Abstract
Respiratory burst function of neutrophils is thought to play a pivotal role in the development of pathologies such as indirect acute lung injury (iALI) as well as sepsis. The current study was conducted to determine the effect of an HIV transactivator of transcription (TAT)-fusion protein containing a soluble N-ethylmaleimide-sensitive factor (NSF) attachment protein receptor (SNARE) domain from Synaptosome-associated protein-23 (SNAP-23) on the shock/sepsis- and sepsis-enhanced neutrophil burst capacity using the clinical relevant two-hit indirect acute lung injury (iALI) mouse model and the classical cecal ligation and puncture (CLP) septic model. Results showed that TAT-SNAP-23 significantly decreased the blood neutrophil respiratory burst not only in vitro, but also in vivo in CLP and haemorrhaged (Hem) mice. We found that the neutrophil influx to the lung tissue as measured by myeloperoxidase (MPO) levels and neutrophil-specific esterase+ cells were also decreased in TAT-SNAP-23-treated group comparing with their respective controls. Consistent with this, treatment of TAT-SNAP-23 reduced significantly the disruption of lung tissue architecture and protein concentration of bronchoalveolar lavage (BAL) fluid in iALI mice compared to vehicle-treated iALI mice. In addition, although TAT-SNAP-23 did not alter the extent of local cytokine/chemokine expression, the in vitro migration capacity of neutrophils was blunted from septic and Hemorrhagic mice. These data suggest that TAT-SNAP-23 reduces neutrophil dysfunction in iALI and sepsis. We indicate that administration of TAT-SNAP-23 inhibits neutrophil respiratory burst in in vivo sepsis and Hem (shock)-primed iALI. Targeting respiratory burst is a potential therapeutic strategy to ameliorate neutrophil-dependent critical complications as iALI and sepsis.
Keywords: Neutrophil influx, Respiratory burst capacity, Migration
Introduction
Sepsis is a progressive, inflammatory response to overwhelming infection associated with tissue hypoperfusion and multiorgan dysfunction[1,2]. Despite the development of novel treatments such as early appropriate antibiotherapy, and early goal-directed therapy, severe sepsis remains a common and frequently fatal condition with high associated costs and a mortality rate approaching 50% in the case of septic shock[1,2,3]. Acute lung injury (ALI) and its most severe form, acute respiratory distress syndrome (ARDS) are characterized by increased capillary and alveolar permeability, hypoxemia, decreased lung compliance, and diffuse bilateral pulmonary infiltrates[4]. At present, therapeutic interventions to treat ALI or ARDS remain limited. Thus far, no real pathophysiologic-driven therapeutic interventions has been available[5,6]. Based on pathophysiologically oriented views, ALI and ARDS can be of pulmonary (direct) or extrapulmonary (indirect) origin[7]. Indirect acute lung injury (iALI) accounts for approximately 20% of all ALI cases, meanwhile sepsis is the most commonly encountered condition underlying the development of ALI, with severe sepsis accounting for 33% of indirect ALI cases[8]. There are several studies indicating that direct and indirect ALI are truly different[9,10,11,12,13]. As the failure of many supportive therapies in ARDSnet[5,6], novel therapeutic strategies with respect to etiology and pathophysiologic-driven are still needed.
Neutrophils comprise one of the major cellular components of the innate immune system. They are thought to contribute directly to antimicrobial killing via expression of a range of antimicrobial peptides, proteases, and oxidants, which are proposed to have an important role in sepsis and inducing iALI[14,15,16]. There is compelling evidence indicating that neutrophil function during severe sepsis and iALI is substantially dysregulated, resulting in impaired directed migration of neutrophils to infectious foci and inadequate antimicrobial responses. The ability of neutrophils to destroy bacteria or other pathogens is dependent upon the ability to mount an effective respiratory burst response. Respiratory burst function resulting in the release of reactive oxygen species such as superoxide anion (O2−) from neutrophils is one of the mechanisms of the innate immune response to a microbial challenge. Maladaptive control of this mechanism is thought to play a pivotal role in the development of iALI/ARDS as well as sepsis[17,18,19], both are reported to be the most common forms of organ dysfunction seen in trauma/surgical intensive care unit patients. Using our clinically relevant hemorrhagic shock (Hem; a ‘priming’ insult) followed by polymicrobial septic challenge (CLP; ‘triggering’ event) (a double-hit model which has been demonstrated to induce iALI) animal model, we reported previously that neutrophils isolated from donor animals that had undergone hemorrhagic shock induced iALI when adoptively transferred into naive animals that were subsequently subjected to a septic challenge[20,21,22]. In this setting, in vivo neutrophil ‘priming’ by hemorrhage (Hem) was not only associated with an increase in respiratory burst capacity ex vivo, but also with a decrease in neutrophil apoptosis[20,21,22], a finding confirmed by others in experimental iALI[23]. We further found that these primed neutrophils were recruited into the lungs to mediate the development of ALI upon exposure to the second hit, while the severity of iALI was reduced by neutrophil depletion[24] or the blockade of CXCR2 signaling[25]. Although a role for neutrophil respiratory burst capacity in sepsis and iALI is well established, strategies to control neutrophil activation to prevent tissue and organ injury have not followed.
To kill microbial organisms more effectively, the neutrophil respiratory burst, based upon the assembly and activation of nicoinamide adenine dinucleotide phosphate (NADPH) oxidase which then catalyses the univalent reduction of molecular oxygen (O2) to O2−, is augmented by a variety of biological agents through a process termed “priming”[15,26]. The respiratory burst can be primed by exocytosis of neutrophil granule subsets[27,28]. Soluble NSF attachment protein receptor (SNARE) proteins play a central role in intracellular membrane trafficking by mediating fusion of membranes from different cellular compartments[29,30,31]. It has been suggested that the selective SNARE proteins in different granule membranes could have their independent mobilization of the different granule populations present in neutrophils during activation[32,33]. Studies have also shown that synaptosome-associated protein-23 (SNAP-23), one of the SNARE proteins which had been identified on neutrophil granules and plasma membranes, plays a role in neutrophil granule exocytosis[33]. Uriarte et al recently developed an HIV transactivator of transcription (TAT)-fusion protein containing a SNARE domain from SNAP-23 (TAT-SNAP-23). They demonstrated that TAT-SNAP-23’s ability to inhibit tumor necrosis factor-α (TNF-α) and platelet-activating factor-induced priming of the normal human neutrophil respiratory burst by up to 80% in vitro by blocking exocytosis selectively. They further confirmed that neutrophil granule exocytosis contributing to phagocytosis-induced respiratory burst played a critical role in priming of the respiratory burst by increasing expression of membrane components of the NADPH oxidase[34]. They recently showed that intravenous administration of TAT-SNAP-23 resulted in amelioration of injury in a rat model of direct ALI by inhibiting neutrophil exocytosis[35]. However, to our knowledge, the action of TAT-SNAP-23 on neutrophil respiratory burst in a murine model of sepsis or iALI has not been elucidated. Therefore, it is quite reasonable to hypothesize that TAT-SNAP-23 could have a role in regulating neutrophil respiratory burst induced by murine models of sepsis and hemorrhage-induced priming for iALI. The goal of the present study was to identify TAT-SNAP-23 as an inhibitor of neutrophil respiratory burst in vivo using our two-hit model of iALI or CLP alone. Our results indicate that TAT-SNAP-23 plays a crucial role in blocking the neutrophil respiratory burst not only in septic insult, but also in Hem primed iALI, during which neutrophil influx (as determined by myeloperoxidase [MPO] activity) in the lung was also suppressed while not altering the change in local cytokine/chemokine levels.
Materials and Methods
Mice
Male C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice aged 8–10 weeks were used for experiments. Experiments were performed in accordance with National Institutes of Health guidelines and with approval from the Animal Use Committee of Rhode Island Hospital.
Reagents
Keratinocyte-derived chemokine (KC) and macrophage inflammatory protein-2 (MIP-2) antibodies for ELISA assays were purchased from R&D Systems, Minneapolis, MN. Mouse interleukin (IL)-6, IL-10, monocyte chemoattractant protein-1 (MCP-1) and TNF-α ELISA kits were purchased from BD Bioscience, San Diego, CA. The TAT-SNAP-23 fusion protein was provided by Uriarte et al[34,35]. All other chemicals were of analytical reagent grade and purchased from Sigma Chemical, St Louis, MO.
Rodent Models
Hemorrhage (Hem)
The non-lethal fix-pressure Hem model used for these experiments has been previously described in our laboratory[20,21,22,24,25]. In brief, mice were anesthetized with isoflurane, restrained in supine position, and catheters were inserted into both femoral arteries. Anesthesia was discontinued, and blood pressure was continuously monitored through one catheter attached to a blood pressure analyzer (BPA; MicroMed, Louisville, KY). When fully awake, as determined by a mean blood pressure of ~95 mmHg, the mice were bled over a 5 to 10 min period to a mean blood pressure of 35 mmHg (±5 mmHg) and kept stable for 90 min. Immediately following Hem, mice were resuscitated with Ringer’s lactate at four times drawn blood volume. After resuscitation, arteries were ligated, catheters removed, and catheter sites bathed with lidocaine and sutured closed. Sham mice were anesthetized, restrained in a supine position for the same duration, and blood vessels were ligated, not bled[20,21,22,24,25].
Polymicrobial Sepsis/Cecal Ligation and Puncture (CLP)
Mice were anesthetized with isoflurane and restrained in supine position. A 1 cm midline incision was made; the cecum was ligated with 5-0 silk thread and punctured twice with a 22-gauge needle. The cecum was then replaced, and the incision was closed. Mice were resuscitated with 1 ml Ringer’s lactate s.c. and returned to their cages. Sham mice were anesthetized, restrained in a supine position for the same duration, and the cecum was exposed, but not ligated nor punctured[20,21,22,24,25].
iALI (Hem/Sepsis Model)
Mice subjected to Hem procedures 24 hours later, sepsis was induced as a secondary challenge via CLP as previously described[20,21,22,24,25].
Neutrophil Isolation
Neutrophil isolation from individual mouse blood was performed as previously described[20]. In summary, whole blood was collected from Naïve, sham CLP, CLP, Hem alone or iALI mice via cardiac puncture into a heparin rinsed syringe. An equal volume of 3% Dextran in PBS was added to the heparinized blood, mixed vigorously and left to stand at room temperature for 45 minutes. The top leukocyte rich layer was removed, transferred to a 15ml tube, added PBS to 15 ml in total and centrifuged 10 minutes at 400 × g (4°C). Cells were resuspended in 3 ml PBS with 0.1% BSA and layered over a discontinuous density percoll gradient (2 ml of 1.097 density in the bottom layer carefully overlaid with 3 ml of 1.077 density) and centrifuged 1 hour at 500 × g and 25°C. Neutrophils were removed from lower interface, transferred to a 15 ml tube and filled up with PBS to 15 ml, centrifuged 15 minutes at 2,000 rpm and 4°C. The number of viable cells per sample was determined using Trypan blue exclusion.
Respiratory Burst Assay
In Vitro
Isolated neutrophils (3 × 105 cells) from naïve or sham mice were pre-treated with increasing concentration of TAT-SNAP-23 (0.2 to 0.6ug/assay), in the presence or absence of TNF-α, as a priming agent (2 ng/ml, at 37°C for 10 minutes) followed by phorbol 12-myristate 13-acetate (PMA, 20 nM). Superoxide release was assessed by measuring the superoxide dismutase-inhibitable reduction of ferricytochrome C. After reading at duel wavelength 550, 630 (reference wavelength) at 37°C, data was analyzed using the following formula: Respiratory burst = 13.258 × (OD at X minutes − OD at 0 minutes)[20].
In Vivo
For the in vivo studies, Sham and CLP mice were given 6 ug or 12 ug of TAT-SNAP-23 in 200ul HBSS/mouse by intravenous tail injection right after surgery. Hem mice were given 12 ug or 24 ug of TAT-SNAP-23 in 200ul HBSS/mouse by intravenous infusion during resuscitation period. The dose of TAT-SNAP-23 used for these experiments was determined based on the results of TAT-SNAP-23 dose study in vitro. Blood was collected and neutrophils were isolated at various times after surgery, and the respiratory burst activity was assessed as described in the in vitro section.
Neutrophil chemotaxis assay
The modified Boyden chamber/48 well micro-chemotaxis assay was performed as per manufacturer’s protocol (Neuro Probe, Inc, Gaithersburg, MD). Briefly, 25 ul of fMLP (stock 5nM) or media control (DMEM) was pipetted into the 48 wells in the bottom chamber. Isolated neutrophils from CLP and hemorrhaged mice were pipetted into the top wells (50 ul media containing 1 × 104 cells/well). The chamber was incubated for 60 min at 37 °C. Following incubation, the chamber was inverted and the filter removed. Non-migrated cells were wiped off from the cell solution side of the membrane and the migrated cells on the membrane were stained with modified Giemsa stain (Sigma-Aldrich, MO) and counted at 100 × magnification on at least five random fields. Results were expressed as number of neutrophils per field. The number of migrating cells present in the containing media alone condition were used as controls for random migration[21].
Lung Myeloperoxidase (MPO) Activity
As an assessment of neutrophil influx, MPO activity was measured according to established protocols[21,22]. Lung tissue was homogenized in 0.3 ml 50 mM Potassium Phosphate Buffer (0.1 M of KH2PO4 and 0.1 M of K2HPO4), centrifuged at 500 × g, at 4°C for 5 minutes, supernatant was collected and centrifuged at 20,000 × g, at 4°C for 60 minutes, then pellet was resuspended in 0.3 ml sonicate buffer (50 mM Potassium Phosphate Buffer [0.1 M of KH2PO4, 0.1 M of K2HPO4 and 0.5% of Hexadecyltrimethyl-ammonium bromide]) for 30 seconds. After three times freeze-thaw cycle, lysate was centrifuged 12,000 × g, at 4°C for 10 minutes. Supernatants were then assayed for MPO activity at a 1:20 dilution in the reaction buffer (50 mM Potassium Phosphate Buffer [0.1 M of KH2PO4, 0.1 M of K2HPO4, 530 nM of O-diansidine and 0.0005% of H2O2]) and read at 490 nm.
BAL Protein Assay
The acquisition of bronchoalveolar lavage (BAL) was done as previously described[21,22]. This lavage fluid was centrifuged at 1,500 × g for 10 min at 4°C for protein concentration assay[21,22].
Plasma Cytokine Measurement by Cytometric Bead Array (CBA)
Blood was collected via cardiac puncture into heparinized syringes, centrifuged, and plasma samples were collected and stored at −70°C for cytokine analysis. Mice TNF-α, MCP-1, IL-10 and IL-6 levels in plasma were determined by using CBA technique (BD™ Cytometric Bead Array Mouse Inflammation Kit, BD Biosciences). Procedures are according to the manufacturer’s instructions.
Lung Tissue Cytokine Measurement by ELISA
Lung tissue samples were homogenized in the lysis buffer (50mM Tris-HCl, pH7.5, 5mM EDTA, 150mM NaCl, 10mM NaPhosphate, 10mM NaF, 1mM NaOrthovanadate, 0.5% TritonX100 and protease inhibitor cocktail) for 30 seconds, kept on ice for 30 minutes, centrifuged, and lysate was collected. Protein content was determined by a standard protein assay using BIO-RAD reagents. Mouse KC, MIP2, IL-6, MCP-1, IL-10 and TNF-α levels in lung tissue were determined with ELISA (BD Bioscience, San Diego, CA.) as described previously[20,21,22].
Immunohistochemical Staining for Assessment of Neutrophil Influx and Tissue Architecture
For histological assessment, the trachea was cannulated, and lungs were gently inflated with 10% formalin through the cannula by a continuous release pump under pressure and volume-controlled conditions (12 ml/h; 5 min) and then paraffin embedded. Samples were then stained with hematoxylin and eosin and examined by light microscopy for lung morphology. Staining for leukocyte-specific esterase, Naphthol AS-D chloroacetate esterase (Sigma Diagnostics, St Louis, Mo) was performed on tissue sections fixed in citrate-acetone-formaldehyde[21,22]. Slides were incubated in a solution of sodium nitrate, fast red violet BL base solution, TRIZMAL 6.3 buffer, and naphthol AS-D chloroacetate solution in deionized water for 15 min at 37°C. After rinsing, slides were counter-stained with Gills hematoxylin solution and cover-slipped. Stained lung sections were examined microscopically for morphology and positively stained cells. To establish the percentage (%) of cells (per field) that were esterase+ (neutrophils) present in the sample, tissue sections were randomly screened (seven to eight fields/slide) at 400 × (25 um2/field)[14,15].
Statistical Analysis
Data were expressed as mean ± SEM and analyzed using GraphPad Prism 5 statistical analysis and graphing software. Unpaired student t test was used to determine differences between the two groups according to Microsoft Excel (Microsoft Corp, Bethesda, MD). Multiple group comparisons were performed using one-way AVONA with the post hoc test of Tukey's. P <0.05 was considered significant.
Results
Inhibition of neutrophil respiratory burst by TAT-SNAP-23
TAT-SNAP-23 inhibits TNF-induced priming of mouse blood neutrophil respiratory burst in vitro
It has been shown in vitro that blocking human neutrophil granule exocytosis by TAT-SNAP-23 inhibited TNF-α-induced priming of the respiratory burst[34]. In the current study, we wanted to test the effect of TAT-SNAP-23 in a mouse model of in vivo ‘priming’ by hemorrhage followed by septic challenge. First, we performed a dose response to determine an effective concentration of TAT-SNAP-23 using neutrophils isolated from naïve mice. Mouse neutrophils were pretreated with TAT-SNAP-23 at 0.4 ug/ml and 0.6 ug/ml for 10 min, then respiratory burst capacity following TNF-α priming was measured using phorbol myristate acetate (PMA) as the stimulus. The results showed that 0.6 ug /3×105 cells/ml was effective to inhibit respiratory burst induced by TNF-α priming (Supplemental Figure 1).
In vivo administration TAT-SNAP-23 in mice alters ex vivo respiratory burst capacity of blood neutrophils from mice subjected to experimental septic challenge or hypotensive shock
CLP model
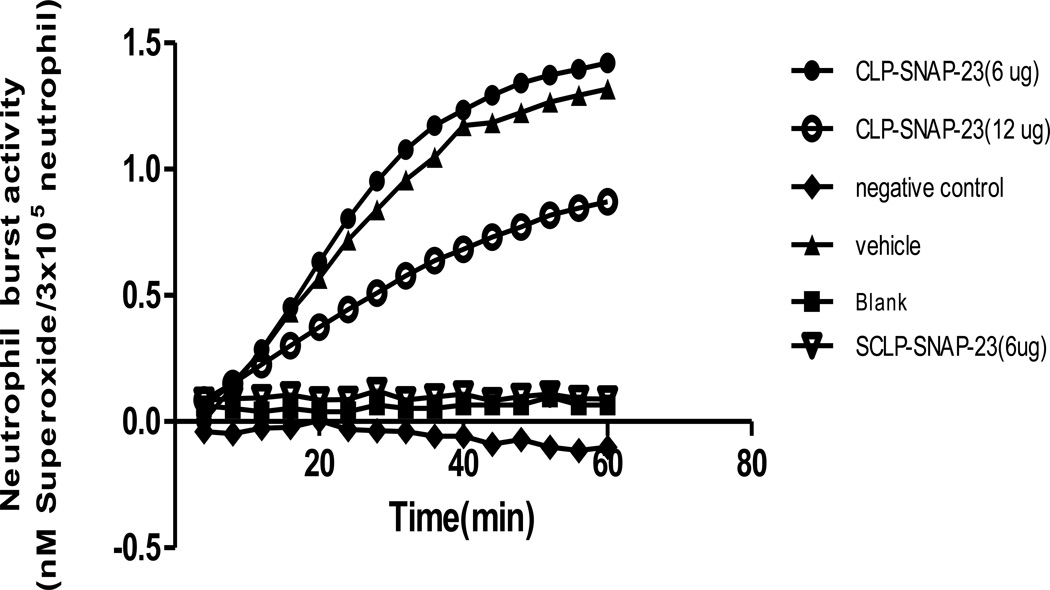
After establishing that TAT-SNAP-23 was able to inhibit the TNF-induced respiratory burst in vitro using mouse neutrophils; since it has been shown that the cells are not only affected by sepsis but are proposed to serve as mediators (via dysfunctional regulation of their respiratory burst function) of by-stander organ injury seen in sepsis, we hypothesized that TAT-SNAP-23 should have a role in regulating neutrophil respiratory burst induced by septic challenge in vivo. To address this hypothesis, CLP mice were divided into two groups with or without treatment of 6 or 12 ug/mouse of TAT-SNAP-23 (ten or twenty times the in vitro dose). Four hours post-surgery, blood neutrophils were isolated and respiratory burst was tested similar to the in vitro studies described above. Figure 1 shows that PMA stimulated respiratory burst capacity was significantly inhibited by 12 ug/mouse dose of TAT-SNAP-23, but not by 6 ug/mouse.
Figure 1. In vivo dose response of TAT-SNAP-23 in CLP model.
CLP mice were given TAT-SNAP-23(6 ug or 12 ug) in 200 ul HBSS/mouse, or vehicle (200 ul HBSS/mouse) by intravenous tail injection right after surgery. Blood was collected 4 hours post surgery. Neutrophils were isolated and resuspended in 100 ul sterial HBSS, then added to 100 ul of 0.398% Cytochrome C and 20 nM of PMA (as activating agonists) for burst test. The blank control was set as no cells; the negative control was set as without 20 nM of PMA. Sham CLP mice were given 6 ug TAT-SNAP-23 in 200 ul HBSS/mouse and neutrophils were also isolated for respiratory burst test as baseline. Sham mice had small/no neutrophil respiratory burst test. The dose of 12 ug/mouse TAT-SNAP-23, not 6 ug/mouse significantly inhibits the neutrophil respiratory burst capacity in CLP mice (n=6–10/group, *p < 0.05 12 ug/mouse vs. Sham CLP, HBSS/vehicle and 6 ug/mouse CLP group, determined by one-way AVONA for multiple group comparisons). A typical result of three groups is provided.
Hem alone model
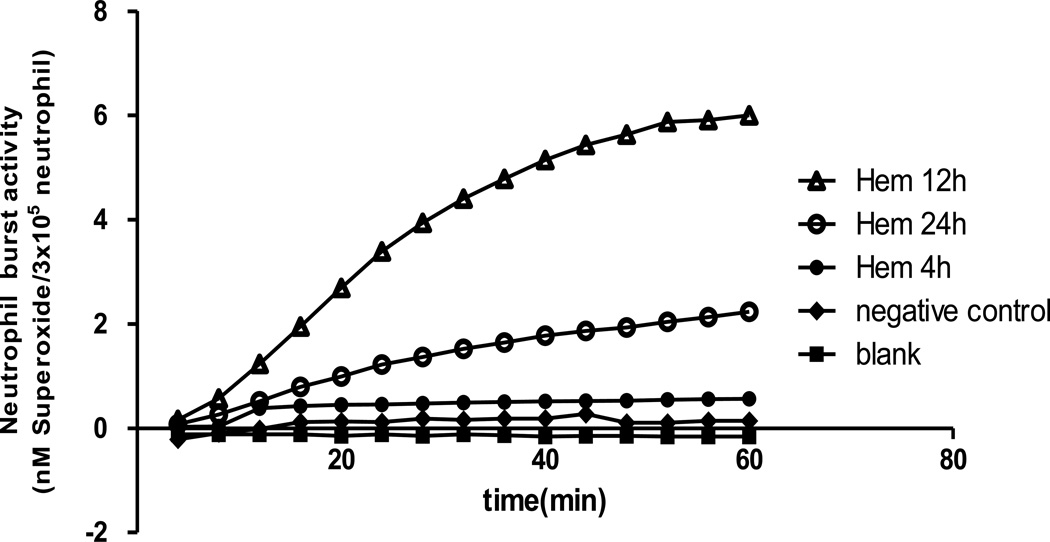
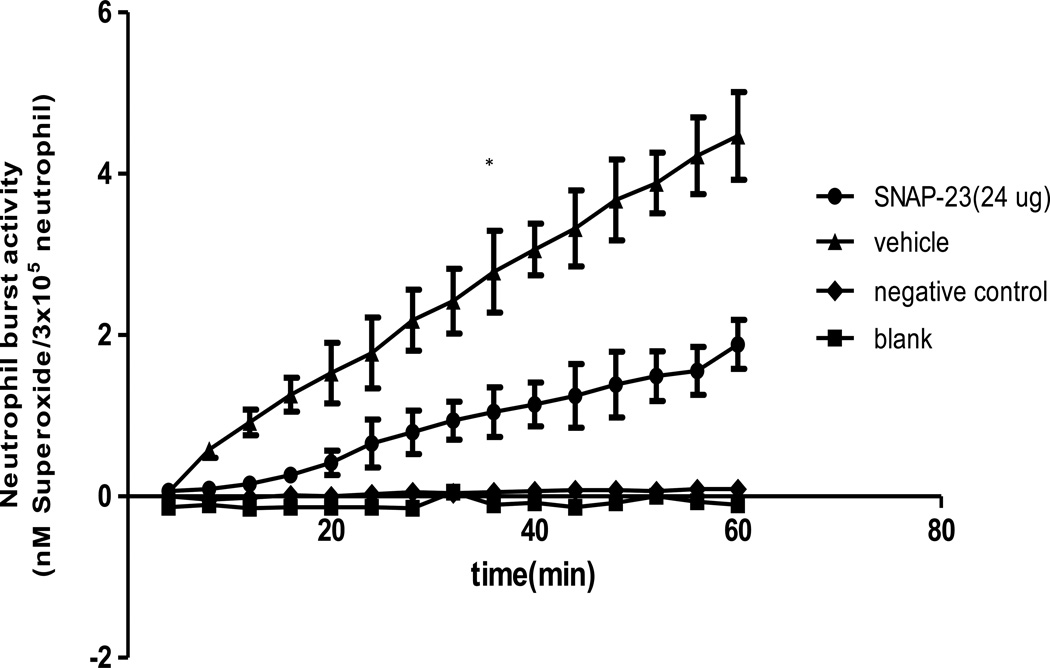
In previously published experiments we have shown that non-lethal Hem serves to transiently prime for enhanced respiratory burst capacity of neutrophils, which appears to contribute to the induction of lung injury in response to a subsequent septic challenge[20]. To determine if intravenous administration of TAT-SNAP-23 inhibited Hem-induced priming of the neutrophil respiratory burst, we first set out to establish the kinetics of the change in ex vivo blood neutrophil respiratory burst capacity over time following 4, 12 and 24 hours after Hem/resuscitation. Figure 2 shows that there is no priming of PMA stimulated superoxide release at 4 hours post Hem, however, when cells were taken for assessment at 12 hours, a priming response is observed after PMA stimulation, which is reduced if cells are obtained at 24 hours. These data indicate that the 12 hour time point is the optimum time for assessing the in vivo capacity of TAT-SNAP-23 administration to effect neutrophil priming ex vivo. That said; when we initially examined the in vivo capacity of 12 ug/mouse (the effective dose that alter the septic mouse blood neutrophil rise in ex vivo respiratory burst capacity) of TAT-SNAP-23 we found that this dose in Hem mice (data not shown) only slightly decreased the respiratory burst capacity, while 24 ug/mouse (Figure 3) significantly (p<0.05) inhibited the respiratory burst comparing them with vehicle treatment group. Based on these results, 24 ug/mouse TAT-SNAP-23 was selected for use in the following studies using the iALI model induced by Hem-priming followed by CLP.
Figure 2. Optimal timepoints for neutrophil respiratory burst testing after Hem alone.
Blood was collected at three different timepoints (4, 12 and 24 hours) after Hem. Neutrophils were isolated and resuspended in 100 ul sterial HBSS, then added to 100 ul of 0.398% Cytochrome C and 20 nM of PMA (as activating agonists) for burst test. The blank control was set as no cells; the negative control was set as without 20 nM of PMA. Twelve hours after Hem is the optimum time to give TAT-SNAP-23. A typical result of three independent experiments is provided.
Figure 3. TAT-SNAP-23 inhibits neutrophil respiratory burst in Hem model.
Hem mice were given 24 ug TAT-SNAP-23 in 200 ul HBSS/mouse, or vehicle (200 ul HBSS/mouse) by intravenous infusion during resuscitation period. Blood was collected 12 hours post surgery. Neutrophils were isolated and resuspended in 100 ul sterial HBSS, then added to 100 ul of 0.398% Cytochrome C and 20 nM of PMA (as activating agonists) for burst test. The blank control was set as no cells; the negative control was set as without 20 nM of PMA. 24 ug/mouse TAT-SNAP-23 significantly inhibits the neutrophil respiratory burst 12 hours after Hem comparing with vehicle-treated control group (n=6–8/group, *p<0.05 TAT-SNAP-23 vs. vehicle treatment group, determined by Unpaired student t test), All data are expressed as mean ± SEM.
Neutrophil influx to the lungs is significantly blocked by in vivo treatment with TAT-SNAP-23
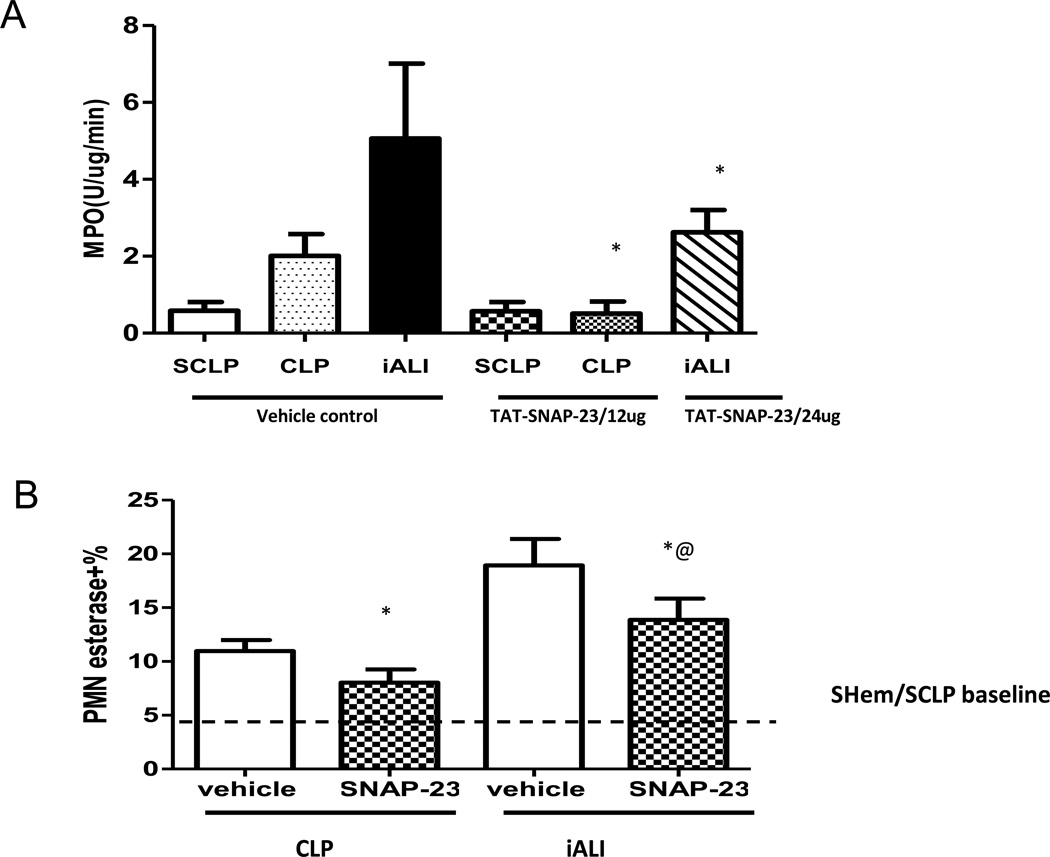
In both the CLP and the iALI model, TAT-SNAP-23 significantly inhibited PMA induced respiratory burst capacity. We next wanted to evaluate if inhibition of respiratory burst capacity affected neutrophil infiltration into the lungs. Our data indicate that neutrophil influx, as assessed by myeloperoxidase (MPO) activity, was significantly reduced in lung tissue of CLP mice that received 12 ug /mouse TAT-SNAP-23 after 4 hours treatment (Fig 4A). Figure 4A shows that TAT-SNAP-23 treatment significantly decreased tissue MPO activity by 48% when compared with control mice in the iALI model (Hem/CLP). Supporting the MPO data, lung tissue histology from both CLP and iALI mice that received TAT-SNAP-23 showed a significant reduction in the percentage of neutrophil-specific esterase+ cells compared with their respective vehicle treatment control group (Fig 4B).
Figure 4. Neutrophil influx significantly blocked by TAT-SNAP-23.
Lung tissue MPO activity (A), a measure of neutrophil influx to lung, was reduced in lungs from both CLP and iALI model comparing to respective control groups (n=6–8/group, * p < 0.05 TAT-SNAP-23 vs. vehicle treatment CLP group; TAT-SNAP-23 vs. vehicle treatment iALI group, determined by Unpaired student t test). (B) This is consistent with the percentage of esterase+ (neutrophil specific) cells compared with lung tissue from vehicle treatment control group (n=4–8/group, * p<0.05 vs. equivalent vehicle treatment group, @ p<0.05 TAT-SNAP-23 treatment iALI group vs. TAT-SNAP-23 treatment CLP group, determined by Unpaired student t test), All data are expressed as mean ± SEM. Dashed line represents Sham Hem/Sham CLP (SHem/SCLP) baseline level.
In vivoTAT-SNAP-23 administration supresses ex vivo neutrophil migratory capacity
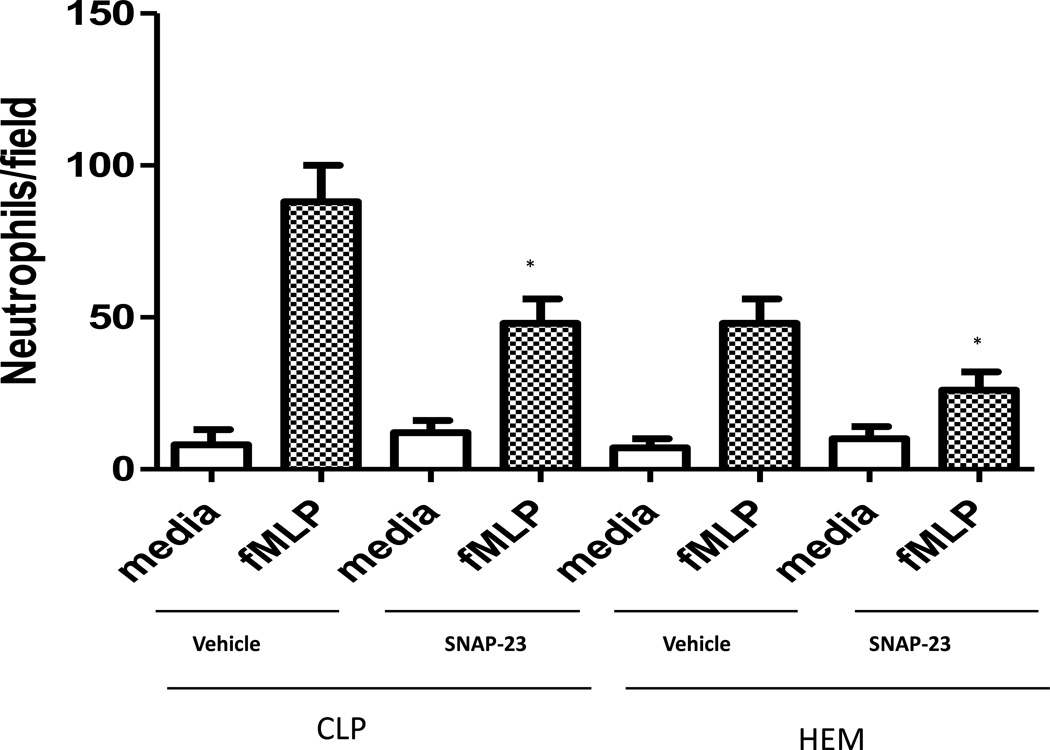
To further investigate the potential mechanisms by which inhibition of neutrophil influx was induced by TAT-SNAP-23 as seen in data above (Fig. 4), blood neutrophils were isolated at 4 hours post-CLP, or 24 hours post-Hem alone, and their corresponding control groups were evaluated ex vivo for their ability to migrate toward classical chemotactic agent fMLP (20uM) using the modified Boyden chamber in vitro assay. As shown in Fig 5, neutrophil migration from control CLP mice was 88 ± 12 cells/field and this was significantly reduced in the TAT-SNAP-23 treated animals (48 ± 8 cells/field). Similarly, neutrophil migration from control Hem mice was 48 ± 8 cells/field, and TAT-SNAP-23 treatment significantly reduced neutrophil migration (26 ± 6 cells/field). The significant reduction in neutrophil migration in both mouse models treated with TAT-SNAP-23 is consistent with the MPO data. These data indicate that TAT-SNAP-23 treatment blunted the in vitro chemotactic capacity of neutrophils isolated from CLP and Hem mice.
Figure 5. Effects of TAT-SNAP-23 on neutrophil chemotaxis ability in vitro.
Isolated Neutrophils (1 × 104 cells) from CLP and Hem mice with or without treatment with TAT-SNAP-23 were pipetted into the top wells of a modified Boyden chamber, while the DMEM medium with fMLP (20 µM) was placed in the lower chambers. Migration was carried out for 1 h. After the incubation, the number of migrated cells was counted at 100 × magnification on at least five random fields. Each data represents the mean ± SEM from four experiments in each group (N= 4/ each group, * p < 0.05 TAT-SNAP-23 treatment groups vs. equivalent vehicle treatment control groups, determined by Unpaired student t test), All data are expressed as mean ± SEM.
In vivo TAT-SNAP-23 treatment preserves lung architecture in iALI mice
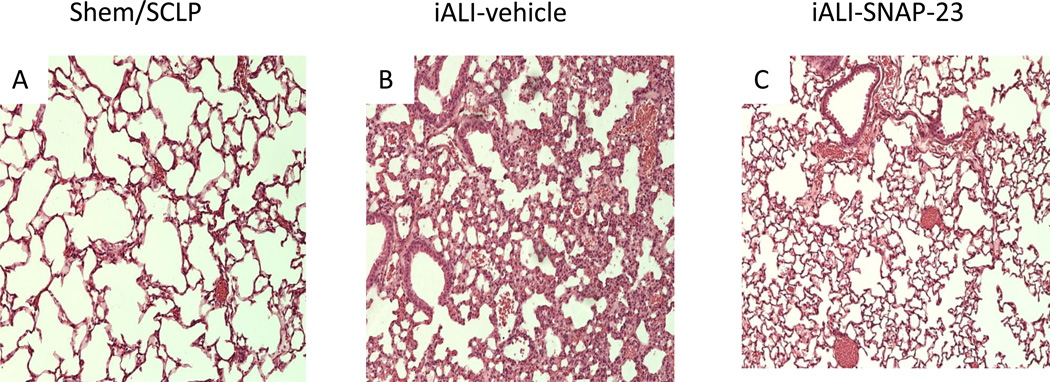
To the extent that the administration of TAT-SNAP-23 decreased the neutrophil infiltration into the lungs of iALI mice, we further want to know whether TAT-SNAP-23 would reduce the degree of lung injury. Figure 6 shows representative lung histology from iALI mice with or without treatment of TAT-SNAP-23. Disruption of lung tissue architecture, cellularity and septal thickening were reduced in lung tissue sections from TAT-SNAP-23-treatment mice subjected to Hem/CLP. Histologic changes were less evident in the CLP mice sections compared with their respective controls (Figure not shown), this is consistent with our former findings and those of Iskander et al[36], that CLP alone is not a strong inducer of ALI[20].
Figure 6. TAT-SNAP-23 treatment preserves lung architecture in iALI mice.
Hematoxylin and eosin stain of representative sections (100 × magnification) of the lungs of SHem/SCLP group (A), vehicle control (B) and TAT-SNAP-23 (C) treatment mice 24 hours post-iALI. Histological findings confirmed that TAT-SNAP-23 significantly reduced lung tissue septal thickening and cellular infiltrate of mice subjecting to iALI (C) compared with vehicle treatment control group (B) and SHem/SCLP group(A).
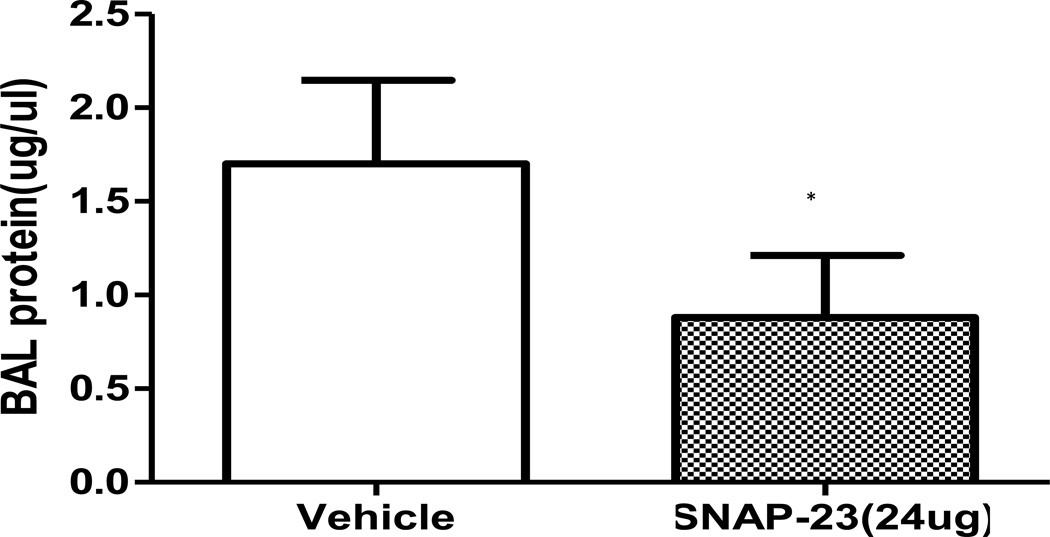
To quantify the amelioration of increased vascular permeability observed in the iALI mice treated with TAT-SNAP-23, protein concentration in bronchio-alveolar lavage (BAL) fluids were assessed in iALI groups. The protein levels in BAL from TAT-SNAP-23-treated animals were significantly reduced in comparison with the control group (Fig 7). This was consistent with our histological findings of a reduction in the disruption of lung tissue architecture and congestion seen in the TAT-SNAP-23 treatment group (Fig 6B, 6C).
Figure 7.
Protein concentration in bronchoalveolar lavage fluid from TAT-SNAP-23 treated iALI mice decreased significantly compared with control group (n= 6–8/group, * p < 0.05 TAT-SNAP-23 vs. vehicle treatment control iALI group, determined by Unpaired student t test), All data are expressed as mean ± SEM.
In vivo TAT-SNAP-23 administration has no effect on blood or lung tissue neutrophil chemotactic protein and inflammatory cytokine levels in CLP or iALI mice
The data above showed that administration of TAT-SNAP-23 resulted in amelioration of lung injury, significant reduction of protein levels in BAL fluids, reduced ex vivo neutrophil migratory function, as well as their respiratory burst capacity. Thus, we wanted to determine whether the local tissue or systemic blood levels of pro-inflammatory cytokines/chemokines were also affected by administration of TAT-SNAP-23. Interestingly, levels of the neutrophil chemotactic proteins, KC and MIP-2, were not reduced in the lung of either CLP or iALI mice treated with TAT-SNAP-23 post 24 hours when compared with their respective vehicle-treated mice (Table 1). In addition, no differences in TNF-α, IL-6, IL-10 and MCP-1 levels were observed in lung tissue or plasma from mice with/without treatment of TAT-SNAP-23 (Table 2). Taken together, these results suggest that administration of TAT-SNAP-23 has no effect on proinflammatory cytokines/ chemokines production/release.
Table 1.
Administration of TAT-SNAP-23 had no effect on neutrophil chemokines production in lung in both CLP and iALI model
| Chemokine protein (pg/ml) | ||
|---|---|---|
| KC | MIP-2 | |
| CLP-vehicle | 191±59 | 911±386 |
| CLP-SNAP | 179±48 | 864±289 |
| iALI-vehicle | 310±62 | 1311±586 |
| iALI-SNAP | 291±59 | 1478±301 |
Data are expressed as mean ± SD.
Table 2.
Administration of TAT-SNAP-23 had no effect on inflammatory cytokines release in both CLP and iALI model
| Cytokines in lung(pg/ml) | Cytokines in plasma(pg/ml) | |||||||
|---|---|---|---|---|---|---|---|---|
| TNF-α | IL-6 | IL-10 | MCP-1 | TNF-α | IL-6 | IL-10 | MCP-1 | |
| CLP-vehicle | 166±19 | 1234±256 | 143±41 | 191±75 | 312±43 | 2341±365 | 124±35 | 491±115 |
| CLP-SNAP | 152±28 | 1026±345 | 121±38 | 182±71 | 289±61 | 2176±499 | 138±28 | 383±185 |
| iALI-vehicle | 225±17 | 1578±431 | 156±29 | 234±55 | 448±67 | 2701±712 | 184±41 | 823±305 |
| iALI-SNAP | 201±29 | 1643±512 | 138±38 | 207±60 | 412±89 | 2532±865 | 167±55 | 764±215 |
Data are expressed as mean ± SD.
Discussion
Neutrophils contribute to the primary cellular defense against bacterial and fungal infections. Neutrophils ingest microbes by a process known as phagocytosis, and the ingested microorganisms are destroyed by the combination of reactive oxygen species (ROS) and cytotoxic components of granules[37]. Martins et al. had reported that generation of nitric oxide (NO) and ROS by neutrophils was increased in septic patients, and their persistence was associated with poor outcome[38,39]. Unfortunately, no effective drug is currently available that controls and terminates the neutrophils burst activity and prevents neutrophil-dependent inflammation without compromising other aspects of the innate immune response.
In this respect, soluble NSF attachment protein receptor (SNARE) proteins could represent just such a potential selective inhibitor of neutrophil function as these proteins play a central role in intracellular membrane trafficking by mediating fusion of membranes from different cellular compartments of these cells[29,30,31]. This, in turn, affects processes related to neutrophil function such as granule exocyctosis, migration and/or their respiratory capacity. Interestingly, it has been reported that inhibition of SNARE protein function with TAT-SNAP-23 was able to selectively block neutrophil degranulation and reduced TNF-mediated respiratory burst priming in human neutrophils[34]. However, until now, no studies have focused on these proteins or the effect of TAT-SNAP-23 inhibition of these proteins on the sepsis-induced neutrophil respiratory burst response. Thus, we hypothesized that TAT-SNAP-23 would decrease sepsis-induced neutrophil respiratory burst in an experimental model of this condition. In this study, we show for the first time that in vivo septic-challenge priming of neutrophil respiratory burst capacity is significantly inhibited by the intravenous administration of TAT-SNAP-23 compared to vehicletreated controls.
Based on these data, we sought to further investigate the role of TAT-SNAP-23 in Hem-induced priming for neutrophil respiratory burst capacity, as this is thought to set the stage for the development of full-blown indirect acute lung injury similar to that seen in critically injured trauma patients with ARDS when the animal is subjected to a secondary inflammatory/infectious challenge like peritoneal sepsis[20,21,22,24,25]. According to our previous studies, peripheral blood neutrophils from C3H/HeN mice isolated 24 hours post-Hem (Hem alone), but not sham Hem, exhibited an increase in ex vivo respiratory burst capacity, consistent with in vivo “priming” for iALI[20]. As we used different strain of mice (C57BL/6) to test the respiratory burst in this study, it was important to re-assess the kinetics of optimal neutrophil priming following shock. The time course results show that importantly, while there was still an increase in ex vivo respiratory burst capacity of blood neutrophils isolated from these C57BL/6 mice at 24 hours post-hemorrhage, the peak increase in capacity was seen at 12 hours post-Hem. Consistent with the result from CLP model, we also found that in vivo TAT-SNAP-23 treatment of mice significantly inhibited their ex vivo neutrophil respiratory burst capacity when assessed at 12 hours post-Hem.
Having documented that TAT-SNAP-23 significantly decreased the ex vivo blood neutrophil respiratory burst both in CLP and Hem alone mice, we sought to further investigate the role of TAT-SNAP-23 in our dual-insult (hemorrhage priming subsequent to septic challenge) model of iALI. We found that lung tissue sections from TAT-SNAP-23 treated iALI group showed significantly less disruption of normal lung tissue architecture, including reduced tissue congestion. In addition, BAL fluid protein concentration (an index of lung injury/permeability) from the TAT-SNAP-23-treated group was reduced compared to the vehicle-treated control group. This observation is supported, in part, by the recent findings by Uriarte et al, using an immune complex mediated deposition direct lung injury model in rats[35], in which they showed that TAT-SNAP-23 treatment resulted in a reduction in lung edema, vascular hemorrhage, vascular permeability, total BAL fluid protein levels as well as a decline in lung tissue MPO. Taken together, we broadened the potential treatment indications of TAT-SNAP-23 in protecting against lung injury of indirect causes in mice model, which is thought to be better reflect the clinical scenario of ALI encountered in the severely injured patients and pathophysiologically distinct from the direct insult to the lung[9,10,11,12,13].
In the current study, we found that MPO (an index of neutrophil influx) levels in lung tissue were decreased in TAT-SNAP-23-treated mice compared with the vehicle-treated controls. Consistent with the reduced tissue MPO levels, we found that TAT-SNAP-23 treatment also suppressed the number of neutrophil-specific esterase staining cells seen in lung tissue sections from both CLP and iALI mice. Morrell et al[39] had shown that intravenous administration of a TAT-fusion protein containing an inhibitory peptide derived from NSF inhibited vascular endothelial cell exocytosis and blocked neutrophil infiltration into the peritoneum in a mouse model of abdominal sepsis. These findings are similar to our results showing that administration of TAT-SNAP-23 reduced the neutrophil influx into lungs both in septic insult and iALI. Our finding of reduced neutrophil recruitment differs from the work of Uriarte et al who indicated that neutrophil infiltration was not affected by TAT-SNAP-23 treatment following immune complex (IC) deposition in a rat ALI model, which is thought to be dependent on complement activation, cytokine production, and/or chemokine-induced neutrophil infiltration[35]. One possible explanation for the difference could be that in the current study we used a two-hit mouse model in which the indirect-pulmonary insults of Hem-induced neutrophil priming is followed by peritoneal (another indirect-lung insult) polymicrobial septic challenge, which may be pathophysiologically distinct insults from those changes seen in response to immune complex deposition induced (direct-pulmonary insult) ALI in rats.
Neutrophils are the earliest immune cells to be recruited to the site of injury/inflammation, and their activation and recruitment are thought to play key roles in the progression of iALI and sepsis associated organ injury/dysfunction[40, 41]. Here we sought to further investigate the potential mechanism by which targeting neutrophil SNARE proteins with TAT-SNAP-23 affected neutrophil influx. A number of studies in mice have provided evidence supporting the concept that the most relevant chemokines for neutrophil recruitment into lungs are KC and MIP-2[25,42, 43]. Further, our previous antibody inhibition studies had shown that anti-MIP-2α, but not anti-KC treatment reduced neutrophil recruitment into lungs in iALI model[21]. It has also been reported by other laboratories that SNAP-23 is required for the release of all chemokines by mature human mast cells and the regulation of macrophage adhesion, spreading and migration on fibronectin[44,45,46,47]. In light of this, we attempted to assess whether in vivo treatment with TAT-SNAP-23 affect on neutrophil influx could be explained by its direct/indirect effect on lung tissue or circulating levels of these two chemokines. However, our data showed that in vivo treatment with TAT-SNAP-23 did not reduce either KC or MIP-2 levels in either CLP or iALI model.
Interestingly, we found irrespective of the inability to affect these chemokine levels, that TAT-SNAP-23 treatment markedly blunted the ex vivo migratory capacity of neutrophils from both the septic challenged and Hem alone mice, which may provide a partial explanation for the capacity of TAT-SNAP-23 treatment to reduce neutrophil influx to lungs of these mice. Four distinct phases of neutrophil migration have been identified: mobilization, margination and rolling, adherence, and transmigration through the vessel wall in response to chemotactic gradients, all of which are impacted by inflammatory activation[40,48]. Multiple mechanisms contribute to sepsis and ALI/ARDS-induced impairment of neutrophil migration; however, these processes are complex and our understanding remains incomplete[40,48]. It is now believed that injurious oxidants increase endothelial and/or epithelial cell permeability via disruption of tight junctions and redistribution of junction proteins during neutrophil transmigration. This is thought to be a key factor in the pathogenesis of sepsis and ALI, thus, neutrophils are thought to be the culprits causing the high levels of plasma and lung oxidants[49,50]. As previous studies had confirmed TAT-SNAP-23’s effects on the inhibition of exocytosis, reduction of the NADPH oxidase activity and production of ROS[34,51]; whether TAT-SNAP-23 has some effect on the neutrophil migration into the lung through a process dependent of oxidant-induced junctional disruption remains to be determined in the future experiments.
Here we first showed that TAT-SNAP-23 had an effect on reducing neutrophil recruitment and/or migration, in turn suppressing injury to the lung in a model of indirect-/extra-pulmonary insult that are believed to more closely mirror the clinical scenario. However, TAT-SNAP-23 did not affect the cytokine response to sepsis and iALI. This observation is supported, in part, by the recent findings in a rat ALI model[35], in which TAT-SNAP-23 had no marked effect on the cytokine production and release by resident macrophages, lung parenchymal cells or infiltrating neutrophils.
In summary, our data demonstrate that administration of TAT-SNAP-23 resulted in a significant reduction of neutrophil respiratory burst capacity in sepsis and hemorrhage, with concomitant amelioration of tissue damage in Hem-primed iALI, and decreased neutrophil recruitment into lung in both CLP and indirect ALI mice. Activated neutrophils mount a vicious respiratory burst that contributes to the initiation of iALI by increasing both the inflammatory response (neutrophils recruitment) and lung (microvascular endothelial and epithelial cells) permeability. These results shed a new light on the potential mechanisms of neutrophil-dependent problems and might contribute to the development of future concepts for the pathophysiologically oriented therapeutic remedies for severe clinical conditions such as sepsis and iALI/ARDS.
Supplementary Material
Acknowledgments
The authors acknowledge funding from NIH grants HL107149 (to A.A.), K99/R00 HL087924 (to S.M.U), and BX001838 from the Department of Veterans Affairs (to K.R. M.). We thank Mr. Paul Monfils at the Core Laboratories Facilities at Rhode Island Hospital for the assistance with the histological preparation/staining done in this study.
Abbreviations
- ALI
Acute lung injury
- ARDS
Acute respiratory distress syndrome
- BALF
Bronchoalveolar lavage fluid
- CLP
Cecal ligation and puncture
- iALI
Indirect-Acute lung injury
- IL-6
Interleukine-6
- IL-10
Interleukine-10
- KC
Keratinocyte-derived chemokine
- MIP-2
Macrophage inflammatory protein-2
- MCP-1
Monocyte chemoattractant protein-1
- MPO
Myeloperoxidase
- fMLP
N-formylmethionyl-leucyl-phenylalanine
- NADPH
Nicoinamide adenine dinucleotide phosphate
- ROS
Reactive oxygen species
- SNARE
Soluble NSF Attachment Protein receptor
- SNAP-23
Synaptosome-associated protein-23
- TNF-α
Tumor necrosis factor-α
Footnotes
Authorship
J.W.B., L.X.T,, Y.P.C., K.R.M. and S.L.M. performed the experiments. A.A., C.S.C, J.L.N, K.R.M. and S.L.M. provided guidance and critical review of methods. J.W.B. and L.X.T. wrote the manuscripts.
Conflict of interest Disclosure
The authors declare no conflict of interest
References
- 1.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb S, Beale RJ, Vincent JL, Moreno R. Surviving Sepsis Campaign Guidelines Committee including The Pediatric Subgroup. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock 2012. Intensive. Care. Med. 2013;9:165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huttunen Reetta, Aittoniemi Janne. New concepts in the pathogenesis, diagnosis and treatment of bacteremia and sepsis. J. Infect. 2011;63:407–419. doi: 10.1016/j.jinf.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Puskarich MA. Emergency management of severe sepsis and septic shock. Curr. Opin. Crit. Care. 2012;18:295–300. doi: 10.1097/MCC.0b013e328354dc16. [DOI] [PubMed] [Google Scholar]
- 4.Ware LB. Pathophysiology of acute lung injury and the acute respiratory distress syndrome. Semin. Respir. Crit. Care. Med. 2006;27:337–349. doi: 10.1055/s-2006-948288. [DOI] [PubMed] [Google Scholar]
- 5.ARDS Definition Task Force. Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 6.Calfee CS, Eisner MD, Ware LB, Thompson BT, Parsons PE, Wheeler AP, Korpak A, Matthay MA. Acute Respiratory Distress Syndrome Network, National Heart, Lung, and Blood Institute. Trauma-associated lung injury differs clinically and biologically from acute lung injury due to other clinical disorders. Crit. Care. Med. 2007;35:2431–2432. doi: 10.1097/01.ccm.0000280434.33451.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perl M, Lomas-Neira J, Venet F, Chung CS, Ayala A. pathogenesis of indirect (secondary) acute lung injury. Expert Rev Respir Med. 2011 Feb;5(1):115–26. doi: 10.1586/ers.10.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zimmerman JJ, Akhtar SR, Caldwell E, Rubenfeld GD. Incidence and outcomes of pediatric acute lung injury. Pediatrics. 2009 Jul;124(1):87–95. doi: 10.1542/peds.2007-2462. [DOI] [PubMed] [Google Scholar]
- 9.Menezes SL, Bozza PT, Neto HC, Laranjeira AP, Negri EM, Capelozzi VL, Zin WA, Rocco PR. Pulmonary and extrapulmonary acute lung injury: inflammatory and ultrastructural analyses. J Appl Physiol. 2005 May;98(5):1777–1783. doi: 10.1152/japplphysiol.01182.2004. [DOI] [PubMed] [Google Scholar]
- 10.Suntharalingam G, Regan K, Keogh BF, Morgan CJ, Evans TW. Influence of direct and indirect etiology on acute outcome and 6-month functional recovery in acute respiratory distress syndrome. Crit Care Med. 2001 Mar;29(3):562–566. doi: 10.1097/00003246-200103000-00016. [DOI] [PubMed] [Google Scholar]
- 11.Rocco PR, Zin WA. Pulmonary and extrapulmonary acute respiratory distress syndrome: are they different? Curr Opin Crit Care. 2005 Feb;11(1):10–17. doi: 10.1097/00075198-200502000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Pelosi P, D'Onofrio D, Chiumello D, Paolo S, Chiara G, Capelozzi VL, Barbas CS, Chiaranda M, Gattinoni L. Pulmonary and extrapulmonary acute respiratory distress syndrome are different. Eur Respir J Suppl. 2003 Aug;42:48s–56s. doi: 10.1183/09031936.03.00420803. [DOI] [PubMed] [Google Scholar]
- 13.Kovach MA, Standiford TJ. The function of neutrophils in sepsis. Curr. Opin. Infect. Dis. 2012;25:321–327. doi: 10.1097/QCO.0b013e3283528c9b. 2012. [DOI] [PubMed] [Google Scholar]
- 14.Grommes J, Soehnlein O. Contribution of neutrophils to acute lung injury. Mol. Med. 2011;17:293–307. doi: 10.2119/molmed.2010.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perl M, Hohmann C, Denk S, Kellermann P, Lu D, Braumüller S, Bachem MG, Thomas J, Knöferl MW, Ayala A, Gebhard F, Huber-Lang MS. Role of activated neutrophils in chest trauma-induced septic acute lung injury. Shock. 2012;38:98–106. doi: 10.1097/SHK.0b013e318254be6a. [DOI] [PubMed] [Google Scholar]
- 16.Wang L, Taneja R, Razavi HM, Law C, Gillis C, Mehta S. Specific role of neutrophil inducible nitric oxide synthase in murine sepsis-induced lung injury in vivo. Shock. 2012;37:539–547. doi: 10.1097/SHK.0b013e31824dcb5a. [DOI] [PubMed] [Google Scholar]
- 17.Carey PD, Leeper-Woodford SK, Walsh CJ, Byrne K, Fowler AA, Sugerman HJ. Delayed cyclo-oxygenase blockade reduces the neutrophil respiratory burst and plasma tumor necrosis factor levels in sepsis-induced acute lung injury. J. Trauma. 1991;31:733–740. doi: 10.1097/00005373-199106000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Tung JP, Fraser JF, Wood P, Fung YL. Respiratory burst function of ovine neutrophils. BMC. Immunol. 2009;10:25. doi: 10.1186/1471-2172-10-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ayala A, Chung CS, Lomas JL, Song GY, Doughty LA, Gregory SH, Cioffi WG, LeBlanc BW, Reichner J, Simms HH, Grutkoski PS. Shock induced neutrophil mediated priming for acute lung injury in mice: divergent effects of TLR-4 and TLR-4/FasL deficiency. Am. J. Pathol. 2002;161:2283–2294. doi: 10.1016/S0002-9440(10)64504-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lomas JL, Chung CS, Grutkoski PS, LeBlanc BW, Lavigne L, Reichner J, Gregory SH, Doughty LA, Cioffi WG, Ayala A. Differential effects of macrophage inflammatory protein-2 and keratinocyte-derived chemokine on hemorrhage-induced neutrophil priming for lung inflammation: assessment by adoptive cell transfer in mice. Shock. 2003;19:358–365. doi: 10.1097/00024382-200304000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Lomas JL, Perl M, Venet F, Chung CS, Ayala A. The Role and Source of TNF-α in Hemorrhage Induced Priming for Septic Lung Injury. Shock. 2012;37:611–620. doi: 10.1097/SHK.0b013e318254fa6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parsey MV, Kaneko D, Shenkar R, Abraham E. Neutrophil apoptosis in the lung after hemorrhage or endotoxemia: apoptosis and migration are independent of IL-1 beta. Clin. Immunol. 1999;91:219–225. doi: 10.1006/clim.1999.4693. [DOI] [PubMed] [Google Scholar]
- 23.Boussetta T, Gougerot-Pocidalo MA, Hayem G. The prolyl isomerase Pin1 acts as a novel molecular switch for TNF-alpha-induced priming of the NADPH oxidase in human neutrophils. Blood. 2010;116:5795–5802. doi: 10.1182/blood-2010-03-273094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lomas-Neira J, Chung CS, Perl M, Gregory S, Biffl W, Ayala A. Role of alveolar macrophage and migrating neutrophils in hemorrhage-induced priming for ALI subsequent to septic challenge. Am J Physiol Lung Cell Mol Physiol. 2006 Jan;290(1):L51–L58. doi: 10.1152/ajplung.00028.2005. [DOI] [PubMed] [Google Scholar]
- 25.Lomas-Neira JL, Chung CS, Grutkoski PS, Miller EJ, Ayala A. CXCR2 inhibition suppresses hemorrhage-induced priming for acute lung injury in mice. J Leukoc Biol. 2004 Jul;76(1):58–64. doi: 10.1189/jlb.1103541. [DOI] [PubMed] [Google Scholar]
- 26.El-Benna J, Dang PM, Gougerot-Pocidalo MA. p47phox, the phagocyte NADPH oxidase/NOX2 organizer: structure, phosphorylation and implication in diseases. Exp. Mol. Med. 2009;41:217–225. doi: 10.3858/emm.2009.41.4.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nauseef WM. How human neutrophils kill and degrade microbes: an integrated view. Immunol. Rev. 2007;219:88–102. doi: 10.1111/j.1600-065X.2007.00550.x. [DOI] [PubMed] [Google Scholar]
- 28.Martín-Martín B, Nabokina SM, Blasi J, Lazo PA, Mollinedo F. Involvement of SNAP-23 and syntaxin 6 in human neutrophil exocytosis. Blood. 2000;96:2574–2583. 2000. [PubMed] [Google Scholar]
- 29.Wang P, Chintagari NR, Gou D, Su L, Liu L. Physical and Functional Interactions of SNAP-23 with Annexin A2. Am. J. Respir. Cell. Mol. Biol. 2007;37:467–476. doi: 10.1165/rcmb.2006-0447OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stow JL, Manderson AP, Murray RZ. SNAREing immunity: the role of SNAREs in the immune system. Nat. Rev. Immunol. 2006;6:919–929. doi: 10.1038/nri1980. [DOI] [PubMed] [Google Scholar]
- 31.Mollinedo F, Lazo PA. Identification of two isoforms of the vesicle-membrane fusion protein SNAP-23 in human neutrophils and HL-60 cells. Biochem. Biophys. Res. Commun. 1997;231:808–812. doi: 10.1006/bbrc.1997.6196. [DOI] [PubMed] [Google Scholar]
- 32.Martín-Martín B, Nabokina SM, Lazo PA, Mollinedo F. Co-expression of several human syntaxin genes in neutrophils and differentiating HL-60 cells: variant isoforms and detection of syntaxin 1. J. Leuk. Biol. 1999;65:397–406. doi: 10.1002/jlb.65.3.397. [DOI] [PubMed] [Google Scholar]
- 33.Uriarte SM, Rane MJ, Luerman GC, Barati MT, Ward RA, Nauseef WM, McLeish KR. Granule Exocytosis Contributes to Priming and Activation of the Human Neutrophil Respiratory Burst. J. Immunol. 2011;187:391–400. doi: 10.4049/jimmunol.1003112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uriarte SM, Rane MJ, Merchant ML, Jin S, Lentsch AB, Ward RA, McLeish KR. Inhibition of neutrophil exocytosis ameliorates acute lung injury in rats. Shock. 2013;39:286–292. doi: 10.1097/SHK.0b013e318282c9a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iskander KN, Craciun FL, Stepien DM, Duffy ER, Kim J, Moitra R, Vaickus LJ, Osuchowski MF, Remick DG. Cecal ligation and puncture-induced murine sepsis does not cause lung injury. Crit. Care. Med. 2013;41:159–170. doi: 10.1097/CCM.0b013e3182676322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kallas EG, Neto MC, Dalboni MA, Blecher S, Salomaõ R. Upregulation of reactive oxygen species generation and phagocytosis, and increased apoptosis in human neutrophils during severe sepsis and septic shock. Shock. 2003;20:208–212. doi: 10.1097/01.shk.0000079425.52617.db. [DOI] [PubMed] [Google Scholar]
- 37.Martins PS, Brunialti MK, Martos LS, Machado FR, Assunçao MS, Blecher S, Salomao R. Expression of cell surface receptors and oxidative metabolism modulation in the clinical continuum of sepsis. Crit. Care. 2008;12:R25. doi: 10.1186/cc6801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Santos SS, Brunialti MK, Rigato O, Machado FR, Silva E, Salomao R. Generation of nitric oxide and reactive oxygen species by neutrophils and monocytes from septic patients and association with outcomes. Shock. 2012;38:18–23. doi: 10.1097/SHK.0b013e318257114e. [DOI] [PubMed] [Google Scholar]
- 39.Morrell CN, Matsushita K, Lowenstein CJ. A novel inhibitor of Nethylmaleimide-sensitive factor decreases leukocyte trafficking and peritonitis. J. Pharmacol. Exp. Ther. 2005;314:155–161. doi: 10.1124/jpet.104.082529. [DOI] [PubMed] [Google Scholar]
- 40.Zemans RL, Colgan SP, Downey GP. Transepithelial migration of neutrophils: mechanisms and implications for acute lung injury. Am. J. Respir. Cell. Mol. Biol. 2009;40:519–535. doi: 10.1165/rcmb.2008-0348TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 42.Heine SJ, Olive D, Gao JL, Murphy PM, Bukrinsky MI, Constant SL. Cyclophilin A cooperates with MIP-2 to augment neutrophil migration. J. Inflamm. Res. 2011;4:93–104. doi: 10.2147/JIR.S20733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang XW, Wang Y, Liu Q, Thorlacius H. Redundant function of macrophage inflammatory protein-2 and KC in tumor necrosis factor-alpha-induced extravasation of neutrophils in vivo. Eur. J. Pharmacol. 2001;427:277–283. doi: 10.1016/s0014-2999(01)01235-3. [DOI] [PubMed] [Google Scholar]
- 44.Gillitzer A, Peluso M, Bültmann A, Münch G, Gawaz M, Ungerer M. Effect of dominant negative SNAP-23 expression on platelet function. J. Thromb. Haemost. 2008;6:1757–1763. doi: 10.1111/j.1538-7836.2008.03108.x. [DOI] [PubMed] [Google Scholar]
- 45.Frank SP, Thon KP, Bischoff SC, Lorentz A. SNAP-23 and syntaxin-3 are required for chemokine release by mature human mast cells. Mol. Immunol. 2011;49:353–358. doi: 10.1016/j.molimm.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 46.Veale KJ, Offenhäuser C, Lei N, Stanley AC, Stow JL, Murray RZ. VAMP3 regulates podosome organisation in macrophages and together with Stx4/TAT-SNAP-23 mediates adhesion, cell spreading and persistent migration. Exp. Cell. Res. 2011;317:1817–1829. doi: 10.1016/j.yexcr.2011.04.016. [DOI] [PubMed] [Google Scholar]
- 47.Sakurai C, Hashimoto H, Nakanishi H, Arai S, Wada Y, Sun-Wada GH, Wada I, Hatsuzawa K. SNAP-23 regulates phagosome formation and maturation in macrophages. Mol. Biol. Cell. 2012;23:4849–4863. doi: 10.1091/mbc.E12-01-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ishii M, Asano K, Namkoong H, Tasaka S, Mizoguchi K, Asami T, Kamata H, Kimizuka Y, Fujiwara H, Funatsu Y, Kagawa S, Miyata J, Ishii K, Nakamura M, Hirai H, Nagata K, Kunkel SL, Hasegawa N, Betsuyaku T. CRTH2 is a critical regulator of neutrophil migration and resistance to polymicrobial sepsis. J. Immunol. 2012;188:5655–5564. doi: 10.4049/jimmunol.1102330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Auten RL, Jr, Mason SN, Tanaka DT, Welty-Wolf K, Whorton MH. Anti-neutrophil chemokine preserves alveolar development in hyperoxia-exposed newborn rats. Am. J. Physiol. Lung. Cell. Mol. Physiol. 2001;281:L336–L344. doi: 10.1152/ajplung.2001.281.2.L336. [DOI] [PubMed] [Google Scholar]
- 50.Gao XP, Standiford TJ, Rahman A, Newstead M, Holland SM, Dinauer MC, Liu QH, Malik AB. Role of NADPH oxidase in the mechanism of lung neutrophil sequestration and microvessel injury induced by Gram-negative sepsis: studies in p47phox−/− and gp91phox−/− mice. J. Immunol. 2002;168:3974–3982. doi: 10.4049/jimmunol.168.8.3974. [DOI] [PubMed] [Google Scholar]
- 51.McLeish KR, Uriarte SM, Tandon S, Creed TM, Le J, Ward RA. Exocytosis of neutrophil granule subsets and activation of prolyl isomerase 1 are required for respiratory burst priming. J. Innate. Immun. 2013;5:277–289. doi: 10.1159/000345992. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.