Abstract
The Hippo pathway, a cascade of protein kinases that inhibits the oncogenic transcriptional coactivators YAP and TAZ, was discovered in Drosophila as a major determinant of organ size in development. Known modes of regulation involve surface proteins that mediate cell-cell contact or determine epithelial cell polarity which, in a tissue specific manner, use intracellular complexes containing FERM domain and actin-binding proteins to modulate the kinase activities or directly sequester YAP. Unexpectedly, recent work demonstrates that GPCRs, especially those signaling through Galpha12/13 such as the Protease Activated Receptor PAR1, cause potent YAP dephosphorylation and activation. This response requires active RhoA GTPase and increased assembly of filamentous (F-)actin. Morever, cell architectures that promote F-actin assembly per se also activate YAP by kinase-dependent and independent mechanisms. These findings unveil the ability of GPCRs to activate the YAP oncogene through a newly recognized signaling function of the actin cytoskeleton, likely to be especially important for normal and cancerous stem cells.
The Drosophila Hippo pathway is an anti-proliferative, proapoptotic two-tiered protein kinase cascade
The Hippo pathway was discovered through genetic screens in Drosophila seeking elements that control organ size [1–3]. In addition to many members of the insulin/IGF/MTOR pathway, these screens retrieved several functionally interrelated negative regulators of growth that appeared to constitute a novel pathway, including the protein kinases Lats/warts and Hippo and the noncatalytic proteins Salvador and MATS. Elimination of any of these elements results in dramatic overgrowth due to upregulation of cell proliferation and resistance to developmentally programmed apoptosis. Biochemical experiments showed that the noncatalytic scaffold protein Salvador binds both Lats/warts, an AGC family kinase and Hippo, a Ste20 family protein kinase of the GC kinase type. Thus approximated, Hippo phosphorylates Lats/warts; independently of Salvador, Hippo also phosphorylates MATS. Phospho-MATS binds to phospho-Lats/warts and promotes Lats autophosphorylation and activation. The transcriptional coactivator yorkie was retrieved through a two-hybrid screen with Lats and proved to be the crucial target of the “Hippo” kinase cassette; loss of yorkie reverses the overgrowth phenotypes caused by inactivation of Lats, Salvador, Hippo or MATS [4]. Inhibition of yorkie by the pathway occurs through Lats phosphorylation of yorkie, which promotes its binding to14-3-3 and forces yorkie nuclear exit, terminating its transcription regulatory function.
The control of Drosophila Hippo by cell contact and polarity is complex
In contrast to the strong phenotypes and very clearcut wiring of the pathway from Hippo to yorkie, the inputs upstream of Hippo identified through Drosophila genetics give weaker phenotypes and are less confidently arranged [5–8]. Overall, however the elements identified point to regulatory inputs arising from cell contact and cell polarity, transmitted to the kinase cassette at least in part through the cytoskeleton. Thus inactivation of the cell surface adhesion molecules Fat, an atypical cadherin, or Echinoid, an immunoglobulin domain, adherens junction protein, each promotes modest yorkie-dependent overgrowth. Fat acts through the atypical myosin Dachs which binds the LIM domain protein zyxin and modulates a zyxin-Lats interaction, whereas Echinoid binds directly to Salvador. The transmembrane apicobasal polarity protein Crumbs also regulates yorkie activity, but in a more complex fashion, inasmuch as depletion or overexpression can each result in yorkie activation. Such complexity may reflect the intricate signaling between the competing apicobasal polarity-determining complexes involving the apical complexes involving Crumbs and aPKC vs the lateral Scribble/Lgl/Dlg complex; mutations in the latter result in yorkie-dependent hyperproliferation. Crumbs regulates the level of Expanded, a FERM domain protein that acts in a complex with the related FERM domain protein Merlin and the WW domain protein Kibra; this complex interacts with, and somehow activates the Hippo/Salvador module. Considerable evidence supports a reciprocal regulation between F-actin content and Hippo pathway activity; increased F-actin activates yorkie-dependent transcription whereas pathway activity decreases F-actin content, independently of yorkie [7,8] (discussed further below). Although the genetic relationships are generally consistent across reports, the biochemical mechanisms by which these multiple protein-protein interactions control pathway function are much less clear. Finally, The Ste20-related protein kinase dTAO is able to phosphorylate and activate Hippo [9,10], however the upstream input(s) to dTAO is(are) not currently known.
Hippo signaling is conserved but diversified in mammals
The core components and the architecture of the Hippo kinase cassette are conserved in mammalians; the kinases Mst1(STK4)/Mst2(STK3) and Lats1/Lats2 are homologous with Hippo and Lats/warts respectively and WW45/SAV1 and Mob1A/Mob1B are homologs of Salvador and MATS [11]. The oncogenic transcriptional coactivator YAP and its paralog TAZ are homologs of yorkie [12]. Nevertheless, considerable tissue-specific diversification in the modes of YAP/TAZ regulation is evident as compared with canonical picture provided by Drosophila screens; e.g., 1) kinases other than Mst1/Mst2 and perhaps Lats1/Lats2 can function as negative regulators upstream of YAP [13,14]; 2) inhibition of YAP nuclear residence can occur through sequestration by adhesion complex-associated proteins such as α-catenin [14,15] or angiomotin [16–18] (which can also activate Lats1/2 [19]), in addition to 14-3-3 [20]; 3) in addition to YAP nuclear localization, YAP abundance emerges as a critical determinant of YAP’s transcriptional output [21], pointing to the importance of YAP gene transcription and phosphorylation-dependent ubiquitination/degradation [22]. Overexpression of YAP [4,23] or inactivation of the upstream inhibitory kinases [13,21] produces a marked expansion of stem cell compartments, which if sustained results in multifocal carcinogenesis. YAP nuclear residence and abundance are the key regulated parameters and varied mechanisms have evolved for their control; the Mst/Sav1/Lats kinase cassette, as defined through Drosophila screens is not the ubiquitous determinant of YAP activation in the way that a MAP3K/MAP2K is almost invariably operative upstream of MAPKs. The use of Drosophila genetics to identify elements that regulate yorkie, however powerful, may have biased discovery toward inputs operative during development.
G Protein Coupled Receptors regulate YAP in a cell autonomous manner
Several recent reports [24–26] in mammalian systems have uncovered GPCR regulation of the YAP transcriptional activity, a regulatory input not detected in the Drosophila screens. This discovery arose from the observation that the cytoplasmic retention and phosphorylation of YAP observed in proliferating, subconfluent mammalian cells can be substantially increased by reducing the serum concentration, i.e., serum inhibits YAP phosphorylation in proliferating cells [24,25]. This implies the presence in serum of an inhibitor of the YAP kinase, presumably Lats1/Lats2, and various treatments and extractions pointed to an acidic lipid. Active fractions contained sphingosine-1 phosphate (S1P), and direct addition of S1P or lysophosphatidic acid (LPA) each inhibits the site specific phosphorylation of YAP (and TAZ) and promotes YAP nuclear abundance in a dose dependent fashion, with an EC50 of 17nM for S1P and 317nM for LPA [24], both well within the concentration range found in serum. In addition the use of inhibitors and siRNA demonstrated that the action of these lipids in mediated by their cell surface G-protein coupled receptors; S1P action on YAP in 293A (or HaCaT) cells is mediated by S1P2 rather than S1P1 or S1P3 (of five known S1P receptors) and LPA receptors 1 and 3 (of six) [25]. A variety of individual GPCRs were coexpressed transiently with YAP, whose phosphorylation state was assessed on phos-tag gels; the large majority promoted YAP dephosphorylation, especially Purinergic receptors 1 (P2YR1) and 9 (LPAR4) and the Platelet Activating Factor receptor (PTAFR). A few GPCRs however gave a weak increase in YAP phosphorylation, e.g., the receptors of Glucagon GCGR), Endothelin type A (EDNRA) Dopamine (DRD1) and CCR4. The response of endogenous TAZ generally paralleled that of recombinant YAP [25]. Inasmuch as GPCRs are generally capable of signaling through mutiple heterotrimeric proteins, Yu et. al. [25] examined the response to representatives of the major G alpha subunit gene families (Gs, Gq/11, G12/13, Gi/o), both in wildtype and mutant, activated form. Among the wildtype G alpha polypeptides, only G12/13 elicited a strong response, YAP dephosphorylation. Mutant activated members of the Gq family also gave strong YAP dephosphorylation, whereas much weaker YAP dephosphorylation was elicted by activated Gi/o variants. Only Gs gave increased YAP phosphorylation, and as with overexpression of the glucagon receptor, only to a modest extent. Notwithstanding, treatment of cultured cells with glucagon, epinephrine or the adenyl cyclase stimulator Forkolin each stimulated YAP phosphorylation and mice injected with epinephrine exhibited increased YAP phosphorylation in the heart [25].
Galpha12/13, a potent oncogene that activates YAP through RhoA
Mutant, activated variants from all G alpha subfamilies have been retrieved from human tumors and/or have been shown capable of causing cell transformation in vitro. Notably however, only Galpha12/13 are capable of eliciting oncogenic transformation when overexpressed in their wildtype form [27,28]. The potent ability of wildtype Galpha12/13 to promote the dephosphorylation and activation of the YAP/TAZ oncogenes raises the possibility that YAP and/or TAZ contribute significantly to Galpha12/13-induced transformation. Although little information addressing this question is as yet available, Mo et. al., [26] have carried out a detailed examination of YAP regulation by the thrombin receptor PAR1, a potent activator of Galpha12/13. PAR1 expression is upregulated in a variety of cancers and has been shown to promote to tumor cell proliferation, invasion and metastasis [29–31]. Moreover, a substantial body of clinical and laboratory research indicates that thrombin, acting through PAR1 promotes tumor cell proliferation, migration and contributes to the metastatic potential of breast, prostate, gastrointestinal cancers and melanoma [32,33]. In HEK293A and MCF10A cells, PAR1 activation by a peptide ligand results in transient YAP dephosphorylation, nuclear localization, and increased expression of known YAP target genes, such as CTGF and Cyr61. PAR1-induced YAP dephosphorylation is paralleled by dephosphorylation and inactivation of Lats1, whereas the activity of Mst1, the kinase most closely related to Drosophila Hippo and a known Lats1 activator, is completely unaffected by PAR1 [26]. Depletion of YAP suppresses PAR1-stimulated migration and invasiveness of MCF10A cells, a surrogate of metastatic potential, pointing to the importance of YAP in PAR1’s carcinogenic actions.
PAR1–induced dephosphorylation and activation of YAP and its outputs is prevented by depletion of G12/13 but not Gq/11 [26]. Among the candidate Galpha 12/13 effectors critical to Galpha 12/13 transforming ability [27,28], substantial evidence points to the importance of the family of Rho-GEFs that contain an RGS-domain, i.e. p115Rho-GEF, PDZ-Rho-GEF and the Leukemia-Associated Rho-GEF (LARG) [34,35]. Consistent with this, overexpression of wildtype RhoA promotes YAP dephosphorylation and inhibitors of endogenous RhoA interfere with PAR1 signaling to YAP; RhoA[T19N], a dominant inhibitor of RhoA, or the RhoA-inactivating toxin C3 each completely inhibit PAR1-induced YAP dephosphorylation [26]. Thus PAR1 activation of YAP requires G12/13 activation of RhoA, which inhibits Lats1/2 activation independently of Mst1/2, and is both necessary and sufficient for YAP dephosphorylation. Although several Rho-GEFs are well established human oncogenes (e.g., Dbl, LARG, etc), mutant activated forms of RhoA/B/C have not been retrieved from human cancers and overexpression of constitutively active RhoA/B/C is only weakly transforming. Nevertheless, RhoA is required for transformation by Ras or Raf-CAAX, suggesting that RhoA/B/C individually may activate both pro- and anti-proliferative outputs. Thus it’s likely that outputs of the Rho-GEFs and/or the Galpha12/13 in addition to YAP/TAZ are needed for cell transformation.
RhoA requires F-actin to promote Lats/YAP dephosphorylation
Although RhoA/B/C mediate a wide variety of morphologic and transcriptional responses, cardinal among these is the generation of F-actin stress fibers; here, of the numerous candidate RhoA effectors [36,37], the formin mDia, which initiates F-actin assembly, and the Rho kinases (ROCK1/2), through their ability to activate myosin, play the central roles [38]. Inhibition of ROCK does not interfere with PAR1 induced YAP dephosphorylation, however F-actin depolymerization with Lantrunculin or Cytochalasin D is strongly inhibitory [26]. The finding that PAR1-induced YAP dephosphorylation requires F-actin mirrors an enlarging body of evidence which shows that the state of the actin cytoskeleton, whether determined by cell attachment to matrix [39], the stiffness of the matrix to which it is attached [40] or the shape imposed on the cell [41], signals to YAP through Lats1/2-dependent and/or independent means. Thus, transient expression of mDia, which initiates F-actin assembly, or depletion of the actin capping protein CapZα/β, which limits F-actin assembly, each results in YAP dephosphorylation that is inhibitable by Lantrunculin or Cytochalasin D, but is insensitive to inhibitors of ROCK, Myosin ATPase or MLCK [42,43]. Complete detachment from matrix, the most draconian intervention, results in activation of Lats1/2 and YAP nuclear exclusion, whereas reattachment results in rapid YAP dephosphorylation, independent of the formation of focal adhesions [39]. Detachment leads to apoptotic death (anoikis) in noncancerous cells but is frequently minimal in malignant or metastatic cells. Zhao et. al., [39] find that combined depletion of YAP and TAZ can promote anoikis in a subset of cancer cell lines; presumably the nonresponders employ antiapoptotic effectors other than YAP to avoid anoikis, e.g., PI-3 kinase/Akt [44]. Cells forced into increasingly smaller sizes by growth in patterned microdomains exhibit progressive loss of YAP nuclear localization, independent of the area of surface attachment; conversely flattened cells with abundant F-actin exhibit abundant nuclear YAP which can be inhibited by overexpression of Lats but minimally by Mst1/2 [41].
F-actin can also activate YAP independently of Lats1/Lats2
Growth on matrices of increasing stiffness also causes increasing F-actin and nuclear YAP/TAZ; the response to matrix stiffness however appears to be regulated differently than that seen with cell detachment or crowding, in that it depends on cytoskeletal tension and the generation of stress fibers rather than just the increased F-actin abundance and is therefore sensitive to inhibitors of myosin activity (ROCK inhibitors or blebbistatin) [40]. The loss of nuclear YAP/TAZ that occurs with growth on softer matrices is not accompanied by Lats1/2 activation and is also seen with a non-phosphorylatable TAZ mutant. Thus the actin cytoskeleton is a critical intermediate in RhoA regulation of YAP; the mechanism by which the actin cytoskeleton controls Lats1/2 activity and the Lats1/2-independent nuclear-cytoplasmic transport of YAP/TAZ remains to be defined. Mechanotransduction is an emerging area of cell regulation of special importance to organogenesis and cell migration [45–47].
Conclusions and outlook
Thus, GPCRs join the various cell surface adhesion proteins as physiologic regulators of YAP/TAZ. It’s likely that the range of GPCRs that regulate YAP/TAZ function will continue to enlarge to include those that are strongly coupled through Galpha12/13 such as LPAR1,2,4,5, PGER1-4, S1P2,4,5, The Angiotensin II receptor, the Endothelin receptors A/B, the Ca sensing receptor and others [48,49]. The potency of Galpha12/13 in YAP/TAZ regulation should not detract from consideration of Gq/11-coupled GPCRs, which can also recruit RhoA through other Rho-GEFs (e.g., p63 Rho-GEF, Trio) [34]. Moreover, immune [50] and cytokine [51] receptors have recently been shown to signal to YAP through mechanisms less well explored. The elucidation of the effectors by which the actin cytoskeleton regulates YAP/TAZ nuclear localization, whether through Lats1/2 or independently of those kinases is eagerly awaited. The physiologic roles of GPCR regulation of the YAP/TAZ through Galpha12/13 remains to be established. The functions of these transcriptional modulators has been examined predominantly in stem cells, where YAP/TAZ are strongly proliferative and inhibitory to differentiation [52]. YAP exerts potent effects on cell motility and migration in transformed cells, and this output is likely relevant to stem cell behavior [53,54] as well as to metastatic potential [55,56]. Although this review has emphasized the control of the Lats/YAP by the actin cytoskeleton, it is to be emphasized that the elements of the pathway upstream of YAP, i.e., the FERM domain proteins and the kinases, also control the the actin cytoskeleton, independently of YAP. By example, Lats1, in a kinase-independent manner, was shown to modulate cytokinesis by binding LIM kinase at the contractile ring and inhibiting LIMK phosphorylation of cofilin [57]. In addition, the cells of the immune system, which are highly migratory in executing their physiologic functions, have numerous chemokine and other receptors that regulate actin polymerization to control their adhesive and motile behaviors. The Mst1/2 kinases, in response to antigen, chemokine and S1P receptors, control Rho family GTPases, actin assembly and migratory behavior in mature thymocytes [58,59] and naïve T cells [60,61] independently of YAP. Thus there a feedback cycle between the F-actin network and the hippo pathway that remains to be fully elucidated.
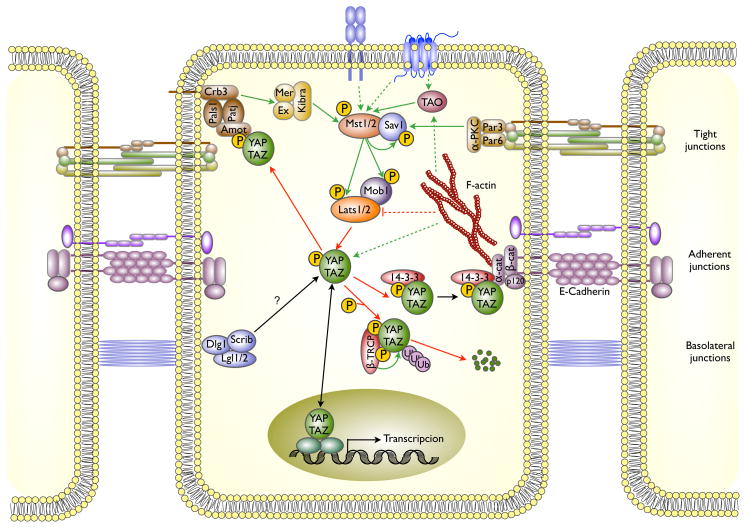
Figure 1. Regulation of the Hippo pathway by cell adhesion and cell polarity complexes.
Cell membrane complexes located at tight junctions (Par3/Par6/aPKC), adherens junctions (cadherins/catenins) and the apical (Crumbs, Pals1, Patj) and basolateral (Scribble/Dlg1/Lgl) polarity complexes each signal to the core kinase cassette through poorly understood mechanism. The kinase cassette consists of Mst1/Mst2, orthologs of Drosophila Hippo, the scaffold Sav1 and Lats1/Lats2 and Mob1A/B an Mst1/Mst2 substrate and Lats1/Lats2 activator. Lst1/Lats2 directly phosphorylates the YAP and TAZ transcriptional coactivators, enabling the binding of 14-3-3 which promotes nuclear exit. Cytoplasmic sequestration of YAP is also caused by binding to Angiomotin or alpha-Catenin. Solid lines indicate direct interactions, dashed lines indicate regulation by unknown mechanisms and/or intermediaries; green indicates an activating input, red inhibitory.
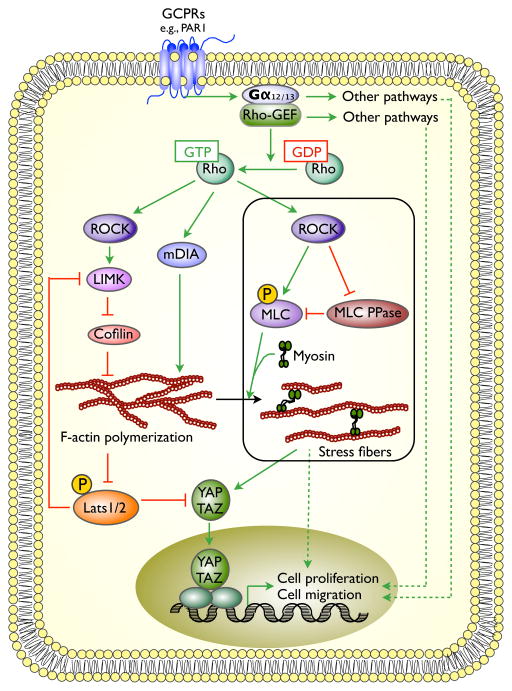
Figure 2. Regulation of YAP by GPCRs and F-actin.
Although many classes of GPCRs appear capable of promoting YAP activation, those like PAR1 that signal strongly through the Gα12/13 family are especially potent. Gα12/13 recruitment of Rho-GEFs causes RhoA activation and F-actin assembly, which promotes Lats1/Lat2 inactivation by an unknown, but myosin-independent mechanism. The generation of stress fibers, which entails both F-actin and myosin activation, also promotes YAP activation, however independently of Lats1/Lats2 inhibition. Not shown is the ability of some Gαs-linked GPCRs to promote the phosphorylation and inactivation of YAP. Solid and dashed lines and colors are used as in figure 1.
Acknowledgments
Work cited from the author’s lab was supported by NIH grants DK17776, CA136567 and DK057521.
References
- 1.Pan D. The Hippo Signaling Pathway in Development and Cancer. Dev Cell. 2010;19:491–05. doi: 10.1016/j.devcel.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halder G, Johnson RL. Hippo signaling: growth control and beyond. Development. 2011;138:9–22. doi: 10.1242/dev.045500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tumaneng K, Russell RC, Guan KL. Organ size control by Hippo and TOR pathways. Curr Biol. 2012;22:R368–79. doi: 10.1016/j.cub.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang J, Wu S, Barrer J, Matthews K, et al. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell. 2005;122:421–34. doi: 10.1016/j.cell.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 5.Grusche FA, Richardson HE, Harvey KF. Upstream regulation of the hippo size control pathway. Curr Biol. 2010;20:R574–82. doi: 10.1016/j.cub.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 6.Genevet A, Tapon N. The Hippo pathway and apico-basal cell polarity. Biochem J. 2011;436:213–24. doi: 10.1042/BJ20110217. [DOI] [PubMed] [Google Scholar]
- 7.Boggiano JC, Fehon RG. Growth control by committee: intercellular junctions, cell polarity and cytoskeleton regulate Hippo signaling. Dev Cell. 2012;22:695–02. doi: 10.1016/j.devcel.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schroeder MC, Halder G. Regulation of the Hippo pathway by cell architecture and mechanical signals. Semin Cell Dev Biol. 2012;23:803–11. doi: 10.1016/j.semcdb.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 9.Poon CL, Lin JL, Zhang X, Harvey KF. The sterile 20-like kinase Tao-1 controls tissue growth by regulating the Salvador-Warts-Hippo pathway. Dev Cell. 2011;21:896–06. doi: 10.1016/j.devcel.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 10.Boggiano JC, Vanderzalm PJ, Fehon RG. Tao-1 Phosphorylates Hippo/MST kinases to Regulate the Hippo-Salvador-Warts Tumor Suppressor Pathway Tao-1 Phosphorylates Hippo/MST kinases to Regulate the Hippo-Salvador-Warts Tumor Suppressor Pathway. Dev Cell. 2011;21:888–95. doi: 10.1016/j.devcel.2011.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Avruch J, Zhou D, Fitamant J, Bardeesy N, et al. Protein kinases of the Hippo pathway: Regulation and substrates. Semin Cell Dev Biol. 2012;23:770–84. doi: 10.1016/j.semcdb.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hong W, Guan KL. The YAP and TAZ transcription co-activators: key downstream effectors of the mammalian Hippo pathway. Semin Cell Dev Biol. 2012;23:785–93. doi: 10.1016/j.semcdb.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou F, Conrad C, Xia F, Park JS, et al. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the YAP1 oncogene. Cancer Cell. 2009;16:425–38. doi: 10.1016/j.ccr.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schlegelmilch K, Mohseni M, Kirak O, Pruszak J, et al. YAP1 acts downstream of α-catenin to control epidermal proliferation. Cell. 2011;144:782–95. doi: 10.1016/j.cell.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silvis MR, Kreger BT, Lien WH, Klezovitch O, et al. α-catenin is a tumor suppressor that controls cell accumulation by regulating the localization and activity of the transcriptional coactivator YAP1. Sci Signal. 2011;4:ra33. doi: 10.1126/scisignal.2001823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao B, Li L, Lu Q, Wang LH, et al. Angiomotin is a novel Hippo pathway component that inhibits YAP oncoprotein. Genes Dev. 2011;25:51–63. doi: 10.1101/gad.2000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan SW, Lim CJ, Chong YF, Pobbati AV, et al. Hippo pathway-independent restriction of TAZ and YAP by angiomotin. J Biol Chem. 2011;286:7018–26. doi: 10.1074/jbc.C110.212621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oka T, Schmitt AP, Sudol M. Opposing roles of angiomotin-like-1 and zona occludens-2 on pro-apoptotic function of YAP. Oncogene. 2012;31:128–34. doi: 10.1038/onc.2011.216. [DOI] [PubMed] [Google Scholar]
- 19.Paramasivam M, Sarkeshik A, Yates JR, 3rd, Fernandes MJ. Angiomotin family proteins are novel activators of the LATS2 kinase tumor suppressor. Mol Biol Cell. 2011;22:3725–33. doi: 10.1091/mbc.E11-04-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao B, Wei X, Li W, Udan RS, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–61. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou D, Zhang Y, Wu H, Barry E, et al. Mst1 and Mst2 protein kinases restrain intestinal stem cell proliferation and colonic tumorigenesis by inhibition of Yes-associated protein (YAP) overabundance. Proc Natl Acad Sci U S A. 2011;108:E1312–20. doi: 10.1073/pnas.1110428108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao B, Li L, Tumaneong K, Wang CY, et al. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF (beta-TRCP) Genes Dev. 2010;24:72–85. doi: 10.1101/gad.1843810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Camargo FD, Gokhale S, Johnnidis JB, Fu D, et al. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol. 2007;17:2054–60. doi: 10.1016/j.cub.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 24.Miller E, Yang J, DeRan M, Wu C, et al. Identification of serum-derived sphingosine-1-phosphate as a small molecule regulator of YAP. Chem Biol. 2012;19:955–62. doi: 10.1016/j.chembiol.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 25.Yu FX, Zhao B, Panupinthu N, Jewell JL, et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. 2012;150:780–91. doi: 10.1016/j.cell.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mo JS, Yu FX, Gong R, Brown JH, et al. Regulation of the Hippo-YAP pathway by protease-activated receptors (PARs) Genes Dev. 2012;26:2138–43. doi: 10.1101/gad.197582.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Juneja J, Casey PJ. Role of G12 proteins in oncogenesis and metastasis. Br J Pharmacol. 2009;158:32–40. doi: 10.1111/j.1476-5381.2009.00180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suzuki N, Hajicek N, Kozasa T. Regulation and physiological functions of G12/13-mediated signaling pathways. Neurosignals. 2009;17:55–70. doi: 10.1159/000186690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eck SM, Blackburn JS, Schmucker AC, Burrage PS. Matrix metalloproteinase and G protein coupled receptors: co-conspirators in the pathogenesis of autoimmune disease and cancer. J Autoimmun. 2009;33:214–21. doi: 10.1016/j.jaut.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bar-Shavit R, Turm H, Salah Z, Maoz M, et al. PAR1 plays a role in epithelial malignancies: transcriptional regulation and novel signaling pathway. IUBMB Life. 2011;2011:397–402. doi: 10.1002/iub.452. [DOI] [PubMed] [Google Scholar]
- 31.Zigler M, Kamiya T, Brantley EC, Villares GJ, et al. PAR-1 and thrombin: the ties that bind the microenvironment to melanoma metastasis. Cancer Res. 2011;71:6561–6. doi: 10.1158/0008-5472.CAN-11-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nierodzik ML, Karpatkin S. Thrombin induces tumor growth, metastasis, and angiogenesis: Evidence for a thrombin-regulated dormant tumor phenotype. Cancer Cell. 2006;10:355–62. doi: 10.1016/j.ccr.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 33.Green D, Karpatkin S. Role of thrombin as a tumor growth factor. Cell Cycle. 2010;94:656–61. doi: 10.4161/cc.9.4.10729. [DOI] [PubMed] [Google Scholar]
- 34.Aittaleb M, Boguth CA, Tesmer JJ. Structure and function of heterotrimeric G protein –regulated Rho guanine nucleotide exchange factors. Mol Pharmacol. 2010;77:111–25. doi: 10.1124/mol.109.061234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kozasa T, Hajicek N, Chow CR, Suzuki N. Signalling mechanisms of RhoGTPase regulation by the heterotrimeric G proteins G12 and G13. J Biochem. 2011;150:357–69. doi: 10.1093/jb/mvr105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wheeler AP, Ridley AJ. Why thre Rho proteins? RhoA, RhoB, RhoC, and cell motility. Experimental Cell Research. 2004;301:43–9. doi: 10.1016/j.yexcr.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 37.Karlsson R, Pedersen ED, Wang Z, Brakebusch C. Rho GTPase function in tumorigenesis. Biochimica et Biophysica Acta. 2009;1796:91–98. doi: 10.1016/j.bbcan.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 38.Narumiya S, Tanji M, Ishizaki T. Rho signaling, ROCK and mDia1, in transformation, metastasis and invasion. Cancer Metastasis Rev. 2009;28:65–76. doi: 10.1007/s10555-008-9170-7. [DOI] [PubMed] [Google Scholar]
- 39.Zhao B, Li L, Wang L, Wang CY, et al. Cell detachment activates the Hippo pathway via cytoskeleton reorganization to induce anoikis. Genes Dev. 2012;26:54–68. doi: 10.1101/gad.173435.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dupont S, Morsut L, Aragona M, Enzo E, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–83. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 41.Wada K, Itoga K, Okano T, Yonemura S, et al. Hippo pathway regulation by cell morphology and stress fibers. Development. 2011;138:3907–14. doi: 10.1242/dev.070987. [DOI] [PubMed] [Google Scholar]
- 42.Sansores-Garcia L, Bossuyt W, Wada K, Yonemura S, et al. Modulating F-actin organization induces organ growth by affecting the Hippo pathway. EMBO J. 2011;30:2325–35. doi: 10.1038/emboj.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fernández BG, Gaspar P, Brás-Pereira C, Jezowska B, et al. Actin-Capping Protein and the Hippo pathway regulate F-actin and tissue growth in Drosophila. Development. 2011;138:2337–46. doi: 10.1242/dev.063545. [DOI] [PubMed] [Google Scholar]
- 44.Taddei ML, Giannoni E, Fiaschi T, Chiarugi P. Anoikis: an emerging hallmark in health and diseases. J Pathol. 2012;226:380–93. doi: 10.1002/path.3000. [DOI] [PubMed] [Google Scholar]
- 45.Mammoto T, Ingber DE. Mechanical control of tissue and organ development. Development. 2010;137:1407–20. doi: 10.1242/dev.024166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang H, Landmann F, Zahreddine H, Rodriguiz D, et al. A tension-induced mechanotransduction pathway promotes epithelial morphogenesis. Nature. 2011;471:99–103. doi: 10.1038/nature09765. [DOI] [PubMed] [Google Scholar]
- 47.Halder G, Dupont S, Piccolo S. Transduction of mechanical and cytoskeletal cues by YAP and TAZ. Nat Rev Mol Cell Biol. 2012;13:591–600. doi: 10.1038/nrm3416. [DOI] [PubMed] [Google Scholar]
- 48.Alexander SP, Mathie A, Peters JA. Br J Pharmacol. 2. Suppl 1. Vol. 50. Macmillan Press LTD; 2007. 7 TM Receptors: Guide to Receptors and Channels (GRAC) pp. S1–S68. (2007 Revision) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Siehler S. Regulation of RhoGEF proteins by G12/13-coupled receptors. British Journal of Pharmacology. 2009;158:41–9. doi: 10.1111/j.1476-5381.2009.00121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thaventhiran JE, Hoffmann A, Magiera L, de la Roche M, et al. Activation of the Hippo pathway by CTLA-4 regulates the expression of Blimp-1 in the CD8+ T cell. Proc Natl Acad Sci U S A. 2012;109:E2223–9. doi: 10.1073/pnas.1209115109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen D, Sun Y, Wei Y, Zhang P, et al. LIFR is a breast cancer metastasis suppressor upstream of the Hippo-YAP pathway and a prognostic marker. Nat Med. 2012;18:1511–7. doi: 10.1038/nm.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ramos A, Camargo FD. The Hippo signaling pathway and stem cell biology. Trends cell Biol. 2012;22:339–46. doi: 10.1016/j.tcb.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Inglesias-Bartolome R, Gutkind JS. Signaling circuitries controlling stem cell fate: to be or not to be. Curr Opin Cell Biol. 2011;23:716–23. doi: 10.1016/j.ceb.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sahin AO, Buitenhuis M. Molecular mechanisms underlying adhesion and migration of hematopoietic stem cells. Cell Adh Migr. 2012;6:39–48. doi: 10.4161/cam.18975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Menke A, Giehl K. Regulation of adherens junctions by Rho GTPases and p120-catenin. Arch Biochem Biophys. 2012;524:48–55. doi: 10.1016/j.abb.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 56.Balkwill FR. The chemokine system and cancer. J Pathol. 2012;226:148–57. doi: 10.1002/path.3029. [DOI] [PubMed] [Google Scholar]
- 57.Yang X, Yu K, Hao Y, Li DM, Stewart R, et al. LATS1 tumour suppressor affects cytokinesis by inhibiting LIMK1. Nat Cell Biol. 2004;6:609–17. doi: 10.1038/ncb1140. [DOI] [PubMed] [Google Scholar]
- 58.Mou F, Praskova M, Xia F, Van Buren D, et al. The Mst1 and Mst2 kinases control activation of rho family GTPases and thymic egress of mature thymocytes. J Exp Med. 2012;209:741–59. doi: 10.1084/jem.20111692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ueda Y, Katagiri K, Tomiyama T, Yasuda K, et al. Mst1 regulates integrin-dependent thymocyte trafficking and antigen recognition in the thymus. Nat Commun. 2012;3:1098. doi: 10.1038/ncomms2105. [DOI] [PubMed] [Google Scholar]
- 60.Katagiri K, Imamura M, Kinashi T. Spatiotemporal regulation of the kinase Mst1 by binding protein RAPL is critical for lymphocyte polarity and adhesion. Nat Immunol. 2006;7:919–28. doi: 10.1038/ni1374. [DOI] [PubMed] [Google Scholar]
- 61.Zhou D, Medoff BD, Chen L, Li L, et al. The Nore1B/Mst1 complex restrains antigen receptor-induced proliferation of naïve T cells. Proc Natl Acad Sci USA. 2008;105:20321–6. doi: 10.1073/pnas.0810773105. [DOI] [PMC free article] [PubMed] [Google Scholar]