Abstract
Mediators of anti-hypertensive actions of docosahexaenoic acid (DHA) are largely unknown. The omega-3 epoxide of DHA, 19, 20-EDP (epoxydocosapentaenoic acid) is metabolized by soluble epoxide hydrolase (sEH), which also metabolizes the anti-inflammatory and anti-hypertensive arachidonic acid (ARA) epoxides, EETs (epoxyeicosatrienoic acids). Based in part on plasma levels of EDPs following a DHA-rich diet, we hypothesized that 19, 20-EDP contributes to the anti-hypertensive actions of DHA in angiotensin-II dependent hypertension. Treatment individually with 19, 20-EDP, and a potent sEH inhibitor (sEHI) TPPU (1-trifluoromethoxyphenyl-3-(1-propionylpiperidin-4-yl) urea) significantly lowered blood pressure (BP) as compared to angiotensin-II infused animals. The largest reduction in BP was obtained with the combination of 19, 20-EDP and TPPU, which was more efficacious than the combination of 14, 15-EET and TPPU. Oxylipin profiling revealed that 19, 20-EDP and 14, 15-EET infusion affected mostly metabolites of the P450 pathway but also renal levels of prostaglandin-E2. Our findings suggest that 19, 20- EDP is a mediator of the anti-hypertensive effects of DHA in angiotensin-II dependent hypertension. It appears that 19, 20- EDP requires metabolic stabilization with a sEHI to be most effective in lowering BP, although both TPPU and 19, 20- EDP are so effective on their own that demonstrating additive or synergistic interactions is difficult.
Keywords: Docosahexaenoic acid (DHA); 19, 20-Epoxydocosapentaenoic acid; soluble epoxide hydrolase inhibitors; omega-3 polyunsaturated fatty acids; angiotensin-II dependent hypertension
INTRODUCTION
The long chain omega-3 polyunsaturated fatty acids (ω-3 PUFAs) have blood pressure (BP) lowering effects. 1–3 These fatty acids have key structural roles and may alter BP by increasing membrane fluidity, altering lipid rafts or other structural attributes, which can potentially increase the permeability of the membranes and leakage of cellular components.4, 5 The ω-3 PUFAs are also substrates for beta-oxidation and enter metabolic energy pathways.5, 6
Among the two major ω-3 PUFAs, docosahexaenoic acid (DHA, 22:6n3) is more efficacious than eicosapentaenoic acid (EPA, 20:5n3) in lowering BP.2, 7–13 However, the mechanisms by which ω-3 PUFAs such as DHA exert their anti-hypertensive effects are still unclear.12, 14, 15 A widely accepted mechanism by which omega 3 lipids reduce inflammation and BP is by competing with ARA (20:4n6) for the cyclooxygenase enzymes. DHA is reported to have a lower Km and lower kcat than ARA resulting competition with ARA at the cyclooxygenase catalytic site and thus in lower concentrations of inflammatory and hypertensive cyclooxygenase products and presumably products that are less active than the prostaglandins and thromboxanes from ARA.16, 17 DHA also competes with ARA for oxidation by the Cytochromes P450 where CYP2C and 2J are stereoselective for epoxidation of the last double bond of these fatty acids generating epoxy fatty acids (EpFAs).18–20 BP reduction by the epoxides of ARA known as epoxyeicosatrienoic acids or EETs is well established. DHA competes for ARA to generate epoxides known as EDPs (epoxydocosapentaenoic acid).18, 21 As with the EETs, the EDPs are hydrolyzed by the soluble epoxide hydrolase (sEH) to the corresponding diols which are far more polar and rapidly conjugated than the epoxides. All EpFAs tested are turned over rapidly by the sEH. In general, the omega-3 derived EDPs are turned over more rapidly than the corresponding omega-6 derived EETs with the exception of the relatively stable 19, 20-EDP.22 Thus, an additional mechanism by which dietary omega-3 lipids could reduce BP is by the biosynthesis of EDPs which may be intrinsically more active than EETs, produced at higher levels or more stable to degradation. In pre-constricted coronary arteries, both DHA and the EDPs are potent activators of BKCa channels and also are more potent vasodilators than EETs.23–27 However, it is unclear whether EDPs indeed mediate the anti-hypertensive effects of DHA in vivo.
We recently demonstrated that a diet rich in ω-3 PUFAs (EPA and DHA) lowers systolic BP in angiotensin-II (Ang-II) dependent hypertension when compared to animals on a diet rich in ω-6 PUFAs. This reduction in BP is enhanced by treatment with a sEH inhibitor. Among the regioisomers of DHA epoxides that we quantified, increased tissue levels of 19, 20- EDP correlated well with the reduction in BP. When compared to other regioisomers, 19, 20-EDP is a preferred metabolite for synthesis by P450s due to the epoxide position at the terminal (ω-olefin) as in 14, 15-EET, which are both substrates of sEH. As introduced above, all of the EDP and EET regioisomers are rapidly metabolized by the sEH, and the EDPs are more rapidly metabolized than the similar EET regioisomers. However, 19, 20- EDP is an exception in being more slowly metabolized than most other fatty acid epoxides tested (Table S1, Supplementary Digital Content 1). 21, 22 Because inhibition of sEH stabilizes EETs, and because most EDPs are better substrates of sEH than EETs, inhibition of sEH should also stabilize and thereby prolong the effects of EDPs. In addition, EETs have been previously shown to lower BP in Ang-II dependent hypertension. Thus, we hypothesize that the reduction in Ang-II driven BP by ω-3 PUFAs is due in part to the generation of epoxides of the ω-3 fatty acid DHA. Since our recent data suggest that EDPs mediate the anti-hypertensive effects of DHA, and that 19, 20- EDP is the predominant regioisomer that increases with sEH inhibition, here we hypothesized that 19, 20-EDP exhibits anti-hypertensive and anti-inflammatory properties in Ang-II dependent hypertension. We further tested the hypothesis that co-treatment with 19, 20- EDP and a sEH inhibitor (sEHI) would have a larger effect as compared to either treatment alone. Considering the similarities in the vasodilator effects of 19, 20- EDP and 14, 15-EET, we also compared the anti-hypertensive effects of 19, 20-EDP and 14, 15- EET.
METHODS
Animals, diet and treatments
Experiments including animals were approved by the University of California Davis Animal Use and Care Committee, and were conducted compliant with the National Institutes of Health Guide for the care and use of laboratory animals. Animals (male Swiss Webster mice, 8 weeks old, Charles River Laboratories, Wilmington, MA) were acclimated to their new housing environment for a week and were maintained under a 12 hour light-dark cycle with free access to water and food. Considering the effect of diet on BP,28 animals were put on a diet that has low omega-3 fatty acids (Harlan Teklad standard diet). Briefly, the diet is composed of 2.7 % omega-3 PUFAs (α-linolenic acid, C18:3n3) and 31% omega-6 PUFAs (linoleic acid, C18:2n6). The detailed fatty acid composition of the diets can be found in the supporting information by Ulu et al.28
After this acclimation period, baseline BP was recorded for each animal. Then, a potent sEHI, 1-trifluoromethoxyphenyl-3-(1-propionylpiperidin-4-yl) urea (UC 1770 or TPPU),29 was administered in the drinking water at a dose of 0.02, 0.06 and 0.2 mg/kg, which was dissolved in PEG400 (polyethylene glycol) and added to drinking water to give a 1% v/v final solution of PEG (see Supplementary Digital Content 1, detailing the preparation of the inhibitor in drinking water). The dose of sEHI in subsequent analyses was selected based on the dose-dependent effects of TPPU in Ang II dependent hypertension (Figure S1–S4). Dosing with TPPU started five days before the induction of hypertension, which was induced by infusion of Ang II at a constant rate (20 ng/min or 1 mg/kg/day) for 14 days using subcutaneously implanted osmotic mini pumps (Model 1002-Alzet, Cupertino, CA). The DHA and ARA epoxides, 19, 20- EDP and 14, 15-EET were formulated in 25% DMSO in PEG400 and infused together with Ang II in designated groups. Implantation of osmotic mini-pumps delivering this solvent did not influence the BP in Ang II treated animals (Figure S5).
Animals were randomly divided into designated groups: All animals received Ang II, which was indicated by ‘Ang II’ in groups. One group served as Ang II infused controls. The other groups receiving Ang II were randomly assigned to receive 19, 20-EDP, TPPU at 0.02, 0.06 and 0.2 mg/kg doses, combination of 19, 20-EDP + TPPU (0.02, 0.06, 0.2 mg/kg) or combination of 14, 15- EET and TPPU (0.2 mg/kg).
Measurement of Blood Pressure
Systolic blood pressure (SBP) was measured from conscious animals using a non-invasive tail cuff BP system (Kent Scientific Corporation, Torrington, CT), as previously described. 28, 30, 31 Because implanting both mini-pumps and telemetry transmitters into small mice leads to stress and inflammation, which could bias the resulting data, direct measurement of BP such as radiotelemetry method was not preferred in our study.
After the animals were acclimated to the tail cuff and restraining procedure for 7 consecutive days, a total of 20 cycles were performed for each animal every other day always between 12:00–17:00 by the same qualified operator (A.U.). The values that are associated with excess noise or animal movement were discarded, and the average of the remaining readings were used to establish SBP as described.30 Tail cuff measurements using a volume pressure recording (VPR) system were validated in mice in 2008 by Feng et al.32, which showed that the tail cuff VPR method underestimates SBP by 0.25 mmHg, whereas it underestimates diastolic BP by 12.2 mmHg when compared to direct telemetry measurements. Therefore, only the SBP values were reported in our study. In addition, we did not include heart rate data due to the high number of missing values per animal per group, which limits the statistical analysis and interpretation of those data.
Quantification of Oxylipins and TPPU in the Kidney
The levels of oxylipins and TPPU were determined in the kidney and plasma using a liquid chromatography electrospray ionization tandem mass spectrometry (LC/MS/MS) as described.28, 33, 34 Briefly, a hundred mg tissue was homogenized in the presence of an anti-oxidant solution (0.2 mg/mL BHT (butyl hydroxytoluene) and EDTA) and an appropriate internal standard (1-(1-acetypiperidin-4-yl)-3-adamantanylurea). Then, tissue homogenates were extracted using SPE cartridges (Waters Oasis HLB C18 cartridge), and reconstituted in an additional standard solution (1-adamantan-1-yl-3-decyl-urea). Extracted samples were quantified for oxylipins using LC/MS/MS analysis (Applied Biosystems, 4000 QTRAP tandem mass spectrometer, Foster City, CA).
Renal mRNA expression of the angiotensin converting enzyme-2 (ACE-2) and Ang-II receptor 1a (AT1A)
Total RNA was extracted from the renal cortex and mRNA expression of the ACE-2 and AT1A was determined in a two-step process using TaqMan gene expression assays (Applied Biosystems, Foster City, CA) as described before.28 The following TaqMan gene expression assays were employed: Ace-2, Mm01159003_m1, Agtr1a, Mm01957722_s1 and a housekeeping gene beta-actin, Actb, Mm00607939_s1.
Statistical Analyses
All data are presented as mean ± standard error of the mean (SEM). Because multiple measurements were taken from the same mouse, a mixed effects model with ‘subject’ as a random effect was used to analyze the effects of Ang II, 19, 20- EDP, and TPPU on blood pressure.35 We analyzed the SBP data using a mixed effects model, which included ‘group’ as the between-subjects factor and ‘time’ as repeated measures and pairwise comparisons. An additional statistical analysis using a model comparison approach to analyze the BP data are also provided in the Supplemental Digital Content 1. Both the mixed effects model and model comparison analyses supported the conclusions presented here. The differences in oxylipins among all the groups were analyzed for the effects of treatment (EpFAs, TPPU or the combination) on dependent measures. To determine whether differences among treatments were significant, one-way ANOVA was performed, followed by Fisher’s Protected LSD (least significant difference) pairwise comparisons when P <0.05.36
RESULTS
To test if EDPs act as potential mediators of the anti-hypertensive effects of DHA, we synthesized the 19, 20-EDP regioisomer in our laboratory. To compare with the efficacy of 19, 20-EDP on BP, we also examined the anti-hypertensive effects of 14, 15-EET. Administration of TPPU or infusion with either EpFAs did not alter body weight gain (see supplemental digital content 1 for body weight data).
Co-administration of the 19, 20- EDP and TPPU lowers systolic blood pressure in Ang II induced hypertension
Considering the previously reported anti-hypertensive effects of sEHIs,37–40 we first optimized the dose of the sEHI, TPPU. Based on results from the doses that we have previously used to lower BP (0.2 and 0.6 mg/kg), we selected 0.02, 0.06 and 0.2 mg/kg doses, and examined the dose dependent effects of TPPU on changes in BP and on the efficacy of 19, 20-EDP in Ang-II dependent hypertension (Figure 1A–C, Figure S1–S4). The mixed effects model analysis showed clear main effects of group (F = 3.95, P = 0.004) and time (F = 5.87, P < 0.001). In the overall mixed effects analysis (including data from all days), the SBP (percent change from baseline) in Ang II infused animals that are treated with TPPU alone at 0.02 mg/kg did not differ from their Ang II counterparts (P > 0.05, Figure 1A); however, those treated with TPPU alone at 0.06 mg/kg (Figure 1B) or 0.2 mg/kg (Figure 1C) showed statistically lower SBP as compared to Ang II infused controls (P < 0.05). In addition, comparison of SBP between the animals treated with 0.02 and 0.06 mg/kg TPPU alone (P = 0.06) or comparison of their 19, 20-EDP combination equivalents (P = 0.08) missed statistical significance. Furthermore, we found that Ang II infused animals that are treated with the combination of 19, 20- EDP and TPPU at all doses exhibit statistically lower SBP as compared to Ang II infused controls (F= 3.49, P < 0.05) (Figure 1A–C, Figure S4). Consistent with our previous results 28 Figure 1 shows that BP reached a plateau at day 6 after infusion with Ang II. Supporting this observation, our mixed effects model indicates a major time dependent effect. Therefore, we further focused our analyses on the SBP data obtained from day 6 to 12. As expected, this analysis showed no significant main effects of time during days 6–12 (F = 1.03, P = 0.385). Similar to the overall mixed effects model (including SBP data from all days), between day 6–12, we obtained a significant main effect of ‘group’ (F = 4.9, P = 0.03). Further pairwise comparisons revealed statistically significant differences in SBP between Ang II infused animals and those treated with the combination of 19, 20- EDP and TPPU at all doses (P = 0.02 for 0.02 mg/kg and P < 0.01 for 0.06 mg/kg, and 0.2 mg/kg). We also observed a significant difference between the Ang II infused animals and of those treated with 19, 20- EDP alone (P = 0.02), TPPU alone at 0.06 mg/kg (P = 0.1) and 0.2 mg/kg (P = 0.005), but not at 0.02 mg/kg (P = 0.4). In addition, SBP differed between the 19, 20- EDP and 19, 20-EDP + TPPU treatment only at 0.02 mg/kg (P = 0.02), suggesting that a higher dose of TPPU is necessary to inhibit sEH metabolism of 19, 20-EDP. Consistent with this finding, evaluation of the dose-response relationship of the changes in BP and dose of TPPU or combination of TPPU and 19, 20- EDP indicated that BP reduction with 19, 20- EDP is most effective at the 0.2 mg/kg dose of TPPU (Figure 2, Figure S4A and S4B).
FIGURE 1.
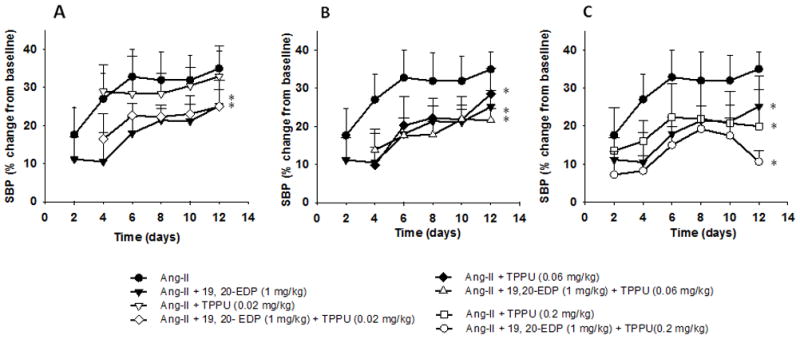
Comparison of the blood pressure-time course of Ang II infused animals and those treated with: A. 19, 20- EDP alone, TPPU alone (0.02 mg/kg) and combination of 19, 20-EDP and TPPU at 0.02 mg/kg dose, B. 19, 20- EDP alone, TPPU alone (0.06 mg/kg) and the combination of 19, 20-EDP and TPPU at 0.06 mg/kg dose, C. 19, 20- EDP alone, TPPU alone (0.2 mg/kg) and the combination of 19, 20-EDP and TPPU at 0.2 mg/kg dose. Data are mean ± SEM. Error bars are only shown unidirectional. Ang II alone, n= 6–8; Ang II + 19, 20- EDP, n= 8–9; Ang-II + TPPU (0.02 mg/kg), n = 7–9, Ang-II + 19, 20-EDP + TPPU (0.02 mg/kg), n = 6, Ang-II + TPPU (0.06 mg/kg), n = 8; Ang-II + 19, 20-EDP + TPPU (0.06 mg/kg), n = 9–10; Ang II + TPPU (0.2 mg/kg), n= 4–6, Ang II + 19, 20- EDP + TPPU (0.2 mg/kg), n= 7–9. The asterisk indicates a statistically significant difference in SBP between treatment groups (TPPU, 19, 20-EDP or the combination) and the Ang-II infused animals.
FIGURE 2.
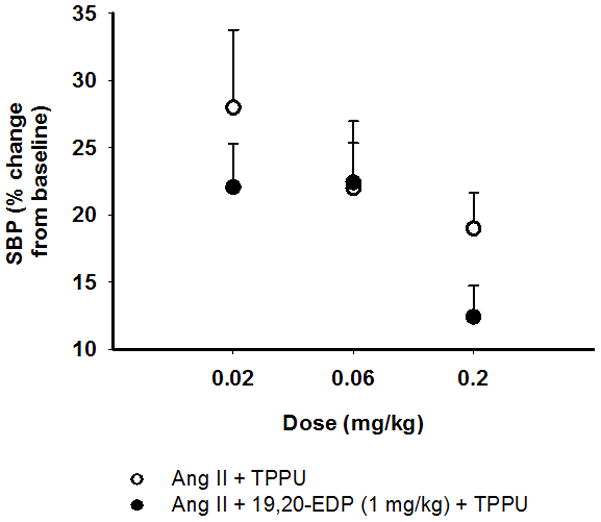
Dose- response relationship associated with the sEH inhibitor TPPU and 19, 20-EDP in Ang II dependent hypertension. Data are mean ± SEM. Error bars are only shown unidirectional.
On the last day of BP measurements (day 12), we compared the SBP among the Ang II infused animals with those treated with the combination of 19, 20- EDP and TPPU at different doses. Animals treated with 19, 20-EDP and TPPU at either 0.06 or 0.2 mg/kg, but not at 0.02 mg/kg exhibited lower BP as compared to Ang II infused controls on day 12 (P = 0.04). Also, pairwise group comparisons revealed a statistically significant difference in SBP between the 19, 20- EDP + TPPU combination treatments at 0.06 mg/kg and 0.2 mg/kg doses (P = 0.04), and approximated a significance in SBP between the combination treatments at 0.02 and 0.2 mg/kg doses (P = 0.06). This is likely due to the low number of animals (n=4–6) in the latter group on day 12. These results are consistent with the dose-response plot (Figure 2). Therefore, subsequent experiments that we performed included the 0.2 mg/kg dose of TPPU.
Next, we compared the anti-hypertensive effects of 19, 20- EDP with that of an ARA epoxide, 14, 15- EET in Ang II dependent hypertension. We examined the effects of Ang II, 19, 20- EDP, 14, 15-EET and TPPU treatments on BP using a mixed effects model, which included ‘group’ as the between-subjects factor and ‘time’ as repeated measures. As in previous analyses, the mixed effects model demonstrated significant main effects of both group (F = 7. 29, P < 0.01) and time (F = 3.48, P < 0.01). Pairwise group comparisons revealed that Ang II infusion increased BP significantly compared to all four treatment groups (P < 0.05) (Figure 3A), indicating that all of the treatments lowered BP as compared to Ang-II alone. Treatment with the combination of 19, 20-EDP and TPPU (0.2 mg/kg) resulted in lower SBP as compared to treatment with the combination of 14, 15-EET and TPPU (0.2 mg/kg) (P = 0.009), or treatment with either 19, 20- EDP (P = 0.07) or TPPU alone (P = 0.06) (Figure 3A). Marginal means of the sum of six day measurements revealed that the combination treatment with 19, 20- EDP and TPPU lowers BP by 2.4 fold in hypertensive animals, while the effect size for other treatments is between 1.4– 1.6 fold as compared to Ang-II infused animals. Examination of the area under the blood pressure-time curve (AUC) revealed that treatment with the combination of 19, 20- EDP and TPPU but not 19, 20-EDP alone or the combination of 14, 15-EET and TPPU resulted in lower SBP between days 2–12 and 6–12 as compared to Ang II controls (P < 0.05) (Figure S6). Of note, both the 19, 20- EDP and the 14, 15- EET are quite stable at 37°C at neutral pH (Figure S7A and S7B). The better effectiveness of the 19, 20-EDP in reducing BP as compared to 14, 15-EET is consistent with the stability of the compound to metabolism to the corresponding diols by the sEH (Table S1).22
FIGURE 3.
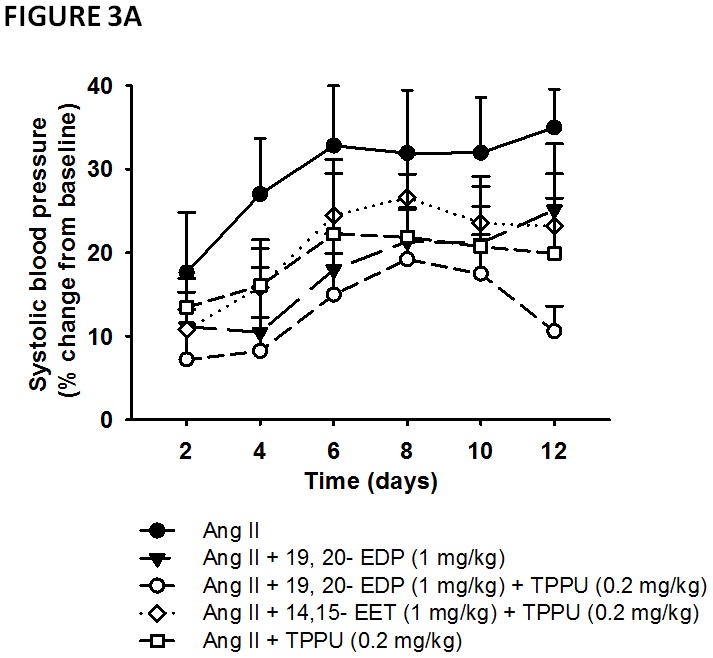
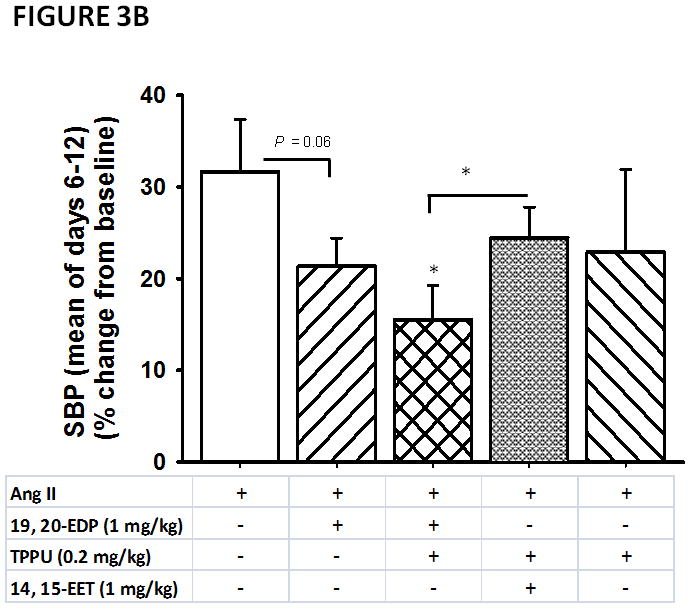
Anti-hypertensive effects of 19, 20- EDP and 14, 15- EET in angiotensin-II dependent hypertension. A. Systolic blood pressure (SBP) is shown as percent change from baseline. Blood pressure readings commenced with initiation of Ang II infusion. TPPU was administered in drinking water at a 0.2 mg/kg dose each day for five days before the induction of hypertension by Ang II. B. Marginal group means of days 6–12 are shown. Statistically significant differences (P < 0.05) are indicated with an asterisk as compared to Ang II infused animals. Data are mean ± SEM. Ang II alone, n=6–8; Ang II + 19, 20- EDP, n= 8–9; Ang II + 19, 20- EDP + TPPU (0.2 mg/kg), n=7–9; Ang II + 14, 15- EET + TPPU (0.2 mg/kg), n= 6–8; Ang II + TPPU (0.2 mg/kg), n= 4–6, Ang-II + 19, 20-EDP + TPPU (0.02 mg/kg), n =6, Ang-II + 19, 20-EDP + TPPU (0.06 mg/kg), n =9. The symbol (*) on Panel B indicates P < 0.05 as compared to all other groups. Differences in SBP between Ang II infused animals and other treatment groups are indicated with an asterisk.
To improve the generality and the interpretability of our results, we performed an additional statistical analysis of the SBP data using a model comparison framework 41 (see Supplemental Digital Content 1 explaining the statistical analysis in detail). Overall, the model comparison analysis of the SBP data with Akaike’s Information Criterion (AIC)42 showed a time dependence in the generation of hypertension with Ang II (parameter estimate for ‘day’ is 4.04, 95% Cl: 2 to 5.8 and parameter estimate for ‘day*day’ is −0.19, 95% Cl: −0.3 to −0.06) as one would expect from a subpressor dose of Ang II. AICc strongly favored a model with a quadratic effect of time (Table S2A). The ‘time’ parameter estimate was 8.2 (95% Cl: −1.5 to 18), while the ‘time2’ parameter was −0.19, 95% Cl: −0.3 to −0.06) (Table S2A). We observed only a minor effect of time after day six (Table S2 B). Therefore, subsequent analysis of these data focused on these last four days unless indicated otherwise. Briefly, we built a set of models including a model for each hypothesis, and a null (intercept) model. Of these models, the top two were very similar and received the majority of the AICc weight (Table S2 B). The top-ranked model included Ang II with a parameter estimate of 9 (95% Cl: −1.3 to 19.2), indicating that Ang II raised BP reliably in the experiment. The second ranked model included Ang II, 19, 20-EDP and TPPU with a parameter estimate of −7.2 (95% Cl: −17.5 to 3) (Table S 2B), suggesting that this treatment fairly consistently lowers BP. These results are also depicted in Figure 3B, showing a significant difference in SBP between Ang II infused animals and those treated with the combination of 19, 20-EDP and TPPU combination on days 6 – 12. We further made group comparisons (Tables S3 – S6). Comparison of SBP in Ang II infused animals that are treated with 19, 20-EDP alone and those treated with both 19, 20-EDP and TPPU resulted in similar AICc values with comparable weights, indicating no predictive differences between these two groups (Table S4). No differences were observed in SBP between the TPPU alone and the combination of TPPU and 19, 20- EDP treatments (Table S5). Of note, this difference missed statistical significance when considering the overall marginal means (6 day average SBP) (P = 0.06). We also observed possibly predictive differences in SBP between treatment with the combination of 19, 20-EDP +TPPU and treatment with 14, 15-EET + TPPU (Table S6 A, Figure 3B).
In comparing the SBP data for Ang II and for the apparently most efficacious treatment of the combination of 19, 20- EDP and TPPU, the quadratic drivers are equally important in the model of both the treatment data and the Ang II data (Table S2 A), suggesting that the treatment becomes more effective with time. Thus, we also compared the SBP among all the groups on day 12, which revealed a significant main effect of ‘group’ (F = 3.39, P = 0.02). Further analysis using pairwise group comparisons revealed that treatment with the combination of 19, 20- EDP and TPPU results in lower BP as compared to Ang II alone (P < 0.01) as well as to treatment with 19, 20- EDP alone (P = 0.03). We further examined the differences in the last day SBP values between the groups using model comparison, which supported the results of the mixed effects model (Table S6 B). In addition, this analysis revealed a strong predictive effect when comparing SBP in Ang II infused animals receiving the combination of 19, 20- EDP and TPPU treatment and of those receiving the combination of 14, 15- EET and TPPU treatments (Table S6 B).
Effects of 19, 20-EDP and 14, 15- EET infusion on the P450 metabolites of the ARA cascade
To examine the interactions between the reduction in BP and modulation of the ARA cascade by 19, 20- EDP and 14, 15-EET, we determined plasma concentration and renal levels of the EpFAs across all the groups (Figure 4). Because the BP reduction is most effective when Ang II infused animals were administered with 19, 20-EDP and TPPU at 0.2 mg/kg, we did not further examine the changes in the ARA cascade in groups treated with the 0.02 and 0.06 mg/kg dose of TPPU.
FIGURE 4.
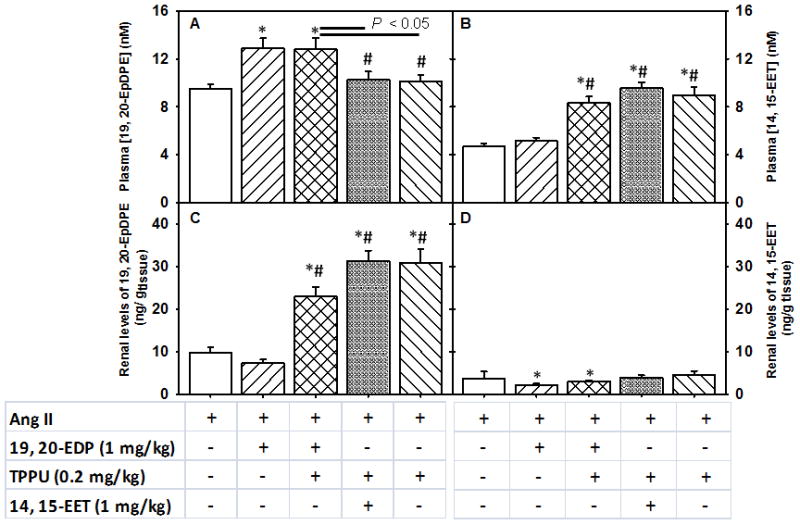
Plasma and renal levels of 19, 20- EDP and 14, 15-EET upon subcutaneous infusion of EpFAs in angiotensin-II dependent hypertension. Plasma and renal levels are shown in separate Panels. Panel A. Plasma concentration of 19, 20-EDP, Panel B. Plasma concentration of 14, 15- EET, Panel C. Renal levels of 19, 20-EDP and Panel D. Renal levels of 14, 15- EET. Statistically significant differences were determined by one-way ANOVA followed by pairwise comparisons. P < 0.05, *compared with Ang II animals, #compared with Ang II + 19, 20- EDP animals. Ang II, n=8; Ang II + 19, 20- EDP, n= 9; Ang II + 19, 20- EDP + TPPU, n=10; Ang II + 14, 15- EET + TPPU, n= 9; Ang II + TPPU, n= 9. Data are mean ± SEM.
Administration of 19, 20-EDP and 14, 15-EET by subcutaneous infusion resulted in approximately 2 fold increase in plasma levels of both EpFAs in corresponding groups of mice as compared to Ang II infused animals (P < 0.05, Figure 4, Panels A and B). Similarly, renal levels of 19, 20-EDP significantly increased in Ang II infused animals that are treated with the combination of 19, 20- EDP and TPPU, and with TPPU alone as compared to Ang II alone or 19, 20-EDP treated animals (Figure 4, Panel C). While renal levels of 14, 15-EET did not change in Ang II infused animals that are treated with the combination of 14, 15- EET and TPPU, it significantly decreased in those treated with 19, 20-EDP and combination of 19, 20- EDP and TPPU when compared to Ang II infused animals (Figure 4, Panel D). Comparing the tissue levels of the two EpFAs, we observed that the tissue levels of 19, 20- EDP were 6 times higher than the tissue levels of 14, 15-EET in animals receiving these EpFAs. Plasma concentration of both EpFAs was comparable.
In order to further examine the modulation of the P450 pathway of the ARA cascade, we quantified other P450 metabolites of DHA, EPA, ARA and linoleic acid (LA) (Figures 5 and 6). Similar to the changes in the plasma concentration of each EpFA, the summed plasma EDPs and EETs showed a similar pattern of change across all the groups (Figure 5, Panels A and B), suggesting that infusion with the 19, 20-EDP and 14, 15-EET did not alter the levels of other regioisomeric forms (see Supplemental Digital Content 1, Table S7 and S8).
FIGURE 5.
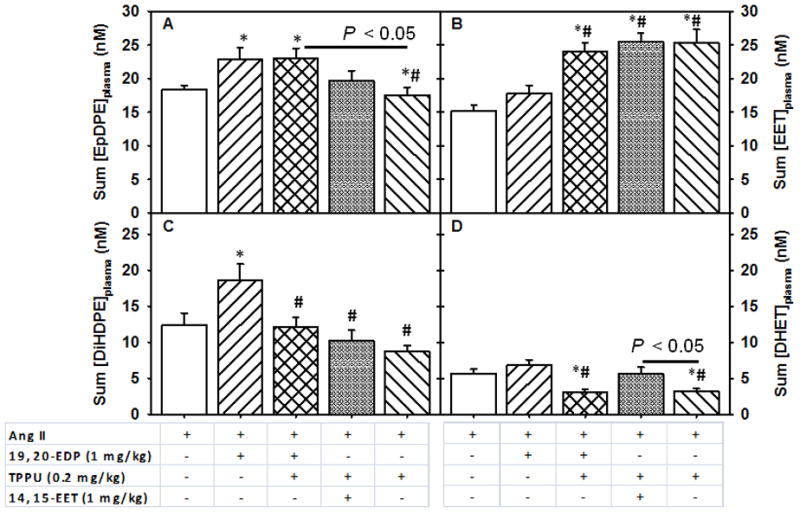
Changes in plasma levels of summed EDPs and EETs and their corresponding diols upon subcutaneous infusion of EpFAs in angiotensin-II dependent hypertension. Summed plasma concentrations of Panel A. EDPs, which includes 10, 11-, 13, 14-, 14, 15- and 19,20-EDP, Panel B. EETs, which include 8, 9-, 11, 12- and 14, 15-EET, Panel C. DiHDPEs, which includes 10, 11-, 13, 14-, 14, 15- and 19,20-DiHDPE and Panel D. DHETs, which include 8, 9-, 11, 12- and 14, 15-DHETs. Statistically significant differences were analyzed by one-way ANOVA followed by pairwise comparisons. P < 0.05, *compared with Ang II animals, #compared with Ang II + EDP animals. Ang II, n=8; Ang II + 19, 20- EDP, n= 9; Ang II + 19, 20- EDP + TPPU, n=10; Ang II + 14, 15- EET + TPPU, n= 9; Ang II + TPPU, n= 9. Data are mean ± SEM.
FIGURE 6.
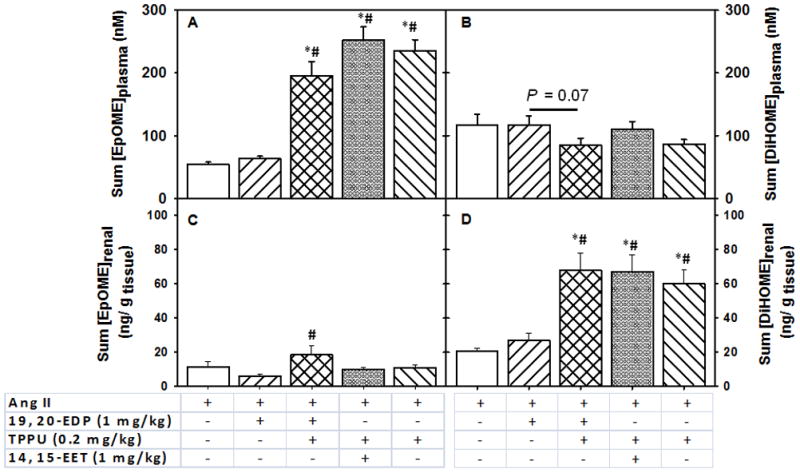
Changes in the summed linoleic acid P450 and sEH metabolites in the plasma and kidney upon subcutaneous infusion of EpFAs in angiotensin-II dependent hypertension. Summed EpOMEs include 9, 10- EpOME and 12, 13- EpOME, and summed DiHOMEs include 9, 10- DiHOME and 12, 13- DiHOME. Summed plasma concentrations of Panel A. EpOMEs, Panel B. DiHOMEs, and summed renal levels of Panel C. EpOMEs, Panel D. DiHOMEs. Statistically significant differences were analyzed by one-way ANOVA followed by pairwise comparisons. P < 0.05, *compared with Ang II animals, #compared with Ang II + 19, 20- EDP animals. Ang II, n=8; Ang II + 19, 20- EDP, n= 9; Ang II + 19, 20- EDP + TPPU, n=10; Ang II + 14, 15- EET + TPPU, n= 9; Ang II + TPPU, n= 9. Data are mean ± SEM.
Next, we evaluated how administration of TPPU with either of the EpFAs affected the levels of sEH metabolites (diol metabolites). The 19, 20-DiHDPE (dihydroxydocosapentaenoic acid) and 14, 15-DHET (dihydroxyeicosatrienoic acid) are generated by sEH metabolism of 19, 20-EDP and 14, 15-EET. Consistent with inhibition of sEH, 19, 20-DiHDPE significantly decreased upon treatment with the combination of 19, 20- EDP and TPPU as compared to Ang II (P < 0.05) or treatment with 19, 20- EDP (P > 0.05) in the kidney. Similarly, the summed plasma concentration of DiHDPEs decreased significantly with the treatment of the combination of 19, 20- EDP and TPPU, the combination of 14, 15 EET and TPPU or with TPPU alone when compared to 19, 20- EDP treatment alone (Figure 5, Panel C). We observed slight changes in other regioisomeric forms of DiHDPEs in the plasma and kidney (Tables S7 and S8). The sEH metabolites of ARA, the DHETs tend to decrease in the plasma, but not in the kidney with TPPU treatment as compared to Ang II infusion alone (Figure 5-Panel D, Table S7).
In the western diet, levels of LA are very high as are their epoxide and diol metabolites, which are excellent markers of LA oxidative metabolism.43 The LA epoxides, 9, 10- and 12, 13-EpOMEs (epoxyoctadecenoic acid) increased significantly in plasma and kidney upon treatment with the combination of 19, 20-EDP and TPPU, combination of 14, 15-EET and TPPU, or TPPU alone (Figure 6, Panel A) as compared to treatment with 19, 20- EDP alone or Ang II infusion (P < 0.05) (Tables S7 and S8). The diols that are produced from EpOMEs by sEH metabolism, DiHOMEs; decreased slightly with the combination treatment of 19, 20-EDP and TPPU as compared to treatment with 19, 20-EDP alone (P = 0.07, Figure 6, Panel B). In contrast, in the kidney EpOMEs increased only with the combination of 19, 20-EDP and TPPU as compared to treatment with 19, 20-EDP alone (Figure 6, Panel C). Renal DiHOMEs increased approximately 3 fold with the combination of 19, 20- EDP and TPPU, combination of 14, 15-EET and TPPU, or TPPU alone as compared to treatment with 19, 20- EDP alone or Ang II infusion (P < 0.05, Figure 6, Panel D).
Only slight changes were found in the tissue or plasma levels of other P450 and sEH products of ARA and EPA across all the groups (Tables S7 and S8).
In vivo target engagement of sEH inhibition
To test the hypothesis that inhibition of sEH protects the EpFAs from hydrolysis while maintaining their potency, animals were provided TPPU in their drinking water along with subcutaneous infusion of 19, 20-EDP or 14, 15-EET. One group received TPPU alone (Ang II + TPPU animals) to control for the previously reported anti-hypertensive effects associated with sEHIs.37, 38, 44 In order to provide evidence for sEH inhibition, we determined the TPPU concentration in blood samples that were collected at the end of each week after Ang II infusion. Even though we observed a mild accumulation of TPPU, the difference in TPPU levels on day 7 vs. 14 did not reach statistical significance (Table 1), indicating the TPPU levels were near steady state and sufficient to largely inhibit the sEH. The renal levels of TPPU were comparable among the groups (Table 1). Overall, both blood and renal levels of TPPU was at least 300 fold above the Ki of TPPU (2.5 nM or 2.5e10−4 μg/gtissue). 45
TABLE 1.
Plasma concentration and tissue level of the sEH inhibitor TPPU.
| [TPPU]plasma (nmol/L) | [TPPU]kidney (nmol/g tissue) | ||||
|---|---|---|---|---|---|
|
| |||||
| n | Day 71 | Day 14 | P value (day 7 vs. 14) | Day 14 | |
| Ang II + TPPU | 5 | 630 ± 60 | 800 ± 50 | 0.09 | 1.3 ± 0.1 |
| Ang II + 19, 20-EDP + TPPU | 5 | 680 ± 60 | 850 ± 90 | 0.08 | 1.3 ± 0.1 |
| Ang II + 14,15-EET + TPPU | 5 | 670 ± 70 | 680 ± 90 | 0.8 | 1.1 ± 0.1 |
Time after ANG II infusion started. Data are mean ± SEM. The water intake of animals was in average of 5 ± 1, 4.9 ± 0.8 and 5.1 ± 0.7 ml/day in animals treated with Ang II + TPPU, Ang II + 19, 20- EDP + TPPU and Ang II + 14, 15- EET + TPPU for the duration of the experiment. See Table S12 for TPPU concentrations in the drinking water.
To provide further evidence for inhibition of sEH, we examined epoxide-to-diol ratio in the plasma and the kidney. The 19, 20-EDP-to-19, 20-DiHDPE ratio increased about 3.4–4 fold in the kidney and the 14, 15-EET-to-14, 15-DHET ratio increased approximately 2–3.5 fold in the plasma with the combination of 19, 20- EDP and TPPU, TPPU alone and combination of 14, 15-EET and TPPU as compared to Ang II infusion or treatment with 19, 20- EDP alone. While the epoxide-to-diol ratio of the ARA, EPA and DHA was comparable among the groups, we observed that EpOME-to-DiHOME ratio was 2 fold higher in the presence of the combination of 19, 20-EDP and TPPU as compared to the combination of 14, 15-EET and TPPU.
We also examined correlations between last day SBP values and 19, 20-EDP, 14, 15-EET and summed plasma or tissue levels of the EpFAs. We observed a significant correlation between the last day BP and tissue levels of 19, 20-EDP (R = −0.44, P < 0.01) as well as summed tissue levels of EDPs (R = −0.38, P = 0.02). Also, we observed a strong correlation between the tissue levels of TPPU and an increase in tissue levels of 19, 20- EDP (R = 0.65, P < 0.01), which is consistent with sEH inhibition. Furthermore, plasma concentration of 14, 15-EET (R = −0.39, P = 0.02) and summed plasma EpOMEs (R = −0.34, P = 0.04) showed a statistically significant correlation with the reduction in SBP.
Effects of 19, 20- EDP and 14, 15- EET on the COX pathway
To examine anti-inflammatory effects of the administered EpFAs, we quantified the major prostaglandins that are produced from ARA in the COX pathway in the kidney. The plasma concentrations of PGE2 and PGD2 did not differ across all the groups (P > 0.05) (Figure 7, Panels A and B). However, the tissue levels of PGE2 significantly decreased by half upon treatment with 19, 20 EDP, combination of 19, 20- EDP and TPPU and the combination of 14, 15-EET and TPPU as compared to Ang II infusion alone (P < 0.05) (Figure 7, Panel C). Another major prostaglandin, PGD2 decreased only with the combination of 14, 15-EET and TPPU, or TPPU alone as compared to Ang II infusion and treatment with 19, 20- EDP alone (P < 0.05) (Figure 7, Panel D). No changes were observed in other prostaglandins either in the plasma or in the kidney (Tables S9 and S10).
FIGURE 7.
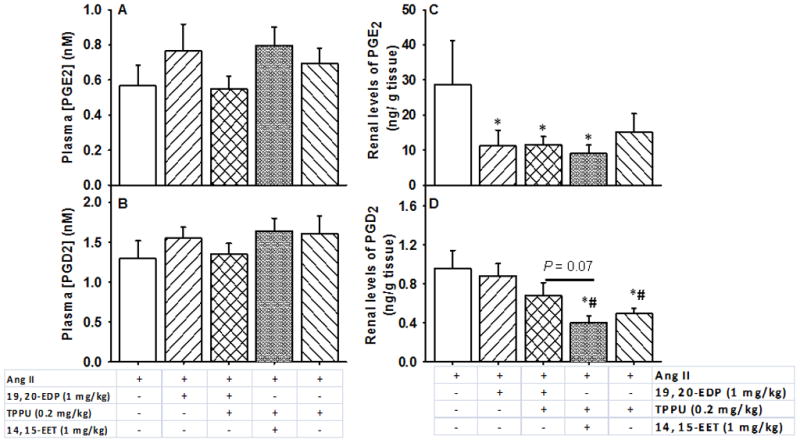
Effects of 19, 20- EDP and 14, 15-EET on plasma and renal prostaglandins. Plasma concentrations of A. PGE2, B. PGD2 and renal levels of C. PGE2, D. PGD2 are shown. Statistically significant differences were determined by one-way ANOVA followed by pairwise comparisons. P < 0.05, *compared with Ang II animals, #compared with Ang II + 19, 20- EDP animals. Ang II, n=8; Ang II + 19, 20- EDP, n= 9; Ang II + 19, 20- EDP + TPPU, n=10; Ang II + 14, 15- EET + TPPU, n= 9; Ang II + TPPU, n= 9. Data are mean ± SEM.
LOX pathway
To examine whether the LOX metabolites in the ARA cascade are altered in response to given treatments, we determined both the tissue levels and plasma concentration of HETEs (hydroxyeicosatetraenoic acid). Among the HETEs, tissue levels of 15-HETE and 15(S)-HETrE increased upon treatment with the combination of 19, 20- EDP and TPPU, the combination of 14, 15-EET and TPPU or TPPU alone as compared to 19, 20- EDP treatment alone (Table S10); however plasma concentration of none of these metabolites were affected by any of the treatments (Table S9).
We have also provided a comparison of the renal oxylipin levels of the sham operated controls to that of Ang-II infused animals in Table S11. In general, while the tissue levels of the P450 metabolites of the ARA, LA, EPA and DHA decreased with Ang-II treatment, prostaglandins tended to increase with the Ang-II infusion.
Effects of 19, 20-EDP and 14, 15- EET infusion on the renal expression of ACE-2 and AT1a message
We have previously shown that supplementation of a diet rich in omega-3 fatty acids (EPA and DHA) in hypertensive mice up-regulated the ACE-2 (the message for angiotensin converting enzyme-2). This enzyme degrades Ang-II and counteracts the vasoconstrictor action of Ang-II in the renin-angiotensin-aldosterone system (RAAS). Therefore, we examined whether the DHA epoxide 19, 20- EDP would also affect the gene expression of the ACE-2 in the kidney. As shown in Figure 8A we evaluated various combinations of Ang-II, 19, 20-EDP, the sEHI TPPU, and 14, 15-EET on levels of the AT1a message. Pairwise group comparisons using one-way ANOVA revealed a significant main effect of ‘group’ for AT1a (F: 10.36, P < 0.01) (Figure 8A). We also found that treatment with the combination of TPPU and 19, 20-EDP down-regulated the mRNA expression of the AT1a more than the 19, 20-EDP treatment alone (P = 0.04). In addition, the expression pattern of the AT1a message across all the groups correlated well with the last day SBP (as percent change from baseline) with a Pearson’s correlation coefficient of 0.49 (P = 0.01, two-tailed t-test).
FIGURE 8.
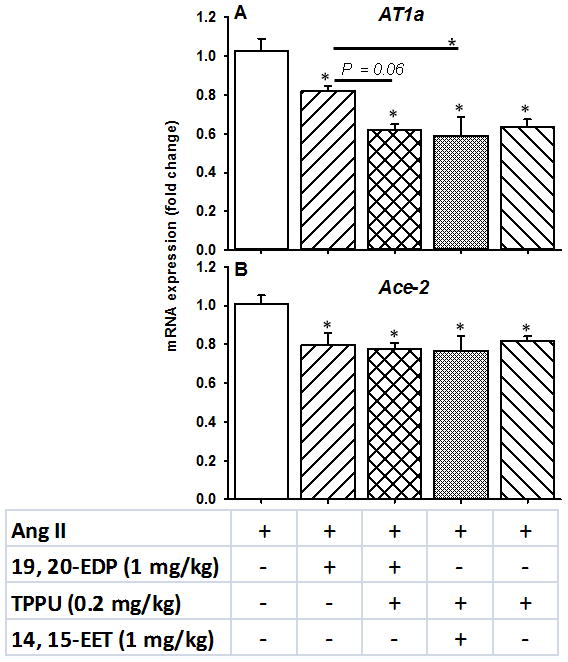
Changes in the renal mRNA expression of the A. AT1a and B. Ace-2 of the renin-angiotensin-aldosterone system upon treatment with 19, 20-EDP, 14, 15-EET and TPPU. Statistically significant differences were determined by one-way ANOVA followed by pairwise group comparisons. P < 0.05, *compared with Ang II animals. Ang II, n=7; Ang II + 19, 20-EDP, n= 6; Ang II + 19, 20- EDP + TPPU, n=7; Ang II + 14, 15- EET + TPPU, n= 6; Ang II + TPPU, n= 6. Data are mean ± SEM.
The same analysis was run with the Ace-2 message (Figure 8B) where surprisingly all three treatments had about the same effect on reduction of the message level (significant main effect of group, F: 7.38, P < 0.01). The levels of circulating Ang-II should be addressed in future studies, because the plasma samples required for those measurements were inadequate in our study.
DISCUSSION
Our primary goal was to test if 19, 20-EDP is a mediator of the anti-hypertensive actions of DHA in Ang II dependent hypertension. The main finding of our study is that 19, 20- EDP exhibits anti-hypertensive properties alone and shows a larger effect in the presence of a sEH inhibitor presumably due to stabilization of this epoxide from degradation by sEH. These data support the activity of EDP and EET being due to the epoxides and not their diol metabolites. In addition, our data suggest that 19, 20-EDP is more efficacious than 14, 15-EET in lowering Ang II induced increase in SBP either alone or when combined with TPPU. Because 14, 15-EET is a much better substrate for sEH than is 19, 20-EDP this comparison was made in the presence of a potent sEHI. We found that the sEHI improves the effect of 19, 20- EDP more than 14, 15-EET. Based on the renal eicosanoid profiling, our results also suggest that 19, 20- EDP like 14, 15-EET modulates the COX pathway by reducing major pro-inflammatory prostaglandins in the kidney.
Studies on the mechanisms of the anti-hypertensive effects of the long chain ω-3 PUFAs are limited. We show that 19, 20- EDP alone lowers SBP in Ang II infused mice (Figure 1–3), which supports our hypothesis that 19, 20-EDP is an in vivo mediator of the anti-hypertensive effects of DHA in angiotensin-II dependent hypertension. This finding is also in line with previous studies conducted on isolated aortic rings and BK channel studies indicating that EDPs contribute to the anti-hypertensive effects of ω-3 PUFAs. 23, 24, 46, 47 We also show that infusion with 19, 20- EDP results in a lower SBP as compared to 14, 15- EET, which agrees well with previous findings that EDPs are more potent vasodilators and activators of the BK channels than EETs in coronary microvessels.24 Of note, our results indicate that 14, 15-EET infusion is effective at lowering BP when administered with a potent sEHI. This approach provides a mechanism to circumvent the previously experienced difficulties in the delivery and study of EETs.26, 48–50 A recent study has shown that CYP1A1 (−/−) mice, which are hypertensive, exhibit normal vasorelaxation in response to DHA and EPA epoxide metabolites 19, 20-EDP and 17, 18-EpETE, respectively, but reduced vasorelaxation in response to EPA and DHA.14 Consistent with our findings, Agbor et al concluded that CYP 1A1 as well as CYP 2C/2J are involved in the metabolism of ω-3 PUFAs, and that 19, 20-EDP and 17, 18-EpETE might be mediators of the DHA and EPA action. However, potential stabilization of these epoxides in the presence of a sEH inhibitor was not explored in the studies by Agbor et al. Among other DHA epoxides, 16, 17-EDP and 13, 14- EDP are also promising candidates for lowering BP, particularly in the presence of a sEH inhibitor. This is because inhibition of sEH would likely affect the plasma and tissue levels of these epoxides more than 19, 20-EDP due to the higher kcat, kcat/Km and preference index of these EpFAs (Table S1). For example, EDPs reduce inflammatory pain in accordance with their kinetic parameters for sEH.22 Also, we have recently shown that EDPs can be stabilized by inhibition of sEH in vivo and in vitro.51
One of the difficulties in testing the hypothesis that 19, 20- EDPs lower blood pressure as the epoxide and not the diol metabolite is that 19, 20- EDP alone as well as the sEHI alone reduce Ang II driven blood pressure. The latter has been shown by multiple studies.37–40, 52 Since there is a narrow range between normal BP and the BP which leads to mortality and confounding effects, several studies were run to optimize the dose of the sEHI. We previously published that TPPU at the doses of 0.2 and 0.6 mg/kg lower blood pressure to near baseline levels and small additional blood pressure reduction is obtained with still higher doses.28 Thus, we determined steady state blood levels of TPPU following administration of 0.02, 0.06 and 0.2 mg/kg in the drinking water (Figure S2). We then examined the dose-responses of these levels of TPPU alone and in combination with 19, 20-EDP in reducing BP in Figures S1 and S4. The overall dose response of TPPU in combination with 19, 20- EDP (Figure 2) shows a clear dose response of TPPU in reducing BP in the absence and presence of 1 mg/kg 19, 20- EDP. The response of TPPU alone appears dose linear from 0.02 to 0.2 mg/kg but only 0.2 mg/kg of TPPU seemed to interact (additive effect) with 19, 20- EDP to lower BP. Because the sEH is such an efficient enzyme,22 a high percentage inhibition and a high level of enzyme occupancy by the sEHI is anticipated necessary for efficacy in significantly reducing EpFA hydrolysis.45 Based on these data we selected a dose of 0.2 mg/kg for oral administration of TPPU to use with the infused EETs and EDPs in this study (Figure 2).
We showed that the combination of 19, 20-EDP and the sEH inhibitor TPPU (0.2 mg/kg) results in a lower BP when compared to Ang II infused animals that are treated with 19, 20-EDP or TPPU alone. Although the P values that are associated with these differences were close to the threshold, mainly due to the efficacy of TPPU in lowering BP, 28, 34, 37, 38, 44 further analyses of the BP data supported these observations. The AUC analysis of the SBP data supported that the combination treatment is effective on both days 2–12 and days 6–12 (Figure S6). Moreover, this finding is consistent with the significant increase in the renal levels of 19, 20- EDP with the combination treatment of 19, 20-EDP and TPPU (0.2 mg/kg), but not with 19, 20-EDP alone. This difference was significant on the last day of the study, where we expected the effects of treatment to be maximal. In addition, model comparison of the SBP data revealed clear time dependence and therefore we focused our analyses on the SBP between days 6–12. Also, model analysis revealed large effects of Ang II and Ang II + 19, 20-EDP + TPPU (0.2 mg/kg) treatments on BP. Consistent with this finding, marginal means associated with day 6–12 indicated a larger effect size for the combination treatment with 19, 20- EDP and TPPU (0.2 mg/kg) when compared to Ang II infusion alone and treatment with Ang II + 14, 15- EET + TPPU (0.2 mg/kg) (Figure 3A). This analysis also revealed a predictive difference in SBP between 14, 15-EET and 19, 20- EDP treatments, suggesting that 19, 20- EDP is more effective than 14, 15-EET in combination with TPPU. Furthermore, these results suggest that 19, 20-EDP should be infused with a potent sEHI to be most effective in lowering BP.
Our major goal in this study was to test the hypothesis that the reduction in hypertension observed with an omega-3 based diet is at least in part on the increased EDP metabolites. However, there are therapeutic implications of this work. For example, angiotensin converting enzyme inhibitors and angiotensin II receptor blockers such as losartan are major therapeutics for hypertension. Also, there is increased prevalence of omega-3 lipids as value added products, and sEH inhibitors are being considered as possible therapeutics. Thus, we provide a brief comparison of losartan and sEHIs. The effectiveness of sEHIs and losartan has been compared previously in studies where both losartan and sEHIs lower BP and prevent hypertension induced renal damage in rodent models of hypertension.44,53 While losartan acts through the RAAS system and does not affect the bioavailability of EETs, sEHIs target the ARA cascade through an increase in EETs and EDPs and influence RAAS only indirectly. In addition, EETs not only regulate BP but also display other beneficial effects including prevention of inflammation, pain, Ang-II induced cardiac hypertrophy and renal injury.54,39, 53, 55 Losartan and related compounds as well are increasingly used to reduce renal inflammation. Of note, losartan, but not sEHI treatment results in reduced BP in normotensive animals.44 This finding suggests that sEHIs but not Ang-II receptor blockers provide a compensatory regulation of BP. Such feature of sEHIs has also been demonstrated in LPS induced hypotension, where sEHIs reverse BP to control levels in hypotensive animals rather than a decrease as seen in hypertensive animals.56 Losartan has the advantage of being more effective than sEHI or diet at giving a profound reduction in BP. This of course comes with a cost of a limited therapeutic index, while in theory drug induced hypotension will be hard to achieve with either an omega-3 diet, sEHI or a combination. Additionally, BP reduction with current anti-hypertensive drugs might require a combination of different anti-hypertensive drugs to lower BP back to control levels, which may not be achieved without side effects.57 Losartan can cause hyperkalemia while sEHI and epoxy fatty acids lead to natriuresis.58 Since sEHI modulate epithelial sodium channels, this may contribute to long term anti-hypertensive therapy. Thus, the drugs could be considered, in part, complementary with regard to monovalent cation balance. Increasingly hypertension is addressed by drug combinations. Hopefully in the future dietary intervention, particularly dietary intervention combined with metabolomic evaluation could be integrated with these drug combinations. Possibly there is a future role for sEHI or mimics of EETs and EDPs in these combined therapies.
Regarding the underlying mechanisms of the anti-hypertensive effects of the sEHIs and DHA epoxides, down-regulation of the AT1a mRNA expression correlates well with the last day BP, suggesting that both ARA and DHA P450 metabolites directly affect the components of the RAAS. In contrast to our previous study, we did not observe an up-regulation in the renal expression of the Ace-2 message by any of the treatments, possibly due to the differences in the diet, which included both EPA and DHA in our previous study.28
The administration of 19, 20- EDP resulted in changes mostly in the metabolites of the P450 pathway of the ARA cascade (Figures 4–7, Tables S7 and S8). Overall, the combination of 19, 20- EDP and TPPU (0.2 mg/kg) increased the tissue levels of DHA and LA epoxides, and decreased ARA epoxides, whereas the combination of 14, 15-EET and TPPU (0.2 mg/kg) increased the tissue levels of only DHA epoxides in Ang II infused animals (Figure 4, Panel A). Also, we expected that the plasma concentration of 19, 20- EDP would be higher in animals treated with 19, 20-EDP and TPPU (0.2 mg/kg) as compared to 19, 20-EDP treated animals due to stabilization of the epoxide by sEH inhibition. In the kidney, the results were similar to our expectation, except for the surprising increase in 19, 20- EDP in groups treated with TPPU (0.2 mg/kg) and 14, 15-EET + TPPU (0.2 mg/kg) as compared to animals treated with 19, 20-EDP alone. This could result from the sEHI stabilizing the endogenously available 19, 20-EDP. Similarly, we also observed an unexpected increase in 14, 15-EET in plasma of animals treated with the combination of 19, 20-EDP and TPPU (0.2 mg/kg). Considering the presence of endogenous 19, 20-EDP and 14, 15-EET in addition to the infused fatty acid epoxide, inhibition of sEH seems to explain the unexpected increase in renal 19, 20-EDP and plasma 14, 15-EET in animals treated with 14, 15-EET + TPPU (0.2 mg/kg) and 19, 20-EDP + TPPU (0.2 mg/kg), respectively. Still, we observed that the reduction in SBP is most effective in animals treated with 19, 20-EDP and TPPU (0.2 mg/kg) when compared to animals treated with 14, 15-EET + TPPU (0.2 mg/kg), 19, 20-EDP alone or TPPU (0.2 mg/kg) alone. Altogether, these findings suggest that both 19, 20- EDP and 14, 15-EET might contribute to the reduction in SBP in groups treated with 19, 20-EDP + TPPU (0.2 mg/kg) and 14, 15-EET + TPPU (0.2 mg/kg). Since the changes in 19, 20-EDP are more pronounced in the kidney than the plasma and changes in 14, 15- EET are more pronounced in the plasma than in the kidney, the reduction in Ang II augmented SBP might be a concerted effect of 19, 20-EDP and 14, 15- EET acting locally in the kidney and systemically. The tissue levels of EDP regioisomers upon infusion with 19, 20- EDP or with oral administration of TPPU were comparable to our previous study, where the ω-3 PUFAs were administered in the diet.28 Because we did not attempt to minimize the levels of endogenously available EpFAs, we cannot disregard the contribution of a combined effect of EpFAs to the reduction in Ang II induced increase in BP.
In addition to ARA and DHA epoxides, the increase in EpOMEs upon treatment with TPPU (Figure 6, Panel A) is consistent with the fact that EpOMEs are substrates of sEH. Thus inhibition of sEH also stabilizes EpOMEs as it does the EETs or EDPs,22, 59 and inhibition of sEH should lead to decreased levels of DiHOMEs. However, we observed a two fold increase in renal DiHOMEs in groups treated with TPPU (0.2 mg/kg) (Figure 6, Panel D). While we do not have a full explanation for these surprising results, this increase might be a homeostasis mechanism related to a biological activity of DiHOMEs in the kidney. Also, the total renal EpOME-to-DiHOME ratio is higher with the combination treatment of 19, 20-EDP + TPPU (0.2 mg/kg) as compared to 14, 15-EET + TPPU (0.2 mg/kg), which seems to be a major difference between these two groups with differing SBP values. We have shown before that an increased EpOME-to-DiHOME ratio is an indication of sEH inhibition, target engagement and moderately correlates with the anti-atherosclerotic effects of sEHI.59 Such evidence suggests a complex biological process involving both inflammation and regulation of BP with a delicate balance among the EDPs, EpOMEs, EETs and their corresponding diols. Although the role of these diols is largely unknown in the kidney and in regulation of BP, the sEH metabolism of the EpOMEs to DiHOMEs has been suggested to be a detoxification pathway at the mitochondrial level in the kidney.60
The oxylipin profiling of the ω-3 and ω-6 PUFAs in the ARA cascade revealed that both 19, 20- EDP and 14, 15- EET infusion results in reduced renal markers of inflammation such as decreased renal PGE2 and PGD2 when compared to Ang II infused animals. These results are consistent with our previous study where the animals were provided with dietary DHA and EPA.28 Also, inhibition of sEH has been shown to reduce renal cytokine levels, increase the bioavailability of EETs, which have been linked to lower BP, reduced renal inflammation and injury in rodent models of inflammation and hypertension.39, 61–63 Here, we did not examine the underlying mechanisms of the potential anti-inflammatory properties of the 19, 20- EDP. Instead, we focused on the mechanisms of the anti-hypertensive effects of the 19, 20-EDP, especially the changes in the ARA cascade.
Despite the decrease in renal PGs, SBP decreased with the infusion of EpFAs in our study. This is contrary to the effects of the non-steroidal anti-inflammatory drugs (NSAIDs) on blood pressure, which is mildly increased with the use of NSAIDs.64
CONCLUSION
Our results support the hypothesis that 19, 20- EDP is a mediator of the anti-hypertensive effects observed with dietary DHA in angiotensin-II dependent hypertension. Also, we showed that 19, 20- EDP requires metabolic stabilization using a sEH inhibitor to be most effective in lowering BP. However, we cannot disregard endogenously available EETs or other EpFAs, which could be enhanced by sEH inhibition and therefore might have contributed to the reduction of BP in our study. We anticipate that the relative effects of sEHI on other regioisomers of EDPs will be larger than for the 19, 20- EDP since they are better substrates of the sEH. Future studies are required to isolate the biological actions of 19, 20-EDP either with a dose adjustment of a sEHI or using EET and EDP antagonists. Furthermore, our results indicate more pronounced changes in EDPs in the kidney than the plasma and the opposite effect with EETs, suggesting that renal versus systemic effects of these EpFAs might be different and should be tested in future studies.
Supplementary Material
Acknowledgments
SOURCES OF FUNDING
This work was supported by NIEHS grant R01 ES002710 and NIEHS Superfund Research Program grant P42 ES004699. Analytical work was partially supported by the NIH and NIDDK grant U24 DK097154. BDH is a George and Judy Marcus senior fellow of the American Asthma Foundation.
Abbreviations
- ARA
arachidonic acid
- DHA
docosahexaenoic acid
- DHET
dihydroxyeicosatrienoic acid
- DiHOME
dihydroxyoctadecenoic acid
- DiHDPE
dihydroxydocosapentaenoic acid
- DiHETE
dihydroxyeicosatetraenoic acid
- EET
epoxyeicosatrienoic acid
- EPA
eicosapentaenoic acid
- EDP
epoxydocosapentaenoic acid
- EpETE
epoxyeicosatetraenoic acid
- EpOME
epoxyoctadecenoic acid
- HETE
hydroxyeicosatetraenoic acid
- HEPE
hydroxy-eicosapentaenoic acid
- PG
prostaglandin
- TXB2
thromboxane B2
Footnotes
DISCLOSURES
The University of California, Davis has filed patents in the area of soluble epoxide hydrolase inhibitors for the treatment of diseases.
References
- 1.Cabo J, Alonso R, Mata P. Omega-3 fatty acids and blood pressure. Br J Nutr. 2012;107 (Suppl 2):S195–200. doi: 10.1017/S0007114512001584. [DOI] [PubMed] [Google Scholar]
- 2.Mori TA. Omega-3 fatty acids and hypertension in humans. Clin Exp Pharmacol Physiol. 2006;33(9):842–846. doi: 10.1111/j.1440-1681.2006.04451.x. [DOI] [PubMed] [Google Scholar]
- 3.Liu JC, Conklin SM, Manuck SB, Yao JK, Muldoon MF. Long-chain omega-3 Fatty acids and blood pressure. Am J Hypertens. 2011;24(10):1121–1126. doi: 10.1038/ajh.2011.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calder PC. Mechanisms of action of (n-3) fatty acids. The Journal of nutrition. 2012;142(3):592S–599S. doi: 10.3945/jn.111.155259. [DOI] [PubMed] [Google Scholar]
- 5.Jump DB, Depner CM, Tripathy S. Omega-3 fatty acid supplementation and cardiovascular disease. J Lipid Res. 2012;53(12):2525–2545. doi: 10.1194/jlr.R027904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arterburn LM, Hall EB, Oken H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. The American journal of clinical nutrition. 2006;83(6 Suppl):1467S–1476S. doi: 10.1093/ajcn/83.6.1467S. [DOI] [PubMed] [Google Scholar]
- 7.Mavrommatis Y, Ross K, Rucklidge G, Reid M, Duncan G, Gordon MJ, Thies F, Sneddon A, de Roos B. Intervention with fish oil, but not with docosahexaenoic acid, results in lower levels of hepatic soluble epoxide hydrolase with time in apoE knockout mice. Br J Nutr. 2010;103(1):16–24. doi: 10.1017/S0007114509991450. [DOI] [PubMed] [Google Scholar]
- 8.Mori TA, Watts GF, Burke V, Hilme E, Puddey IB, Beilin LJ. Differential effects of eicosapentaenoic acid and docosahexaenoic acid on vascular reactivity of the forearm microcirculation in hyperlipidemic, overweight men. Circulation. 2000;102(11):1264–1269. doi: 10.1161/01.cir.102.11.1264. [DOI] [PubMed] [Google Scholar]
- 9.Mori TA, Bao DQ, Burke V, Puddey IB, Beilin LJ. Docosahexaenoic acid but not eicosapentaenoic acid lowers ambulatory blood pressure and heart rate in humans. Hypertension. 1999;34(2):253–260. doi: 10.1161/01.hyp.34.2.253. [DOI] [PubMed] [Google Scholar]
- 10.Theobald HE, Goodall AH, Sattar N, Talbot DC, Chowienczyk PJ, Sanders TA. Low-dose docosahexaenoic acid lowers diastolic blood pressure in middle-aged men and women. The Journal of nutrition. 2007;137(4):973–978. doi: 10.1093/jn/137.4.973. [DOI] [PubMed] [Google Scholar]
- 11.Cazzola R, Russo-Volpe S, Miles EA, Rees D, Banerjee T, Roynette CE, Wells SJ, Goua M, Wahle KW, Calder PC, Cestaro B. Age- and dose-dependent effects of an eicosapentaenoic acid-rich oil on cardiovascular risk factors in healthy male subjects. Atherosclerosis. 2007;193(1):159–167. doi: 10.1016/j.atherosclerosis.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 12.Engler MM, Engler MB, Pierson DM, Molteni LB, Molteni A. Effects of docosahexaenoic acid on vascular pathology and reactivity in hypertension. Exp Biol Med (Maywood) 2003;228(3):299–307. doi: 10.1177/153537020322800309. [DOI] [PubMed] [Google Scholar]
- 13.Diep QN, Amiri F, Touyz RM, Cohn JS, Endemann D, Neves MF, Schiffrin EL. PPARalpha activator effects on Ang II-induced vascular oxidative stress and inflammation. Hypertension. 2002;40(6):866–871. doi: 10.1161/01.hyp.0000037969.41360.cc. [DOI] [PubMed] [Google Scholar]
- 14.Agbor LN, Walsh MT, Boberg JR, Walker MK. Elevated blood pressure in cytochrome P4501A1 knockout mice is associated with reduced vasodilation to omega-3 polyunsaturated fatty acids. Toxicol Appl Pharmacol. 2012;264(3):351–360. doi: 10.1016/j.taap.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Engler MB, Engler MM. Docosahexaenoic acid--induced vasorelaxation in hypertensive rats: mechanisms of action. Biological research for nursing. 2000;2(2):85–95. doi: 10.1177/109980040000200202. [DOI] [PubMed] [Google Scholar]
- 16.Vecchio AJ, Simmons DM, Malkowski MG. Structural basis of fatty acid substrate binding to cyclooxygenase-2. J Biol Chem. 2010;285(29):22152–22163. doi: 10.1074/jbc.M110.119867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maldonado-Rojas W, Olivero-Verbel J. Potential interaction of natural dietary bioactive compounds with COX-2. Journal of molecular graphics & modelling. 2011;30:157–166. doi: 10.1016/j.jmgm.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Fer M, Dreano Y, Lucas D, Corcos L, Salaun JP, Berthou F, Amet Y. Metabolism of eicosapentaenoic and docosahexaenoic acids by recombinant human cytochromes P450. Arch Biochem Biophys. 2008;471(2):116–125. doi: 10.1016/j.abb.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 19.Lucas D, Goulitquer S, Marienhagen J, Fer M, Dreano Y, Schwaneberg U, Amet Y, Corcos L. Stereoselective epoxidation of the last double bond of polyunsaturated fatty acids by human cytochromes P450. J Lipid Res. 2010;51(5):1125–1133. doi: 10.1194/jlr.M003061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Westphal C, Konkel A, Schunck WH. CYP-eicosanoids--a new link between omega-3 fatty acids and cardiac disease? Prostaglandins Other Lipid Mediat. 2011;96(1–4):99–108. doi: 10.1016/j.prostaglandins.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Arnold C, Markovic M, Blossey K, Wallukat G, Fischer R, Dechend R, Konkel A, von Schacky C, Luft FC, Muller DN, Rothe M, Schunck WH. Arachidonic acid-metabolizing cytochrome P450 enzymes are targets of {omega}-3 fatty acids. J Biol Chem. 2010;285(43):32720–32733. doi: 10.1074/jbc.M110.118406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morisseau C, Inceoglu B, Schmelzer K, Tsai HJ, Jinks SL, Hegedus CM, Hammock BD. Naturally occurring monoepoxides of eicosapentaenoic acid and docosahexaenoic acid are bioactive antihyperalgesic lipids. J Lipid Res. 2010;51(12):3481–3490. doi: 10.1194/jlr.M006007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang RX, Chai Q, Lu T, Lee HC. Activation of vascular BK channels by docosahexaenoic acid is dependent on cytochrome P450 epoxygenase activity. Cardiovasc Res. 2011;90(2):344–352. doi: 10.1093/cvr/cvq411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ye D, Zhang D, Oltman C, Dellsperger K, Lee HC, VanRollins M. Cytochrome p-450 epoxygenase metabolites of docosahexaenoate potently dilate coronary arterioles by activating large-conductance calcium-activated potassium channels. J Pharmacol Exp Ther. 2002;303(2):768–776. doi: 10.1124/jpet.303.2.768. [DOI] [PubMed] [Google Scholar]
- 25.Konkel A, Schunck WH. Role of cytochrome P450 enzymes in the bioactivation of polyunsaturated fatty acids. Biochim Biophys Acta. 2011;1814(1):210–222. doi: 10.1016/j.bbapap.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 26.Imig JD, Hammock BD. Soluble epoxide hydrolase as a therapeutic target for cardiovascular diseases. Nat Rev Drug Discov. 2009;8(10):794–805. doi: 10.1038/nrd2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fleming I. Cytochrome P450 epoxygenases as EDHF synthase(s) Pharmacol Res. 2004;49(6):525–533. doi: 10.1016/j.phrs.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 28.Ulu A, Harris TR, Morisseau C, Miyabe C, Inoue H, Schuster G, Dong H, Iosif AM, Liu JY, Weiss RH, Chiamvimonvat N, Imig JD, Hammock BD. Anti-inflammatory Effects of Omega-3 Polyunsaturated Fatty Acids and Soluble Epoxide Hydrolase Inhibitors in Angiotensin-II Dependent Hypertension. J Cardiovasc Pharmacol. 2013 doi: 10.1097/FJC.0b013e318298e460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rose TE, Morisseau C, Liu JY, Inceoglu B, Jones PD, Sanborn JR, Hammock BD. 1-Aryl-3-(1-acylpiperidin-4-yl)urea inhibitors of human and murine soluble epoxide hydrolase: structure-activity relationships, pharmacokinetics, and reduction of inflammatory pain. J Med Chem. 2010;53(19):7067–7075. doi: 10.1021/jm100691c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daugherty A, Rateri D, Hong L, Balakrishnan A. Measuring blood pressure in mice using volume pressure recording, a tail-cuff method. Journal of visualized experiments: JoVE. 2009;(27) doi: 10.3791/1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elmarakby AA, Williams JM, Imig JD, Pollock JS, Pollock DM. Synergistic actions of enalapril and tempol during chronic angiotensin II-induced hypertension. Vascular pharmacology. 2007;46(2):144–151. doi: 10.1016/j.vph.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feng M, Whitesall S, Zhang Y, Beibel M, D’Alecy L, DiPetrillo K. Validation of volume-pressure recording tail-cuff blood pressure measurements. Am J Hypertens. 2008;21(12):1288–1291. doi: 10.1038/ajh.2008.301. [DOI] [PubMed] [Google Scholar]
- 33.Yang J, Schmelzer K, Georgi K, Hammock BD. Quantitative profiling method for oxylipin metabolome by liquid chromatography electrospray ionization tandem mass spectrometry. Anal Chem. 2009;81(19):8085–8093. doi: 10.1021/ac901282n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koeners MP, Wesseling S, Ulu A, Sepulveda RL, Morisseau C, Braam B, Hammock BD, Joles JA. Soluble epoxide hydrolase in the generation and maintenance of high blood pressure in spontaneously hypertensive rats. Am J Physiol Endocrinol Metab. 2011;300(4):E691–698. doi: 10.1152/ajpendo.00710.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38(4):963–974. [PubMed] [Google Scholar]
- 36.Holm S. A simple sequentially rejective multiple test procedure. Scandinavian journal of statistics. 1979;6 (2):65–70. [Google Scholar]
- 37.Imig JD, Zhao X, Capdevila JH, Morisseau C, Hammock BD. Soluble epoxide hydrolase inhibition lowers arterial blood pressure in angiotensin II hypertension. Hypertension. 2002;39(2 Pt 2):690–694. doi: 10.1161/hy0202.103788. [DOI] [PubMed] [Google Scholar]
- 38.Kopkan L, Huskova Z, Sporkova A, Varcabova S, Honetschlagerova Z, Hwang SH, Tsai HJ, Hammock BD, Imig JD, Kramer HJ, Burgelova M, Vojtiskova A, Kujal P, Vernerova Z, Cervenka L. Soluble Epoxide Hydrolase Inhibition Exhibits Antihypertensive Actions Independently of Nitric Oxide in Mice with Renovascular Hypertension. Kidney & blood pressure research. 2012;35(6):595–607. doi: 10.1159/000339883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manhiani M, Quigley JE, Knight SF, Tasoobshirazi S, Moore T, Brands MW, Hammock BD, Imig JD. Soluble epoxide hydrolase gene deletion attenuates renal injury and inflammation with DOCA-salt hypertension. Am J Physiol Renal Physiol. 2009;297(3):F740–748. doi: 10.1152/ajprenal.00098.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neckar J, Kopkan L, Huskova Z, Kolar F, Papousek F, Kramer HJ, Hwang SH, Hammock BD, Imig JD, Maly J, Netuka I, Ostadal B, Cervenka L. Inhibition of soluble epoxide hydrolase by cis-4-[4-(3-adamantan-1-ylureido)cyclohexyl-oxy]benzoic acid exhibits antihypertensive and cardioprotective actions in transgenic rats with angiotensin II-dependent hypertension. Clin Sci (Lond) 2012;122(11):513–525. doi: 10.1042/CS20110622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Das K, Li R, Sengupta S, Wu R. A Bayesian semiparametric model for bivariate sparse longitudinal data. Statistics in medicine. 2013 doi: 10.1002/sim.5790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burnham KP, Anderson DR. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. 2. Springer-Verlag; 2002. [Google Scholar]
- 43.Blasbalg TL, Hibbeln JR, Ramsden CE, Majchrzak SF, Rawlings RR. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. The American journal of clinical nutrition. 2011;93(5):950–962. doi: 10.3945/ajcn.110.006643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Honetschlagerova Z, Sporkova A, Kopkan L, Huskova Z, Hwang SH, Hammock BD, Imig JD, Kramer HJ, Kujal P, Vernerova Z, Chabova VC, Tesar V, Cervenka L. Inhibition of soluble epoxide hydrolase improves the impaired pressure-natriuresis relationship and attenuates the development of hypertension and hypertension-associated end-organ damage in Cyp1a1-Ren-2 transgenic rats. J Hypertens. 2011;29(8):1590–1601. doi: 10.1097/HJH.0b013e328349062f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee KS, Morisseau C, Yang J, Wang P, Hwang SH, Hammock BD. Forster resonance energy transfer competitive displacement assay for human soluble epoxide hydrolase. Anal Biochem. 2013;434(2):259–268. doi: 10.1016/j.ab.2012.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lai LH, Wang RX, Jiang WP, Yang XJ, Song JP, Li XR, Tao G. Effects of docosahexaenoic acid on large-conductance Ca2+-activated K+ channels and voltage-dependent K+ channels in rat coronary artery smooth muscle cells. Acta Pharmacol Sin. 2009;30(3):314–320. doi: 10.1038/aps.2009.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lauterbach B, Barbosa-Sicard E, Wang MH, Honeck H, Kargel E, Theuer J, Schwartzman ML, Haller H, Luft FC, Gollasch M, Schunck WH. Cytochrome P450-dependent eicosapentaenoic acid metabolites are novel BK channel activators. Hypertension. 2002;39(2 Pt 2):609–613. doi: 10.1161/hy0202.103293. [DOI] [PubMed] [Google Scholar]
- 48.VanRollins M, Kochanek PM, Evans RW, Schiding JK, Nemoto EM. Optimization of epoxyeicosatrienoic acid syntheses to test their effects on cerebral blood flow in vivo. Biochim Biophys Acta. 1995;1256(3):263–274. doi: 10.1016/0005-2760(95)00029-c. [DOI] [PubMed] [Google Scholar]
- 49.Rosolowsky M, Falck JR, Willerson JT, Campbell WB. Synthesis of lipoxygenase and epoxygenase products of arachidonic acid by normal and stenosed canine coronary arteries. Circ Res. 1990;66(3):608–621. doi: 10.1161/01.res.66.3.608. [DOI] [PubMed] [Google Scholar]
- 50.Carroll MA, Schwartzman M, Capdevila J, Falck JR, McGiff JC. Vasoactivity of arachidonic acid epoxides. Eur J Pharmacol. 1987;138(2):281–283. doi: 10.1016/0014-2999(87)90445-6. [DOI] [PubMed] [Google Scholar]
- 51.Zhang G, Panigrahy D, Mahakian LM, Yang J, Liu JY, Stephen Lee KS, Wettersten HI, Ulu A, Hu X, Tam S, Hwang SH, Ingham ES, Kieran MW, Weiss RH, Ferrara KW, Hammock BD. Epoxy metabolites of docosahexaenoic acid (DHA) inhibit angiogenesis, tumor growth, and metastasis. Proc Natl Acad Sci U S A. 2013;110(16):6530–6535. doi: 10.1073/pnas.1304321110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Imig JD, Zhao X, Zaharis CZ, Olearczyk JJ, Pollock DM, Newman JW, Kim IH, Watanabe T, Hammock BD. An orally active epoxide hydrolase inhibitor lowers blood pressure and provides renal protection in salt-sensitive hypertension. Hypertension. 2005;46(4):975–981. doi: 10.1161/01.HYP.0000176237.74820.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ai D, Pang W, Li N, Xu M, Jones PD, Yang J, Zhang Y, Chiamvimonvat N, Shyy JY, Hammock BD, Zhu Y. Soluble epoxide hydrolase plays an essential role in angiotensin II-induced cardiac hypertrophy. Proc Natl Acad Sci U S A. 2009;106(2):564–569. doi: 10.1073/pnas.0811022106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chiamvimonvat N, Ho CM, Tsai HJ, Hammock BD. The soluble epoxide hydrolase as a pharmaceutical target for hypertension. J Cardiovasc Pharmacol. 2007;50(3):225–237. doi: 10.1097/FJC.0b013e3181506445. [DOI] [PubMed] [Google Scholar]
- 55.Inceoglu B, Jinks SL, Schmelzer KR, Waite T, Kim IH, Hammock BD. Inhibition of soluble epoxide hydrolase reduces LPS-induced thermal hyperalgesia and mechanical allodynia in a rat model of inflammatory pain. Life Sci. 2006;79(24):2311–2319. doi: 10.1016/j.lfs.2006.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu JY, Yang J, Inceoglu B, Qiu H, Ulu A, Hwang SH, Chiamvimonvat N, Hammock BD. Inhibition of soluble epoxide hydrolase enhances the anti-inflammatory effects of aspirin and 5-lipoxygenase activation protein inhibitor in a murine model. Biochem Pharmacol. 2009 doi: 10.1016/j.bcp.2009.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kjeldsen SE, Lyle PA, Kizer JR, Oparil S, Hoieggen A, Os I. Fixed combination of losartan and hydrochlorothiazide and reduction of risk of stroke. Vascular health and risk management. 2007;3(3):299–305. [PMC free article] [PubMed] [Google Scholar]
- 58.Imig JD. 20-HETE or EETs: which arachidonic acid metabolite regulates proximal tubule transporters and contributes to pressure natriuresis? Am J Physiol Regul Integr Comp Physiol. 2004;287(1):R3–5. doi: 10.1152/ajpregu.00151.2004. [DOI] [PubMed] [Google Scholar]
- 59.Ulu A, Davis BB, Tsai HJ, Kim IH, Morisseau C, Inceoglu B, Fiehn O, Hammock BD, Weiss RH. Soluble epoxide hydrolase inhibitors reduce the development of atherosclerosis in apolipoprotein e-knockout mouse model. J Cardiovasc Pharmacol. 2008;52(4):314–323. doi: 10.1097/FJC.0b013e318185fa3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moran JH, Nowak G, Grant DF. Analysis of the toxic effects of linoleic acid, 12,13-cis-epoxyoctadecenoic acid, and 12,13-dihydroxyoctadecenoic acid in rabbit renal cortical mitochondria. Toxicol Appl Pharmacol. 2001;172(2):150–161. doi: 10.1006/taap.2001.9149. [DOI] [PubMed] [Google Scholar]
- 61.Manhiani MM, Quigley JE, Socha MJ, Motamed K, Imig JD. IL6 suppression provides renal protection independent of blood pressure in a murine model of salt-sensitive hypertension. Kidney & blood pressure research. 2007;30(4):195–202. doi: 10.1159/000104094. [DOI] [PubMed] [Google Scholar]
- 62.Liu JY, Lin YP, Qiu H, Morisseau C, Rose TE, Hwang SH, Chiamvimonvat N, Hammock BD. Substituted phenyl groups improve the pharmacokinetic profile and anti-inflammatory effect of urea-based soluble epoxide hydrolase inhibitors in murine models. Eur J Pharm Sci. 2013;48(4–5):619–627. doi: 10.1016/j.ejps.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Imig JD. Epoxides and soluble epoxide hydrolase in cardiovascular physiology. Physiol Rev. 2012;92(1):101–130. doi: 10.1152/physrev.00021.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rios A, Vargas-Robles H, Gamez-Mendez AM, Escalante B. Cyclooxygenase-2 and kidney failure. Prostaglandins Other Lipid Mediat. 2012;98(3–4):86–90. doi: 10.1016/j.prostaglandins.2011.11.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


