Abstract
Background
More than 400 preclinical studies report ≥ 1 compound as cytotoxic to multiple myeloma (MM) cells; however, few of these agents became relevant in the clinic. Thus, the utility of such assays in predicting future clinical value is debatable.
Patients and Methods
We examined the application of early-phase trial experiences to predict future clinical adoption. We identified 129 drugs explored as single agents in 228 trials involving 7421 patients between 1961 and 2013.
Results
All drugs in common use in MM (melphalan, dexamethasone, prednisone, cyclophosphamide, bendamustine, thalidomide, lenalidomide, pomalidomide, bortezomib, carfilzomib, and doxorubicin) demonstrated a best reported response rate of ≥ 22%. Older agents, including teniposide, fotemustine, paclitaxel, and interferon, also appear active by this criterion; however, if mean response rates from all reported trials for an agent are considered, then only drugs with a mean response rate of 15% partial response are in clinical use.
Conclusion
Our analysis suggests that thresholds of 20% for best or 15% for mean response are highly predictive of future clinical success. Below these thresholds, no drug has yet reached regulatory approval or widespread use in the clinic. Thus, this benchmark provides 1 element of the framework for guiding choice of drugs for late-stage clinical testing.
Keywords: Drugs, Early clinical trials, Multiple myeloma, Phase 1, Single-agent activity
Introduction
After decades of minor improvements, the introduction of 5 new United States Food and Drug Administration (FDA)–approved therapeutic agents since 1999 marks a milestone in the history of multiple myeloma (MM) therapy. Overall response rates to induction therapy are now consistently > 90% in recent trials, and progression-free and overall survival have more than doubled, with a minority of MM patients likely being cured. Nevertheless, a majority of patients still relapse and develop drug resistance. The commercial success of novel drugs in MM has attracted significant pharmaceutical industry attention for a less common malignancy, and a large number of potentially useful therapeutics have been explored in preclinical and early- and late-phase clinical trials. It is encouraging that a multitude of new compounds and drug classes have been identified to be cytotoxic to multiple myeloma (MM) cells in cell lines, primary tumor samples, or mouse models. However, preclinical assays and models tend to overemphasize antitumor effects, and indeed, it seems there is no limit to the number of compounds that are cytotoxic to MM cell lines at high enough concentration. Recently, a number of therapeutics with promising preclinical studies have disappointed in later-stage clinical testing (eg, vorinostat,1 siltuximab2). By Internet- and library-based literature search, we identified > 400 compounds that were pre-clinically tested in MM, of which 129 went to early-phase clinical studies in MM patients. In retrospect, it has been unclear what basis there is for deciding which agents should advance to late-phase clinical testing or which agents further the likelihood of a preclinical investigation predicting clinical success. In this analysis, we explore these issues and provide some guidance for determining the likelihood of ultimate clinical success, which might be employed at least as a positive predictor for ongoing clinical trial analysis.
Methods
To identify preclinical studies and early-phase single-agent trials of drugs tested in MM, we performed an extensive literature search, utilizing the Mayo Clinic library, Internet-based research tools, and the US National Institutes of Health National Library of Medicine (pubmed.gov), as well as screening annual meeting abstracts from the American Society of Hematology (ASH), the American Society of Clinical Oncology (ASCO), the European Hematology Association (EHA), and the International Myeloma Workshop (IMW). Any early-phase study, irrespective of age group, study population, or eligibility, that utilized a drug as a single agent was assessed. We excluded trials using concomitant drugs (eg, steroids) or high-dose regimens that require autologous stem cell rescue. We also excluded any study with < 5 evaluable patients. We documented the quantity and the quality of published response for each trial, as well as the number of evaluable patients, the study phase, and the year of publication. Because trials were conducted over many decades and definitions have changed over time, partial response (PR) rates (> 50% reduction in measureable paraprotein) or better were counted as true responses, as this metric and its laboratory protein electrophoresis measurement has remained constant. When we identified > 1 study for a drug, we noted the best reported response and also calculated the mean response rate across all studies. To establish a comparative ranking of drug activity that was less dependent on patient cohort selection, we aimed to assess the activity under the most beneficial conditions for each drug. We therefore additionally screened for subgroup analyses and considered the highest activity reported for each single drug, if the minimum requirements (eg, patient cohort size > 5) were met.
Results
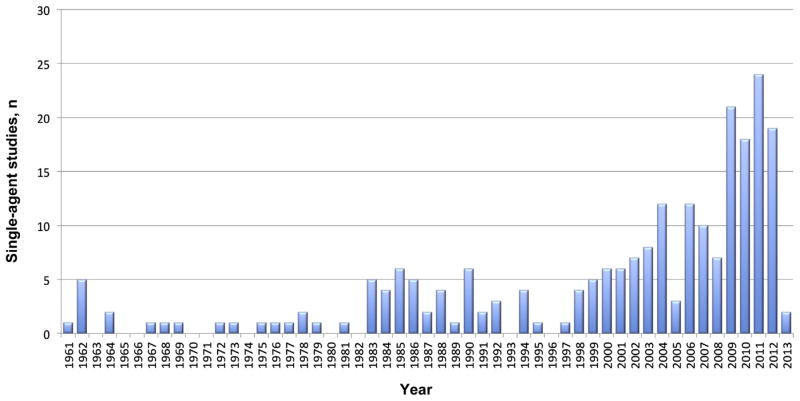
We identified 129 single-agent drugs, explored in 228 early clinical trials (phase I/Ib/II) published between 1961 and 2013 (see Supplemental Table 1 and Supplemental References in the online version). In aggregate, these trials treated a total of 7421 MM patients. The trials identified included a mean of 32 patients per trial (median, 22) with a mean number of patients tested per drug of 58 (median, 24). A remarkable increase in single-agent trials in MM was seen in the past decade (Fig. 1), most frequently utilizing small molecules (especially kinase and histone deacetylase inhibitors, as well as heat shock proteins), followed by monoclonal antibodies, proteasome inhibitors, and immunomodulatory drugs (Table 1).
Figure 1.
Distribution of Single-Agent Studies Over Time
Table 1.
Drug Classes Investigated in Early-Phase Clinical Trials in Multiple Myeloma
| Classical Cytostatic Agents | Novel Agents | Others | Total | |||
|---|---|---|---|---|---|---|
| n | Type | n | Type | n | Type | n |
| 5 | Alkaloids | 42 | Small molecules | 2 | Steroids | |
| 5 | Topoisomerase inhibitors | 4 | Proteasome inhibitors | 5 | Antibiotics | |
| 2 | Taxanes | 22 | Monoclonal antibodies | 3 | Immuno-suppressants | |
| 7 | Nucleoside analogues | 3 | Immunomodulatory drugs | 14 | Others | |
| 6 | Anthracyclines | |||||
| 8 | Alkylators | |||||
| 1 | Platinum-based agent | |||||
| 34 | Total | 71 | Total | 24 | Total | 129 |
Interestingly, only 26% of early-phase single-agent trials published in MM over the past 52 years investigated classical cytostatic agents. Nevertheless, the most potent drug in our ranking of clinical activity was an alkylating agent, melphalan,3 that has been continuously used in MM therapy since the 1960s and remains the backbone of many therapeutic regimens.
Most of the trials identified were performed in relapsed or relapsed and refractory patients (203 of 228, 89%), only 26 (11%) in previously untreated patients. The proportion of trials reporting active drugs was significantly higher in studies with untreated patients than in trials with relapsed and refractory patients (23 of 27, 84.6% vs. 114 of 203, 56.1%, P = .005). Additionally, the mean response rate differed significantly whether a targeted agent or conventional compound was tested. Trials using a targeted agent reported greater activity in pretreated patients (15.67% vs. 9.68%, P = .016) as well as in untreated patients (41.11% vs. 25.18%, P = .034).
The Majority of Tested Drugs Show No Anti-MM Activity
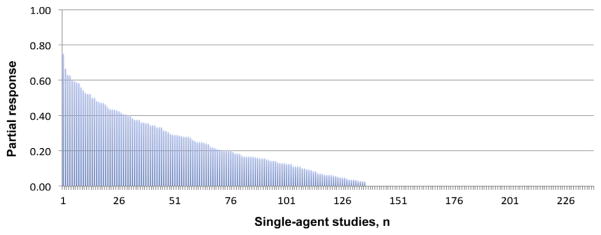
Figure 2 shows that anti-MM activity was described in ≥ 1 patient in nearly 60% of the 228 reported trials, with a mean response rate on all trials of 15.30%.
Figure 2.
Single-Agent Activity of 228 Early Clinical Trials in Multiple Myeloma
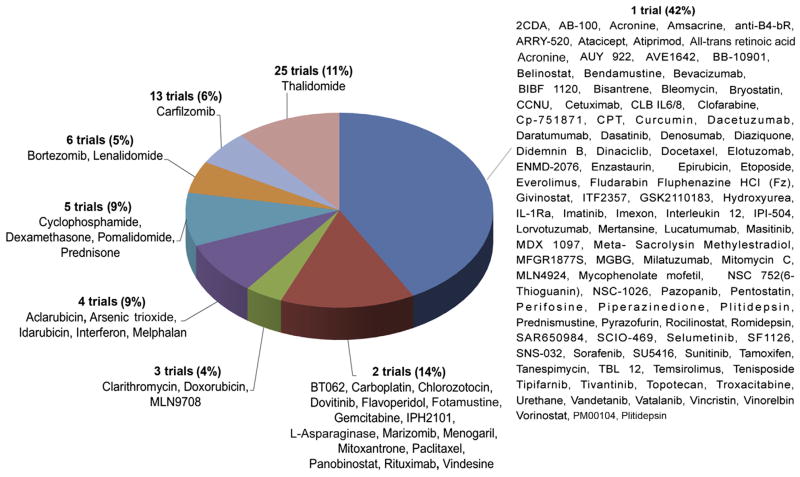
However, this result is biased by differences in publication frequency within the compounds, as preferentially more trials were performed in active agents than in nonactive agents. The most frequently tested single agent was thalidomide (25 of 228, 11% of all trials) followed by the novel agents carfilzomib (13 of 228, 5.7%), lenalidomide, and bortezomib (6 of 228, 2.6% each). Of the drugs studied, 75.2 (97 of 129) were studied in only 1 single agent study, representing 42.5% (97 of 228) of the trials. (Fig. 3).
Figure 3.
Frequency of Single-Agent Trials per Drug
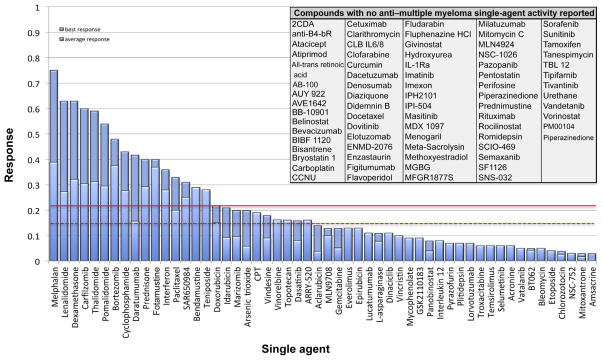
In contrast, the activity of the 129 tested drugs ranged from 0% to 75% patient response. The mean response rate was poor at 6.03%, which was only marginally improved (8.90%) when the best response reported in any trial for each drug was considered. This low response rate was partly due to a high percentage of nonactive drugs in our analysis. When we excluded these nonactive compounds, the response rate of the 54 remaining active drugs improved to a mean of 14.40% (median, 9.76%), and the mean response rate rose to 21.3% (median, 13.50%) when the best response reported for each drug was considered. Disappointingly, even under these most beneficial conditions, 72.86% of all drugs did not reach 10% activity when tested as a single agent in early-phase MM trials, and 58.13% of the 129 drugs did not demonstrate any anti-MM activity at all (Fig. 4).
Figure 4.
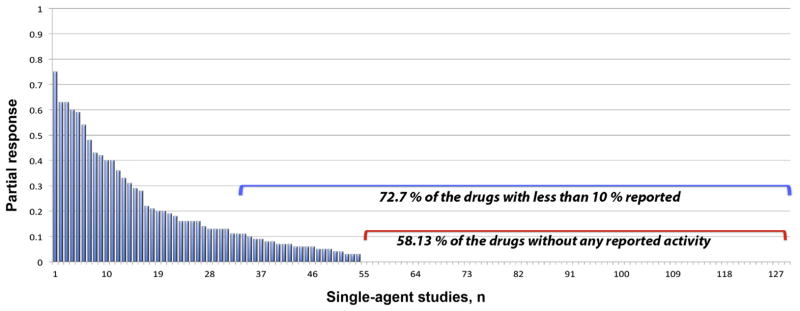
Activity of 129 Drugs in Early Clinical Trials in Multiple Myeloma (Best Reported Response)
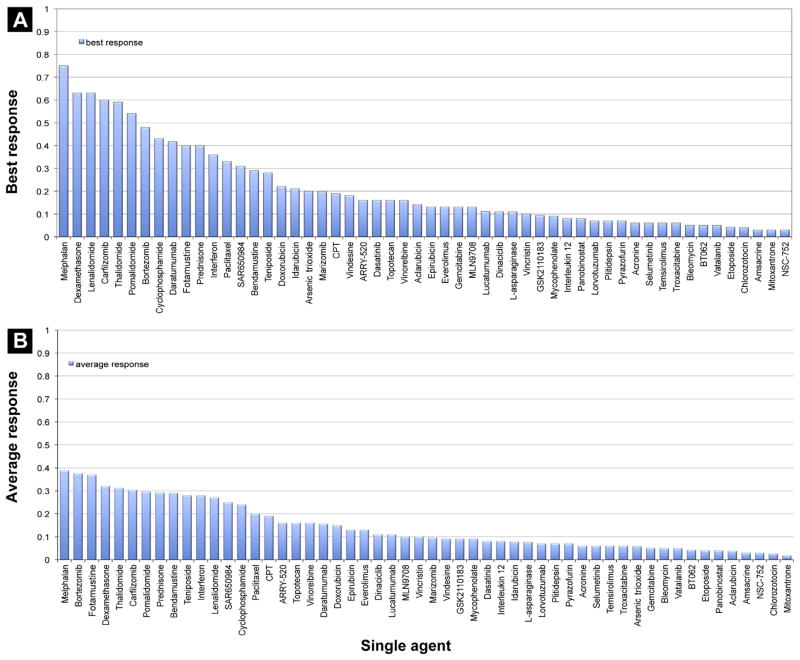
All FDA-Approved Drugs Have a Single-Agent Activity > 22%
From 228 early phase studies, 54 drugs were identified as having any anti-MM properties when used as a single agent. When evaluated according to their best reported activity, all 10 FDA-approved drugs had a single-agent activity of ≥ 22% (Fig. 5). Of these, melphalan was found to be the most potent (best result reported) single agent in MM treatment (75%),3 followed by dexamethasone (63%),4 lenalidomide (63%),5 carfilzomib (60%),6 thalidomide (59%),7 pomalidomide (54%),8 bortezomib (48%),9 cyclophosphamide (43%),10 prednisone (40%),11 and doxorubicin (22%).12 Additionally, 7 non–FDA-approved drugs met the threshold and showed activity > 22%:
Figure 5.
Active Drugs Sorted by (A) Best Response and (B) Mean Response Rate
Daratumumab13 and SAR65098414 are the first monoclonal antibodies (targeting CD38) that display promising single-agent activity and rapid responses in heavily pretreated MM (42% and 31% PR or better).
Fotemustine (40%)15 showed promising activity in alkylator-pretreated patients in 2 single-agent trials and was judged to be safely administrable, but no further data have been published.
Interferon (36%)16 and paclitaxel (33%)17 have proven to be active but have fallen from favor because of toxicities. Interferon trials are hard to judge overall, as different formulations, dosing schedules, and disease measurements are quite variable across reported studies. Paclitaxel and related taxanes have both positive and negative reported studies, so again further testing in the modern era is indicated.
Bendamustine (29%),18 a highly effective drug in certain lymphomas, is already approved in European countries for MM treatment by the European Medicines Agency and is under clinical investigation in the United States.
Teniposide (28%)19 was judged to be relatively effective and safe in 1988, but little additional information is available.
Discussion
We identified > 400 published compounds that were pre-clinically tested in MM cell lines, primary tumor samples, and animal studies. The real number of agents, including unpublished results, is likely much higher because of publication bias. Of these 400 compounds, 228 proceeded into early-phase clinical trials; however, only 10 are FDA approved. Obviously, preclinical models used so far are inefficient in predicting later clinical success in human disease. Fortunately, improvements are being reported, such as the Vk*Myc mouse model that recently demonstrated an impressive positive predictive value of compounds’ clinical activity of 68%.20 Nevertheless, rodent models did not predict the potential of the immunomodulatory agents,21 which constitute 1 of the most important drug classes used in MM therapy today. We propose a framework that might be part of a package of criteria used in guiding the future choice of drugs for late-stage clinical testing in MM based on results of the 228 existing early-phase clinical trials in MM over a time-frame of > 50 years.
To determine this framework, we chose the least active FDA-approved drug, doxorubicin (an anthracycline with a best reported single-agent activity rate of 22%12) as our baseline, and in fact, no drug with an activity rate below this threshold has yet made it into common clinical use or is regarded today to be a major benefit in MM therapy. Still, the role of anthracyclines in MM therapy has been intensely debated, and the drugs are generally regarded as having a minor role in MM treatment. Only 3 single-agent trials with doxorubicin have been published, 2 resulting in an activity rate of 13% and 1 with 22%, and the trial with the highest response rate was chosen to be the benchmark activity in our paper because of the rationale described in the Methods section. However it should be noted that this trial, by Alberts and Salmon from 1975, used unusual response criteria, in that the authors calculated a total myeloma cell number as the marker of response, based on a body M-component synthetic rate divided by the cellular M-component synthetic rate. In 2 of 9 patients, a remarkable reduction of more than 60% of the total body myeloma cell number was observed, and we concluded that these 2 patients were most likely PR patients, based on the accepted definition. The statistical variability in studying only 9 patients should also be noted. In all, 4 single-agent trials in MM have been reported for the anthracycline idarubicin.22–25 Of these, 3 studies demonstrated poor single-agent activity (0%–4%), whereas 1 reported an extraordinarily high potency, 59%.22 We revisited this study and counted only 3 of 8 published PR cases as clearly matching IMWG criteria, resulting in a single-agent activity of 21%, which is in the range of its analogue doxorubicin as described by Alberts and Salmon.
Given the plethora of regimens MM patients now endure, one could argue that the benchmark for predicting future clinical success should be lower, to not miss a potentially active agent. However, the most recent additions—carfilzomib, pomalidomide, and bendamustine—all met the 20% criterion. Therefore, we think that agents that meet this benchmark in early-phase testing should receive priority for late-phase study over agents whose activity is below the benchmark. Of note, a number of newer agents that are in or that recently completed randomized phase II or phase III testing, such as siltuximab and vorinostat,26 show little or no single-agent activity. Similarly, vincristine is highly active in many preclinical MM models and was until recent years commonly used in MM. Today, we know that it lacks sufficient clinical efficacy in human disease, and with a best reported single-agent activity of 10%, our approach does not rank it within the active drugs.27
No clinical single-agent activity data are available for cisplatin, which today is used as part of some combination regimens in newly diagnosed28 and resistant late-stage MM patients.29 However, 2 early clinical trials of its analogue carboplatin have been published, both without any single-agent activity.30,31 As this especially renally toxic agent is used in patients who have the highest risk to present with kidney failure, a revisit of the efficacy and role of cisplatin in MM regimens should be considered. Of note, etoposide, a combination partner of cisplatin, showed high activity rates in the high-dose setting32 but clearly failed to reach our benchmark when used as a single agent in conventional doses (4%).33
Of interest, paclitaxel is located within the active drugs although taxanes were not adopted in MM treatment, because of concerns of toxicity and efficiency. Additionally, the activities of fotemustine, bendamustine, and teniposide range above the proposed threshold, suggesting that these agents may be worth revisiting.
Limitations of the Analysis
This analysis is limited by a number of features. First, our data were obtained from early-phase (I/II) single-agent trials that were not primarily conducted to determine efficacy but were performed to gain information about safety and optimal dosage in small cohorts of human patients. Statistical variation in those small trials is high. The benchmark set by the Alberts and Salmon trial on doxorubicin did not include > 9 patients, having a “true” PR rate with a wide 95% CI from 3% to 60%, or 0 to 5 patients.12 Additionally, changes in the measurement of response over time might bias the results, especially in favor of older studies that were less stringent in the definition of PR. We also see the possibility that the chance of responding to a new drug has likely slowly declined as therapies have improved and patients have entered such trials at increasingly late stages of disease. Because the trials were conducted over a 50-year period, testing, reporting, patient makeup, and willingness to place highly refractory patients in trial versus hospice may as well produce a bias in favor of drugs studied many decades ago.
Our approach cannot rule out the possibility that cytostatic drugs may emerge that are not individually active but that are synergistic when used in combination (eg, recent clinical data suggest this may be true for elotuzumab in combination with lenalidomide). It should be noted, however, that despite the hypothetical validity of combination therapies and cytostatic drugs no drug without single-agent activity has yet been approved for use. Thus, despite the limitations, we believe this analysis provides valuable positive guidance and merits discussion and debate.
Conclusion
A cutoff of 22% single-agent best reported PR activity has in the past been highly predictive of future clinical success. No drug below this threshold has yet reached regulatory approval or widespread use in the clinic, except vincristine, which has subsequently fallen from favor. A more stringent analysis that includes all trials of a single-agent suggests that a mean response across all trials of 15% may be another marker for predicting future clinical utility that could be employed (Fig. 6). Thus, only drugs with 22% PR as best activity or 15% as mean activity level are in widespread use, and this benchmark provides a historical basis guiding choice of drugs for late-stage clinical testing that can be added to traditional preclinical evaluations of success likelihood. However, one must remember that the past may not always be predictive of the future.
Figure 6.
Single-Agent Activities of 129 Drugs in MM Sorted by Best Response
Clinical Practice Points
Single agent activities in humans of drugs used in MM are widely unknown.
We provide single agent activity data of 129 drugs from 228 early clinical trials.
A cutoff of 22% single-agent best reported PR activity has in the past been highly predictive of future clinical success.
This finding provides some guidance for the likelihood of ultimate clinical success, which might be employed at least as a positive predictor for ongoing clinical trial analysis.
Supplementary Material
Acknowledgments
KMK was supported by a research grant of the deutsche Forschungsgemeinschaft (Ko 4604/1-1).
KMK and AKS were the principal investigators and took responsibility for the article; KMK, KZ, SRS, MLK, VHJ-Z, JRM, RF, and AKS participated in the data acquisition; KMK, JRM, and AKS wrote the article.
Footnotes
Supplemental material accompanying this article can be found in the online version at http://dx.doi.org/10.1016/j.clml.2013.12.015.
The authors KMK, SRS, MLK and KZ have stated that they have no conflicts of interest.
Disclosure
VHJ-Z has received honoraria from Celgene and Janssen and research funding from the Multiple Myeloma Research Foundation. JRM has received research funding from Celgene, Onyx, and Sanofi. RF has received honoraria from Medtronic, Otsuka, Celgene, BMS, Lilly, Onyx, Binding Site, Millennium, and AMGEN and research funding from Cylene and Onyx. AKS has received honoraria from Millennium, Celgene, Novartis, and Amgen and research funding from Millennium and Onyx.
References
- 1.Siegel DS, Dimopoulos MA, Yoon S-S, et al. Vantage 095: vorinostat in combination with bortezomib in salvage multiple myeloma patients: final study results of a global phase 2b trial. Blood (ASH Annu Meet Abstr) 2011;118:abstract 480. [Google Scholar]
- 2.Orlowski RZ, Gercheva L, Williams C, et al. Phase II, randomized, double blind, placebo-controlled study comparing siltuximab plus bortezomib versus bortezomib alone in pts with relapsed/refractory multiple myeloma. ASCO Annu Meet Abstr. 2012;30(suppl 15):abstract 8018. [Google Scholar]
- 3.Back H, Lindblad R, Rodjer S, Westin J. Single-dose intravenous melphalan in advanced multiple myeloma. Acta Haematol. 1990;83:183–6. doi: 10.1159/000205210. [DOI] [PubMed] [Google Scholar]
- 4.Kumar S, Lacy MQ, Dispenzieri A, et al. Single-agent dexamethasone for pre-stem cell transplant induction therapy for multiple myeloma. Bone Marrow Transpl. 2004;34:485–90. doi: 10.1038/sj.bmt.1704633. [DOI] [PubMed] [Google Scholar]
- 5.Baz R, Alsina M, Shain KH, et al. Response adapted therapy using single-agent lenalidomide in older adults with newly diagnosed standard risk multiple myeloma. Blood (ASH Annu Meet Abstr) 2012;120:abstract 4060. [Google Scholar]
- 6.Papadopoulos KP, Lee P, Singhal S, et al. A phase 1b/2 study of prolonged infusion carfilzomib in patients with relapsed and/or refractory (R/R) multiple myeloma: updated efficacy and tolerability from the completed 20/56mg/m2 expansion cohort of PX-171-007. Blood (ASH Annu Meet Abstr) 2011;118:abstract 2930. [Google Scholar]
- 7.Fenk R, Hoyer B, Steidl U, et al. Single-agent thalidomide for treatment of first relapse following high-dose chemotherapy in patients with multiple myeloma. Leukemia. 2005;19:156–9. doi: 10.1038/sj.leu.2403564. [DOI] [PubMed] [Google Scholar]
- 8.Schey SA, Fields P, Bartlett JB, et al. Phase I study of an immunomodulatory thalidomide analog, CC-4047, in relapsed or refractory multiple myeloma. J Clin Oncol. 2004;22:3269–76. doi: 10.1200/JCO.2004.10.052. [DOI] [PubMed] [Google Scholar]
- 9.Dispenzieri A, Jacobus S, Vesole DH, Callandar N, Fonseca R, Greipp PR. Primary therapy with single-agent bortezomib as induction, maintenance and re-induction in patients with high-risk myeloma: results of the ECOG E2A02 trial. Leukemia. 2010;24:1406–11. doi: 10.1038/leu.2010.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lenhard RE, Jr, Oken MM, Barnes JM, Humphrey RL, Glick JH, Silverstein MN. High-dose cyclophosphamide. An effective treatment for advanced refractory multiple myeloma. Cancer. 1984;53:1456–60. doi: 10.1002/1097-0142(19840401)53:7<1456::aid-cncr2820530705>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 11.McIntyre OR, Pajak TF, Kyle RA, Cornwell GG, 3rd, Leone L. Response rate and survival in myeloma patients receiving prednisone alone. Med Pediatr Oncol. 1985;13:239–43. doi: 10.1002/mpo.2950130502. [DOI] [PubMed] [Google Scholar]
- 12.Alberts DS, Salmon SE. Adriamycin (NSC-123127) in the treatment of alkylator-resistant multiple myeloma: a pilot study. Cancer Chemother Rep. 1975;59(2 Pt 1):345–50. [PubMed] [Google Scholar]
- 13.Plesner T, Lokhorst HM, Gimsing P, Nahi H, Lisby S, Richardson PGG. Daratumumab, a CD38 mab, for the treatment of relapsed/refractory multiple myeloma patients: preliminary efficacy data from a multicenter phase I/II study. ASCO Annu Meet Abstr. 2012;30(suppl 15):abstract 8019. [Google Scholar]
- 14.Strickland SA, Glenn M, Zheng W, Daskalakis N, Mikhael JR. SAR650984, a CD38 monoclonal antibody in patients with selected CD38+ hematological malignancies–data from a dose-escalation phase I study. Blood. 2013;122:abstract 284. [Google Scholar]
- 15.Mangiacavalli S, Pica G, Varettoni M, Lazzarino M, Corso A. Efficacy and safety of fotemustine for the treatment of relapsed and refractory multiple myeloma patients. Eur J Haematol. 2009;82:240–1. doi: 10.1111/j.1600-0609.2008.01184.x. [DOI] [PubMed] [Google Scholar]
- 16.Ahre A, Bjorkholm M, Osterborg A, et al. High doses of natural alpha-interferon (alpha-IFN) in the treatment of multiple myeloma–a pilot study from the Myeloma Group of Central Sweden (MGCS) Eur J Haematol. 1988;41:123–30. doi: 10.1111/j.1600-0609.1988.tb00881.x. [DOI] [PubMed] [Google Scholar]
- 17.Miller HJ, Leong T, Khandekar JD, Greipp PR, Gertz MA, Kyle RA. Paclitaxel as the initial treatment of multiple myeloma: an Eastern Cooperative Oncology Group Study (E1A93) Am J Clin Oncol. 1998;21:553–6. doi: 10.1097/00000421-199812000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Knop S, Straka C, Haen M, Schwedes R, Hebart H, Einsele H. The efficacy and toxicity of bendamustine in recurrent multiple myeloma after high-dose chemotherapy. Haematologica. 2005;90:1287–8. [PubMed] [Google Scholar]
- 19.Tirelli U, Carbone A, Zagonei V, et al. Phase II study of teniposide (VM-26) in multiple myeloma. Am J Clin Oncol. 1985;8:329–31. doi: 10.1097/00000421-198508000-00011. [DOI] [PubMed] [Google Scholar]
- 20.Chesi M, Matthews GM, Garbitt VM, et al. Drug response in a genetically engineered mouse model of multiple myeloma is predictive of clinical efficacy. Blood. 2012;120:376–85. doi: 10.1182/blood-2012-02-412783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yaccoby S, Johnson CL, Mahaffey SC, Wezeman MJ, Barlogie B, Epstein J. Anti-myeloma efficacy of thalidomide in the SCID-hu model. Blood. 2002;100:4162–8. doi: 10.1182/blood-2002-03-0939. [DOI] [PubMed] [Google Scholar]
- 22.Chisesi T, Capnist G, de Dominicis E, Dini E. A phase II study of idarubicin (4-demethoxydaunorubicin) in advanced myeloma. Eur J Cancer Clin Oncol. 1988;24:681–4. doi: 10.1016/0277-5379(88)90299-4. [DOI] [PubMed] [Google Scholar]
- 23.Rodjer S, Nilsson B, Westin J. Do anthracyclines have a role in the therapy of multiple myeloma? Hematol J. 2000;1:422–6. doi: 10.1038/sj.thj.6200064. [DOI] [PubMed] [Google Scholar]
- 24.Errante DZV, Lestuzzi C, Freshci A, Saracchin S, Tirelli U. Studio di face II con 4-demetossidaunorubicina orale nei pazzienti anziani affetti da lifoma non Hodgkin e mieloma multiplo. Folio Oncologica. 1988;11:214. [Google Scholar]
- 25.Sumpter K, Powles RL, Raje N, et al. Oral idarubicin as a single-agent therapy in patients with relapsed or resistant multiple myeloma. Leuk Lymphoma. 1999;35:593–7. doi: 10.1080/10428199909169624. [DOI] [PubMed] [Google Scholar]
- 26.Richardson P, Mitsiades C, Colson K, et al. Phase I trial of oral vorinostat (sub-eroylanilide hydroxamic acid, SAHA) in patients with advanced multiple myeloma. Leuk Lymphoma. 2008;49:502–7. doi: 10.1080/10428190701817258. [DOI] [PubMed] [Google Scholar]
- 27.Jackson DV, Case LD, Pope EK, et al. Single-agent vincristine by infusion in refractory multiple myeloma. J Clin Oncol. 1985;3:1508–12. doi: 10.1200/JCO.1985.3.11.1508. [DOI] [PubMed] [Google Scholar]
- 28.Barlogie B, Anaissie E, van Rhee F, et al. Incorporating bortezomib into upfront treatment for multiple myeloma: early results of total therapy 3. Br J Haematol. 2007;138:176–85. doi: 10.1111/j.1365-2141.2007.06639.x. [DOI] [PubMed] [Google Scholar]
- 29.D’Sa S, Yong K, Kyriakou C, et al. Etoposide, methylprednisolone, cytarabine and cisplatin successfully cytoreduces resistant myeloma patients and mobilizes them for transplant without adverse effects. Br J Haematol. 2004;125:756–65. doi: 10.1111/j.1365-2141.2004.04981.x. [DOI] [PubMed] [Google Scholar]
- 30.Omura GA, Perri RT, Peterson B, Schiffer CA. Carboplatin in previously treated multiple myeloma. A Cancer and Leukemia Group B phase II trial. Am J Clin Oncol. 1994;17:196–8. doi: 10.1097/00000421-199406000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Barlogie B, Crowley J, Salmon SE, Bonnet J, Weick JK, Hayden K. Phase II study of carboplatin (CBDCA) in refractory multiple myeloma. A Southwest Oncology Group study. Invest New Drugs. 1994;12:53–5. doi: 10.1007/BF00873237. [DOI] [PubMed] [Google Scholar]
- 32.Long GD, Chao NJ, Hu WW, Negrin RS, Wong RM, Blume KG. High dose etoposide-based myeloablative therapy followed by autologous blood progenitor cell rescue in the treatment of multiple myeloma. Cancer. 1996;78:2502–9. doi: 10.1002/(sici)1097-0142(19961215)78:12<2502::aid-cncr9>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 33.Gockerman JP, Bartolucci AA, Nelson MO, Silberman H, Velez-Garcia E, Stein R. Phase II evaluation of etoposide in refractory multiple myeloma: a Southeastern Cancer Study Group Trial. Cancer Treat Rep. 1986;70:801–2. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.