophila melanogaster.
Results
A behavioral screen for neurons that inhibit consumption
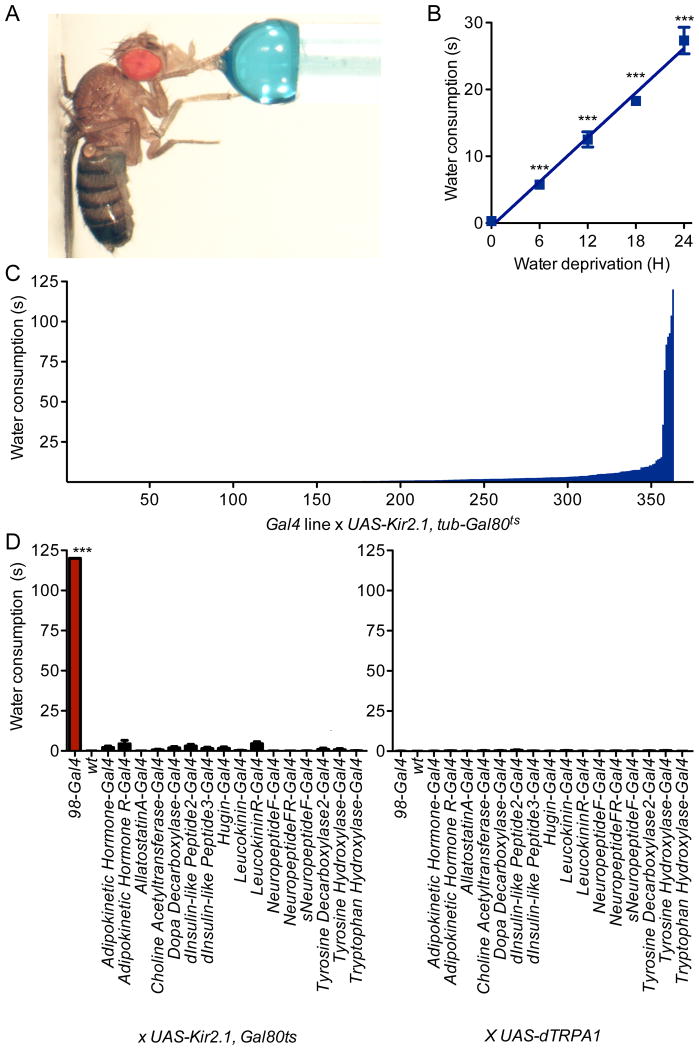
To identify neurons that regulate consumption, we carried out a behavioral screen in which we inactivated neural subsets within the Drosophila central nervous system and monitored effects on water consumption time. Single flies were fed water until they became unresponsive to further stimulation and total consumption time was monitored (Figure 1A). Water-satiated control flies consumed no water, whereas water-deprived controls increased intake in proportion to water deprivation time (Figure 1B).
Figure 1. Neuronal inactivation screen identifies flies with insatiable behavior.
A. Water consumption time of single flies was monitored. Blue dye was included for illustration.
B. Wild-type flies consumed water in proportion to water deprivation time. n=20-31 flies; mean±SEM; one-way ANOVA, Tukey post-hoc, ***p<0.001.
C. 363 Gal4 lines, expressing Kir2.1 conditionally in the adult, were assayed for water consumption under non-deprived conditions (mean, n=20 flies). See also Figure S1, showing the neural expression of the 6 Gal4 lines that overconsumed.
D. Neuropeptide/neurotransmitter-Gal4 lines were tested for water consumption upon neural inactivation with Kir2.1, tub-Gal80ts (left) or activation with dTRPA1 (right) under non-deprived conditions. n=20 flies; mean±SEM; t-test; ***p<0.001.
We performed a behavioral screen for flies that consumed water under water-replete conditions. An inwardly-rectifying potassium channel that prevents depolarization, Kir2.1 (Baines et al., 2001), was expressed in different neurons using a collection of Gal4 enhancer trap lines. A ubiquitous temperature-sensitive Gal80ts (McGuire et al., 2004) was used to repress Kir2.1 expression until adulthood and then was inactivated by a two-day temperature-shift to 30°C, allowing Kir2.1 induction. Neurons in 363 Gal4 lines (Gohl et al., 2011) were conditionally inactivated upon Kir2.1 expression and flies were monitored for water consumption time under water-replete conditions. The vast majority of Gal4 lines (349/363) drank water for less than ten seconds. Strikingly, the screen yielded six transgenic lines that continued to consume water for more than a minute (Figure 1C), with assay termination at two minutes. The lack of water satiety in six Gal4 lines suggests that these lines mark neurons essential for consumption regulation.
Five of the six Gal4 lines were broadly expressed in the brain and ventral nerve cord (Figure S1). One line, 98-Gal4, showed sparse expression and was further characterized. Wild-type water-deprived flies readily engaged in water consumption for a brief period, leading to meal termination and failure to initiate further consumption (Video S1). In contrast, inactivation of 98-Gal4 neurons caused a complete absence of water satiety, resulting in extreme bloating and regurgitation. Nevertheless, the flies continued to initiate new meals (Video S2).
To examine whether the water overconsumption phenotype was similar to previously identifed feeding phenotypes, we tested whether manipulation of identified neuropeptide/neurotransmitter systems altered water consumption (Al-Anzi et al., 2010; Alekseyenko et al., 2010; Bharucha et al., 2008; Cole et al., 2005; Colombani et al., 2003; Friggi-Grelin et al., 2003; Hergarden et al., 2012; Lee and Park, 2004; Li et al., 2000; Melcher and Pankratz, 2005; Nassel et al., 2008; Rulifson et al., 2002; Salvaterra and Kitamoto, 2001; Wen et al., 2005). Neither conditional inactivation with UAS-Kir2.1, tub-Gal80ts or acute activation with the UAS-dTRPA1 heat-activated cation channel (Hamada et al., 2008) elicited water overconsumption using these Gal4 lines (Figure 1D). Thus, the 98-Gal4 behavioral phenotype is distinct from other feeding phenotypes and is unlikely to result from altered activity of neuromodulatory systems previously associated with feeding regulation.
Inactivation of four neurons causes dramatic overconsumption
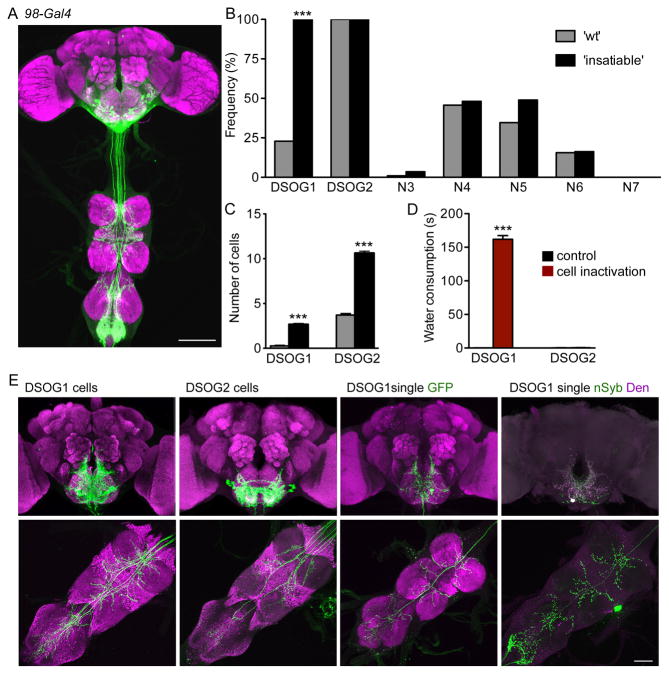
The 98-Gal4 line labels 32 neurons in the brain, six neurons in the ventral nerve cord and approximately ten peripheral neurons projecting to the abdominal ganglion (Figure 2A). We identified seven different morphological neural classes labeled by 98-Gal4 (Figure S2A). To determine the causal neurons for overconsumption, we used a molecular genetic approach to limit reporter expression by stochastic excision of the Gal80 repressor of Gal4, resulting in mosaic expression of Gal4 and Gal4-dependent reporters, tetanus toxin C (Sweeney et al., 1995) (to inhibit synaptic transmission, TNT) and GFP in 98-Gal4 cell subsets (Gordon and Scott, 2009). Mosaic animals were tested for water consumption and categorized into two groups: animals in the ‘insatiable’ cohort (n=44) were behaviorally indistinguishable from 98-Gal4, UAS-TNT flies, whereas ‘wt’ animals (n=105) consumed water for less than 5 seconds. Cells causal for the overconsumption phenotype were identified based on two criteria: (1) they were labeled and consequently silenced in all the ‘insatiable’ cohort brains and (2) they were underrepresented in the ‘wt’ cohort.
Figure 2. A subset of 98-Gal4 neurons influences consumption.
A. 98-Gal4 drives expression of UAS-GFP in the brain and ventral nerve cord (scale 100 μm). See Figure S2 for the seven different cell-types labeled by 98-Gal4.
B. Frequency distribution of cell-types in wild-type ‘wt’ (grey bars; n=105) and ‘insatiable’ (black bars; n=44) mosaic animals (hs-FLP122/ tub>Gal80>; 98-Gal4/UAS-TNT; UASCD8:GFP). mean±SEM; Fisher's test, ***p<0.001.
C. The number of DSOG1 cells (4 cells total) or DSOG2 cells (16 total) labeled in ‘wt’ (grey bars) or ‘insatiable’ (black bars) mosaic animals.
D. Water consumption under non-deprived conditions was measured for flies with DSOG1 or DSOG2 inactivated. 98-Gal4 expression was restricted to DSOG1 (276B-FLP) or DSOG2 (934-FLP) with tub>Gal80> and cells were inactivated in UAS-Kir2.1, tub-Gal80ts animals at 30°C (cell inactivation) or kept at 22°C (control). n=20; mean±SEM; t-test, ***p<0.001.
E. GFP expression in brain (top) and VNC (bottom) for DSOG1 (left) and DSOG2 (second). Single-cell labeling of DSOG1 with GFP (third) or dendritic (DenMark; magenta) and synaptic (SYT-GFP; green) markers (right). Scale bar is 50μm.
Five of the seven cell types were silenced in less than half of the brains in either group with no significant overrepresentation in the ‘insatiable’ cohort, demonstrating that they do not underlie the overconsumption phenotype (Figure 2B). Two populations of descending suboesophageal neurons (DSOG) were always present in the ‘insatiable’ group: DSOG1 (4 cells total) and DSOG2 cells (16 cells total) (Figure 2B). The frequency of DSOG1 cells was overrepresented in the ‘insatiable’ cohort, whereas DSOG2 cells were labeled in all animals. In addition, the number of DSOG1 and DSOG2 cells labeled in the ‘insatiable’ cohort was greater than in the control group (Figure 2C). Eight of 44 ‘insatiable’ brains had only DSOG1 and DSOG2 cells silenced, arguing that DSOG1 or DSOG2 influences consumption.
To further determine whether DSOG1 or DSOG2 cells were causal for the behavioral phenotype, we restricted expression to smaller subsets by screening for FLP enhancer trap lines (Bohm et al., 2010) that excised tub>Gal80> in 98-Gal4 subsets. This screen identified two informative FLP lines: 276B-FLP that restricted Gal4 activity to the four DSOG1 cells and 934-FLP that restricted activity to 10-12 DSOG2 cells (Figure 2E). Exclusively silencing DSOG1 cells resulted in insatiable behavior similar to silencing all 98-Gal4 neurons, with animals consuming water in non-deprived states (Figure 2D). Silencing DSOG2 cells did not cause overconsumption (Figure 2D). These studies demonstrate that inactivation of the four DSOG1 cells is sufficient to elicit overconsumption.
Single-cell labeling of DSOG1 revealed the cell body in the ventral suboesophageal zone (SEZ), with wide-field bilateral arborizations in the SEZ and ventral nerve cord (VNC) (Figure 2E, Video S3). Labeling individual DSOG1 cells with a photoactivatable GFP showed that all DSOG1 cells have a similar morphology (Figure S2B). Single-cell clones showed dendrites (labeled with DenMark) (Nicolai et al., 2010) in the SOG and axons (labeled with synaptotagmin-GFP, SYT-GFP) (Zhang et al., 2002) in the SEZ and VNC (Figure 2E). The SEZ contains axons from gustatory sensory neurons and dendrites of motor neurons that drive feeding (Stocker, 1994), suggesting that DSOG1 neurons are well-positioned to modulate food intake.
Flies with inactivated DSOG1 neurons overconsume independent of taste quality or nutritional state
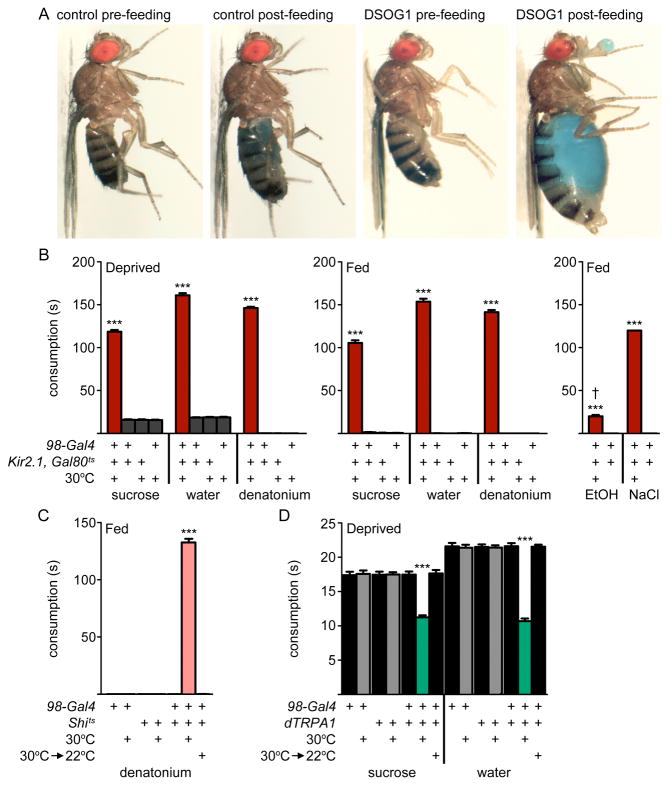
To examine whether DSOG1 cells selectively inhibit water consumption or generally regulate intake, we tested whether the overconsumption phenotype depended on category of the taste stimulus or satiety state. Unlike wild-type flies which terminate feeding after a brief meal, flies with DSOG1 neurons expressing Kir2.1 exhibited insatiable ingestion culminating in regurgitation, at which time measurements were terminated (Figure 3A). They consumed appetitive compounds (sucrose and water) as well as aversive compounds (denatonium, 6M sodium chloride, 100% ethanol) for approximately 150 seconds, approximately one μl volume, in food-deprived and non-deprived conditions (Figure 3AB, and Figure S3A). In contrast, flies without Kir2.1 induction and controls did not consume under fed conditions, but consumed water and sucrose for approximately 17 seconds, 150 nl volume, after 24 hour deprivation and did not consume aversive compounds (Figure 3B and Figure S3A). In addition, flies expressing Kir2.1 in DSOG1 neurons showed increased consumption on solid food (crystallized sucrose) (Figure S3BC), arguing against a specific defect in liquid consumption. Rapid inactivation of DSOG1 neurons using a temperature-sensitive dominant-negative dynamin (UAS-shibirets) (Kitamoto, 2001), which acts within minutes to inhibit neurotransmission, also led to overconsumption, showing that output from DSOG1 cells is acutely required to mediate meal rejection (Figure 3C).
Figure 3. Activity in DSOG1 neurons controls consumption.
A. Controls and flies expressing Kir2.1 in DSOG1 before (pre-feeding) and after (post-feeding) water consumption. The genotype for DSOG1 inactivation was 98-Gal4, 276B-FLP, tub>Gal80>, tub-Gal80ts, UAS-Kir2.1. Flies were kept two days at 30°C for Kir2.1 induction or remained at 22°C for controls.
B. DSOG1 inactivation increased consumption in deprived (24h wet-starved for 1M sucrose and 1mM denatonium; 24h dry-starved for water) and non-deprived (fed) conditions. 100% ethanol (EtOH) and 6M sodium chloride (NaCl) were consumed by non-deprived animals. n=20; mean±SEM; one-way ANOVA, Tukey post-hoc, ***p<0.001. † flies died after consumption.
C. DSOG1 inactivation with shibirets (Shits) caused 1mM denatonium consumption in non-deprived animals. n=20; mean±SEM; one-way ANOVA, Tukey post-hoc, ***p<0.001.
D. dTRPA1 activation of DSOG1 (98-Gal4, 276B-FLP, tub>Gal80>, UAS-dTRPA1) reduced sucrose and water consumption in deprived flies. n=61-71; mean+/-s.e.m.; one-way ANOVA, square root transformation, Tukey post-hoc, ***p<0.001. See Figure S3 for additional feeding phenotypes.
As inactivation of DSOG1 neurons promoted consumption, we tested whether inducing activity in these cells would inhibit feeding. The temperature-sensitive cation channel dTRPA1 was expressed in DSOG1 cells and flies were monitored for water and sucrose consumption at temperatures at which dTRPA1 was not active (22°C) or active (30°C). dTRPA1-induced activation of DSOG1 cells reduced sucrose consumption by a third and water consumption by half as compared to controls in food- and food- and water-deprived flies, respectively (Figure 3D and Figure S3D), demonstrating that acute activation of DSOG1 neurons directly inhibits consumption.
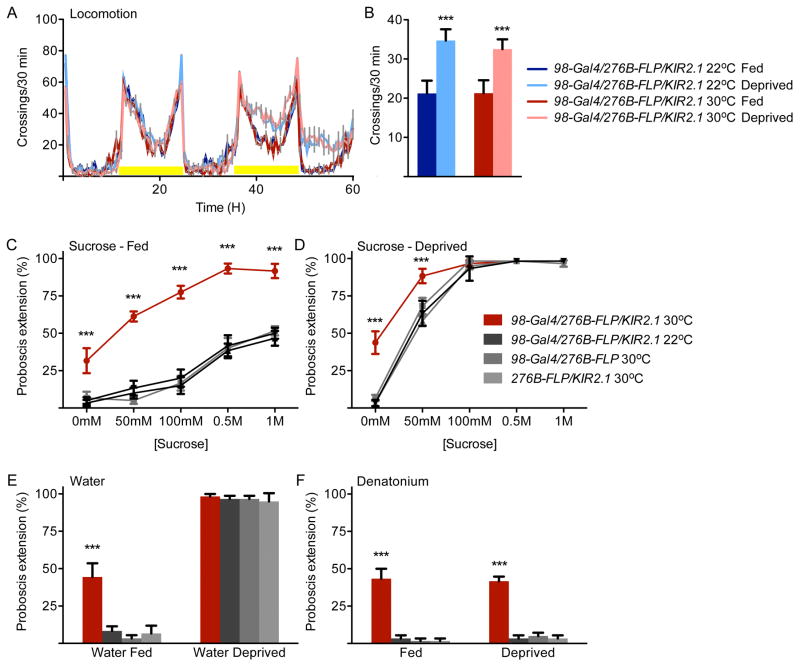
Feeding in Drosophila comprises a number of steps, including foraging to identify food at a distance, proboscis extension to allow feeding initiation, and ingestion. We tested whether flies with inactivated DSOG1 neurons altered the thresholds for all aspects of feeding behavior, by examining effects on starvation-induced locomotion and proboscis extension. The activity of single flies was monitored for 60 hours under fed and deprived conditions. Flies expressing the potassium channel Kir2.1 in DSOG1 neurons showed normal increased locomotion under food-deprived conditions (Figure 4AB) and normal locomotor behavior (Figure S3E), demonstrating that DSOG1 is not required for starvation-induced foraging. In contrast, flies lacking DSOG1 activity showed increased proboscis extension to nutrients and bitter compounds in fed and deprived states as well increased extension to water in non-deprived states (Figure 4C-F). These experiments argue that DSOG1 activity is required for rejection of aversive substances and rejection of appetitive substances upon satiation, affecting both feeding initiation and consumption.
Figure 4. DSOG1 neurons influence proboscis extension but not starvation-induced foraging.
A. Locomotor activity measurements in a single fly beam-crossing assay. Starvation-induced locomotor changes were unaffected in flies with DSOG1 neurons inactivated.
B. Average locomotor activity of starved and unstarved flies during the second day (36-60H) activity peak. n=19-32 flies per genotype and condition; mean±SEM; t-test to same genotype control; ***p<0.001.
C. Flies with DSOG1 neurons expressing Kir2.1 showed increased proboscis extension to sugar under fed conditions. All flies with Kir2.1 also contain tub>Gal80> and tub-Gal80ts and were incubated at 30°C for 2 days for Kir2.1 induction prior to behavioral testing or remained at 22°C for same genotype controls. n=60 flies per data point; mean±SEM; t-test to same genotype control; ***p<0.001.
D. Proboscis extension was significantly different in flies with DSOG neurons expressing Kir2.1 upon 24 hr food-deprivation. n=60 flies per data point; mean±SEM; t-test to same genotype control; ***p<0.001.
E. Flies with DSOG1 neurons expressing Kir2.1 showed increased proboscis extension to water in non-deprived states. n=60 flies per line; t-test to same genotype control; mean±SEM; ***p<0.001.
F. Flies with silenced DSOG1 neurons showed increased proboscis extension to bitter compounds in fed and deprived states. n=60 flies per line; mean±SEM; t-test to same genotype control; ***p<0.001.
DSOG1 cells are GABAergic interneurons that are not regulated by taste quality or physiological state
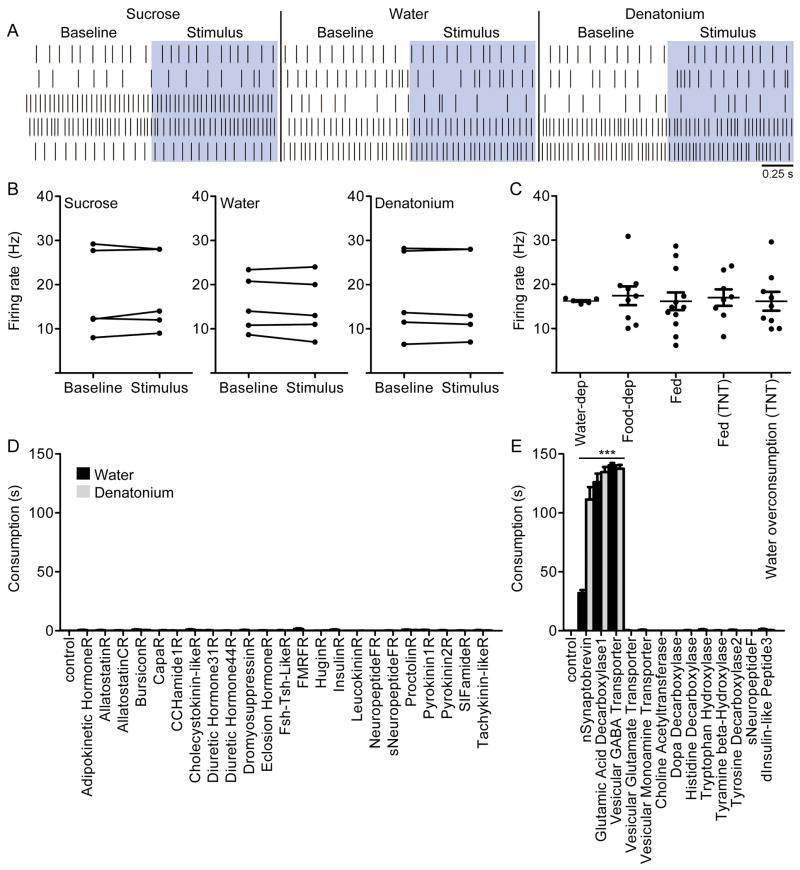
In the absence of DSOG1 activity, flies consume substances that are normally rejected as well as compounds that are rejected in sated conditions, suggesting that DSOG1 acts downstream of bitter cues and satiety signals to inhibit consumption. To test whether the activity of DSOG1 neurons is altered by gustatory detection or satiety state, we performed cell-attached recordings of DSOG1 in live flies (Marella et al., 2012).
DSOG1 neurons showed an average baseline firing rate of ∼17Hz, with a standard deviation of 6Hz. DSOG1 neurons did not respond to gustatory stimulation with 1M sucrose, 1mM denatonium or water in fed states (Figure 5AB) or in deprived states (Figure S4A). The baseline activity of DSOG1 neurons was not significantly different in flies that were water-deprived, food-deprived or non-deprived (Figure 5C). We also monitored the steady state activity of DSOG1 neurons in flies with over-distended or non-distended abdomens. 98-Gal4, UAS-TNT flies were fed water until bloated and activity in DSOG1 cells was monitored. TNT was used to block activity as it selectively inhibits synaptic vesicle release without altering action potential firing. The steady state activity of DSOG1 neurons in 98-Gal4, UAS-TNT flies with non-distended or over-distended abdomens was also not significantly different (Figure 5C), arguing that gut distention does not activate DSOG1 neurons.
Figure 5. DSOG1 neurons are GABAergic interneurons that do not respond to gustatory stimulation or satiety state.
A. Raster plots showing firing rates of DSOG1 cells 1s prior to (Baseline, white) and 1s after activation (Stimulus, blue) of proboscis gustatory neurons with 1M sucrose, water or 1mM denatonium. Five flies/taste compound, different flies for each stimulation.
B. DSOG1 firing rate in A before (Baseline) and during 1s stimulation (Stimulus) with 1M sucrose, water or 1mM denatonium in non-deprived conditions. Baseline rate was averaged 10s pre-stimulus. n=5 flies, Wilcoxon matched pairs test.
C. DSOG1 activity in wt animals that are water-deprived, food-deprived or non-deprived (fed) and flies expressing TNT in 98-Gal4 under non-deprived conditions (fed) or after water overconsumption. Activity was averaged for 30-200s. n=7-12; mean±SEM; Kruskal Wallis. See Figure S4 for additional experiments monitoring the activity of DSOG1.
D. UAS-RNAi and UAS-shRNA lines targeting transcripts encoding neuropeptide receptors implicated in food intake regulation were used to knock down gene expression in DSOG1 neurons in 98-Gal4; UAS-dcr2 and 98-Gal4 genetic background respectively. Water and 1mM denatonium consumption was monitored. n=20 per tastant per genotype; mean±SEM; t-test to control (no RNAi).
E. RNAi screen for neurotransmitters in 98-Gal4 that influence consumption. UAS-RNAi lines were crossed to 98-Gal4; UAS-dcr2 and UAS-shRNA lines were crossed to 98-Gal4. n=20; mean±SEM; t-test to control (no RNAi); ***p<0.001.
We performed additional tests to examine activation of DSOG1 cells by sensory stimuli. Using a dissected brain plus ventral nerve cord preparation, we electrically stimulated (10V) the major nerves of the ventral nerve cord and brain that contain gustatory and mechanosenory inputs and monitored responses of DSOG1 by GCaMP calcium imaging (Mann et al., 2013; Tian et al., 2009) (Figure S4B). In addition, we expressed the dTRPA1 heat-activated ion channel in different sensory classes, stimulated the sensory classes with heat and monitored activity in DSOG1 by GCaMP calcium imaging in a live fly preparation (Figure S4C). Neither nerve stimulation nor activation of sugar, bitter, water or pheromone gustatory inputs with dTRPA1 elicited responses in DSOG1. Taken together with the electophysiology studies, these results demonstrate that DSOG1 cells are not directly activated by gustatory stimuli.
The observation that DSOG tonic activity does not change in deprived or non-deprived states suggests that DSOG1 is not regulated by satiety signals. To further investigate molecular mechanisms within DSOG1 cells that might influence consumption, we carried out an RNAi screen of candidate receptors, neuropeptide/ neurotransmitter synthesis and trafficking genes in DSOG1 neurons and examined effects on consumption (Figure 5D). RNAi against neuropeptide receptors implicated in feeding regulation did not elicit overconsumption, consistent with the model that DSOG1 is not directly regulated by internal state cues. Inhibiting GABAergic signaling by RNAi against glutamate decarboxylase 1 or vesicular GABA transporter dramatically increased consumption, as did inhibiting synaptic transmission with nSynaptobrevin RNAi (Figure 5E). Immunostaining against GABA confirmed the GABAergic identity of DSOG1 neurons (Figure S4D).
Taken together, our data are inconsistent with the model that DSOG1 acts downstream of gustatory and internal state cues. Instead, this data argues that DSOG1 cells, gustatory cues and physiological state signals likely independently impinge on a common feeding pathway, with DSOG1 activity required to gate the response to gustatory and satiety cues.
DSOG1 does not act upstream of a putative feeding command neuron to regulate feeding
How does DSOG1 interact with the feeding circuit to inhibit consumption? Although very little of the central pathway that processes gustatory cues has been characterized, recent studies suggested that an SOG interneuron (FDG) may act as a feeding command neuron to drive multiple subprograms of feeding (Flood et al., 2013). As DSOG1 inhibits multiple feeding subprograms, an attractive hypothesis is that it acts on FDG to inhibit its activity and inhibit feeding.
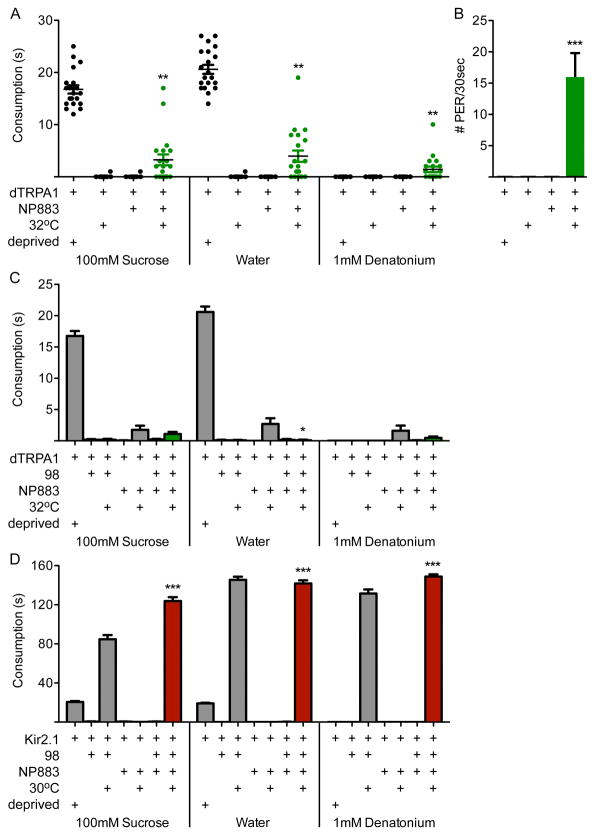
We first compared the behavioral phenotypes of activating FDG with those of inactivating DSOG1. We expressed the heat-activated ion channel dTRPA1 in NP883-Gal4 neurons (which contains FDG) and monitored consumption in sated animals (Figure 6A). Unlike DSOG1 inactivation which results in consumption times of ∼150s, FDG activation resulted in consumption times of ∼3s. This is significantly greater than fed controls (∼0s) and significantly less than starved controls (∼20s). In addition, activating NP883-Gal4 neurons elicited spontaneous proboscis extensions in the absence of food (Figure 5B). Activation of a second Gal4 line with selective expression in the FDG neuron had a similar proboscis extension phenotype but had no effect on consumption (Figure S5). These results argue that the FDG neuron drives proboscis extension but does not cause dramatic consumption.
Figure 6. The feeding phenotype of a putative feeding command neuron (FDG) is different from DSOG1 inactivation.
A. Flies with NP883-Gal4 neurons activated with dTRPA1 (32°C, green) showed mild consumption under fed conditions. Deprived controls are shown as a reference. In contrast, 98-Gal4, UAS-Kir2.1 flies consumed ∼150s. n=20 flies/genotype, each data point is one fly; mean±SEM; t-test to NP883-dTRPA1 (22°C); **p<0.01.
B. Spontaneous proboscis extensions were observed in NP883-Gal4, UAS-dTRPA1 flies upon dTRPA1 activation (32°C). n=20 flies/genotype; mean±SEM; t-test to NP883-Gal4, UAS-dTRPA1 (22°C); ***p<0.001.
C. The consumption of NP883-Gal4, UAS-dTRPA1 flies was not significantly different when 98-Gal4 neurons were also activated (green bars), with the exception of the water response. n=20-22 flies; mean±SEM; t-test to NP883-dTRPA1 (22°C); *p<0.05.
D. Consumption of 98-Gal4, UAS-Kir2.1 flies was not different when NP883-Gal4 neurons were also inactivated (red bars), demonstrating that the putative feeding command neuron is dispensable for the DSOG1 overconsumption phenotype. n=10-22 flies; mean±SEM; t-test to NP883-Kir2.1 (22°C); ***p<0.001. See Fiugre S5 for feeding phenotypes of a second Gal4 line with selective expression in FDG.
Although the behavioral phenotypes of FDG activation and DSOG1 inactivation differ, we tested for neural pathway interactions by simultaneously activating or silencing both neural classes. There was no impact on the consumption phenotype of NP883-dTRPA1 upon DSOG1 activation (Figure 6C), and no impact on the overconsumption behavior of DSOG1-Kir2.1 flies when FDG was also inactivated (Figure 6D). These experiments demonstrate that the putative feeding command neuron is dispensable for the DSOG1 overconsumption phenotype.
The recurrent nerve inhibits nutrient intake independently of DSOG1 function
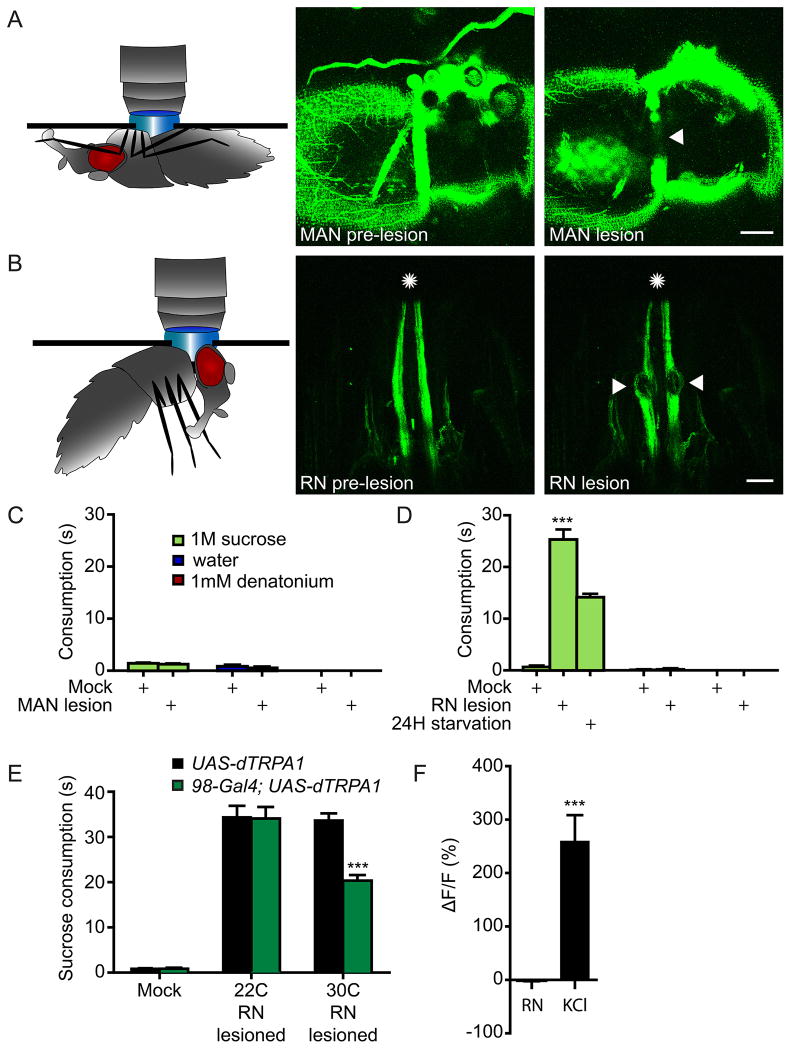
A hyperphagic phenotype similar to DSOG1 inactivation has previously been described in blowflies. Severing the recurrent nerve (RN) or the medial abdominal nerve connecting the digestive tract to the brain elicited dramatic overconsumption of carbohydrate solutions (Dethier and Gelperin, 1967), suggesting that gut-derived signals terminate nutrient consumption. We tested whether DSOG1 might act downstream of the recurrent nerve or abdominal nerve to inhibit feeding.
We used two-photon microscopy to target UV light to the nerves for lesioning. Flies with pan-neuronal GFP (nSyb-Gal4; UAS-CD8:GFP) were used to identify nerves and successful lesioning was indicated by GFP disappearance (Figure 7AB). Severing the medial abdominal nerve did not increase consumption (Figure 7C), whereas severing the recurrent nerve elevated consumption of sucrose but not water or bitter solutions (Figure 7D). Flies with RN lesions consumed sucrose for ∼26s, significantly less than seen with DSOG1 inactivation (∼110s). Nevertheless, the lesioning studies are consistent with previous studies in blowflies that argue that the recurrent nerve inhibits nutrient intake.
Figure 7. Recurrent nerve lesions influence carbohydrate intake independently of DSOG1.
A-B. Flies with pan-neuronal GFP (nSyb-Gal4; UAS-CD8:GFP) were used to target lesions to the medial abdominal nerve (MAN) (A) or the recurrent nerve (RN) (B). A 2-photon laser was used for lesioning and the disappearance of GFP was used to indicate successful lesioning. Arrowheads mark the lesioned nerves. Scale is 50 μm for panel A and 20 μm for panel B.
C. MAN lesion did not affect consumption of nutrients, water and bitters solutions. For mock lesions, the laser was directed to tissue adjacent to the nerve. n=10; mean±SEM; t-test to mock lesion; ***p<0.001.
D. Flies with RN lesions showed increased 1M sucrose consumption but no consumption of water or 1mM denatonium. Consumption of 24H food deprived flies is shown as a reference.
E. DSOG1 activation partially suppressed the 1M sucrose consumption induced by severing the RN. n=20; mean±SEM; t-test to 22°C same genotype; ***p<0.001.
F. Electrical stimulation of the RN did not induce calcium increases in DSOG1. The RN was stimulatedin a dissected brain preparation and activity was monitored in DSOG1 using 98-Gal4, UAS-GCaMP5 flies. 1M KCl elicited a calcium change. n=5; mean±SEM; ***p<0.001.
To examine if DSOG1 receives signals from the recurrent nerve, we tested whether acute activation of DSOG1 neurons could rescue the overconsumption upon RN lesioning. Consistent with this notion, activation of DSOG1 partially suppressed consumption in RN-lesioned animals (Figure 7E). To more directly test if the RN activated DSOG1, we applied an electrical stimulus to the RN and monitored activity in DSOG1 by GCaMP5 calcium imaging in a dissected brain plus ganglia preparation (Figure 7F). Activation of the RN did not activate DSOG1, aguing that they do not function in a linear pathway. Instead, our results argue that the RN and DSOG1 neurons inhibit feeding by independent convergence onto feeding circuits.
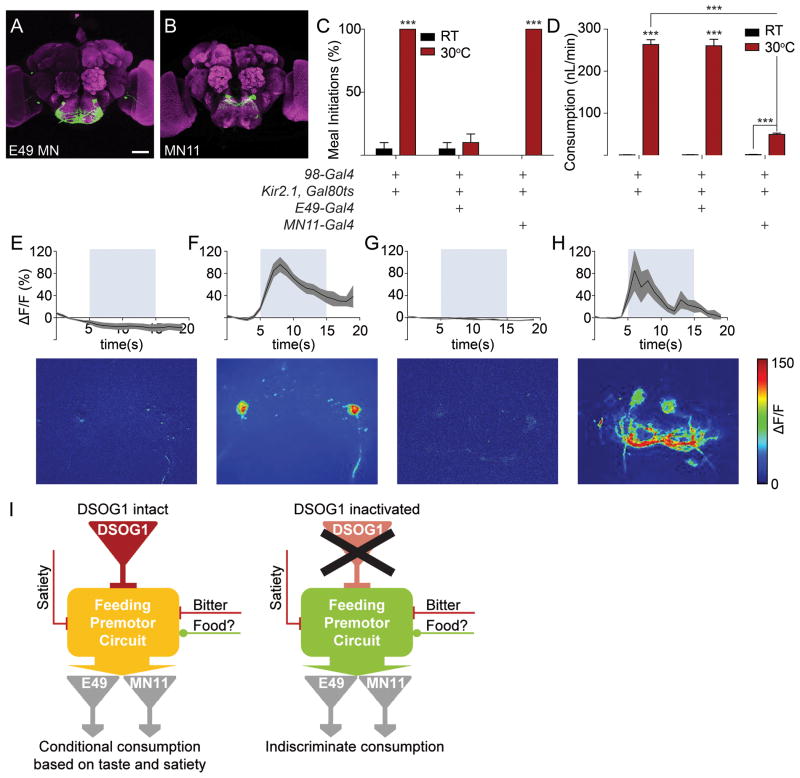
DSOG1 neurons gate taste evoked activity in feeding motor neurons
The motor outputs for feeding include E49 motor neurons that drive proboscis extension to initiate feeding (Gordon and Scott, 2009) and MN11 motor neurons that control food ingestion (Manzo and Scott, 2012) (Figure 8AB). To test whether DSOG1 acts uptream of specific motor neurons, we generated flies containing Kir2.1 in DSOG1 neurons and E49 MNs or MN11. DSOG1 inactivation alone resulted in robust proboscis extension and ingestion of bitter compounds. Blocking activity in E49 MNs in DSOG1-silenced flies selectively abolished proboscis extension to bitter solutions without affecting the overconsumption phenotype (Figure 8CD). Conversely, flies with DSOG1 neurons and MN11 inactivated still showed proboscis extension to bitter compounds but significantly decreased the volume ingested (Figure 8CD). These results argue that DSOG1 is upstream of multiple motor pathways and that the behavioral phenotypes of DSOG1 can be separated into one pathway that requires the E49 MN for increased proboscis extension and a second pathway that requires MN11 for increased consumption.
Figure 8. DSOG1 gates taste-evoked activation of feeding motor neurons.
A. E49 motorneurons controlling feeding initiation (VT201861), scale 50μm.
B. MN11 motorneurons controlling ingestion volume (NP543-Gal4).
C. E49 motorneurons are required for proboscis extension to bitter in DSOG1-silenced flies. n=20; mean±SEM; t-test to temperature control; ***p<0.001.
D. MN11 motorneurons are required for bitter overconsumption in DSOG1-silenced flies. n=20, t-test to temperature and genetic controls; ***p<0.001.
E. Bitter taste does not evoke activation of E49 MNs (UAS-TNT; E49-Gal4/UAS-GCaMP6). n=5, mean±SEM.
F. Bitter stimulation of the proboscis activates E49 MNs in the absence of DSOG1 output (98-Gal4/UAS-TNT; E49-Gal4/UAS-GCaMP6). n=5, mean±SEM. t-test to maxΔF/F data in E, ***p<0.001.
G. Bitter taste does not evoke activation of MN11 (UAS-TNT; NP534-Gal4/UAS-GCaMP6). n=5, mean±SEM.
H. Bitter stimulation of the proboscis activates MN11 in the absence of DSOG1 output (98-Gal4/UAS-TNT; NP534-Gal4/UAS-GCaMP6). n=5, mean±SEM. t-test to maxΔF/F data in G, ***p<0.001.
I. Model for DSOG1 function. DSOG1 inhibits feeding probability to enable regulation by satiety and bitter cues (left). In the absence of DSOG1, bitter and satiety cues are not sufficient to inhibit feeding, leading to uncontrolled food intake mediated by feeding motor neurons E49 and MN11 (right). Food indicates a positive sensory drive.
To test more directly if taste-evoked activity in feeding motor neurons is altered in flies lacking DSOG1 activity, we monitored activity in E49 or MN11 by GCaMP calcium imaging. Stimulation with denatonium produced strong GCaMP responses in E49 and MN11 in DSOG1-inactivated flies but not in controls (Figure 8E-H), arguing that output from DSOG1 cells serves to suppress bitter-evoked activity in feeding motor neurons. In the absence of DSOG1 neurons, even aversive taste compounds readily engage feeding motor neurons. GCaMP responses of Gr5a sugar-sensing or Gr66a bitter-sensing sensory neurons were unaffected in 98-Gal4, UASKir2.1 flies, arguing that activation of E49 and MN11 occurs despite normal sensory detection of bitter cues (Figure S6A). Anatomical studies co-labeling DSOG1 and E49 or MN11 showed that DSOG1 does not directly contact E49 MN or MN11, arguing that it does not directly modify MN activity but controls circuits upstream of MNs (Figure S6BC). These experiments demonstrate that DSOG1 influences multiple motor subprograms, acting upstream of motor neurons to gate activity in feeding output circuits.
Discussion
Our studies reveal a novel layer of feeding regulation in Drosophila, in which four GABAergic interneurons establish and maintain a central feeding threshold. These cells act as an essential brake to suppress a latent state of ubiquitous and non-selective consumption. Animals with inactivated DSOG1 neurons lack any feeding threshold, excessively overconsuming and failing to reject any substance. They are behaviorally taste-blind although their peripheral taste detection is intact. The activity of DSOG1 neurons is not influenced by taste detection or satiety state, arguing that the central nervous system has distinct mechanisms for establishing feeding thresholds that are independent from gustatory and metabolic state pathways. Our studies suggest that the drive to feed is under constant inhibition and this inhibition dampens activity of mutiple feeding subprograms to enable dynamic regulation (Figure 8I).
DSOG1 is necessary to establish a threshold for feeding
Inhibitory mechanisms controlling food intake have been described in both vertebrate and invertebrate systems (Carter et al., 2013; Hergarden et al., 2012; Jennings et al., 2013), yet there are several aspects that distinguish the mode of inhibition mediated by DSOG1 neurons from previous reports. In contrast to other regulators of feeding, DSOG1 activity is required to inhibit nutrient intake, water intake, uptake of noxious substances, and consumption in sated animals. Thus, DSOG1 does not act selectively in a homeostatic pathway that regulates nutrient intake or osmotic balance nor is it part of a taste pathway that processes gustatory cues. Instead, DSOG1 neurons establish a central threshold for feeding that is necessary for regulated consumption of all compounds, to enable rejection of aversive substances as well as rejection of appetitive substances in sated states.
The function of DSOG1 starkly contrasts to other systems that have been shown to negatively regulate feeding in Drosophila. Recurrent nerve lesions in Drosophila and blowflies specifically influence caloric intake and do not elicit indiscriminate consumption (Belzer, 1978; Dethier, 1976). Several neuropeptide systems like allatostatin, drosulfakinin, hugin, and leukokinin also negatively regulate feeding (Al-Anzi et al., 2010; Hergarden et al., 2012; Melcher and Pankratz, 2005; Söderberg et al., 2012). However, the effect of these feeding inhibitory systems is more nuanced and distinct from the robust hyperphagic phenotypes of DSOG1 inactivation. The majority of central inhibitory mechanisms appears to finetune the expression of a single or a subset of feeding programs. For example, leucokinin signaling selectively decreases meal duration (Al-Anzi et al., 2010) whereas hugin inhibits feeding on novel food sources (Melcher and Pankratz, 2005). Inhibiting allatostatin and drosulfakinin signaling increased consumption of sugar mixed with deterrents, but did not cause unregulated consumption (Hergarden et al., 2012; Söderberg et al., 2012). In contrast, DSOG1 appears to provide a universal baseline inhibition of feeding, preventing unselective hyperphagia and polydipsia.
Feeding inhibition by DSOG1 differs substantially from inhibitory mechanisms described in mammalian feeding circuits where suppression arises from acute post-ingestive signals, taste processing pathways or central mechanisms that sense energetic state and are specific for a particular homeostatic category like osmoregulation or caloric intake (Bourque, 2008; Morton et al., 2006; Murphy and Bloom, 2006; Sternson, 2013). Whether a similar neural mechanism for establishing central feeding thresholds exists in other animals, remains to be determined.
DSOG1 imparts an inhibitory tone on feeding
Our experimental data suggests that DSOG1 neurons impose an inhibitory tone within the feeding circuit. We found that DSOG1 activity is not regulated by taste detection, food and water deprivation, gut distention, or recurrent nerve activation. In addition, RNAi against neuropeptide receptor genes in DSOG1 neurons did not alter consumption, arguing that the phenotype of DSOG1 inactivation is not driven by a single peptidergic system. It is feasible that some internal or external cue that we did not test influences the activity of DSOG1 neurons. However, our studies show that inhibition of GABAergic signaling in DSOG1 elicited overconsumption, arguing that DSOG1 neurons are inhibitory interneurons necessary to maintain any feeding threshold.
We tested whether DSOG1 acts on putative feeding command neurons to inhibit feeding and found that the putative feeding command neuron is not required for the DSOG1 overconsumption phenotype. E49 motor neurons that drive proboscis extension and MN11 motor neurons that control ingestion are aberrantly activated by bitter sensory stimulation when DSOG1 neurons are silenced, arguing that DSOG1 decreases activity of multiple feeding subprograms. As DSOG1 does not directly contact these motor neurons, more of the feeding circuit will have to be elucidated to determine the site of DSOG1 action.
The function of DSOG1 enables plasticity within the feeding circuit
How is plasticity achieved in a feeding circuit that is tonically inhibited? Although DSOG1 neurons appear to impart constant inhibition on circuits mediating meal initiation and ingestion, these behaviors are clearly modulated by nutritional state and feeding history (Edgecomb et al., 1994). This implies that DSOG1 inhibition is overcome or bypassed in deprived states. The lack of feeding behaviors in sated states may reflect a general level of higher inhibition on feeding circuits stemming from systems detecting nutritional status and inhibitory feedback from the foregut (Hergarden et al., 2012; Söderberg et al., 2012). Importantly, none of these inhibitory systems is sufficient to suppress feeding behaviors in the absence of DSOG1 neurons. Conversely in deprived states, feeding behaviors may emerge by either a decrease in inhibitory signals or an increase in feeding signals (Hergarden et al., 2012; Lee et al., 2004). Together, our studies argue that DSOG1, bitter cues, satiety cues and the recurrent nerve act on a common pathway for feeding, with DSOG1 providing essential inhibitory control. Our studies show that restraint in feeding is established by four DSOG1 neurons that critically gate the expression of satiety and the evaluation of taste quality, thus establishing a central feeding threshold.
Experimental Procedures
Transgenic flies
The Gal4 collection used for the behavior screen was the InSite collection (Gohl et al., 2011). FDG-Gal4 is Gmr81e10 from the Janelia Farm Gal4 collection (Jenett et al., 2012). E49-Gal4 is VT201861 from the VDRC Gal4 collection. NP534 line was used to selectively label the MN11 motorneurons (Manzo and Scott, 2012).
Immunohistochemistry
Immunofluorescence on fly brains was performed as described (Marella et al., 2012). GABA immunostaining in Figure S4D was performed as reported (Crickmore and Vosshall, 2013), except PBS replaced PBST.
Behavior
Temporal and volumetric consumption assays
Females were collected at eclosion and aged 5-6 days. Fasted cohorts were kept in vials with wet kimwipes for 24 hours (food-deprived). Flies were kept in an empty vial for 24 hours for food- and water-deprivation experiments (water/food-deprived). Sated, non-deprived flies were taken directly from food vials (fed). Flies were mounted onto glass slides with nail polish, allowed to recover in a humidified chamber for two hours. Individual flies were presented with either a syringe or a capillary filled with water, 1M sucrose or 1mM denatonium and consumption for single flies was measured by ingestion time or volume (loss of fluid in the capillary). Measurements were terminated after flies did not initiate consumption to 10 consecutive taste exposures or regurgitated. For illustration only, 0.25 mg/ml FD&C #1 blue dye was included in solutions.
For silencing experiments, Gal4 lines were crossed to UAS-KIR2.1, tub-Gal80ts. Two- to three-day-old females were collected and incubated at 30°C for 48 hours to inactivate Gal80ts then mounted for consumption assays.
For neural activation experiments, Gal4 lines were crossed to UAS-dTRPA1; UAS-dTRPA1. Three to four-day-old flies were fasted or water-deprived for 24 hours and mounted on glass slides. Activity was induced by exposing flies to 120 seconds of elevated temperature (30°C) on a heat block after which consumption was monitored.
Proboscis extension response assays
Assays were carried out with tarsal stimulation as previously described (Marella et al., 2012), except that flies were not water satiated prior to the assay. Proboscis extension assays in Figure 8 were performed with proboscis stimulation.
Dry food consumption assay
Crystallized sucrose was used as the dry food stimulus, using a cotton swab dipped in a saturated sucrose solution and allowed to dry. Flies were prepared as described (Marella et al., 2012). The crystallized sucrose was exposed to the proboscis and feeding attempts were quantified by measuring the duration of pumping. Measurements were terminated if no pumping ensued after 10 consequtive proboscis stimulations.
Foraging locomotor assay
For the locomotor activity assay, we generated flies bearing tub>Gal80>; 98-Gal4/276-FLP; UAS-Kir2.1, tub-Gal80ts. Flies were reared at 22°C for controls whereas flies were reared at 30°C for DSOG1 inactivation for 48 hours prior to the assay. Individual 4-6 days-old female flies were lightly anesthetized by CO2 and introduced into polycarbonate tubes (5 mm (D) X 65 mm (L)). One end of the tubes was filled with 2% agar medium (no supplement for the starved groups, agar supplemented with 5% sucrose for the fed groups). Tubes were inserted in Drosophila activity monitors (DAM2) for the duration of experiments. Experiments were started before the end of a light-on period (Day 0) and lasted for 3 days (Day 1-3). Midline crossing activity was sampled for every minute and pooled into 30-minute bins for analysis. The average midline crossing activity was calculated for Day 1-2. Flies that showed no midline crossing activity were considered dead and removed from analysis. Starvation induced enhanced locomotion was measured by averaging the activity during the 24H starting from day 2 lights-on period (hours 36-60H).
Locomotor assay
Locomotor assay in Figure S5F was performed as described (Mann et al., 2013).
Mosaic Analysis of 98-Gal4
hs-FLP122/ tub>Gal80>; 98-Gal4/UAS-TNT; UAS-CD8:GFP flies were raised at 22 °C and heat-shocked 0-5 min at 37 °C during pupal stages. Eclosed flies were collected and aged for 4-6 days. Flies were separated based on consumption phenotype: those consuming 120 seconds of water and 20 seconds of 1mM denatonium on two separate measurement sessions were classified as ‘insatiable’, those consuming no denatonium and fewer than 5 seconds of water on two sessions were classified as ‘wt’. Animals exhibiting intermediate phenotypes were discarded. Mosaic approaches were used for single-cell labeling of DSOG1, using hs-FLP122, tub>Gal80>, 98-Gal4, UAS-DenMark, UAS-synaptotagmin-GFP flies to label axons and dendrites.
The expression of 98-Gal4 was restricted to DSOG1 using the transgenes 98-Gal4, 276B-FLP, tub>Gal80>, UAS-CD8:GFP or to DSOG2 cells using 934-FLP instead of 276B-FLP. Flies contained UAS-Kir2.1, tub-Gal80ts for neural inactivation experiments or UAS-dTRPA1 for activation experiments instead of UAS-CD8:GFP.
Electrophysiology
Extracellular recordings in live flies were performed as described (Marella et al., 2012). 3-5 day-old females were anesthetized using CO2. The antennae and surrounding cuticle were gently removed using fine forceps, exposing the SOG. The proboscis remained intact and exposed to the environment. The perineural sheath was removed on the lateral side of the SOG.
Electrodes (5-7MOhm) containing AHL were used to carry out extracellular recording in a loose patch configuration with resistances from 50-500 MOhm. DSOG1 was identified by GFP-labelled cell bodies. Spikes were recorded in voltage-clamp mode using a multiclamp 700B recorder at 20kHz and low-pass filtered at 5kHz. Recordings were then bandpass filtered between 100 and 3000Hz using a butterworth type filter. Spikes were identified by threshold detection, typically between 5-10pA, using a custom Python script.
For taste stimulation experiments, 1M sucrose, 1mM denatonium or water was delivered to proboscis. Prestimulus spike rates were calculated using 10 seconds of recording preceeding simulation. Stimulus spike rates were calculated using 1 second of recording post stimulation.
Steady state activity was measured in fed, food-deprived (24 hour wet starvation) and water-deprived (4 hour desiccation in a plastic chamber with Drierite absorbent (Fisher Scientific)) conditions. Tonic gut distention was induced in flies with blocked synaptic transmission in DSOG1 neurons (genotype 98-Gal4/UAS-CD8:GFP4; UAS-TNT) by pre-feeding flies with water for 60 seconds prior to recordings. Steady state activity was estimated from recordings averaging the spike rate across an interval of 30 – 200 seconds of activity.
Labelling neurons by photoactivation
For photoactivation experiments in Figure S2B, brains from tub>Gal80>; 98-Gal4/276-FLP; UAS-C3PA-GFP flies (2-3 days old) were were dissected in Ca2+ and Mg2+ free AHL. Photoactivation was carried out with 760nm laser light (at 11-12 mW of laser power measured at the back apperture of the objective) with 3 intervals of photoactivation of the cell body volume (2 min) and 10 minute diffusion intervals
To evaluate the projection patterns of DSOG1 neurons and feeding motor neurons in the SEZ in Figure S6, we generated flies that expressed photoactivatable GFP in DSOG1 cells and in either E49 motorneurons (98-Gal4/E49-Gal4; UAS-C3PA-GFP) or MN11 motorneurons (98-Gal4/UAS-C3PA-GFP; NP534/UAS-C3PA-GFP). Individual cell clones were were viewed as thick optical stacks at different z-depths.
RNAi screen
UAS-RNAi and UAS-shRNA lines from the TRiP and VDRC collections targeting transcripts encoding neuropeptide receptors and neurotransmitter metabolism related proteins were used to knock down gene expression in DSOG1 neurons. UAS-RNAi lines were crossed to 98-Gal4; UAS-dcr2 and UAS-shRNA lines were crossed to 98-Gal4. Water and 1mM denatonium consumption was monitored as detailed above.
2-photon lesions of the recurrent and medial abdominal nerves
Recurrent nerve (RN) or the medial abdominal nerve (AN) were visualised by pan-neuronal expression of GFP (nSyb-Gal4; UAS-CD8:GFP). Nerve lesions were carried out at 760 nm laser light (at 42-47 mW laser power) with a 3 minute lesioning protocol for RN and a 10 minute lesioning protocol for AN. RN was lesioned between the head and the thoracic segment. AN was lesioned beneath the abdominal cuticle immediately below the thoracic segment. Mock lesions in comparable volumes were made laterally from the targeted nerve. Flies were glued on slides and were allowed to recover for 3 hours in a humid chamber followed by consumption measurements. For DSOG1 activation experiments, UAS-dTRPA1 expression was targeted to DSOG1 neurons by 98-Gal4. DSOG1 activity was induced by temperature shifting flies to 30°C on a heat block for two minutes followed by consumption measurements.
Electrical stimulation of peripheral nerves
The nervous system with the intact proboscis, foregut and partial midgut was dissected in cold Ca2+ and Mg2+ free AHL then transferred to AHL. A peripheral nerve was inserted into a stimulating suction electrode and an electrical stimulus of 10V, 300 μs was delivered at 100 Hz for 100 ms. GCaMP5 responses were monitored in 98-Gal4 flies by spinning disk confocal microscopy.
GCaMP imaging
GCaMP imaging was performed as reported previously (Marella et al., 2006).
For motor neuron imaging, flies were allowed to recover one hour after mounting and imaging was performed on flies that retained behavioral responses to 1mM denatonium after dissection. The following genotypes were used: 98-Gal4/UAS-TNT; E49-Gal4/UAS-GCAMP6 and 98-Gal4/UAS-TNT; NP534-Gal4/UAS-GCaMP6 for experimental conditions and +/UAS-TNT; E49-Gal4/UAS-GCAMP6 and +/UAS-TNT; NP534-Gal4/UAS-GCaMP6 as controls. GCaMP6 responses were measured by spinning disk confocal microscopy.
GCaMP imaging of taste evoked responses in DSOG1 neurons in figure S4C was measured by spinning disk confocal microscopy. lexAop-dTRPA1 was expressed in Gr5a-LexA, Gr66a-LexA, ppk23-LexA or ppk28-LexA gustatory sensory neurons. A custom-made heat probe was used to deliver a short heat pulse at a 5s delay from the start of the imaging sequence, controlled from an Arduino Uno microcontroller board with a custom script.
Supplementary Material
Acknowledgments
The authors thank Daryl M. Gohl, Marion Silies and Tom Clandinin for generating the InSite collection. Dr. Barry Dickson generated and generously provided the ppk23-LexA line. David Harris buit the custom-made heat probe. Members of the Scott lab provided advice and comments on the manuscript. This work was supported by Boehringer-Ingelheim Fonds and Fulbright predoctoral fellowships to A.P. and a grant from the NIH (NIDCD) and a John Merck Award to K.S. K.S. is an HHMI Early Career Scientist.
Footnotes
Author Contributions: A.P. performed the majority of experiments and co-wrote the manuscript. P.K. and K.M. performed electrophysiological studies. S.C. carried out the behavioral screen that isolated 98-Gal4. M.G. performed the intial behavioral screen that identified flies that overconsumed. L.W. performed locomotor assays. K.S. supervised the project and co-wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Anzi B, Armand E, Nagamei P, Olszewski M, Sapin V, Waters C, Zinn K, Wyman RJ, Benzer S. The Leucokinin Pathway and Its Neurons Regulate Meal Size in Drosophila. Curr Biol. 2010;20:969–978. doi: 10.1016/j.cub.2010.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alekseyenko OV, Lee C, Kravitz EA. Targeted manipulation of serotonergic neurotransmission affects the escalation of aggression in adult male Drosophila melanogaster. PLoS One. 2010;5:e10806. doi: 10.1371/journal.pone.0010806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines RA, Uhler JP, Thompson A, Sweeney ST, Bate M. Altered electrical properties in Drosophila neurons developing without synaptic transmission. J Neurosci. 2001;21:1523–1531. doi: 10.1523/JNEUROSCI.21-05-01523.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KD, Thummel CS. Diabetic larvae and obese flies-emerging studies of metabolism in Drosophila. Cell Metab. 2007;6:257–266. doi: 10.1016/j.cmet.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belzer WR. Recurrent nerve inhibition of protein feeding in the blowfly Phormia regina. Physiol Entomol. 1978;3:259–263. [Google Scholar]
- Bharucha KN, Tarr P, Zipursky SL. A glucagon-like endocrine pathway in Drosophila modulates both lipid and carbohydrate homeostasis. J Exp Biol. 2008;211:3103–3110. doi: 10.1242/jeb.016451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohm RA, Welch WP, Goodnight LK, Cox LW, Henry LG, Gunter TC, Bao H, Zhang B. A genetic mosaic approach for neural circuit mapping in Drosophila. Proc Natl Acad Sci U S A. 2010;107:16378–16383. doi: 10.1073/pnas.1004669107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque CW. Central mechanisms of osmosensation and systemic osmoregulation. Nat Rev Neurosci. 2008;9:519–531. doi: 10.1038/nrn2400. [DOI] [PubMed] [Google Scholar]
- Carter ME, Soden ME, Zweifel LS, Palmiter RD. Genetic Identification of a Circuit that Suppresses Appetite. Nature. 2013;503:111–116. doi: 10.1038/nature12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SH, Carney GE, McClung CA, Willard SS, Taylor BJ, Hirsh J. Two functional but noncomplementing Drosophila tyrosine decarboxylase genes: distinct roles for neural tyramine and octopamine in female fertility. J Biol Chem. 2005;280:14948–14955. doi: 10.1074/jbc.M414197200. [DOI] [PubMed] [Google Scholar]
- Colombani J, Raisin S, Pantalacci S, Radimerski T, Montagne J, Leopold P. A nutrient sensor mechanism controls Drosophila growth. Cell. 2003;114:739–749. doi: 10.1016/s0092-8674(03)00713-x. [DOI] [PubMed] [Google Scholar]
- Crickmore MA, Vosshall LB. Opposing dopaminergic and gabaergic neurons control the duration and persistence of copulation in Drosophila. Cell. 2013;155:881–893. doi: 10.1016/j.cell.2013.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dethier VG. The Hungry Fly. Cambridge MA: Harvard University Press; 1976. [Google Scholar]
- Dethier VG, Gelperin A. Hyperphagia in the Blowfly. J Exp Biol. 1967;47:191–200. [Google Scholar]
- Flood TF, Iguchi S, Gorczyca M, White B, Ito K, Yoshihara M. A single pair of interneurons commands the Drosophila feeding motor program. Nature. 2013;499:83–87. doi: 10.1038/nature12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friggi-Grelin F, Coulom H, Meller M, Gomez D, Hirsh J, Birman S. Targeted gene expression in Drosophila dopaminergic cells using regulatory sequences from tyrosine hydroxylase. J Neurobiol. 2003;54:618–627. doi: 10.1002/neu.10185. [DOI] [PubMed] [Google Scholar]
- Gohl DM, Silies MA, Gao XJ, Bhalerao S, Luongo FJ, Lin CC, Potter CJ, Clandinin TR. A versatile in vivo system for directed dissection of gene expression patterns. Nat Methods. 2011;8:231–237. doi: 10.1038/nmeth.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon MD, Scott K. Motor Control in a Drosophila Taste Circuit. Neuron. 2009;61:373–384. doi: 10.1016/j.neuron.2008.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada FN, Rosenzweig M, Kang K, Pulver SR, Ghezzi A, Jegla TJ, Garrity PA. An internal thermal sensor controlling temperature preference in Drosophila. Nature. 2008;454:217–220. doi: 10.1038/nature07001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hergarden AC, Tayler TD, Anderson DJ. Allatostatin-A neurons inhibit feeding behavior in adult Drosophila. Proc Natl Acad Sci. 2012;109:3967–3972. doi: 10.1073/pnas.1200778109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki HK, Jagadish S, Barnea G, Ishimoto H, Ben-Tabou de-Leon S, Wong AM, Kitamoto T, Axel R, Anderson DJ. Visualizing Neuromodulation In Vivo: TANGO-Mapping of Dopamine Signaling Reveals Appetite Control of Sugar Sensing. Cell. 2012;148:1065. doi: 10.1016/j.cell.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Masuda N, Shinomiya K, Endo K, Ito K. Systematic analysis of neural projections reveals clonal composition of the Drosophila. brain Curr Biol. 2013;23:644–655. doi: 10.1016/j.cub.2013.03.015. [DOI] [PubMed] [Google Scholar]
- Jenett A, Rubin GM, Ngo TT, Shepherd D, Murphy C, Dionne H, Pfeiffer BD, Cavallaro A, Hall D, Jeter J, et al. A GAL4-driver line resource for Drosophila neurobiology. Cell Reports. 2012;2:991–1001. doi: 10.1016/j.celrep.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings JH, Rizzi G, Stamatakis AM, Ung RL, Stuber GD. The inhibitory circuit architecture of the lateral hypothalamus orchestrates feeding. Science. 2013;341:1517–1521. doi: 10.1126/science.1241812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamoto T. Conditional modification of behavior in Drosophila by targeted expression of a temperature-sensitive shibire allele in defined neurons. J Neurobiol. 2001;47:81–92. doi: 10.1002/neu.1018. [DOI] [PubMed] [Google Scholar]
- Lee G, Park JH. Hemolymph sugar homeostasis and starvation-induced hyperactivity affected by genetic manipulations of the adipokinetic hormone-encoding gene in Drosophila melanogaster. Genetics. 2004;167:311–323. doi: 10.1534/genetics.167.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KS, You KH, Choo JK, Han YM, Yu K. Drosophila short neuropeptide F regulates food intake and body size. J Biol Chem. 2004;279:50781–50789. doi: 10.1074/jbc.M407842200. [DOI] [PubMed] [Google Scholar]
- Leopold P, Perrimon N. Drosophila and the genetics of the internal milieu. Nature. 2007;450:186–188. doi: 10.1038/nature06286. [DOI] [PubMed] [Google Scholar]
- Li H, Chaney S, Roberts IJ, Forte M, Hirsh J. Ectopic G-protein expression in dopamine and serotonin neurons blocks cocaine sensitization in Drosophila melanogaster. Curr Biol. 2000;10:211–214. doi: 10.1016/s0960-9822(00)00340-7. [DOI] [PubMed] [Google Scholar]
- Mann K, Gordon MD, Scott K. A Pair of Interneurons Influences the Choice between Feeding and Locomotion in Drosophila. Neuron. 2013;79:754–765. doi: 10.1016/j.neuron.2013.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzo A, Scott K. Motor neurons controlling fluid ingestion in Drosophila. PNAS. 2012 doi: 10.1073/pnas.1120305109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marella S, Fischler W, Kong P, Asgarian S, Rueckert E, Scott K. Imaging taste responses in the fly brain reveals a functional map of taste category and behavior. Neuron. 2006;49:285–295. doi: 10.1016/j.neuron.2005.11.037. [DOI] [PubMed] [Google Scholar]
- Marella S, Mann K, Scott K. Dopaminergic Modulation of Sucrose Acceptance Behavior in Drosophila. Neuron. 2012;73:941–950. doi: 10.1016/j.neuron.2011.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire SE, Mao Z, Davis RL. Spatiotemporal gene expression targeting with the TARGET and gene-switch systems in Drosophila. Sci STKE. 2004;2004:16. doi: 10.1126/stke.2202004pl6. [DOI] [PubMed] [Google Scholar]
- Melcher C, Pankratz MJ. Candidate gustatory interneurons modulating feeding behavior in the Drosophila brain. PLoS Biol. 2005;3:e305. doi: 10.1371/journal.pbio.0030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto T, Slone J, Song X, Amrein H. A fructose receptor functions as a nutrient sensor in the Drosophila brain. Cell. 2012;151:1113–1125. doi: 10.1016/j.cell.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- Murphy KG, Bloom SR. Gut hormones and the regulation of energy homeostasis. Nature. 2006;444:854–859. doi: 10.1038/nature05484. [DOI] [PubMed] [Google Scholar]
- Nassel DR, Homberg U. Neuropeptides in interneurons of the insect brain. Cell Tissue Res. 2006;326:1–24. doi: 10.1007/s00441-006-0210-8. [DOI] [PubMed] [Google Scholar]
- Nassel DR, Enell LE, Santos JG, Wegener C, Johard HA. A large population of diverse neurons in the Drosophila central nervous system expresses short neuropeptide F, suggesting multiple distributed peptide functions. BMC Neurosci. 2008;9:90. doi: 10.1186/1471-2202-9-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolai LJ, Ramaekers A, Raemaekers T, Drozdzecki A, Mauss AS, Yan J, Landgraf M, Annaert W, Hassan BA. Genetically encoded dendritic marker sheds light on neuronal connectivity in Drosophila. Proc Natl Acad Sci U S A. 2010;107:20553–20558. doi: 10.1073/pnas.1010198107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen SR, Wilson RI. Cracking neural circuits in a tiny brain: new approaches for understanding the neural circuitry of Drosophila. Trends Neurosci. 2008;31:512–520. doi: 10.1016/j.tins.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajashekhar KP, Singh RN. Organization of Motor Neurons Innervating the Proboscis Musculature in Drosophila melanogaster Meigen (Diptera: Drosophilidae) Int J Insect Morphol Embryol. 1994;23:225–242. [Google Scholar]
- Rulifson EJ, Kim SK, Nusse R. Ablation of insulin-producing neurons in flies: growth and diabetic phenotypes. Science (80-. ) 2002;296:1118–1120. doi: 10.1126/science.1070058. [DOI] [PubMed] [Google Scholar]
- Ruta V, Datta SR, Vasconcelos ML, Freeland J, Looger LL, Axel R. A dimorphic pheromone circuit in Drosophila from sensory input to descending output. Nature. 2010;468:686–690. doi: 10.1038/nature09554. [DOI] [PubMed] [Google Scholar]
- Salvaterra PM, Kitamoto T. Drosophila cholinergic neurons and processes visualized with Gal4/UAS-GFP. Brain Res Gene Expr Patterns. 2001;1:73–82. doi: 10.1016/s1567-133x(01)00011-4. [DOI] [PubMed] [Google Scholar]
- Söderberg JaE, Carlsson Ma, Nässel DR. Insulin-Producing Cells in the Drosophila brain also express satiety-inducing cholecystokinin-like peptide, drosulfakinin. Front Endocrinol (Lausanne) 2012;3:109. doi: 10.3389/fendo.2012.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternson SM. Hypothalamic survival circuits: blueprints for purposive behaviors. Neuron. 2013;77:810–824. doi: 10.1016/j.neuron.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker RF. The organization of the chemosensory system in Drosophila melanogaster: a review. Cell Tissue Res. 1994;275:3–26. doi: 10.1007/BF00305372. [DOI] [PubMed] [Google Scholar]
- Sweeney ST, Broadie K, Keane J, Niemann H, O'Kane CJ. Targeted expression of tetanus toxin light chain in Drosophila specifically eliminates synaptic transmission and causes behavioral defects. Neuron. 1995;14:341–351. doi: 10.1016/0896-6273(95)90290-2. [DOI] [PubMed] [Google Scholar]
- Thorne N, Chromey C, Bray S, Amrein H. Taste perception and coding in Drosophila. Curr Biol. 2004;14:1065–1079. doi: 10.1016/j.cub.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Tian L, Hires SA, Mao T, Huber D, Chiappe ME, Chalasani SH, Petreanu L, Akerboom J, McKinney SA, Schreiter ER, et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat Methods. 2009;6:875–881. doi: 10.1038/nmeth.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissot M, Gendre N, Stocker RF. Drosophila P[Gal4] lines reveal that motor neurons involved in feeding persist through metamorphosis. J Neurobiol. 1998;37:237–250. [PubMed] [Google Scholar]
- Venken KJT, Simpson JH, Bellen HJ. Genetic manipulation of genes and cells in the nervous system of the fruit fly. Neuron. 2011;72:202–230. doi: 10.1016/j.neuron.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Singhvi A, Kong P, Scott K. Taste representations in the Drosophila brain. Cell. 2004;117:981–991. doi: 10.1016/j.cell.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Wen T, Parrish CA, Xu D, Wu Q, Shen P. Drosophila neuropeptide F and its receptor, NPFR1, define a signaling pathway that acutely modulates alcohol sensitivity. Proc Natl Acad Sci U S A. 2005;102:2141–2146. doi: 10.1073/pnas.0406814102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Wen T, Lee G, Park JH, Cai HN, Shen P. Developmental control of foraging and social behavior by the Drosophila neuropeptide Y-like system. Neuron. 2003;39:147–161. doi: 10.1016/s0896-6273(03)00396-9. [DOI] [PubMed] [Google Scholar]
- Zhang YQ, Rodesch CK, Broadie K. Living synaptic vesicle marker: Synaptotagmin-GFP. Genesis. 2002:142–145. doi: 10.1002/gene.10144. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.