Abstract
Problem
Chemerin is a novel chemo-attractant and adipokine involved in leukocyte recruitment, inflammation, adipogenesis, lipid/carbohydrate metabolism, and reproduction. Based on the bioinformatic search for putative small peptides in the conserved region of pre-pro-chemerin, an evolutionary conserved region flanked by potential convertase cleavage sites was identified and we named it as C-20. The binding capacity of C-20 to chemerin receptors and its potential bioactivities were investigated in this study.
Method of study
Radioligand binding assay, receptor internalization assay, and early response gene C-FOS simulation, cAMP assay were carried out in chemokine-like receptor 1 (CMKLR1)/HEK293 transfectants and G protein-coupled receptor 1 (GPR1)/HEK293 transfectants. In vitro transwell chemotaxis assay in CMKLR1/L1.2 transfectants, primary Leydig cell culture, and antral follicle culture was explored to investigate the bioactivity of C-20.
Results
C-20 bound to chemerin receptors CMKLR1 and GPR1 with high affinity triggered CMKLR1 internalization and stimulated subsequent signal C-FOS expression and cAMP production. C-20, such as chemerin, showed CMKLR1-dependent chemotactic property. Furthermore, in primary Leydig cells and antral follicles, C-20 showed similar but less potent suppressive effect on human chorionic gonadotropin-stimulated testosterone production and progesterone production, compared with chemerin.
Conclusion
The novel chemerin-derived C-20 peptide binds to chemerin receptors CMKLR1 and GPR1 and showed similar but less potent bioactivity in chemotaxis and the suppression of gonadal steroidogenesis, suggesting that after optimization, C-20 is possible to be a useful experimental tool for the understanding of the biological functions of chemerin/CMKLR1 and chemerin/GPR1 signaling.
Keywords: Adipokine, chemerin, chemotaxis, progesterone, testosterone
Introduction
Chemerin, was initially identified as a chemo-attractant ligand for the G protein-coupled receptor chemokine-like receptor 1 (CMKLR1, also known as ChemR23 or DEZ).1-3 It was subsequently identified as a novel adipokine that regulates adipogenesis and adipocyte metabolism, with high expression levels in white adipose tissue.4 Chemerin was well studied for its proposed roles in adaptive and innate immunity, inflammation, lipid and carbohydrate metabolism, and its association with obesity and diabetes.5 Recent reports point to a role for chemerin in reproduction as well. Patients with polycystic ovary syndrome have elevated circulating and adipose tissue chemerin levels,6 preeclampsia patients have elevated circulating chemerin levels7,8, and fetuses of obese mothers have increased chemerin concentrations, correlating with maternal insulin sensitivity.9 Expression of chemerin and its receptor CMKLR1 was reported in human granulose cell, and chemerin treatment inhibited IGF-1-induced progesterone and estradiol secretion.10 A similar suppressive effect of chemerin on FSH-stimulated progesterone and estradiol secretion was reported in rat granulose cells.11
Three receptors have so far been described for chemerin: CMKLR1,1-3 chemokine (C-C motif) receptor-like 2 (CCRL2)12, and G protein-coupled receptor 1 (GPR1).13 CMKLR1 is presently the receptor through which most biological activities of chemerin have been described including leukocyte recruitment, inflammation, adipogenesis, lipid and carbohydrate metabolism.5 Chemerin binding to CMKLR1 triggers receptor internalization and promotes leukocyte chemotaxis; chemerin binding to GPR1 stimulates receptor internalization with no reported physiological roles5; chemerin binding to CCRL2 stimulates neither internalization nor chemotaxis, but might present chemerin to nearby cell displaying functional receptors.14 The activation of chemerin/CMKLR1 resulted in intracellular calcium release, inhibition of cAMP accumulation, and phosphorylation of MAPK ERK1/2 or the phosphatidylinositol 3-kinase/Akt pathway.15
Prochemerin gene expression is detected in most tissues with high levels in liver, white adipose tissue, and placenta and, to a less extent to skin, adrenal glands and kidney.2 Prochemerin is present at relatively high concentrations (3–10 nm) in human plasma16,17, while liver and white adipose tissue was proposed to be a source of chemerin.4 The TIG-2 gene encodes 163 amino acid pre-pro-chemerin. After removal of the 20 amino acid signal peptide from the pre-pro-chemerin, prochemerin was secreted as an inactive precursor, which is 143 amino acids long in human. C-terminal proteolytic processing turned out to be very important for the bioactivity of chemerin as prochemerin has very low affinity for CMKLR1. Prochemerin needs to be processed by proteolytic enzymes extracellularly within its C-terminal domain to acquire its bioactivity to become a potent and highly specific agonist of CMKLR1.2 The active protein of 137 amino acids in human was named chemerin. Mouse chemerin shared 65% identity with the human polypeptide.1-3
A computer program previously used to identify the novel hormones obestatin18 and neuronostatin19 was explored to search for unique protein signatures including potential mono- or dibasic cleavage sites in known pre-pro-hormone sequences. Pre-pro-chemerin came to our notice for the possible existence of conserved putative mature peptide region at its C-terminus. After alignment of pre-pro-chemerin amino acid sequences from 9 species, a 20 amino acid peptide was identified and we named it chemerin-derived-peptide C-20 (C-20 for short). We synthesized the peptide and studied its possible interaction with chemerin receptors and subsequent bioactivities.
Materials and methods
Animals and Materials
Three-month-old male and female Sprague-Dawley rats and 21-day-old female Sprague-Dawley rats were obtained from the Laboratory Animal Center, Institutes of Biomedicine and Health, Chinese Academy of Sciences, China. The animals were housed at a constant temperature, humidity, under a 12-hr light–dark cycle. Chow and water were available ad libitum. All procedures related to animal usage were approved by the Committee on the Use of Live Animals for Teaching and Research, Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences. Human CMKLR1 overexpressing HEK293 cell line (huCMKLR1/HEK293), human GPR1 overexpressing HEK293 cell line (huGPR1/HEK293), wild-type HEK293 cell line (WT/HEK293), human CMKLR1 overexpressing L1.2 cell line (huCMKLR1/L1.2), wild-type L1.2 cell line (WT/L1.2), and rat anti-human CMKLR1 monoclonal antibody (BZ332) are kind gifts from Dr Brian Zabel and Dr Eugene Butcher (Stanford University, USA). Human recombinant chemerin (huChemerin) and mouse recombinant chemerin (mChemerin) were purchased from R&D Systems (Minneapolis, MN, USA). Human C-20 (huC-20, VQRAGEDPHSFYFPGQFAFS), human C-15 (huC-15, AGEDPHSFYFPGQFA), human C-9 (huC-9, YFPGQFAFS), mouse C-20 (mC-20, IAQAGED PHGYFLPGQFAFS), and a random synthetic peptide neuronostatin (NTS, LRQFLQKSLAAAT) were synthesized by GL Biochem (Shanghai, China). Iodochemerin was purchased from Fu-Rui Ltd. (Beijing, China). Rabbit anti-C-FOS antibody was purchased from Cell Signaling (Boston, MA, USA). Alexa Fluor® 488 Donkey Anti-Rabbit IgG (H+L) and Alexa-488-Donkey anti-rat IgG were purchased from Invitrogen (New York, NY, USA). Parameter cyclic adenosine monophosphate (cAMP) assay was purchased from R&D Systems. Goat polyclonal antibody for CMKLR1 IHC and goat polyclonal antibody for GPR1 IHC in rat testis/ovary were purchased from Santa Cruz (Dallas, TX, USA). The peroxidase-labeling kit and DAB substrate kit for peroxidase were both purchased from Vector Laboratories (Burlingame, CA, USA).
Search for Possible Conserved Peptide(s) Encoded by Chemerin Gene
GenBank was searched for orthologs of the human chemerin gene, and pre-pro-chemerin sequences from 9 mammalian species (human: NP_002880.1; chimpanzee: JAA28993.1; monkey: NP_001253612.1; rabbit: XP_002722563.1; pig: NP_001116658.1; cattle: NP_001039485.1; rat: NP_001013445.1; hamster: NP_ 001231216.1; mouse: NP_082128.1) were aligned to locate possible evolutionary conserved region(s).
Radioligand Binding Assay
Radioligand binding assays were performed as described previously.18 Briefly, about 1,000,000 per tube huCMKLR1/HEK293 cells or huGPR1/HEK293 cells were used to incubate with 2 nm iodochemerin with or without increasing concentrations of unlabeled huChemerin, huC-20, mC-20, or NTS for 16 h at 23°C. After incubation, cells were centrifuged at 1000 × g for 10 min and washed for three times before counting with a γ-spectrophotometer.
Internalization Assay
HuCMKLR1/HEK293 cells, huGPR1/HEK293 cells, or WT/HEK293 cells were seeded into culture dish with 0.1% w/v gelatin-coated cover slips. Cells were subjected to serum starvation at 50–60% confluent for 4 h. Then, cells were incubated with 100 nm huChemerin, huC-20, or NTS for 30 min at 37°C. Washed twice with PBS, cells attached to the cover slip were fixed with 4% w/v paraformaldehyde for 30 min and then incubated with 0.1% w/v Triton X-100 for 10 min. The cells were washed with PBS and incubated with rat anti-human CMKLR1 monoclonal antibody overnight at 4°C. After incubation for 2 h with secondary antibody, Alexa-488-Donkey anti-rat IgG (Invitrogen) and nuclei staining for 10 s with DAPI, the cover slip were mounted with 100% glycerol and observed under a confocal laser microscope.
Early Response Gene C-FOS Gene Expression
At 70–80% confluence, huCMKLR1/HEK293 cells or WT/HEK293 cells were subjected to serum starvation for 24 h. Then, the cells were incubated with culture medium, or culture medium with 30 nm huChemerin, 30 nm huC-20, or 10% v/v FBS for 30 min at 37°C. Cells were harvested for RNA analysis of C-FOS expression with beta-actin served as the reference gene.
Immunocytochemistry (ICC) of Early Response Gene C-FOS
HuCMKLR1/HEK293 cells or WT/HEK293 cells were seeded into culture dish with 0.1% w/v gelatin-coated cover slips. Cells were subjected to serum starvation at 50–60% confluent for 4 h. Then, cells were incubated with 100 nm huChemerin or huC-20 for 30 min at 37°C. Washed twice with PBS, cells attached to the cover slip were fixed with 4% w/v paraformaldehyde for 30 min and then incubated with 0.1% w/v Triton X-100 for 10 min. The cells were washed with PBS and incubated with rabbit anti-C-FOS monoclonal antibody overnight at 4°C. After incubation for 1 h with secondary antibody, Alexa Fluor® 488 Donkey Anti-Rabbit IgG, and nuclei staining for 10 s with DAPI, the cover slip was mounted with 100% glycerol and observed under a confocal laser microscope.
Cyclic Adenosine Monophosphate (cAMP) Assay
HuCMKLR1/HEK293 cells or WT/HEK293 cells were seeded into NUNC 6-well culture dish at 5 × 105 cells per well and cultured for 24 h. Cells were then subjected to serum starvation for 4 h. Then, cells were incubated with 10 nm huChemerin, 10 nm huC-20, 10 μM forskolin (Sigma, St Louis, MO, USA), or combination of forskolin and huC-20, or forskolin and huChemerin or culture medium only for 30 min at 37°C. Washed twice with cold PBS, cells were subjected to the lysis buffer from the Parameter cAMP assay kit of R&D System and the supernatant was assayed for cAMP and total protein normalization using BCA protein assay kit from Pierce (Thermo Scientific, Rockford, IL, USA). The conditioned medium was directly subjected to cAMP assay.
In Vitro Transwell Chemotaxis Assay
HuCMKLR1/L1.2 cells were treated by 5 mm sodium butyrate overnight and then seeded to the top well of 5 μm pore 96-well Corning Caster transwell inserts (Corning Caster, Corning, NY, USA) at 1 × 106 cell/well density. Culture medium, or culture medium with 0.3, 1, 10, and 100 nm huChemerin or 0.3, 1, 10, and 100 nm huC-20, was added to the bottom well of the inserts. At the end of 4 h migration assay at 37°C, the cells that migrated from the top well through the filter to the lower chamber were stained with CellTiter blue (Promega, Madison, WI, USA) and quantified by fluorescence (560/580 nm). Paralleled experiments of the same procedure were carried out with WT/L1.2 cells to serve as negative controls.
RNA Analysis by QPCR
Total RNA was extracted by TRIZOL Reagent (Invitrogen) according to the manufacturer’s instruction. RNA samples were reverse-transcribed into cDNA and subsequent relative transcript abundances were estimated by quantitative real-time PCR using a SYBR RT-PCR kit (Takara, Shiga, Japan) according to the manufacturer’s introduction. The relative gene expression levels normalized to beta-actin were calculated using the ΔΔCT method, where CT was the cycle threshold. PCR primers and relative references are listed in Table S1.
Immunocytochemistry (IHC) of Chemerin Receptors CMKLR1 and GPR1
Testes or ovaries were dissected out right after decapitation of 3-month-old Sprague-Dawley rats, fixed, and processed for embedding in paraffin, sectioned. Immunohistochemistry of testis was performed on 5 μm sections of paraffin-embedded tissues with a peroxidase-labeling kit from Vector Laboratories, using either goat polyclonal antibody to CMKLR1 (sc-32651 from Santa Cruz) or goat polyclonal antibody to GPR1 (sc-48179 from Santa Cruz). Staining was visualized using a DAB substrate kit for peroxidase from Vector Laboratories and counterstained with hematoxylin.
Primary Leydig Cell Culture
Isolation of Leydig cells and subsequent primary Leydig cell culture were performed as previously described20 with some modifications. Briefly, five Sprague-Dawley male rats at 3~4 months old were killed by decapitation. The testes were collected and decapsulated and were then digested for 15 min shaking at 80 cycles/min at 34°C in DMEM/F12-0.1% w/v BSA supplemented with 0.5 mg/mL collagenase type IA, 0.25 mg/mL soybean trypsin inhibitor (all from Sigma). Ice-cold medium was added to the flask to stop the digestion. The suspension was kept on ice for 5 min to allow tubules to settle and was then filtered through cell strainers (70 μm nylon, Falcon BD Biosciences, Franklin Lakes, NJ, USA). After centrifuging at 350 × g for 20 min at 4°C, the Leydig cells were resuspended and separated by discontinuous Percoll (Amersham Biosciences, Uppsala, Sweden) gradients (with six density fractions ranging from 1.030 to 1.096 g/mL). Leydig cells located at the boundary were collected and maintained at 34°C with 5% v/v CO2. After 24 h incubation, the cell purity was determined by 3β-hydroxysteroid dehydrogenase (3β-HSD) staining. The Leydig cells were treated with 0.01 IU/mL of human chorionic gonadotropin (hCG), 1, 10, and 100 nm of mC-20 or mChemerin, and the combination of hCG and mC-20 or the combination of hCG and mChemerin or culture medium only. After 24 h, conditioned media were collected for testosterone measurement. Cells were lysed for RNA expression analysis of key steroidogenetic enzymes 3β-HSD, 17β-HSD, p450scc, and StAR gene expression.
Superovulation and Antral Follicle Culture
Superovulation was induced in 21–23 day-old immature female Sprague-Dawley rats by intraperitoneal (i.p.) injection with 30 IU per rat pregnant mare serum gonadotropin (PMSG; Sigma). Large antral follicles of a diameter over 900 μm were isolated 48 h post-PMSG treatment as previously described.21 After decapitation, follicles were isolated in ice-cold normal saline under a dissecting microscope using two 26G1/2 inch syringe needles and pre-incubated in 24-well plate with 3 follicles per well in DMEM/F12-0.1% BSA at 37°C. Then, the follicles were treated for 6 h with 0.01 IU/mL of hCG, 1, 10, and 100 nm of mC-20 and mChemerin and the combination of hCG and mC-20 or the combination of hCG and mChemerin or culture media only. Conditioned media were collected for progesterone measurement. Cells were lysed for QPCR analysis.
Testosterone and Progesterone Measurement by RIA
The testosterone level and progesterone levels were measured using commercial Iodine [125I] Radio-immunoassay Kits (Lareneen, Guangzhou, China). The sensitivities of the testosterone and progesterone RIA assay were 20 ng/mL and 0.02 ng/mL. The intra-assay error and interassay error for both assays were less than 10 and 15%.
Statistical Analysis
All data were expressed as mean ±S.E.M., and statistical significance was assessed by one-way anova followed by Student–Newman–Keuls test. Statistical significance was taken at the P < 0.05 level.
Results
Bioinformatic Prediction of Conserved Chemerin-Derived Peptide C-20
We compared the pre-pro-chemerin sequences from 9 mammalian species. Based on bioinformatic search for putative small peptides in the conserved region of pre-pro-chemerin, an evolutionary conserved region flanked by potential convertase cleavage sites (arginine and/or lysine) was identified (Fig. 1). This encodes a 20 amino acids peptide at the C-terminal domain of pre-pro-chemerin. We named this chemerin-derivedpeptide C-20 (C-20). Human C-20 (huC-20) encodes VQRAGEDPHSFYFPGQFAFS (V138-S157). Mouse C-20 (mC-20) encodes IAQAGEDPHGYFLPGQFAFS (I137-S156).
Fig. 1.
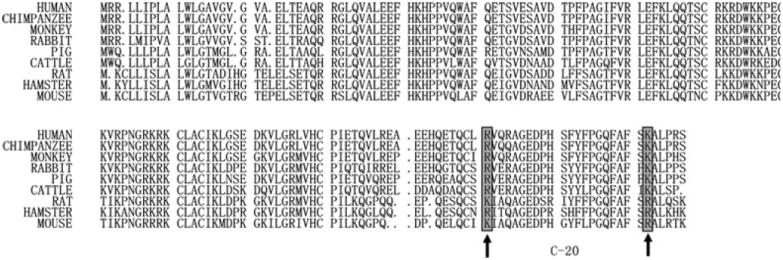
Bioinformatic prediction of conserved chemerin-derived peptide C-20. Amino acid sequences of pre-pro-chemerin from different vertebrates are aligned to search for evolutionary conserved putative peptide regions. Consensus basic residues representing putative convertase cleavage sites are shadowed and indicated by arrows, flanking the predicted chemerin-derived-peptide C-20. GenBank numbers for individual chemerin genes are human: NP_002880.1; chimpanzee: JAA28993.1; monkey: NP_001253612.1; rabbit: XP_002722563.1; pig: NP_001116658.1; cattle: NP_001039485.1; rat: NP_001013445.1; hamster: NP_001231216.1; mouse: NP_082128.1.
C-20 Bound to CMKLR1 and GPR1 and Triggered CMKLR1 Internalization
We synthesized huC-20 and mC-20 and tested their ability to bind to CMKLR1 and GPR1. Radioligand binding assay showed that both huC-20 and mC-20 can bind to HEK293 cells overexpressing either human CMKLR1 or GRP1, using huChemerin as positive control and NTS as negative control (Fig. 2a). The IC50 for huChemerin, huC-20, and mC-20 to huCMKLR1/HEK293 cells were 0.3, 1.6, and 2.5 nm, respectively; the IC50 for huChemerin, huC-20, and mC-20 to huGPR1/HEK293 cells were 0.5, 3.5, and 5.0 nm, respectively. C-20 from human and mouse showed conserved and comparative binding capacity to human CMKLR1 and GPR1 receptors.
Fig. 2.
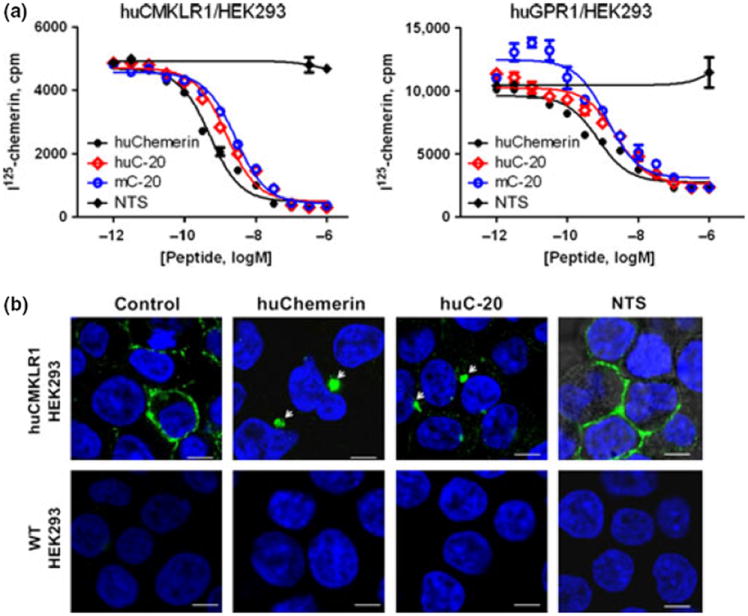
C-20 bound to CMKLR1 and GPR1 and triggered CMKLR1 internalization. (a) Radioligand binding assay showed that both huC-20 and mC-20 can bind to CMKLR1 and GPR1 expressed in HEK293 cells. The IC50 for huChemerin, huC-20, and mC-20 to huCMKLR1/HEK293 cells were 0.3, 1.6, and 2.5 nm, respectively; the IC50 for huChemerin, huC-20, and mC-20 to huGPR1/HEK293 cells were 0.5, 3.5, and 5.0 nm, respectively. Neuronostatin (NTS) was used as negative control. (b) HuC-20 triggered the internalization of huCMKLR1/HEK293 cells using huChemerin as positive control and NTS as negative control. No internalization observed in either huChemerin or huC-20 treated WT/HEK293 cells. Scale bars, 0.75 μm; representative images from four independent experiments.
Also, we tested whether C-20 could stimulate the CMKLR1 and GPR1 internalization. When huCMKLR1/HEK293 cells were stimulated by huC-20, internalization happened, using huChemerin as positive control and NTS as negative control; no such internalization happened in WT/HEK293 cells (Fig. 2b). HuC-20 did not stimulate GPR1 internalization in huGPR1/HEK293 cells (data not shown).
C-20 Stimulated Early Response Gene C-FOS and Suppressed cAMP Production CMKLR1 Dependently
Immunocytochemistry revealed an increase in nuclear C-FOS-positive-staining cells in the huC-20 and huChemerin treatment groups compared with control in huCMKLR1/HEK293 cells, using FBS as positive control (Fig. 3a). A similar stimulation in C-FOS gene expression was found by huC-20 and huChemerin (Fig. 3b). No such stimulation in C-FOS protein expression (Fig. 3a) or gene expression (data not shown) was observed in WT/HEK293 cells by either huC-20 or huChemerin treatment, suggesting that the stimulation of huC-20 and huChemerin on C-FOS was CMKLR1 dependent. In cAMP assay, forskolin at 10 μm stimulated more than 50-fold increase in cAMP production in both huCMKLR1/HEK293 cells and WT/HEK293 cells. HuC-20 and huChemerin cotreatment suppressed cAMP production in huCMKLR1/HEK293 cells, while no effect was observed in WT/HEK293 cells, suggesting that the suppression was CMKLR1 dependent. HuC-20 and huChemerin showed no effect on basal cAMP release (Fig. 3c).
Fig. 3.
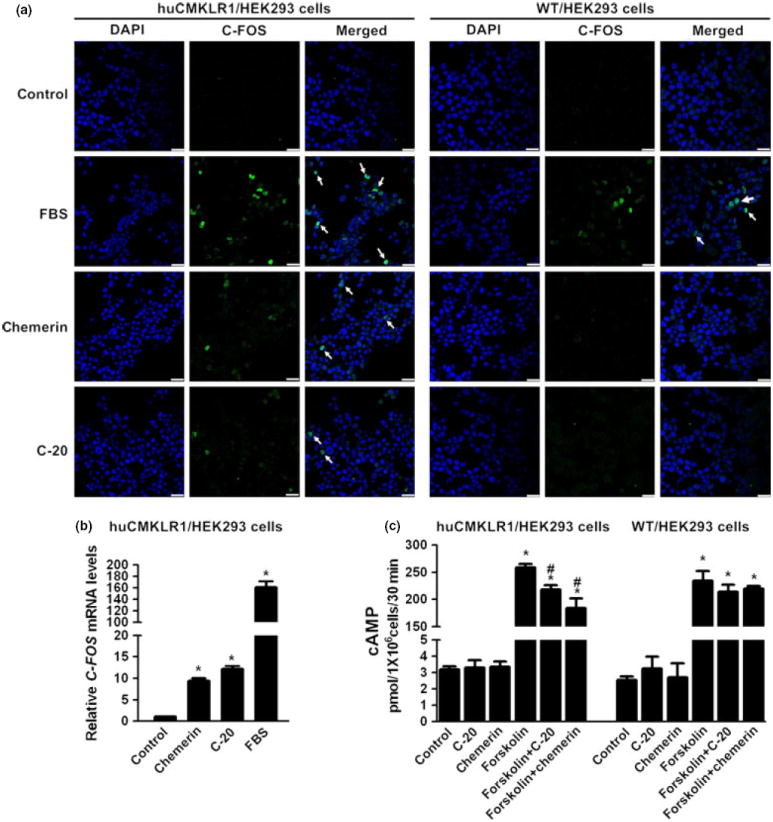
C-20 stimulated early response gene C-FOS and suppressed cAMP production CMKLR1 dependently. (a) Nuclear C-FOS expression was revealed by ICC. HuC-20 and huChemerin both stimulated C-FOS expression in huCMKLR1/HEK293 cells, while no such action was observed in WT/HEK293 cells, both using FBS as positive control. Scale bars, 0.25 μm. (b) HuC-20 stimulated the early response gene C-FOS expression in huCMKLR1/HEK293 cells, using huChemerin and FBS as positive control. Beta-actin served as the reference gene. (c) Forskolin at 10 μm stimulated more than 50-fold increase in cAMP production in both huCMKLR1/HEK293 cells and WT/HEK293 cells. HuC-20 and huChemerin cotreatment suppressed cAMP production in huCMKLR1/HEK293 cells, while no effect was observed in WT/HEK293 cells. HuC-20 and huChemerin showed no effect on basal cAMP production. All data were expressed as mean ± S.E.M.; *P < 0.05 compared with control group, #P < 0.05 compared with forskolin treatment group, by one-way anova followed by Student–Newman–Keuls test.
C-20 Elicited CMKLR1-Dependent Chemotaxis
The chemotactic capacity of C-20 was tested in huCMKLR1/L1.2 cells using huChemerin as positive control by in vitro transwell chemotaxis assays. As showed in Fig. 4, huChemerin stimulated the chemotaxis of huCMKLR1/L1.2 cells at 0.3, 1, 10 nm concentrations, while the dose of 100 nm was not effective. HuC-20 showed no chemotaxis effect at 0.3 nm, while effective at 1, 10, and 100 nm. The optimal dose of chemotaxis for huChemerin was 1 nm compared with the 10 nm of huC-20. WT/L1.2 cells showed no chemotactic response indicating that the chemotaxis was CMKLR1 dependent. Other reported chemerin-derived peptides such as huC-15 and huC-9 were also tested by the in vitro transwell chemotaxis assay by huCMKLR1/L1.2. HuC-15, huC-9, and the negative control peptide NTS showed no chemotactic actions at the concentration of 1 or 10 nm (Figure S1).
Fig. 4.
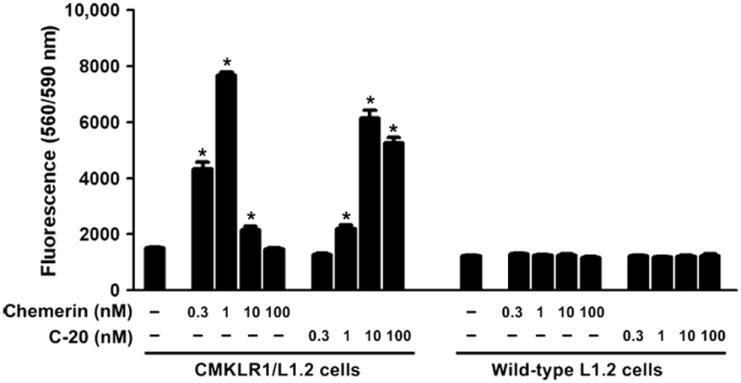
C-20 elicited chemotactic response of huCMKLR1/L1.2 cells. HuChemerin stimulated the chemotaxis of huCMKLR1/L1.2 cells at 0.3, 1, 10 nm concentration, while the dose of 100 nm showed no effect. HuC-20 showed no chemotaxis effect at 0.3 nm, while effective at 1, 10, and 100 nm. The optimal dose of chemotaxis for huChemerin was 1 nm compared with the 10 nm of huC-20. WT/L1.2 cells showed no chemotactic response. *P < 0.05 compared with control group with culture media only, by one-way anova followed by Student-Newman-Keuls test.
C-20 Showed Similar but Less Potent Suppressive Effect on Gonadal Steroid Hormone Production Compared with Chemerin
CMKLR1 and GRP1 were immunolocalized in the testes, majorly in the interstitial Leydig cells (Fig. 5a) and the in the rat ovaries, especially in the theca cells of the follicles (Fig. 6a). In primary Leydig cells, mChemerin suppressed hCG-stimulated testosterone production from Leydig cells at 1, 10, and 100 nm, while mC-20 was only effective at the dose of 100 nm (Fig. 5a). No effect was observed of mChemerin or mC-20 at the basal testosterone production. When treated Leydig cells with another synthetic short 15 amino acid chemerin-derived peptide huC-15, no such kind of suppression can be observed at the dose of 100 nm (data not shown). Furthermore, the suppression of testosterone production was accompanied by the suppression of 3β-HSD gene expression in the Leydig cells, while 17β-HSD, p450scc, and StAR expression levels not affected (Fig. 5b). In antral follicle culture, similar to the Leydig cells, mC-20 effectively suppressed hCG-stimulated progesterone production from the follicle at the concentration of 100 nm, while mChemerin was effective at 10 and 100 nm (Fig. 6b). The suppression of progesterone production from follicles was accompanied by an inhibited gene expression of follicular 3β-HSD as well (Fig. 6b).
Fig. 5.
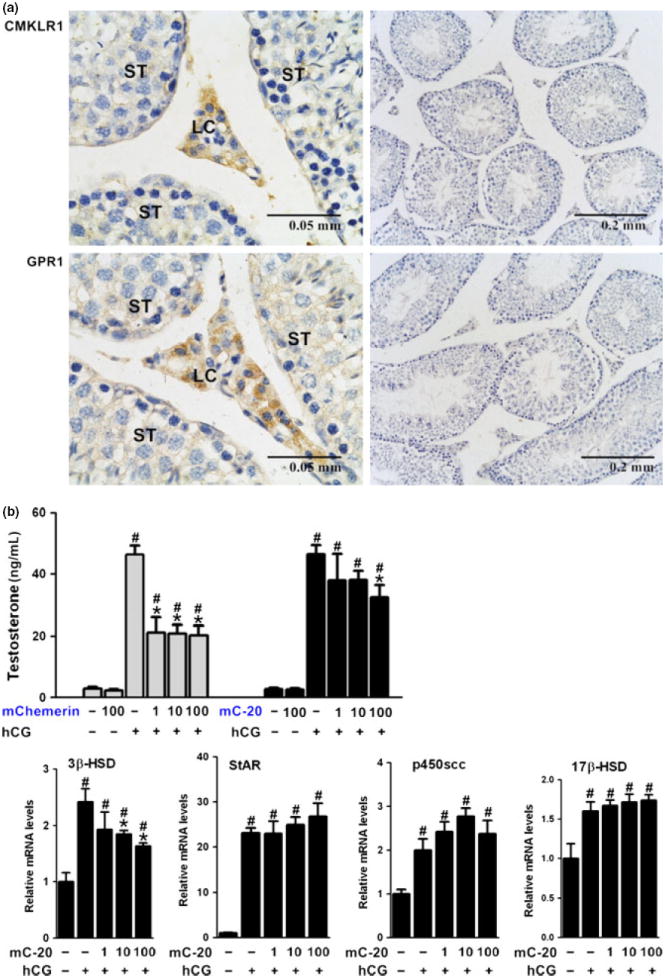
CMKLR1 and GRP1 were immunolocalized in testes, and C-20 showed similar but less potent suppressive effect on testosterone production from Leydig cells. (a) Chemerin and C-20 receptors CMKLR1 and GPR1 were immunolocalized in the rat testes, majorly in the interstitial Leydig cells (left panel). Negative control section was performed in parallel using non-specific IgG instead of primary antibody (right panel). Scale bars, 0.05 or 0.2 mm, as indicated in the picture; LC for Leydig cells, ST for seminiferous tubule; representative sections of n = 5 stain performed on n = 3 rats with similar results. (b) Recombinant mouse chemerin (mChemerin) suppressed hCG-stimulated testosterone production from Leydig cells at 1, 10, and 100 nm, while mC-20 was effective at the dose of 100 nm. The suppression of testosterone production was accompanied by the suppression of 3β-HSD gene expression in the Leydig cells, while 17β-HSD, p450scc, and StAR expression levels not affected. Beta-actin served as the reference gene; all data were expressed as mean ± S.E.M.; #P < 0.05 compared with no treatment control group; *P < 0.05 compared with hCG treatment group by one-way anova followed by Student–Newman–Keuls test.
Fig. 6.
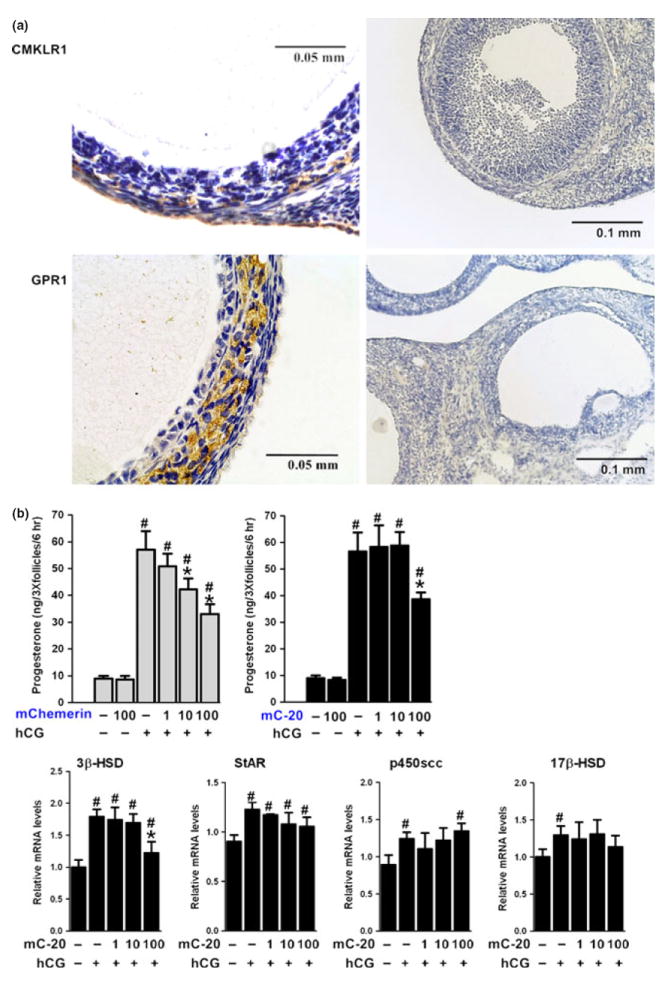
CMKLR1 and GRP1 were immunolocalized in the ovary, and C-20 showed similar but less potent suppressive effect on progesterone production from antral follicles. (a) Chemerin and C-20 receptors CMKLR1 and GPR1 were immunolocalized in the rat ovary, majorly in the theca cells of the follicles (left panel). Negative control section was performed in parallel using non-specific IgG instead of primary antibody (right panel). Scale bars, 0.05 or 0.1 mm as indicated in the picture; representative sections of n = 5 stain performed on n = 3 rats with similar results.(b) Recombinant mouse chemerin (mChemerin) suppressed hCG-stimulated progesterone production from antral follicles at 10 and 100 nm, while mC-20 was effective at the dose of 100 nm. The suppression of progesterone production was accompanied by the suppression of 3β-HSD gene expression in large follicles, while 17β-HSD, p450scc, and StAR expression levels not affected. Beta-actin served as the reference gene; all data were expressed as mean ± S.E.M.; #P < 0.05 compared with no treatment control group; *P < 0.05 compared with hCG treatment group by one-way anova followed by Student–Newman–Keuls test.
Discussion
Based on the bioinformatic prediction of possible mature peptide(s) in the pre-pro-chemerin sequences, we find a 20 amino acid peptide flanked by putative convertase cleavage sites, which is evolutionary conserved among 9 mammalian species and we named it C-20. Then, huC-20 and mC-20 were synthesized to study its pharmacology in receptor binding, subsequent signaling, and bioactivities in vitro. HuC-20 and mC-20 can bind to human CMKLR1 and GPR1 receptors with comparable IC50, suggesting that C-20 can be a potent ligand for CMKLR1 and GPR1 with high affinity. It also demonstrated the C-20 conservation between different species. The binding capacity of C-20 to CMKLR1 was further demonstrated as huC-20 significantly triggered the CMKLR1 internalization expressed on the HEK293 cells. Furthermore, C-20 stimulated both the gene and protein expression of C-FOS in HEK293 cells, depending on the CMKLR1 expressed on the cells. C-FOS belongs to the immediate early gene family of transcription factors involved in important cellular physiological and pathological processes.22-24 The expression level of C-FOS is normally low in quiescent cells, but is rapidly up-regulated with extracellular signals such as diverse hormonal stimulations.25,26 The functional binding of C-20 to CMKLR1 was further demonstrated by the stimulated cAMP production by C-20 treatment using FBS and chemerin as positive controls.
The bioactivity of C-20 was tested in vitro both by transwell chemotaxis assay and primary Leydig cells culture. C-20 elicited the chemotactic of hu-CMKLR1/L1.2 cells as chemerin did, only that C-20 required a higher dose of 1 nm to be effective compared with the 0.3 nm of chemerin. Chemerin was reported for its suppressive roles in steroidogenesis in human and rat granulose cells of the follicles.10,11 So far no role of chemerin in male reproduction was reported. To test the bioactivity of C-20 in gonadal steroidogenesis, we evaluated the effect of C-20 treatment on the testosterone production from primary Leydig cells and the progesterone production from large antral follicles. Our result showed that chemerin receptors CMKLR1 and GPR1 were shown by immunohistochemistry to be expressed in testes especially in the Leydig cells and in ovaries, especially the theca cells. Both recombinant chemerin and mC-20 showed suppressive effect on the hCG-stimulated testosterone production from the Leydig cells and progesterone production from the antral follicles. In consistence with its comparable but less potent binding capacity to CMKLR and GRP1 compared with chemerin, C-20 showed its suppressive effect at 100 nm concentration for both testosterone secretion and progesterone secretion, while 1 and 10 nm was not effective. On the contrary, recombinant chemerin was effective at 1, 10, and 100 nm for testosterone secretion from Leydig cells and 10 and 100 nm for progesterone production from antral follicles. This means that C-20 shared similar but less potent action compared with chemerin. In line with this, we find that both mC-20 and recombinant chemerin elicited significantly chemotactic response of huCMKLR1/L1.2 cells, while chemerin is more than twofold effective than mC-20 at the same dose 1 nm. Also, the inhibitory effect of C-20 upon testosterone/progesterone production was associated with a significant decrease in the gene expression level of the key steroidogenic enzyme −3β-HSD. The causative relationship between these phenomena is yet to be proven. Nevertheless, our data showing that C-20, like chemerin, were able to inhibit hCG-stimulated testosterone/progesterone production, and to decrease mRNA expression levels of 3β-HSD. This together with the immunolocalization of chemerin receptors CMKLR1 and GPR1 to the Leydig cells of testes and the theca cells of ovaries strongly suggests that C-20 and chemerin negatively regulated gonadal steroidogenic function.
The C-terminal proteolytic processing was very important for the bioactivity of chemerin.2 Series short peptides derived from the chemerin C-terminus was reported by different groups with significant bioactivity, mostly based on the intracellular calcium release functional assay.5,27-30 Two groups reported anti-inflammatory activity of the chemerin-derived peptides, mouse C2129 and mouse C1928, in animal disease models. Although none has been reported to be generated in vivo, several proteases have been shown to activate chemerin from prochemerin including elastase, cathepsin G,31 cathepsin L and K,32 plasmin, and tryptase.33,34 To check whether it is possible that C-20 could be a result of in vivo protease cleavage of prochemerin or chemerin. Mouse recombinant chemerin (mChemerin) was incubated for 4 h with different basic proteases (such as carboxypeptidase B, cathepsin C, clostripain, plasmin, and thrombin) and the cleavage products were desalted and analyzed by mass spectrometry. As a result, at the presence of cathepsin C, a peak with a molecular mass of ~2152 Da was observed, corresponding to the peak of synthetic mC-20 (Figure S2).
In summary, based on the bioinformatic search for putative small peptides in the conserved region of pre-pro-chemerin, an evolutionary conserved region flanked by potential convertase cleavage sites was identified and named as C-20. C-20 was shown to bind to CMKLR1 and GPR1 with high affinity, triggered CMKLR1 internalization and stimulated subsequent signal C-FOS expression and cAMP production. In primary Leydig cells and antral follicles, compared with chemerin, C-20 showed similar but less potent suppressive effect on testosterone production and progesterone production, respectively. The estimated plasma and serum concentration for chemerin was 3.0 and 4.4 nm in human and 0.6 and 0.5 nm in mouse, respectively.16 The functional concentration of C-20 in our study is at the nanomolar range. Although less potent compared with recombinant chemerin, considering the high cost from the production of recombinant chemerin, out data suggested that after optimization, C-20 is possible to be a useful experimental tool for the understanding of the biological functions of chemerin/CMKLR1 and chemerin/GPR1 signaling, especially in steroidogenesis.
Supplementary Material
Figure S1. The chemo-attractant capacity of C-20 was compared with other chemerin-derived peptides C-15 and C-9.
Figure S2. Mass spectrometry revealed C-20 in the proteolytic products of mouse chemerin generated by Cathepsin C.
Table S1. Sequences of primers used in qPCR experiments.
Acknowledgments
JV Zhang was supported by the National Major Basic Research Program of China (2013CB945503), One-hundred Talent Program of CAS, Grant 31100850 from NSFC, Grant SW201110059 from Shenzhen City and ShenZhen International Collaborative Grant (GJHZ20130417171414742); L Li was supported by Grant 81200434 from NSFC; B Zabel was supported by National Institutes of Health Grant AI-079320.
Footnotes
Supporting Information
Additional Supporting Information may be found in the online version of this article:
References
- 1.Meder W, Wendland M, Busmann A, Kutzleb C, Spodsberg N, John H, Richter R, Schleuder D, Meyer M, Forssmann WG. Characterization of human circulating TIG2 as a ligand for the orphan receptor ChemR23. FEBS Lett. 2003;555:495–499. doi: 10.1016/s0014-5793(03)01312-7. [DOI] [PubMed] [Google Scholar]
- 2.Wittamer V, Franssen JD, Vulcano M, Mirjolet JF, Le Poul E, Migeotte I, Brezillon S, Tyldesley R, Blanpain C, Detheux M, Mantovani A, Sozzani S, Vassart G, Parmentier M, Communi D. Specific recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluids. J Exp Med. 2003;198:977–985. doi: 10.1084/jem.20030382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zabel BA, Silverio AM, Butcher EC. Chemokine-like receptor 1 expression and chemerin-directed chemotaxis distinguish plasmacytoid from myeloid dendritic cells in human blood. J Immunol. 2005;174:244–251. doi: 10.4049/jimmunol.174.1.244. [DOI] [PubMed] [Google Scholar]
- 4.Bozaoglu K, Curran JE, Stocker CJ, Zaibi MS, Segal D, Konstantopoulos N, Morrison S, Carless M, Dyer TD, Cole SA, Goring HH, Moses EK, Walder K, Cawthorne MA, Blangero J, Jowett JB. Chemerin, a novel adipokine in the regulation of angiogenesis. J Clin Endocrinol Metab. 2007;95:2476–2485. doi: 10.1210/jc.2010-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bondue B, Wittamer V, Parmentier M. Chemerin and its receptors in leukocyte trafficking, inflammation and metabolism. Cytokine Growth Factor Rev. 2011;22:331–338. doi: 10.1016/j.cytogfr.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Tan BK, Chen J, Farhatullah S, Adya R, Kaur J, Heutling D, Lewandowski KC, O’Hare JP, Lehnert H, Randeva HS. Insulin and metformin regulate circulating and adipose tissue chemerin. Diabetes. 2009;58:1971–1977. doi: 10.2337/db08-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duan DM, Niu JM, Lei Q, Lin XH, Chen X. Serum levels of the adipokine chemerin in preeclampsia. J Perinat Med. 2011;40:121–127. doi: 10.1515/JPM.2011.127. [DOI] [PubMed] [Google Scholar]
- 8.Stepan H, Philipp A, Roth I, Kralisch S, Jank A, Schaarschmidt W, Lossner U, Kratzsch J, Bluher M, Stumvoll M, Fasshauer M. Serum levels of the adipokine chemerin are increased in preeclampsia during and 6 months after pregnancy. Regul Pept. 2011;168:69–72. doi: 10.1016/j.regpep.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Barker G, Lim R, Rice GE, Lappas M. Increased chemerin concentrations in fetuses of obese mothers and correlation with maternal insulin sensitivity. J Matern Fetal Neonatal Med. 2012;25:2274–2280. doi: 10.3109/14767058.2012.686540. [DOI] [PubMed] [Google Scholar]
- 10.Reverchon M, Cornuau M, Rame C, Guerif F, Royere D, Dupont J. Chemerin inhibits IGF-1-induced progesterone and estradiol secretion in human granulosa cells. Hum Reprod. 2012;27:1790–1800. doi: 10.1093/humrep/des089. [DOI] [PubMed] [Google Scholar]
- 11.Wang Q, Kim JY, Xue K, Liu JY, Leader A, Tsang BK. Chemerin, a novel regulator of follicular steroidogenesis and its potential involvement in polycystic ovarian syndrome. Endocrinology. 2012;153:5600–5611. doi: 10.1210/en.2012-1424. [DOI] [PubMed] [Google Scholar]
- 12.Zabel BA, Nakae S, Zuniga L, Kim JY, Ohyama T, Alt C, Pan J, Suto H, Soler D, Allen SJ, Handel TM, Song CH, Galli SJ, Butcher EC. Mast cell-expressed orphan receptor CCRL2 binds chemerin and is required for optimal induction of IgE-mediated passive cutaneous anaphylaxis. J Exp Med. 2008;205:2207–2220. doi: 10.1084/jem.20080300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barnea G, Strapps W, Herrada G, Berman Y, Ong J, Kloss B, Axel R, Lee KJ. The genetic design of signaling cascades to record receptor activation. Proc Natl Acad Sci USA. 2008;105:64–69. doi: 10.1073/pnas.0710487105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monnier J, Lewen S, O’Hara E, Huang K, Tu H, Butcher EC, Zabel BA. Expression, regulation, and function of atypical chemerin receptor CCRL2 on endothelial cells. J Immunol. 2012;189:956–967. doi: 10.4049/jimmunol.1102871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshimura T, Oppenheim JJ. Chemokine-like receptor 1 (CMKLR1) and chemokine (C-C motif) receptor-like 2 (CCRL2); two multifunctional receptors with unusual properties. Exp Cell Res. 2011;317:674–684. doi: 10.1016/j.yexcr.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zabel BA, Zuniga L, Ohyama T, Allen SJ, Cichy J, Handel TM, Butcher EC. Chemoattractants, extracellular proteases, and the integrated host defense response. Exp Hematol. 2006;34:1021–1032. doi: 10.1016/j.exphem.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Ernst MC, Sinal CJ. Chemerin: at the crossroads of inflammation and obesity. Trends Endocrinol Metab. 2010;21:660–667. doi: 10.1016/j.tem.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Zhang JV, Ren PG, Avsian-Kretchmer O, Luo CW, Rauch R, Klein C, Hsueh AJ. Obestatin, a peptide encoded by the ghrelin gene, opposes ghrelin’s effects on food intake. Science. 2005;310:996–999. doi: 10.1126/science.1117255. [DOI] [PubMed] [Google Scholar]
- 19.Samson WK, Zhang JV, Avsian-Kretchmer O, Cui K, Yosten GL, Klein C, Lyu RM, Wang YX, Chen XQ, Yang J, Price CJ, Hoyda TD, Ferguson AV, Yuan XB, Chang JK, Hsueh AJ. Neuronostatin encoded by the somatostatin gene regulates neuronal, cardiovascular, and metabolic functions. J Biol Chem. 2008;283:31949–31959. doi: 10.1074/jbc.M804784200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li L, Wong CK. Effects of dexamethasone and dibutyryl cAMP on stanniocalcin-1 mRNA expression in rat primary Sertoli and Leydig cells. Mol Cell Endocrinol. 2008;283:96–103. doi: 10.1016/j.mce.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 21.Li L, O WS, Tang F. Adrenomedullin in rat follicles and corpora lutea: expression, functions and interaction with endothelin-1. Reprod Biol Endocrinol. 2011;9:111. doi: 10.1186/1477-7827-9-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galvis-Alonso OY, Garcia AM, Orejarena MJ, Lamprea MR, Botelho S, Conde CA, Morato S, Garcia-Cairasco N. A combined study of behavior and Fos expression in limbic structures after re-testing Wistar rats in the elevated plus-maze. Brain Res Bull. 2010;81:595–599. doi: 10.1016/j.brainresbull.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 23.Kim KW, Kim HW, Li J, Kwon YB. Effect of bee venom acupuncture on methamphetamine-induced hyperactivity, hyperthermia and Fos expression in mice. Brain Res Bull. 2011;84:61–68. doi: 10.1016/j.brainresbull.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Kang SM, Lim S, Won SJ, Shin YJ, Lim YS, Ahn BY, Hwang SB. c-Fos regulates hepatitis C virus propagation. FEBS Lett. 2011;585:3236–3244. doi: 10.1016/j.febslet.2011.08.041. [DOI] [PubMed] [Google Scholar]
- 25.Hoffman GE, Smith MS, Verbalis JG. c-Fos and related immediate early gene products as markers of activity in neuroendocrine systems. Front Neuroendocrinol. 1993;14:173–213. doi: 10.1006/frne.1993.1006. [DOI] [PubMed] [Google Scholar]
- 26.Zhang JV, Jahr H, Luo CW, Klein C, Van Kolen K, Ver Donck L, De A, Baart E, Li J, Moechars D, Hsueh AJ. Obestatin induction of early-response gene expression in gastrointestinal and adipose tissues and the mediatory role of G protein-coupled receptor, GPR39. Mol Endocrinol. 2008;22:1464–1475. doi: 10.1210/me.2007-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wittamer V, Gregoire F, Robberecht P, Vassart G, Communi D, Parmentier M. The C-terminal nonapeptide of mature chemerin activates the chemerin receptor with low nanomolar potency. J Biol Chem. 2004;279:9956–9962. doi: 10.1074/jbc.M313016200. [DOI] [PubMed] [Google Scholar]
- 28.Cash JL, Hart R, Russ A, Dixon JP, Colledge WH, Doran J, Hendrick AG, Carlton MB, Greaves DR. Synthetic chemerin-derived peptides suppress inflammation through ChemR23. J Exp Med. 2008;205:767–775. doi: 10.1084/jem.20071601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luangsay S, Wittamer V, Bondue B, De Henau O, Rouger L, Brait M, Franssen JD, de Nadai P, Huaux F, Parmentier M. Mouse ChemR23 is expressed in dendritic cell subsets and macrophages, and mediates an anti-inflammatory activity of chemerin in a lung disease model. J Immunol. 2009;183:6489–6499. doi: 10.4049/jimmunol.0901037. [DOI] [PubMed] [Google Scholar]
- 30.Yamaguchi Y, Du XY, Zhao L, Morser J, Leung LL. Proteolytic cleavage of chemerin protein is necessary for activation to the active form, Chem157S, which functions as a signaling molecule in glioblastoma. J Biol Chem. 2011;286:39510–39519. doi: 10.1074/jbc.M111.258921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wittamer V, Bondue B, Guillabert A, Vassart G, Parmentier M, Communi D. Neutrophil-mediated maturation of chemerin: a link between innate and adaptive immunity. J Immunol. 2005;175:487–493. doi: 10.4049/jimmunol.175.1.487. [DOI] [PubMed] [Google Scholar]
- 32.Kulig P, Kantyka T, Zabel BA, Banas M, Chyra A, Stefanska A, Tu H, Allen SJ, Handel TM, Kozik A, Potempa J, Butcher EC, Cichy J. Regulation of chemerin chemoattractant and antibacterial activity by human cysteine cathepsins. J Immunol. 2011;187:1403–1410. doi: 10.4049/jimmunol.1002352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zabel BA, Allen SJ, Kulig P, Allen JA, Cichy J, Handel TM, Butcher EC. Chemerin activation by serine proteases of the coagulation, fibrinolytic, and inflammatory cascades. J Biol Chem. 2005;280:34661–34666. doi: 10.1074/jbc.M504868200. [DOI] [PubMed] [Google Scholar]
- 34.Du XY, Zabel BA, Myles T, Allen SJ, Handel TM, Lee PP, Butcher EC, Leung LL. Regulation of chemerin bioactivity by plasma carboxypeptidase N, carboxypeptidase B (activated thrombin-activable fibrinolysis inhibitor), and platelets. J Biol Chem. 2009;284:751–758. doi: 10.1074/jbc.M805000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. The chemo-attractant capacity of C-20 was compared with other chemerin-derived peptides C-15 and C-9.
Figure S2. Mass spectrometry revealed C-20 in the proteolytic products of mouse chemerin generated by Cathepsin C.
Table S1. Sequences of primers used in qPCR experiments.


