Abstract
Objective
Chemokines are known to play an important role in the pathophysiology of alcoholic hepatitis (AH), a form of acute-on-chronic liver injury frequently mediated by gut derived lipopolysaccharide (LPS). In our study, we hypothesise that chemokine CCL20, one of the most upregulated chemokines in patients with AH, is implicated in the pathogenesis of AH by mediating LPS induced liver injury.
Design
CCL20 gene expression and serum levels and their correlation with disease severity were assessed in patients with AH. Cellular sources of CCL20 and its biological effects were evaluated in vitro and in vivo in chronic, acute and acute-on-chronic experimental models of carbon tetrachloride and LPS induced liver injury. RNA interference technology was used to knockdown CCL20 in vivo.
Results
CCL20 hepatic and serum levels were increased in patients with AH and correlated with the degree of fibrosis, portal hypertension, endotoxaemia, disease severity scores and short term mortality. Moreover, CCL20 expression was increased in animal models of liver injury and particularly under acute-on-chronic conditions. Macrophages and hepatic stellate cells (HSCs) were identified as the main CCL20 producing cell types. Silencing CCL20 in vivo reduced LPS induced aspartate aminotransferase and lactate dehydrogenase serum levels and hepatic proinflammatory and profibrogenic genes. CCL20 induced proinflammatory and profibrogenic effects in cultured primary HSCs.
Conclusions
Our results suggest that CCL20 upregulation is strongly associated with LPS and may not only represent a new potential biomarker to predict outcome in patients with AH but also an important mediator linking hepatic inflammation, injury and fibrosis in AH.
INTRODUCTION
Alcoholic liver disease (ALD) is a major cause of end stage liver disease worldwide and includes a broad spectrum of disorders, from fatty liver and hepatic inflammation to more severe forms of liver injury, including alcoholic hepatitis (AH), cirrhosis and hepatocellular carcinoma.1 AH is the most severe form of ALD and leads to severe complications related to liver failure, portal hypertension or bacterial infection, and is associated with high short term mortality.1–4 AH episodes are associated with an important inflammatory response and a rapid progression of liver fibrosis.5 Unfortunately, corticosteroid treatment is only effective for a subset of patients,6 and no other efficient therapies are currently available. The development of new therapeutic strategies in AH have been hampered by poor knowledge of the molecular mechanisms1,5,7 and lack of animal models of severe AH, as the available models do not reproduce all of the key histological features found in humans.5,8 However, new animal models reproducing some of the features of AH in humans have been described recently9,10 and will represent new important tools to study the disease.
Alcohol consumption induces disruption of the intestinal barrier and causes enhanced gut permeability with subsequent translocation of bacterial derived lipopolysaccharide (LPS), which leads to elevated serum levels of LPS in patients with AH.11–13 Once it reaches the liver, LPS stimulates innate immune receptors, namely toll-like receptors (TLRs), mostly expressed on Kupffer cells and hepatic stellate cells (HSCs).14 LPS mediated activation of Kupffer cells is a crucial step for both liver inflammation and fibrogenesis by promoting hepatocyte damage, increased leucocyte infiltration, and secretion of reactive oxygen species and proinflammatory and profibrogenic cytokines.15,16 Furthermore, LPS can also directly contribute to HSC activation and promote liver fibrosis.15,17 A previous translational study from our laboratory using liver samples from patients with AH allowed us to identify several deregulated pathways potentially implicated in the pathogenesis of AH, including a cytokine–cytokine receptor interaction pathway.8,18 In the same study, we identified CCL20 as the most upregulated chemokine in patients with AH.
Chemokines are a family of small cytokines which have the properties of both chemotactic mediators and cytokines.19 Chemokines mediate the infiltration of immune cells into the injured liver but can also directly interact with hepatic resident cells during inflammation and fibrosis.20 CCL20 was originally identified in the liver as a liver related and activation related chemokine, and is also known as a macrophage inflammatory protein (MIP-3α).21 CCL20 has been described as the only chemokine interacting and activating CC chemokine receptor 6 (CCR6), a receptor shared only with the antimicrobial β-defensins.22 CCL20 has been shown to be expressed in a broad spectrum of cells and tissue types. Based on the variety of CCL20 inducing agents (LPS, tumour necrosis factor α (TNFα), interleukin (IL)-1β), CCL20 and CCR6 have been described as being involved in both normal and pathological processes,22 including chronic liver injury23,24 and hepatocellular carcinoma.25 However, the role of CCL20 in chronic liver diseases and in the context of an acute-on-chronic liver injury is not known.
In the present translational study, we investigated the potential role of CCL20 as a mediator of LPS induced liver injury in AH. We performed an extensive study in liver samples from well characterised patients with AH, and we demonstrated that CCL20 is upregulated in these patients and correlates with grade of fibrosis, portal hypertension, endotoxaemia, disease severity and mortality. Moreover, as there are no available experimental models of AH, we explored the CCL20 cell sources and functions in experimental models of acute, chronic and acute-on-chronic liver injury induced by LPS, carbon tetra-chloride (CCl4) and their combination to reproduce some of the features of AH.
MATERIALS AND METHODS
Patients
Patients admitted to the Liver Unit, Hospital Clínic of Barcelona, with clinical, analytical and histological features of AH from July 2009 to January 2012 were prospectively included in the study. All patients included in this study gave informed consent and the protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the ethics committee of the Hospital Clinic of Barcelona. CCL20 and LPS serum levels were assessed in 49 patients, and hepatic gene expression analysis was performed in 32 liver samples obtained by transjugular biopsy. Inclusion criteria for AH were: excessive alcohol consumption (>60 g/day) prior to admission, elevated levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ-glutamyl transpeptidase and bilirubin, and histological diagnosis of AH.2,26 Patients with hepatocellular carcinoma or any other potential cause of liver disease were excluded from the study. All patients received nutritional as well as psychological support in achieving alcohol abstinence. Fragments of normal livers were selected as previously described.8 We included patients with HCV induced liver disease (genotype 1) who did not use any previous antiviral therapy, patients with compensated cirrhosis due to HCV or past history of alcohol abuse (abstinence for at least 6 months), and a cohort of patients with morbid obesity and associated non-alcoholic steatohepatitis according to Kleiner’s criteria. Clinical and histological characteristics of these patients have been previously described.7,8
Determination of LPS and CCL20 serum levels in patients with AH
Serum samples were obtained from peripheral blood and stored at −80°C. LPS serum levels were determined using the limulus amoebocyte lysate QCL-1000 test (Lonza Walkersville Inc, Walkersville, Maryland, USA). CCL20 serum levels were measured in patients with AH (n=49), HCV (n=8) and compensated alcoholic cirrhosis (n=15), and in healthy volunteers (n=8), using the Quantikine Human CCL20/MIP-3α Immunoassay Kit (R&D Systems, Minneapolis, Minnesota, USA).
Cell cultures and in vitro assays
Human HSCs were isolated and cultured as previously described.7 To study CCL20 production and biological effects, HSCs were serum starved for 12 h and then incubated with LPS 1 μg/mL (Sigma-Aldrich, St Louis, Missouri, USA), TNFα 1 ng/mL (R&D Systems) and IL-1β 20 ng/mL (Sigma-Aldrich) for 24 h and with CCL20 250 ng/mL and 1 μg/mL (R&D Systems) for 24 h and 48 h, respectively. HSC migration assays were performed using a Boyden chamber, and CCL20 induced extracellular signal regulated kinase (ERK) activation was verified by western blotting (see online supplementary material). RAW264 murine macrophages were incubated with LPS (10 ng/mL, 100 ng/mL and 1 μg/mL) for 24 h, as previously described.7 RNA isolation and PCR analysis were performed as described in the online supplementary material section.
Small hairpin interference inducing constructs
We first tested in RAW264 cells three small interfering RNAs (siRNAs) specific for both isophorm 1 and 2 of CCL20 (s73425, s73427 and s73426; Ambion In Vivo siRNA, Ambion, Life Technologies Corporation, Carlsbad, California, USA) (data not shown), and using positive (Ambion In Vivo GAPDH Positive control siRNA, Ambion) and negative (Ambion In Vivo Negative Control #1 siRNA, Ambion) controls. We chose the siRNA that best inhibited Ccl20 gene expression for the production of CCL20 short hairpin interference inducing construct (shRNA). Starting from the siRNA sequence, shRNAs for in vivo use were constructed and provided by the Gene Silencing Platform at CIC bioGUNE (Bilbao, Spain). Briefly, chemically synthesised oligonucleotides, including the gene target sequence (or a scrambled sequence in the case of the control shRNA), and a 19 nt loop from human miR30 were cloned into the pSM2C vector.
Mouse models of liver injury
Animal procedures were approved by the ethics committee of the University of Barcelona and were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and by the Columbia University Institutional Animal Care and Use Committee, and are in accordance with those set by the National Institutes of Health.
Mice aged 8–10 weeks were administrated CCl4 and ethanol or LPS. To mimic the effects of endotoxaemia in the context of chronic liver disease, we also used a model of acute-on-chronic liver injury by combining the effects of chronic CCl4 with LPS. Different hepatic cell populations were isolated from the livers of mice treated with CCl4 and LPS, and Ccl20 hepatic expression was evaluated. The effects of CCL20 were studied in vivo by injecting mice with control shRNA or shRNA specific for CCL20 and LPS. The effects of shRNA on hepatic inflammatory cell infiltration, and gene and protein expression were assessed by quantitative PCR, immunohistochemistry and western blotting, respectively. For details on methodology, please see the online supplementary material.
Statistical analysis
Continuous variables are described as mean (95% CI) or median (IQR). Categorical variables are described by means of counts and percentages. Comparisons between groups were performed using the Student’s t test or the Mann–Whitney U test when appropriate. Correlations between variables were evaluated using Spearman’s r or Pearson’s r, when appropriate. The area under the receiver characteristic curve (AUROC) analysis was used to determine the best cut-off value and the accuracy (sensitivity and specificity) of continuous variables associated with 90 day mortality. Finally, we performed a survival analysis using the Kaplan–Meier method. Comparisons were performed by the log rank test. All statistical analyses were performed using SPSS V.14.0 for Windows (SPSS Inc, Chicago, Illinois, USA).
RESULTS
General characteristics of patients with AH
Forty-nine patients were included in the study with clinical, analytical and histological characteristics of AH. Seventy-eight per cent (n=38) of patients had severe AH at admission, as defined as a ABIC (Age-Bilirubin-INR-Creatinine) score >6.71.2 Patients were predominantly male (80%), and mean age was 52 years. Overall 90 day mortality was 29%. The main causes of death were multiple organ dysfunction (65%) and severe sepsis (20%). The main epidemiological, clinical, haemodynamic and analytical characteristics of the patients are shown in table 1.
Table 1.
Baseline demographic and clinical parameters of patients with alcoholic hepatitis (n=49)
| Characteristic | Median (25–75 IQR) or n (%) |
|---|---|
| Age (years) | 52 (47–56) |
| Male (n (%)) | 39 (80) |
| Alcohol intake (g/day) | 100 (80–160) |
| Corticosteroids (n (%)) | 25 (51) |
| Laboratory and hemodynamic parameters | |
| Haemoglobin (g/dL) | 11 (10–13) |
| Leucocyte count (×109/L) | 8.4 (6.3–12.5) |
| Platelet count (×109/L) | 113 (77–201) |
| AST (U/L) | 117 (67–157) |
| ALT (U/L) | 37 (25–60) |
| Serum Na (mmol/L) | 135 (132–139) |
| Serum albumin (g/dL) | 2.6 (2.3–3.2) |
| Serum creatinine (mg/dL) | 0.9 (0.60–1.1) |
| Serum bilirubin (mg/dL) | 6.7 (3.0–18.7) |
| International normalised ratio | 1.6 (1.4–1.8) |
| HVPG (mm Hg) | 19 (15–22) |
| Alcoholic hepatitis severity scores at admission | |
| MELD score | 19 (14–24) |
| ABIC score | 7.8 (6.7–8.6) |
| ABIC class (n (%)) | |
| A (<6.71) | 11 (23) |
| B (6.71–8.99) | 30 (61) |
| C (≥9) | 8 (16) |
| Clinical decompensations during hospitalisation | |
| AKI (n (%)) | 20 (41) |
| Infection (n (%)) | 21 (43) |
| Mortality at 90 days (n (%)) | 14 (29) |
ABIC, Age-Bilirubin-INR-Creatinine score; AKI, acute kidney injury; ALT, alanine aminotransferase; AST, aspartate aminotransferase; HVPG, hepatic venous pressure gradient; MELD, Model for End-stage Liver Disease.
Patients with AH show increased CCL20 hepatic expression and serum levels
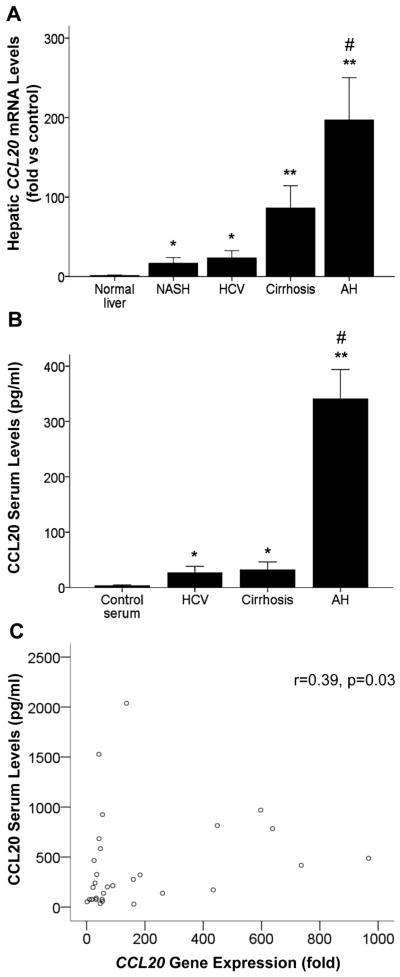
We previously identified CCL20 as the most upregulated CC chemokine in patients with AH.8 To confirm this previous result, we analysed by real time PCR hepatic CCL20 expression in a cohort of patients with AH. The results confirmed marked upregulation of CCL20 in patients with AH (n=32) compared with normal liver (n=8) (p<0.001) and other liver diseases (p<0.001). CCL20 expression was also upregulated, but at a lower extent, in patients with non-alcoholic steatohepatitis (n=8) (p<0.005), chronic hepatitis C (n=8) (p<0.005) and compensated cirrhosis (n=8) (p<0.001) compared with control liver samples (n=8) (figure 1A).
Figure 1.
CCL20 hepatic and serum levels in patients with alcoholic hepatitis (AH). (A) CCL20 hepatic gene expression in patients with AH (n=32), non-alcoholic steatohepatitis (NASH) (n=8), HCV (n=8) and compensated cirrhosis (n=8) compared with normal livers (n=8) (*p<0.005 vs normal livers, **p<0.001 vs normal livers, #p<0.001 vs other groups). (B) CCL20 serum levels (from peripheral blood) in patients with AH (n=49), HCV (n=8), compensated cirrhosis (n=15) and healthy controls (n=8) (*p<0.005 vs controls, **p<0.001 vs controls, #p<0.001 vs other groups). (C) Correlation between CCL20 hepatic gene expression and CCL20 serum levels in patients with AH (n=32) (p=0.03).
We next assessed CCL20 serum levels in patients with AH and other liver diseases. We found that CCL20 serum levels were increased in patients with AH (n=49) (p<0.001), HCV (n=8) and compensated alcoholic cirrhosis (n=15) (p<0.005) compared with healthy controls (n=8). Of note, CCL20 circulating levels were higher in patients with AH compared with patients with other liver diseases (p<0.001, figure 1B). Finally, we observed that hepatic CCL20 mRNA expression and serum levels positively correlated in patients with AH (n=32) (p=0.03, figure 1C), suggesting that the liver may be an important source of CCL20 in these patients.
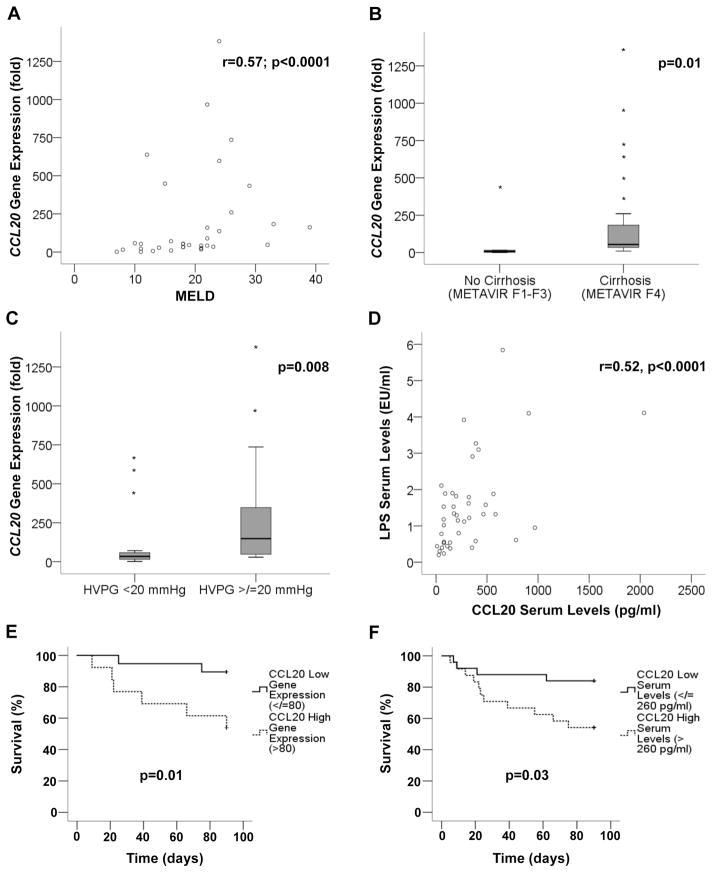
CCL20 expression correlates with disease severity and key features of AH
To gain insight into the pathogenic role of CCL20 in AH, we next explored whether its expression correlated with disease severity. CCL20 hepatic expression positively correlated with important prognostic scores in patients with AH. Hepatic CCL20 correlated with MELD (Model for End-stage Liver Disease) (p<0.0001) (figure 2A), ABIC (p=0.06) and Maddrey’s (p=0.005) (see online supplementary figure S1A, B) scores. Moreover, we observed higher levels of hepatic CCL20 expression in patients with severe AH compared with those with mild to moderate grades of fibrosis and portal hypertension (54 vs 7-fold expression (p=0.01) and 148 vs 34-fold expression (p=0.008), respectively) (figure 2B, C). We next sought to investigate the correlation between circulating CCL20 and LPS, one of the major inducers of CCL20. We observed that CCL20 and LPS serum levels were strongly correlated (p<0.0001, figure 2D) in patients with AH. We also evaluated in our cohort of patients hepatic infiltration of neutrophils (as described in the online supplementary material section), an important hallmark in AH. We found that patients with higher levels of circulating CCL20 showed severe hepatic infiltration of polymorphonuclear cells compared with those with a mild grade of polymorphonuclear cell infiltration (p=0.007, see online supplementary figure S1C).
Figure 2.
CCL20 expression and correlation with clinical features of alcoholic hepatitis (AH). (A) Correlation between CCL20 hepatic gene expression and Model for End-stage Liver Disease (MELD) score in patients with AH (n=32) (p<0.0001). (B) CCL20 hepatic gene expression in patients with AH and METAVIR F4 (patients with cirrhosis, n=27) and METAVIR F1–3 (patients without cirrhosis, n=5) (p=0.01). (C) Comparison of CCL20 hepatic gene expression and the severity of portal hypertension in patients with AH (severe portal hypertension (hepatic venous pressure gradient (HVPG)>20 mm Hg) n=12 and non-severe portal hypertension (HVPG<20 mm Hg) n=20; p=0.008). (D) Correlation between CCL20 and lipopolysaccharide (LPS) serum levels in patients with AH (n=49) (p<0.0001). (E) Kaplan–Meier curve showing 90 day mortality according to CCL20 hepatic gene expression. A value of 80-fold expression (2−ΔΔCt) was identified as the cut-off value with best sensitivity and specificity to define patients with low (≤80-fold) and high (>80-fold) CCL20 gene expression (p=0.01). (F) Kaplan–Meier curve showing 90 day mortality according to CCL20 serum levels in patients with AH. A value of 260 pg/mL was identified as the cut-off with better sensitivity and specificity to define patients with low (≤260 pg/mL) or high (>260 pg/mL) circulating CCL20 serum levels (p=0.03).
Importantly, we observed increased hepatic CCL20 mRNA and serum levels in patients who died within 90 days after admission compared with those who survived (160-fold vs 50-fold induction (p=0.03) and 359 vs 168 pg/mL (p=0.048) respectively) (see online supplementary figure S1D, E). In addition, to determine if CCL20 could be a good predictor of short term mortality, a Kaplan–Meier analysis was performed. As shown in figure 2E and figure 2F, CCL20 hepatic gene expression (receiver operating curve cut-off value of 80-fold (2−ΔΔCt), AUROC 0.72, 95% CI (0.53 to 0.90)) and serum levels (receiver operating curve cut-off value of 260 pg/mL, AUROC 0.68, 95% CI (0.52 to 0.83)) were useful to predict short term mortality in patients with AH. These results suggest that CCL20 may play a role in the pathophysiology of AH and could be used as a biomarker to predict short term mortality.
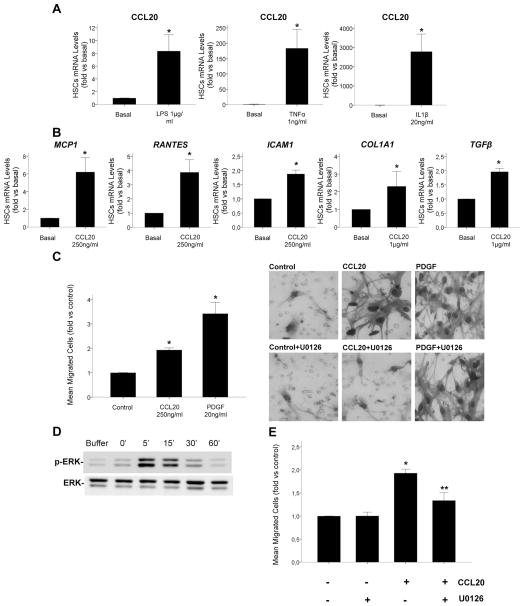
CCL20 proinflammatory and profibrogenic effects on HSCs
As hepatic expression of CCL20 was found to be increased in AH patients with METAVIR F4 compared with those with METAVIR F1–F3 (figure 2B), and as HSCs are key players in the development of liver fibrosis in the injured liver, we next investigated the potential of HSCs to synthesise CCL20 and its biological effects on these cells. We first investigated if mediators known to play a role in ALD and typically present in the AH microenvironment induced CCL20 expression in human primary HSCs. Incubation of HSCs with LPS, TNFα and IL1β induced a marked increase in CCL20 mRNA levels (p<0.05), as shown in figure 3A. On the other hand, to investigate the biological effects of CCL20 on HSCs, cells were incubated with recombinant CCL20. The chemokine induced the expression of proinflammatory (MCP1, RANTES, ICAM1) (p<0.05) and profibrogenic (COL1A1, TGFβ) (p<0.05) genes in HSCs (figure 3B). To investigate if CCL20 had a chemoattractant effect on HSCs, we performed a migration test using a Boyden chamber. We found increased HSC migration after cell stimulation with CCL20 (p<0.005) (figure 3C). Previous studies showed the implication of ERK in HSC migration and activation27 so we tested if CCL20 induced HSC migration occurred in an ERK dependent manner. Interestingly, CCL20 induced transient activation of ERK phosphorylation (figure 3D), and preincubation of HSCs with U0126, a MEK 1/2 specific inhibitor, reduced CCL20 induced migration of HSCs (p=0.014) (figure 3E, C). These results indicate that CCL20 exerts proinflammatory and profibrogenic effects on HSCs and enhances their migration through ERK signalling.
Figure 3.
CCL20 production in hepatic stellate cells (HSCs) and CCL20 effects on HSCs. (A) CCL20 gene expression in HSCs incubated with lipopolysaccharide (LPS) 1 μg/mL, tumour necrosis factor α (TNFα) 1 ng/mL and interleukin 1β (IL-1β) 20 ng/mL for 24 h. (B) HSCs were incubated with CCL20 250 ng/mL and 1 μg/mL for 24 and 48 h, respectively. mRNA expression was determined by quantitative real time PCR and was expressed as fold versus basal (*p<0.05 compared with basal). (C) Effects of CCL20 on HSC migration were evaluated using a Boyden chamber. Both CCL20 250 ng/mL and platelet derived growth factor (PDGF) 20 ng/mL (used as a positive control) increased HSC migration, expressed as mean of migrated cells with respect to controls (*p<0.005). Representative pictures of Giemsa positive migrated cells (×400 magnification) are also shown for control, CCL20 250 ng/mL and PDGF 20 ng/mL stimulated cells in the presence or absence of 10 μM U0126, a specific MEK1/2 inhibitor. (D) Representative western blot of time course stimulation of HSCs with CCL20 250 ng/mL. CCL20 induced a transient extracellular signal regulated kinase (ERK) phosphorylation. (E) Quantification of the number of migrated HSCs incubated with CCL20 250 ng/mL in the presence or absence of U0126 10 μM (*p<0.005 vs vehicle; **p=0.014 vs CCL20 stimulated cells).
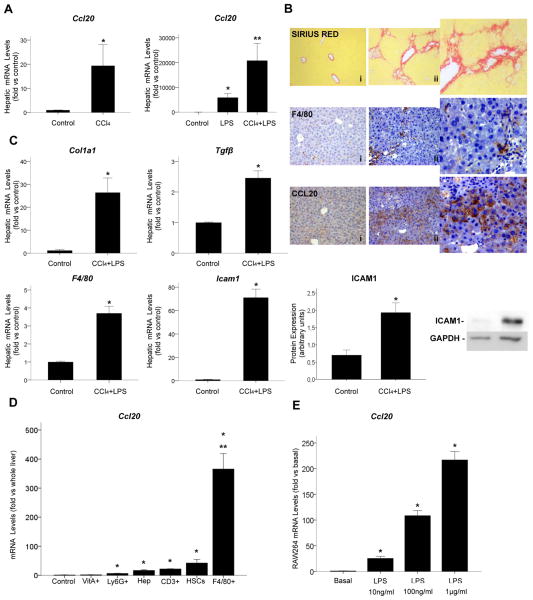
LPS induces hepatic upregulation of CCL20
Our group and others have been working on the development of an animal model of severe AH but, unfortunately, the existing models do not reproduce the pathophysiology of severe AH observed in humans. For this reason, and in order to uncover the mechanisms driving the increase in CCL20 expression in AH and its cellular source, we used different animal models of liver injury representative of some of the key events that occur in AH, such as ethanol consumption, fibrosis and endotoxaemia. We first tested the effect of ethanol on Ccl20 hepatic expression. Mice administered ethanol by gavage did not show increased Ccl20 hepatic levels (data not shown) while other molecules important in AH, such as Fn14, were found to be increased in this model,8 suggesting that ethanol itself may not be directly implicated in the regulation of CCL20. We next investigated if CCl4 administration or LPS induced Ccl20 hepatic expression. We found that CCl4 and LPS significantly increased Ccl20 hepatic gene expression (p<0.05) (figure 4A). Importantly, mice treated with a combination of CCl4 and LPS had a strong increase in Ccl20 hepatic expression compared with mice treated with LPS, CCl4 and control mice (p<0.05, figure 4A). The extent of liver damage in mice injected with the combination of CCl4 and LPS was confirmed by multiple approaches that underlined increased collagen deposition, enhanced hepatic gene expression of Col1a1, Tgfβ, Icam1, and F4/80, and enhanced protein expression of F4/80, CCL20 and ICAM1 (figure 4B, C).
Figure 4.
Ccl20 hepatic expression in animal models of liver injury and CCL20 cell source. (A) Hepatic Ccl20 gene expression in mice treated with carbon tetrachloride (CCl4) (n=6), lipopolysaccharide (LPS) (n=6) and CCl4 plus LPS (n=12) (see online supplementary material) (*p<0.05 compared with controls; **p<0.05 compared with control and other groups). (B) Representative images of sirius red staining in the liver of (i) control (×200 magnification) and (ii) CCl4 plus LPS treated mice (×200 magnification), and representative images of F4/80 and CCL20 immunohistochemistry in the liver of (i) control and (ii) CCl4 plus LPS treated mice (×200 magnification). (C) Hepatic Col1a1, Tgfβ, F4/80 and Icam1 gene expression in mice administered CCl4 plus LPS (*p<0.05) and representative western blot of hepatic ICAM1 protein expression and quantification in mice treated with CCl4 plus LPS compared with the control group (*p<0.05). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an endogenous control. (D) Ccl20 mRNA levels in vitamin A+ HSCs (VitA+), neutrophils (Ly6G+), hepatocytes (Hep), T cells (CD3+), total HSCs (HSCs) and macrophages (F4/80+) isolated from the liver of mice administered CCl4 plus LPS (*p<0.05 compared with controls, **p<0.01 compared with other cell types); as a control to normalise the results, we used whole liver samples from mice treated with CCl4 plus LPS. (E) Ccl20 gene expression in RAW264 cells incubated with LPS 10 ng/mL, 100 ng/mL and 1 μg/mL for 24 h (*p<0.05).
Macrophages are the main cell source of hepatic CCL20 in LPS induced liver injury
In order to identify the main cell source of CCL20 in the injured liver, different hepatic cell populations were isolated from livers of mice subjected to a model of acute-on-chronic liver injury (CCl4 plus LPS), and Ccl20 expression was assessed. As shown in figure 4D, we identified macrophages as the hepatic cell type expressing higher levels of Ccl20 (p<0.001), followed by HSCs, T cells and hepatocytes (p<0.05 for all, compared with whole liver). As macrophages were identified as the main hepatic Ccl20 cell source, and because their activation is a crucial step in liver inflammation and fibrosis, we also explored Ccl20 production in vitro in a RAW264 cell line. We found that LPS induced a strong increase in Ccl20 gene expression in a dose dependent manner in these cells (figure 4E).
Silencing CCL20 ameliorates LPS-induced liver injury
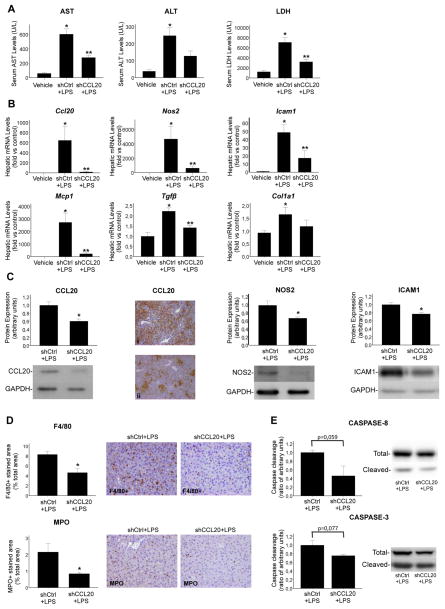
Once LPS was identified as one of the major inducers of Ccl20, we evaluated the effects mediated by CCL20 in LPS induced liver injury. Mice treated with LPS showed an important increase in ALT, AST and lactate dehydrogenase (LDH) levels, which were markedly reduced in animals pretreated with shRNA specific for CCL20 compared with control shRNA (figure 5A). Moreover, LPS induced an important increase in Ccl20, Nos2, Icam1, Mcp1, Tgfβ and Col1a1 gene expression. Animals treated with CCL20 shRNA showed a marked reduction in Ccl20 expression at the mRNA and protein levels, indicating an efficient knockdown by the shRNA treatment (figure 5B, C). Moreover, we observed a clear decrease in Nos2, Icam1, Mcp1 and Tgfβ gene expression (p<0.05) in animals treated with CCL20 shRNA and LPS. Col1a1 also showed a tendency to decrease (figure 5B). We also found a reduction in hepatic protein expression of NOS2 and ICAM1 (p<0.05) in mice injected with CCL20 shRNA and LPS compared with the control group (figure 5C). Furthermore, CCL20 knockdown reduced macrophages and neutrophil hepatic infiltration (p<0.05) (figure 5D), and caspase-8 (p=0.059) and caspase-3 (p=0.077) cleavage (figure 5E). These results suggest that CCL20 mediates LPS induced hepatocellular damage, regulates important genes known to participate in the pathogenesis of AH and modulates the hepatic inflammatory infiltrate.
Figure 5.
CCL20 mediates lipopolysaccharide (LPS) induced liver damage. (A) Aspartate aminotransferase (AST), alanine aminotransferase (ALT) and lactate dehydrogenase (LDH) serum levels in mice treated with control short hairpin interference inducing construct (shRNA) (shCtrl) (n=6) or CCL20 shRNA (shCCL20) (n=6) and LPS (see online supplementary material) (*p<0.05 compared with vehicle; **p<0.05 compared with control shRNA). (B) Hepatic Ccl20, Nos2, Icam1, Mcp1, Tgfβ and Col1a1 gene expression in mice treated with control shRNA (n=6) or CCL20 shRNA (n=6) and LPS (*p<0.05 compared with vehicle; **p<0.05 compared with control shRNA). (C) Representative western blot and quantification of hepatic CCL20 and representative pictures of CCL20 immunohistochemistry in the liver of mice injected with (i) control shRNA and LPS and (ii) CCL20 shRNA and LPS. Representative western blots and protein expression quantification of NOS2 and ICAM1 in the liver of mice treated with control shRNA or CCL20 shRNA and LPS. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an endogenous control (*p<0.05 compared with control shRNA). (D) Representative F4/80 and myeloperoxidase (MPO) immunostainings of liver sections of control shRNA or CCL20 shRNA and LPS treatment (×200 magnification). Quantification of positive stained areas is shown in the graphs (*p<0.05 compared with control shRNA). (E) Representative western blots of total and cleaved caspase-8 and caspase-3 in the liver of mice treated with control shRNA or CCL20 shRNA and LPS. Caspase cleavage is represented as the ratio of cleaved caspase versus total caspase compared with the control group.
DISCUSSION
AH is a form of acute-on-chronic liver damage characterised by hepatocellular damage, inflammatory infiltrate and fibrosis. There is a clear need to identify key drivers of this disease to develop new targeted therapies. Here we investigated the potential role of CCL20, a chemokine that was found to be significantly upregulated in patients with AH. We performed a translational approach, including hepatic and serum studies and molecular–clinical correlations, to evaluate the potential role of CCL20 in the pathogenesis of AH. Because there are no available animal models reproducing all of the features of severe AH, we used experimental models of acute, chronic and acute-on-chronic liver injury, which resemble some of the key hallmarks of AH in humans. A new experimental model to induce severe alcohol liver disease in mice has been described recently,9,10 but its suitability to study AH still needs to be confirmed. Our results strongly suggest that CCL20 is not only a potential biomarker, but also may play a role in the pathogenesis of AH. This conclusion is based on results showing that CCL20 hepatic and serum levels correlate with disease severity and in vitro and experimental data showing that CCL20 mediates fibrosis, inflammation and hepatocellular injury. Obviously, these results needs to be further confirmed in a larger cohort of patients and, when available, in experimental models of severe AH.
AH is characterised by an important inflammatory response that mediates the complex interaction among inflammatory cells, hepatocytes and non-parenchymal cells.5 Here we showed profound upregulation of CCL20 in patients with AH and its correlation with key clinical features of the disease and short term mortality, indicating that CCL20 may represent a good biomarker in patients with AH. Nevertheless, the usefulness of CCL20 to predict outcome in AH patients needs to be further confirmed in a larger cohort of patients. Patients with AH commonly show increased gut permeability and bacterial translocation to the liver, with consequent activation of many hepatic cell types, and activation and perpetuation of hepatic inflammatory and fibrogenic responses.13,28–31 One of the most striking findings of this study is the strong correlation between circulating CCL20 and LPS serum levels, suggesting that hepatic CCL20 upregulation may result from increased levels of circulating pathogen associated molecular patterns that activate macrophages in the injured liver. Supporting this hypothesis, we identified macrophages and activated HSCs as the main hepatic Ccl20 cell sources in an experimental model of acute-on-chronic liver injury where we combined fibrosis and endotoxaemia in order to reproduce two of the main events that occur in AH. The specific role of LPS in CCL20 induction was further confirmed in animal models of LPS induced liver damage, where Ccl20 hepatic levels were strongly upregulated following LPS administration. These results indicate that increased gut permeability, that typically occurs in cirrhotic and AH patients, may result in an increased CCL20 hepatic expression.
In addition to being a potential biomarker, we also suggest a role for CCL20 in the pathophysiology of AH. CCL20 is well known to mediate recruitment of CCR6 positive cells during liver injury,25 which are involved in the amplification of the local inflammatory response.24,32–34 Recently, CCR6 has been shown to exert an important role in the modulation of liver inflammation and fibrosis.24 However, little is known about the direct effects of CCL20 in the injured liver. Although most of the patients included in our study were cirrhotic, hepatic expression of CCL20 was significantly higher in patients with METAVIR F4 compared with those with mild fibrosis (METAVIR F1–F3), suggesting that CCL20 could be related to fibrogenesis. We provided evidence that CCL20 exerts proinflammatory and profibrogenic effects in cultured human primary HSCs and enhances ERK dependent migration in these cells, suggesting a role for this chemokine in the progression of liver fibrosis.
The main limitation to investigation of the mechanisms driving liver injury in AH patients is the lack of an appropriate animal model reproducing the key pathophysiological features of AH. For this reason, we investigated induction of CCL20 expression in animal models of acute-on-chronic liver injury. Interestingly, ethanol administration did not induce by itself Ccl20 hepatic expression. On the contrary, when damaged livers were challenged with an inflammatory insult (LPS), there was strong induction of hepatic Ccl20. Importantly, CCl4 and LPS showed an additive effect, suggesting that endotoxaemia, in the context of liver fibrosis, may enhance expression of CCL20. This observation suggests that ethanol may not be the direct trigger of the CCL20 increase and that endotoxaemia may have the predominant role in the induction of hepatic Ccl20. The most sensitive hepatic cell types to LPS are macrophages, in which LPS promotes activation, M1 polarisation and the burst of inflammatory events35,36 and HSCs. Macrophages and, to a lesser extent HSCs and other liver cell types, were found to be the main cell source of Ccl20 both in vitro and in the acute-on-chronic (CCl4+LPS) liver injury model, suggesting that macrophages and activated HSCs are the main cell types responsible for the cascade of events from LPS-TLR4 activation to Ccl20 induction and consequent worsening of hepatic inflammation and fibrosis.
In order to confirm that CCL20 mediates the effects of LPS induced liver injury, we used a specific shRNA to silence Ccl20 hepatic expression in vivo. Knockdown of Ccl20 reduced AST, ALT and LDH serum levels, caused a reduction in important hepatic proinflammatory and profibrogenic genes and proteins, and decreased macrophage and neutrophil hepatic infiltration. These results provide new important findings in the cascade of events in response to LPS induced liver damage where CCL20 may play an important role inducing both direct damage on liver cells and/or participating through an indirect manner in the LPS cascade that leads to liver injury, hepatic inflammation and fibrosis. The fact that CCL20 regulates expression of other well described molecules involved in the pathogenesis of ALD such as MCP118,37,38 and TGFβ18,39 is an important finding that allows us to include CCL20 into the group of the proinflammatory and profibrogenic molecules that participate in the progression and pathogenesis of AH.
Understanding the role of cytokines in liver disease and their interaction with inflammatory and resident hepatic cells is of the utmost importance to depict the complex inflammatory response that takes place during AH and to define new therapeutic strategies. Our study demonstrates that CCL20 is markedly upregulated in patients with AH and provides evidence that CCL20 may be an important mediator in LPS induced liver inflammation, fibrosis, hepatocellular damage and inflammatory cell recruitment, and could be used as a new biomarker to determine outcome in AH patients. However, further preclinical studies in future models of AH are required to determine if targeting CCL20 is an effective and safe therapeutic strategy to modulate the inflammatory response and liver injury in AH. Moreover, issues regarding CCL20 specificity, modulation of inflammatory cell recruitment and safety will need special attention to evaluate the potential of CCL20 as a therapeutic target in patients with AH.
Supplementary Material
Significance of this study.
What is already known on this subject?
Alcoholic hepatitis (AH) is the most severe form of alcoholic liver disease (ALD) and is associated with a high rate of short term mortality. Current therapies such as corticosteroids are not fully effective, and new targeted therapies for the treatment of this disease are urgently needed.
Alcohol consumption leads to an increase in endotoxin levels in the blood. Once it reaches the liver, endotoxin mostly activates Kupffer cells and hepatic stellate cells, and determines the promotion and perpetuation of hepatic inflammation and fibrosis.
CCL20 is a proinflammatory chemokine strongly induced in different cell types by lipopolysaccharide (LPS), tumour necrosis factor α and interleukin 1β, and is known to recruit chemokine receptor 6 positive cells.
What are the new findings?
CCL20 hepatic expression and serum levels are elevated in patients with AH and are associated with key clinical features of the disease, such as grade of fibrosis, portal hypertension severity, endotoxaemia and hepatic neutrophil infiltration. Increased CCL20 hepatic gene expression and serum levels are associated with short term mortality in patients with AH.
Macrophages and hepatic stellate cells are the main CCL20 producing cell types in experimental acute-on-chronic liver damage induced by the combined treatment of chronic carbon tetrachloride and LPS.
CCL20 exerts proinflammatory and profibrogenic effects on primary human hepatic stellate cells in vitro.
CCL20 knockdown reduces LPS induced liver damage and causes an important decrease in proinflammatory and profibrogenic genes.
How might it impact on clinical practice in the foreseeable future?
The identification of molecular drivers of AH will provide new potential targets for therapy for this severe disease. In our study, we have provided relevant results which show a correlation between CCL20 hepatic and serum levels with grade of fibrosis, portal hypertension, endotoxaemia, neutrophil infiltration and mortality in patients with AH. These findings represent new interesting discoveries in the pathophysiology of ALDs and suggest that CCL20 may play an important role in the pathogenesis of AH. Moreover, the correlation of CCL20 with patient outcome suggests that CCL20 serum levels could be used as a biomarker to predict short term mortality in patients with AH.
Acknowledgments
This work was performed at the Centre Esther Koplowitz (CEK). For their advice and help we thank Dr J P Pradere, Department of Medicine, College of Physicians and Surgeons, Columbia University, New York, New York, USA, and Dr N Beraza, CIC bioGUNE, Bilbao, Spain. We are also grateful to the Citomics Unit of the Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS) for technical help, and to Edurne Berra and Encarnacion Pérez-Andrés, Gene Silencing Platform at CIC bioGUNE, for production of RNAi inducing constructs.
Funding This work was supported by grants from the Instituto de Salud Carlos III (FIS PI041538, FIS PI042380, FIS PI080126 and FIS PI12/01274 to RB, JC, PG and VA, respectively) and European Commission within its FP7 Cooperation Programme and Cosmetics Europe, HeMiBio HEALTH F5 2010 266777, and the NIH (1U01AA021908-01-33490 to PS-B, 1U01AA021908 to RB and U01AA021912 to RFS). SA received a grant from IDIBAPS. DR-T received a grant from the Ministerio de Educación, Cultura y Deporte, FPU program. PS-B is funded by Instituto de Salud Carlos III, Miguel Servet (CP11/00071) and co-financed by Fondo Europeo de Desarrollo Regional (FEDER), Unión Europea, “Una manera de hacer Europa”.
Footnotes
Contributors SA participated in the conception and design of this study, data analysis and interpretation, and performed the majority of the experiments. OM-I, DR-T, DB, DHD, CM, MC and JMC performed the experiments. JA performed the statistical analysis. VA, JC and PG helped with the interpretation of the data and revisions to the final version. RFS contributed by revising the paper critically and helping in data interpretation. RB and PS-B were involved in the conception and design of this paper, analysis and interpretation of the data, and participated in drafting and critically revising the final version of the paper.
Competing interests
Ethics approval The study was approved by the ethics committee of the Hospital Clinic of Barcelona.
Provenance and peer review Not commissioned; externally peer reviewed.
References
- 1.Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572–85. doi: 10.1053/j.gastro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dominguez M, Rincon D, Abraldes JG, et al. A new scoring system for prognostic stratification of patients with alcoholic hepatitis. Am J Gastroenterol. 2008;103:2747–56. doi: 10.1111/j.1572-0241.2008.02104.x. [DOI] [PubMed] [Google Scholar]
- 3.Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med. 2009;360:2758–69. doi: 10.1056/NEJMra0805786. [DOI] [PubMed] [Google Scholar]
- 4.Rosa H, Silverio AO, Perini RF, et al. Bacterial infection in cirrhotic patients and its relationship with alcohol. Am J Gastroenterol. 2000;95:1290–3. doi: 10.1111/j.1572-0241.2000.02026.x. [DOI] [PubMed] [Google Scholar]
- 5.Altamirano J, Bataller R. Alcoholic liver disease: pathogenesis and new targets for therapy. Nat Rev Gastroenterol Hepatol. 2011;8:491–501. doi: 10.1038/nrgastro.2011.134. [DOI] [PubMed] [Google Scholar]
- 6.Porter HP, Simon FR, Pope CE, II, et al. Corticosteroid therapy in severe alcoholic hepatitis. A double-blind drug trial. N Engl J Med. 1971;284:1350–5. doi: 10.1056/NEJM197106172842404. [DOI] [PubMed] [Google Scholar]
- 7.Morales-Ibanez O, Dominguez M, Ki SH, et al. Human and experimental evidence supporting a role for osteopontin in alcoholic hepatitis. Hepatology. 2013;58:1742–56. doi: 10.1002/hep.26521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Affo S, Dominguez M, Lozano JJ, et al. Transcriptome analysis identifies TNF superfamily receptors as potential therapeutic targets in alcoholic hepatitis. Gut. 2012;62:452–60. doi: 10.1136/gutjnl-2011-301146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bertola A, Mathews S, Ki SH, et al. Mouse model of chronic and binge ethanol feeding (the NIAAA model) Nat Protoc. 2013;8:627–37. doi: 10.1038/nprot.2013.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bertola A, Park O, Gao B. Chronic plus binge ethanol feeding synergistically induces neutrophil infiltration and liver injury: a critical role for E-selectin. Hepatology. 2013;58:1814–23. doi: 10.1002/hep.26419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujimoto M, Uemura M, Nakatani Y, et al. Plasma endotoxin and serum cytokine levels in patients with alcoholic hepatitis: relation to severity of liver disturbance. Alcohol Clin Exp Res. 2000;24:48S–54S. [PubMed] [Google Scholar]
- 12.Fukui H, Brauner B, Bode JC, et al. Plasma endotoxin concentrations in patients with alcoholic and non-alcoholic liver disease: reevaluation with an improved chromogenic assay. J Hepatol. 1991;12:162–9. doi: 10.1016/0168-8278(91)90933-3. [DOI] [PubMed] [Google Scholar]
- 13.Rao R. Endotoxemia and gut barrier dysfunction in alcoholic liver disease. Hepatology. 2009;50:638–44. doi: 10.1002/hep.23009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seki E, Schnabl B. Role of innate immunity and the microbiota in liver fibrosis: crosstalk between the liver and gut. J Physiol. 2011;590:447–58. doi: 10.1113/jphysiol.2011.219691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwabe RF, Seki E, Brenner DA. Toll-like receptor signaling in the liver. Gastroenterology. 2006;130:1886–900. doi: 10.1053/j.gastro.2006.01.038. [DOI] [PubMed] [Google Scholar]
- 16.Wheeler MD. Endotoxin and Kupffer cell activation in alcoholic liver disease. Alcohol Res Health. 2003;27:300–6. [PMC free article] [PubMed] [Google Scholar]
- 17.Seki E, De Minicis S, Osterreicher CH, et al. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324–32. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- 18.Colmenero J, Bataller R, Sancho-Bru P, et al. Hepatic expression of candidate genes in patients with alcoholic hepatitis: correlation with disease severity. Gastroenterology. 2007;132:687–97. doi: 10.1053/j.gastro.2006.12.036. [DOI] [PubMed] [Google Scholar]
- 19.Luster AD. Chemokines-chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436–45. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 20.Wasmuth HE, Tacke F, Trautwein C. Chemokines in liver inflammation and fibrosis. Semin Liver Dis. 2010;30:215–25. doi: 10.1055/s-0030-1255351. [DOI] [PubMed] [Google Scholar]
- 21.Hieshima K, Imai T, Opdenakker G, et al. Molecular cloning of a novel human CC chemokine liver and activation-regulated chemokine (LARC) expressed in liver. Chemotactic activity for lymphocytes and gene localization on chromosome 2. J Biol Chem. 1997;272:5846–53. doi: 10.1074/jbc.272.9.5846. [DOI] [PubMed] [Google Scholar]
- 22.Schutyser E, Struyf S, Van Damme J. The CC chemokine CCL20 and its receptor CCR6. Cytokine Growth Factor Rev. 2003;14:409–26. doi: 10.1016/s1359-6101(03)00049-2. [DOI] [PubMed] [Google Scholar]
- 23.Yamauchi K, Akbar SM, Horiike N, et al. Increased serum levels of macrophage inflammatory protein-3alpha in chronic viral hepatitis: prognostic importance of macrophage inflammatory protein-3alpha during interferon therapy in chronic hepatitis C. J Viral Hepat. 2002;9:213–20. doi: 10.1046/j.1365-2893.2002.00354.x. [DOI] [PubMed] [Google Scholar]
- 24.Hammerich L, Bangen JM, Govaere O, et al. Chemokine receptor CCR6-dependent accumulation of gammadelta T-cells in injured liver restricts hepatic inflammation and fibrosis. Hepatology. doi: 10.1002/hep.26697. Published Online First: 19 Aug 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shimizu Y, Murata H, Kashii Y, et al. CC-chemokine receptor 6 and its ligand macrophage inflammatory protein 3alpha might be involved in the amplification of local necroinflammatory response in the liver. Hepatology. 2001;34:311–19. doi: 10.1053/jhep.2001.26631. [DOI] [PubMed] [Google Scholar]
- 26.European Association for the Study of the Liver. EASL clinical practical guidelines: management of alcoholic liver disease. J Hepatol. 2012;57:399–420. doi: 10.1016/j.jhep.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 27.Sancho-Bru P, Juez E, Moreno M, et al. Hepatocarcinoma cells stimulate the growth, migration and expression of pro-angiogenic genes in human hepatic stellate cells. Liver Int. 2009;30:31–41. doi: 10.1111/j.1478-3231.2009.02161.x. [DOI] [PubMed] [Google Scholar]
- 28.Aoyama T, Paik YH, Seki E. Toll-like receptor signaling and liver fibrosis. Gastroenterol Res Pract. 2010 doi: 10.1155/2010/192543. http://dx.doi.org/10.1155/2010/192543. [DOI] [PMC free article] [PubMed]
- 29.Guo J, Friedman SL. Toll-like receptor 4 signaling in liver injury and hepatic fibrogenesis. Fibrogenesis Tissue Repair. 2010;3:21. doi: 10.1186/1755-1536-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petrasek J, Mandrekar P, Szabo G. Toll-like receptors in the pathogenesis of alcoholic liver disease. Gastroenterol Res Pract. 2010 doi: 10.1155/2010/710381. http://dx.doi.org/10.1155/2010/710381. [DOI] [PMC free article] [PubMed]
- 31.Roh YS, Seki E. Toll-like receptors in alcoholic liver disease, non-alcoholic steatohepatitis and carcinogenesis. J Gastroenterol Hepatol. 2013;28(Suppl 1):38–42. doi: 10.1111/jgh.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen KJ, Lin SZ, Zhou L, et al. Selective recruitment of regulatory T cell through CCR6-CCL20 in hepatocellular carcinoma fosters tumor progression and predicts poor prognosis. PLoS ONE. 2011;6:e24671. doi: 10.1371/journal.pone.0024671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karlmark KR, Weiskirchen R, Zimmermann HW, et al. Hepatic recruitment of the inflammatory Gr1+ monocyte subset upon liver injury promotes hepatic fibrosis. Hepatology. 2009;50:261–74. doi: 10.1002/hep.22950. [DOI] [PubMed] [Google Scholar]
- 34.Oo YH, Banz V, Kavanagh D, et al. CXCR3-dependent recruitment and CCR6-mediated positioning of Th-17 cells in the inflamed liver. J Hepatol. 2012;57:1044–51. doi: 10.1016/j.jhep.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benoit M, Desnues B, Mege JL. Macrophage polarization in bacterial infections. J Immunol. 2008;181:3733–9. doi: 10.4049/jimmunol.181.6.3733. [DOI] [PubMed] [Google Scholar]
- 36.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 37.Afford SC, Fisher NC, Neil DA, et al. Distinct patterns of chemokine expression are associated with leukocyte recruitment in alcoholic hepatitis and alcoholic cirrhosis. J Pathol. 1998;186:82–9. doi: 10.1002/(SICI)1096-9896(199809)186:1<82::AID-PATH151>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 38.Mandrekar P, Ambade A, Lim A, et al. An essential role for monocyte chemoattractant protein-1 in alcoholic liver injury: regulation of proinflammatory cytokines and hepatic steatosis in mice. Hepatology. 2011;54:2185–97. doi: 10.1002/hep.24599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen WX, Li YM, Yu CH, et al. Quantitative analysis of transforming growth factor beta 1 mRNA in patients with alcoholic liver disease. World J Gastroenterol. 2002;8:379–81. doi: 10.3748/wjg.v8.i2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.