Abstract
Aims:
There is a cultural barrier to early medical intervention for diabetic foot infections in Trinidad & Tobago, stemming from the strong cultural belief in “soft candle” as effective treatment. We carried out a case-control study to evaluate the outcomes of “soft candle” to treat diabetic foot infections.
Methods:
All consecutive patients admitted with diabetic foot infections were interviewed to collect data on: demographics, medical history, unhealthy lifestyle markers (exposure to risk factors for chronic diseases), chosen treatment and details of “soft candle” use. The hospital records were accessed on discharge to records the main outcome measures: HbA1c readings, duration of hospitalization, amputation and in-hospital mortality. Two groups were defined: The control group included patients who sought medical attention after detecting a foot infection. The study group included patients who recognized their infection but voluntarily chose to utilize “soft candle” regimens. We excluded patients who voluntarily chose to use other forms of non-traditional treatment or sought no treatment at all. Outcomes were compared using SPSS ver 19. A two-tailed P value was calculated for variables of interest in each group using Fisher’s exact test. The duration of hospitalization between the groups was compared using paired T-Test. A P value <0.05 was considered statistically significant.
Results:
There were 442 patients who met inclusion criteria: There were 60 patients in the study group at an average age of 55.2 years (SD ± 11.4; range 43-88): 63% had HBA1c readings >7.0% at presentation and 95% had unhealthy lifestyle habits. There were 382 patients in the control group at an average age of 59.1 years (SD ± 12.6, Range 37-89): 74% with HBA1c readings >7.0% at presentation and 48% with unhealthy lifestyle habits. Patients who used “soft candle” had significantly longer duration of hospitalization (15.5 ± 10.2 vs 9.2 ± 3.9 days; P<0.001) and major amputations (13.3% vs 5.6%; P=0.048) that was considered clinically significant. There was no difference in minor amputations (31.7% vs 34.3%; P=0.770) or in-hospital mortality (1.7% vs 0.52%; P=0.355) between the groups.
Conclusion:
In its current form, the traditional practice of topical “soft candle” application to diabetic foot wounds may be potentially harmful. Persons with diabetes should be warned about these effects. We have identified the target population for educational campaigns.
Keywords: Diabetes, Foot Infection, Alternative, Paraffin, Amputation
BACKGROUND
Persons with diabetes have between 0.75% (1) and 2% (2) annual risk to develop foot infections. When they occur, diabetic foot infections may have disastrous consequences, including amputation and death.
Early and appropriate medical intervention is needed to reduce the need for amputations from severe diabetic foot infections (3). However, there is a cultural barrier in many developing countries stemming from the strong belief in alternative healers and traditional non-medical therapies. One report from the Caribbean revealed that 29% of patients with diabetic foot infections delayed visits to medical doctors in favour of non-medical therapies, the commonest of which was the use of “soft candle” (4).
This study sought to document the outcomes when topical soft candle applications were used to treat diabetic foot infections. There has been no prior report on the use of this remedy for diabetic foot infections in the world literature. These practices have global importance because they are being carried to developed nations with mass migration. Therefore, health care practitioners in developed countries should also be aware of these practices and their implications.
METHODS
This study was carried out in Trinidad & Tobago, a twin island nation in the Caribbean with an estimated population of 1,317,714 persons and approximately 15% prevalence of diabetes mellitus in the general population (1). The study was performed across two tertiary referral hospitals with a combined capacity of 1,850 beds to serve a catchment population of 1,050,000 persons across the western half of the Trinidad.
A prospective evaluation of “soft candle” therapy would not be ethical so we carried out an observational study to evaluate its use to treat diabetic foot infections. The local Institutional Review Board granted permission to collect data from all consecutive patients admitted with diabetic foot infections across these institutions from June 2012 to June 2013. Patients were interviewed within 48 hours of admission to collect data on their demographics, prior hospitalization for diabetic foot infections, prior counseling on foot disease, unhealthy lifestyle markers (regular exposure to recognized risk factors for chronic diseases such as smoking, alcohol use or illicit drug use), chosen treatment and details of “soft candle” use (mechanisms of application, route, dose and duration). In order to standardize data reporting, we used the standardized definitions proposed at the 2007 CARICOM Heads of State Government Summit (5): Regular alcohol intake was >1 alcoholic drink daily on 4 or more days per week; regular tobacco use was smoking >1 cigarette daily; and illicit drug use was any reported use of an illegal drug.
We could not justify withholding standard medical therapy from patients in favor of a trial of “soft candle” therapy. Therefore, the second part of this study was strictly observational. We accessed hospital records and recorded three main outcome measures: duration of hospitalization, amputation and in-hospital mortality. We also recorded the glycosylated hemoglobin (HbA1c) levels on admission as an index of blood glucose control in the preceding eight weeks.
We defined two groups of patients. The control group included patients who sought medical attention after detecting a foot infection. The study group included patients who recognized their infection but voluntarily chose to utilize “soft candle” regimens. We excluded patients who voluntarily chose to use other forms of non-traditional treatment or sought no treatment at all.
We compared the outcome measures in both groups using Statistical Package for Social Sciences (SPSS) version 19. Descriptive statistics were generated as appropriate. A two-tailed P value was calculated for variables of interest in each group using Fisher’s exact test. The duration of hospitalization between the groups was compared using paired T-Test. A P value <0.05 was considered statistically significant.
RESULTS
There were 695 patients admitted with diabetic foot infections over the study period. We excluded 253 patients who either refused to grant consent to participate in the study, embarked on a trial of non-medical therapy other than “soft candle” preparations or for whom data collection was incomplete. Therefore, the study population included 442 patients: 60 patients in the study group and 382 in the control group (Table 1).
Table 1.
A comparison of the demographic characteristics of patients in the Control Group and the Study Group
| Parameter | Control Group (382) | Study Group (60) |
|---|---|---|
|
| ||
| Male to female ratio | 1.0:1 | 5.7:1 |
| Men | 195 | 51 |
| Women | 187 | 9 |
| Age in years | ||
| Mean ± SD (range) | 59.1 ± 12.6 (37-89) | 55.2 ± 11.4 (43-88) |
| Self described ethnicity | ||
| Afro-Caribbean descent | 143 (37.4%) | 34 (56.7%) |
| East Indian descent | 219 (57.3%) | 21 (35.0%) |
| Mixed descent | 20 (5.2%) | 4 (6.7%) |
| Chinese descent | 0 | 1 (1.7%) |
| Diabetes type | ||
| Type 1 diabetes | 12 | 0 |
| Type 2 diabetes | 370 | 60 |
| HBA1c reading at admission | ||
| Mean ± SD (Range) | 7.94% ± 1.54 (4.36-11.23) | 7.62 ± 1.61 (4.32-10.5) |
| Unhealthy lifestyle markers | ||
| Alcohol abuse | 94 | 36 |
| Smoking tobacco | 88 | 20 |
| Illicit drug use | 2 | 1 |
The control group contained 382 patients at a mean age of 59 years. These patients were maintained on oral hypoglycaemic tablets [135], insulin therapy [126] or combination therapy [116] to control their diabetes. Although all patients claimed compliance with their medications, 281 (73.6%) patients had HbA1c readings >7.0% at presentation, indicating poor metabolic control in the preceding 8 week period, and 184 (48.2%) displayed unhealthy lifestyle habits.
The study group contained 60 patients at a mean age of 55 years. These patients were being treated with oral hyoglycaemics [27], insulin [23] or combination therapy [10]. They all claimed compliance to maintenance therapy but 38 (63.3%) had HbA1c readings >7.0% at presentation and 57 (95%) patients had unhealthy lifestyle habits. Table 2 compares the main outcome measures between the groups.
Table 2.
A comparison of the main clinical outcomes between the control group and the study group
| Parameter | Control Group (382) | Study Group (60) | P Value | Odds Ratio (95% CI) |
|---|---|---|---|---|
|
| ||||
| Hospitalization (Mean ± SD) | 9.2 ± 3.9 | 15.5 ± 10.2 | <0.001 | - Continuous variable |
| Minor amputation | 131 (34.3%) | 19 (31.7%) | 0.770 | OR 0.89 (95% CI 0.50 to 1.59) |
| Major amputation | 22 (5.6%) | 8 (13.3%) | 0.048 | OR 2.52 (95% CI 1.07 to 5.95) |
| Hospital mortality | 2 (0.52%) | 1 (1.7%) | 0.355 | OR 3.22 (95% CI 0.29 to 36.05) |
When we enquired about the details of therapy, all patients reported that the “soft candle” was heated with an open flame until the wax melted. The hot candle wax was poured directly onto the wound and left in situ. The wound was then covered with a variety of dressings including brown paper bags, banana leaves, saran wrap and conventional gauze swabs. However, there was no consensus on dosage or frequency of applications. Most applied “soft candle” until the wound was “completely covered” and the wax was generally applied once or twice per day (Figure 1).
Figure 1.
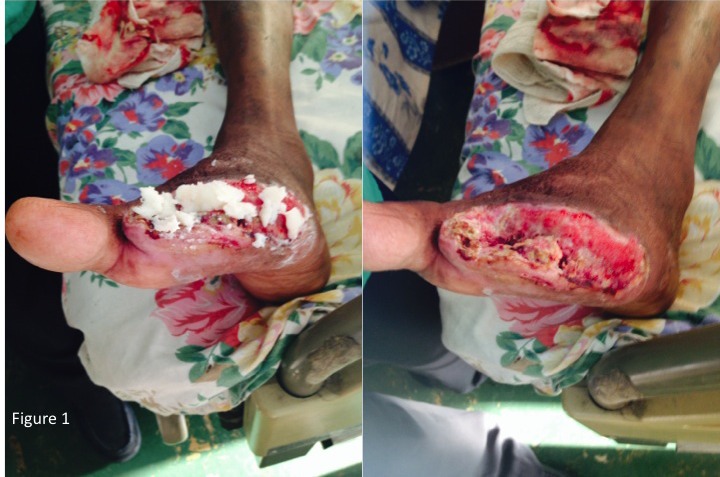
Photograph of an infected diabetic foot wound with soft candle applied topically (1a). The soft candle has been removed and the wound debrided (1b).
DISCUSSION
No attention has been given to the cultural practice of “soft candle” application and its effect on diabetic foot infections in the world literature. This practice deserves attention since persons with diabetes use it to treat skin and soft tissue infections.
Soft candle” is the colloquial name given to container wax candles that were used for several decades in countries with inconsistent or unavailable electricity supply. Although this is no longer the case in most Caribbean urban communities, “soft candle” has persisted on the commercial market driven by a demand for its alleged healing properties.
These candles contain mixtures of paraffin, mineral oil, fragrances, dyes and other ingredients that vary by manufacturer. There are countless anecdotal reports in social media and internet-based resources touting the therapeutic benefits of “soft candle.” We do not contest claims that “soft candle” may have some yet to be elucidated mechanism of action that may prove beneficial, but we found no clinical data in medical literature to substantiate claims that it is useful to treat diabetic foot infections.
More importantly, we demonstrated a relationship between “soft candle” use and worsened outcome measures. There was a significant increase in the duration of hospitalization by 6.3 days when patients opted for a trial of “soft candle” therapy. This has obvious implications on treatment cost and adversely affects the patients’ livelihood since 85% of the users of “soft candle users” were males (more likely to be breadwinners for their families) at an average age of 55 years (still likely to be active in the workforce).
Additionally, “soft candle users” had a significantly increased incidence of major amputations (13.3% vs 5.6%). This is extremely important considering the fact that major amputations lead to reduced quality of life, impaired functionality, depression, social morbidity and increased mortality (3, 6-8).
While the nature of this study does not prove a causal relationship between “soft candle” use and worsened outcome measures, there are plausible mechanisms by which this may theoretically occur.
Delayed commencement of proven medical therapy
Patients who opted for a trial of “soft candle” therapy voluntarily delayed their presentation to conventional medical practitioners by a mean of 8.1 days (SD 4.6; Range 2-21). The delay in itself may have allowed the infections to progress unchecked without the benefit of proven medical therapies.
Absence of Content Standardization
Paraffin wax is a firm, odorless, bland, white substance at room temperature. It is one of the commonest types of wax used to make candles. Chandlers typically add mineral oil, fragrances, dyes and other additives to paraffin wax to make their candles more attractive to purchasers. They usually do not disclose the additives because they are considered trade secrets and disclosure is not legally required. Therefore, the contents of “soft candle” vary significantly. This lack of standardization is not acceptable for any other substance whose use is purported for therapeutic value and it should not be acceptable if “soft candle” is to be used for medicinal purposes.
Potential for Thermal Injury
Although there was no consensus on the dosage and/or frequency of application, “soft candle” was applied in a similar manner by all users. Essentially, the candle is heated on an open flame until the wax melts, the hot wax is poured directly onto the wound and covered by a variety of dressings.
Paraffin wax is a mixture of straight-chain hydrocarbons that are formed as by-products during petroleum distillation. At room temperature, paraffin wax exists in a solid state but its melting point varies widely depending on the ratio of component hydrocarbons (9). There are two types of paraffin wax described: low melt point paraffin has a melting point around 130°F and is used in container candles because it tends to be amorphous at room temperature; high melt point paraffin has a melting point >130°F and is used for pillar candles since it is harder. Because most candles contain both types, the melted wax should have a minimum temperature of 130°F. Additionally, paraffin has a specific heat capacity of 2.5 joules per gram kelvin (10), making it an excellent material to store heat. Application of any material at this temperature to living tissues will generate thermal tissue injury and impair healing.
Burn injuries to the skin have already been reported from hot candle wax (11-15). Diabetics are particularly susceptible to this because they may not be able to appreciate ongoing thermal injuries due to sensory neuropathy. This is compounded by the fact that there is no way for the user to effectively control the temperature of the hot paraffin that is applied to the wound. However, burn wounds may be difficult to diagnose because these patients already have open wounds with infected and ulcerated skin. In our study, we could not find a reliable way to differentiate infected burn wounds and therefore we could not report on this as an individual outcome measure.
Stimulation of chronic inflammatory response
There are descriptions of medical wax applications as heat therapy for arthritis, myalgia and non-specific pain (16-20), but these are all topical therapies applied to intact skin. Spectroscopy studies on in vivo human skin have revealed that the paraffin / base oil elements do not penetrate deeper than the stratum corneum layer (21-25) and so have no direct tissue-related effects.
However, this is not the case with diabetic foot infections. Because these infections are always accompanied by a break in the skin, the paraffin is exposed to sub-cutaneous tissues and may produce unwanted effects. The medical literature contains several reports of patients who develop intense inflammatory reactions after injections of sub-cutaneous paraffin, mineral oil and Vaseline. Collectively, the term “paraffinoma” has been applied to the clinico-pathologic features of the chronic inflammatory response to these oil based substances (26). There have been several reports of foreign body granulomas, marked desmoplasia and destructive ulcers developing after subcutaneous injections at the male genitalia for penile enlargement (26-33), female breasts for augmentation (34-40), gums with dental implants (41) and lower limbs (42-45). We have encountered no reports of this type of reaction specifically in diabetic foot infections, but this might be difficult to recognize as the skin is already infected and ulcerated. At the very least, “soft candle” applications might be retarding healing due to the chronic inflammatory response it generates.
Potential Carcinogenic Effect
Paraffin and related base oils are derived as byproducts of petroleum distillation (46). During this process, alkylated polycyclic aromatic hydrocarbons may remain in these products and are potentially carcinogenic (46). These molecules may be released by vaporization and this is the basis behind suggestions that burning candles in enclosed spaces may increase lung malignancies (47). There have also been suggestions that topical exposure to paraffin, mineral oils and associated impurities may be carcinogenic to skin (46-49). Scott et al (49) studied the skin lesions that resulted from chronic exposure to paraffin products. He described epitheliomas (paraffin workers’ cancers) that were areas of chronic indurated dermatitis with wart-like appearances. Notably, he reported malignant change arising in one of these lesions (49).
Although there is evidence in early studies that paraffin, mineral oils and associated base oil impurities have carcinogenic properties (46-49) especially with lower levels of refinement (50-52), most authorities point out that modern refinery techniques leave significantly less amounts of alkylated polycyclic hydrocarbons and associated impurities, removing any carcinogenic effects (46). Lu et al (53) performed animal experiments on mice pretreated with UV light. They compared mice control mice to mice treated with topical applications of several commercially available crèmes containing paraffin and mineral oils. They noted a significant 69% increase in the incidence of skin malignancies (53). It appears the evidence is still emerging in this area but there have been no studies on the potential carcinogenic effect on open infected wounds in diabetics.
SIGNIFICANCE OF THE STUDY
Governments across the Caribbean region have recognized that the morbidity from diabetic foot infections is insurmountable (1-4). Therefore, they have developed strategies to limit the consequences of diabetic foot infections (5). In Trinidad & Tobago, there is a well-developed policy to limit the consequences of diabetic foot infections (54).
Dedicated diabetes clinics have been placed in high traffic areas within the community. They are staffed by trained multidisciplinary teams capable of meeting all needs of persons with diabetes and there is a well-developed referral system mandating routine referral to tertiary care hospitals for specialist assessment (54). Additionally, the government of Trinidad & Tobago provides these services at no cost to all persons with diabetes (54). Therefore, the target population has affordable, unrestricted, easy access to these services. The diabetes clinics also coordinate educational campaigns and provide primary care services.
Despite the focus on preventive strategies in Trinidad & Tobago, there has been no focus on the use of “soft candle”, although there are demonstrated ill effects. This suggests that our efforts are not yet optimized, especially since 63% of the “soft candle users” had HBA1c readings >7% at presentation and 95% had unhealthy lifestyle habits.
We have demonstrated that this practice is potentially harmful in its current form. We suggest that educational campaigns should be launched to combat this problem by making the patients aware of these potential ill effects. These campaigns should target middle-aged men with Type 2 diabetes. These men require special attention in any event because they tend to have poor metabolic control despite claims of compliance to therapy. These campaigns have been shown to be beneficial for positive behavior modification55 that is important since the majority of these men had poor lifestyle habits.
Campaign messages should convey the need for diabetics to treat foot infections with the appropriate gravity, seeking immediate medical attention instead of home remedies. At the very lease, these campaigns should aim to dissuade soft candle users from this practice or at least to educate them to use medical therapy concomitantly. There should be further research to determine whether this therapeutic method is harmful in itself or whether the outcomes here were due to the delay.
CONCLUSION
In its current form, the traditional practice of topical “soft candle” application to diabetic foot wounds may be potentially harmful. Patients with diabetes should be warned about these ill effects. We have identified the target population for educational campaigns.
FUNDING
No funding has been received for this work
CONFLICT OF INTEREST
The authors do not have any financial or other relationships that might lead to a conflict of interest.
REFERENCES
- 1.Cawich SO, Islam S, Hariharan S, et al. The Economic Impact of Hospitalization for Diabetic Foot infections in a Caribbean Nation. Perm J. 2013 doi: 10.7812/TPP/13-096. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richard JL, Schuldiner S. Epidemiology of diabetic foot problems. Rev. Med. Interne. 2008;29:S222–S230. doi: 10.1016/S0248-8663(08)73949-3. DOI:10.1016/S0248-8663(08)73949-3. [DOI] [PubMed] [Google Scholar]
- 3.Solomon S, Affan AM, Gopie P, et al. Taking the next step in 2005: the year of the diabetic foot. Prim. Care. Diabetes. 2008;2(4):175–180. doi: 10.1016/j.pcd.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Islam S, Harnarayan P, Cawich S, et al. Epidemiology of Diabetic Foot Infections in an Eastern Caribbean Population: A Prospective Study. Perm. J. 2013;17(2):37–40. doi: 10.7812/TPP/12-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strategic plan of action for the prevention and control of non-communicable diseases for countries of the Caribbean community 2011-2015. Georgetown, Guyana: Caribbean Community Secretariat, Pan American Health Organization/World Health Organization. Available: http:// healthycaribbean.org/publications/strategic- plan-of-action.html . [Google Scholar]
- 6.Davidson JH, Khor KE, Jones LE. A cross-sectional study of post-amputation pain in upper and lower limb amputees, experience of a tertiary referral amputee clinic. Disabil Rehabil. 2010;32(22):1855–1862. doi: 10.3109/09638281003734441. [DOI] [PubMed] [Google Scholar]
- 7.Belmont PJ, Davey S, Orr JD, et al. Risk factors for 30-day postoperative complications and mortality after below-knee amputation: A study of 2911 patients from the national surgical quality improvement program. J. Am. Coll. Surg. 2011;213(3):370–378. doi: 10.1016/j.jamcollsurg.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 8.Shah SK, Bena JF, Allemang MT, et al. Lower extremity amputations: factors associated with mortality or contralateral amputation. Vasc. Endovascular Surg. 2013;47(8):608–613. doi: 10.1177/1538574413503715. [DOI] [PubMed] [Google Scholar]
- 9.Nasser WE. Waxes, Natural and Synthetic. In: McKetta, John J, editors. Encyclopedia of Chemical Processing and Design. Vol. 67. New York: Marcel Dekker; 1999. p. 17. [Google Scholar]
- 10.Science and Engineering Encyclopedia. Warwick, England: Dirac Delta Consultants Ltd; 2007. Specific Heat Capacity. Diracdelta.co.uk . [Google Scholar]
- 11.Pickus EJ, Lionelli GT, Parmele JB, et al. Burns and injuries resulting from the use of gel candles. J. Burn. Care. Rehabil. 2001;22(3):243–245. doi: 10.1097/00004630-200105000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Bohnert M, Faller-Marquardt M, Pollak S. Differential burns of head and pubic hair. Arch. Kriminol. 2001;207(1):42–48. [PubMed] [Google Scholar]
- 13.Olaitan PB, Fadiora SO, Agodirin OS. Burn injuries in a young nigerian teaching hospital. Ann. Burns Fire Disasters. 2007;20(2):59–61. [PMC free article] [PubMed] [Google Scholar]
- 14.Erlandsson U, Huss F. Highly important during Christmas holidays. Elderly persons are over-represented when it comes to candle light fires. Lakartidningen. 2005;102(50):3897–3898. [PubMed] [Google Scholar]
- 15.Ang ES, Tan KC. Full-thickness burns of the palm caused by hot wax. Burns. 1997;23(5):458–459. doi: 10.1016/s0305-4179(97)89769-0. [DOI] [PubMed] [Google Scholar]
- 16.Robinson V, Brosseau L, Casimiro L, et al. Thermotherapy for treating rheumatoid arthritis. Cochrane Database Syst. Rev. 2002;2 doi: 10.1002/14651858.CD002826. CD002826. [DOI] [PubMed] [Google Scholar]
- 17.Dellhag B, Wollersjö I, Bjelle A. Effect of active hand exercise and wax bath treatment in rheumatoid arthritis patients. Arthritis Care Res. 1992;5(2):87–92. doi: 10.1002/art.1790050207. [DOI] [PubMed] [Google Scholar]
- 18.Vertree RA, Leeth A, Girouard M, et al. Whole-body hyperthermia: a review of theory, design and application. Perfusion. 2002;17(4):279–290. doi: 10.1191/0267659102pf588oa. [DOI] [PubMed] [Google Scholar]
- 19.Bromley J, Unsworth A, Haslock I. Changes in stiffness following short and long-term application of standard physiotherapeutic techniques. Br. J. Rheumatol. 1994;33(6):555–561. doi: 10.1093/rheumatology/33.6.555. [DOI] [PubMed] [Google Scholar]
- 20.Kim H, Suzuki T, Saito K, et al. Effectiveness of exercise with or without thermal therapy for community-dwelling elderly Japanese women with non-specific knee pain: A randomized controlled trial. Arch. Gerontol. Geriatr. 2013;57(3):352–359. doi: 10.1016/j.archger.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 21.Stamatas GN, de Sterke J, Hauser M, et al. Lipid uptake and skin occlusion following topical application of oils on adult and infant skin. J. Dermatol. Sci. 2008;50(2):135–142. doi: 10.1016/j.jdermsci.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 22.Backhouse L, Dias M, Gorce JP, et al. GAR Field magnetic resonance profiling of the ingress of model skin-care product ingredients into human skin in vitro . J. Pharm. Sci. 2004;93(9):2274–2283. doi: 10.1002/jps.20137. [DOI] [PubMed] [Google Scholar]
- 23.Jiang R, Roberts MS, Prankerd RJ, Benson HA. Percutaneous absorption of sunscreen agents from liquid paraffin: self-association of octyl salicylate and effects on skin flux. J. Pharm. Sci. 1997;86(7):791–796. doi: 10.1021/js960523y. [DOI] [PubMed] [Google Scholar]
- 24.Overgaard Olsen L, Jemec GB. The influence of water, glycerin, paraffin oil and ethanol on skin mechanics. Acta. Derm. Venereol. 1993;73(6):404–406. doi: 10.2340/0001555573404406. [DOI] [PubMed] [Google Scholar]
- 25.Jacobi O. Methodical study on the penetration into stratum corneum of oils and fats used in the manufacture of protective skin ointments and cosmetics. Berufsdermatosen. 1971;19(4):207–215. [PubMed] [Google Scholar]
- 26.Jindarak S, Angspatt A, Loyvirat R, et al. Bilateral scrotal flaps: a skin restoration for penile paraffinoma. J. Med. Assoc. Thai. 2005;88(S4):70–73. [PubMed] [Google Scholar]
- 27.Rintala A. Ulcerating paraffinoma. Ann. Chir. Gynaecol. 1976;65(5):356–360. [PubMed] [Google Scholar]
- 28.Bobik O, Jr, Bobik O., Sr Penile paraffinoma and ulcers of penis. Bratisl Lek Listy. 2011;112(11):653–654. [PubMed] [Google Scholar]
- 29.Pónyai K, Marschalkó M, Hársing J, et al. Paraffinoma. J. Dtsch Dermatol Ges. 2010;8(9):686–688. doi: 10.1111/j.1610-0387.2010.07366.x. [DOI] [PubMed] [Google Scholar]
- 30.Pehlivanov G, Kavaklieva S, Kazandjieva J, et al. Foreign-body granuloma of the penis in sexually active individuals (penile paraffinoma) J. Eur. Acad. Dermatol. Venereol. 2008;22(7):845–851. doi: 10.1111/j.1468-3083.2008.02677.x. [DOI] [PubMed] [Google Scholar]
- 31.Akkus E, Iscimen A, Tasli L, Hattat H. Paraffinoma and ulcer of the external genitalia after self-injection of vaseline. J. Sex Med. 2006;3(1):170–172. doi: 10.1111/j.1743-6109.2005.00096.x. [DOI] [PubMed] [Google Scholar]
- 32.Oñate-Celdrán J, Sanchez-Rodríguez C, Tomás-Ros M, et al. Penile paraffinoma after subcutaneous injection of paraffin. Treatment with a two-step cutaneous plasty of the penile shaft with scrotal skin. Arch. Esp. Urol. 2012;65(5):575–578. [PubMed] [Google Scholar]
- 33.Foxton G, Vinciullo C, Tait CP, Sinniah R. Sclerosing lipogranuloma of the penis. Australas J. Dermatol. 2011;54(3):12–14. doi: 10.1111/j.1440-0960.2010.00665.x. [DOI] [PubMed] [Google Scholar]
- 34.Ho WS, Chan AC, Law BK. Management of paraffinoma of the breast: 10 years’ experience. Br. J. Plast Surg. 2001;54(3):232–234. doi: 10.1054/bjps.2000.3533. [DOI] [PubMed] [Google Scholar]
- 35.Alagaratnam TT, Ng WF. Paraffinomas of the breast: an oriental curiosity. Aust. N. Z. J. Surg. 1996;66(3):138–140. doi: 10.1111/j.1445-2197.1996.tb01141.x. [DOI] [PubMed] [Google Scholar]
- 36.Rintala A. Ulcerating paraffinoma. Ann. Chir. Gynaecol. 1976;65(5):356–360. [PubMed] [Google Scholar]
- 37.Zekri A, Ho WS, King WW. Destructive paraffinoma of the breast and thoracic wall caused by paraffin injection for mammary increase. Apropos of 3 cases with review of the literature. Ann. Chir. Plast. Esthet. 1996;41(1):90–93. [PubMed] [Google Scholar]
- 38.Dimitrowa J, Balabanowa M, Zarnuschanow P, Scharow P. Sclerosing lipogranuloma of the male genitals. Z. Hautkr. 1989;64(3):223–227. [PubMed] [Google Scholar]
- 39.Alagaratnam TT, Ong GB. Paraffinomas of the breast. J. Royal Coll. Surg. Edinb. 1983;28(4):260–263. [PubMed] [Google Scholar]
- 40.Chiu WK, Lee TP, Chen SY, et al. Bilateral breast reconstruction with a pedicled transverse rectus abdominis myocutaneous flap after subcutaneous mastectomy for symptomatic injected breasts. J. Plast. Surg. Hand Surg. 2012;46(3):242–247. doi: 10.3109/2000656X.2012.696263. [DOI] [PubMed] [Google Scholar]
- 41.Kemp HK. Buccal radiopacities associated with cosmetic wax implants. Br. Dent. J. 1982;152(4):134. doi: 10.1038/sj.bdj.4804766. [DOI] [PubMed] [Google Scholar]
- 42.Thiers H, Moulin G, Michel F, Vittori F. Multiple paraffinomas. Bull. Soc. Fr. Dermatol Syphiligr. 1971;78(2):172–173. [PubMed] [Google Scholar]
- 43.Thiers H, Croisille M. Paraffinoma of the soft parts of the leg (apropos of a case) J. Radiol Electrol Med. Nucl. 1969;50(1):83–84. [PubMed] [Google Scholar]
- 44.Giachi LM, Lanfranchi R. On some long-term observations of elaiomas of the lower extremities. Arch. Putti. Chir. Organi Mov. 1968;23:408–412. [PubMed] [Google Scholar]
- 45.Iversen L, Lemcke A, Bitsch M, Karlsmark T. Compression bandage as treatment for ulcers induced by intramuscular self-injection of paraffin oil. Acta. Derm. Venereol. 2009;89(2):196–197. doi: 10.2340/00015555-0583. [DOI] [PubMed] [Google Scholar]
- 46.Mackerer CR, Griffis LC, Grabowski JS, Reitman FA. Petroleum mineral oil refining and evaluation of cancer hazard. Appl. Occup. Environ Hyg. 2003;18(11):890–901. doi: 10.1080/10473220390237467. [DOI] [PubMed] [Google Scholar]
- 47.Duff O. Cancer warning over paraffin-based candles. The Independent. 2009 Available from: http://www.independent.co.uk/life-style/health-and-families/health-news/cancer-warning-over-paraffinbased-candles-1774322.html . [Google Scholar]
- 48.Tolbert PE. Oils and Cancer. Cancer Causes Control. 1997;8(3):386–405. doi: 10.1023/a:1018409422050. [DOI] [PubMed] [Google Scholar]
- 49.Scott A. On The Occupation Cancer Of The Paraffin And Oil Workers Of The Scottish Shale Oil Industry Brit Med J. 1922;2(3232):1108–1109. [Google Scholar]
- 50.Freeman JJ, Federici TM, McKee RH. Evaluation of the contribution of chronic skin irritation and selected compositional parameters to the tumorigenicity of petroleum middle distillates in mouse skin. Toxicology. 1993;81(2):103–112. doi: 10.1016/0300-483x(93)90002-a. [DOI] [PubMed] [Google Scholar]
- 51.Clark CR, Walter MK, Ferguson PW, Katchen M. Comparative dermal carcinogenesis of shale and petroleum-derived distillates. Toxicol Ind. Health. 1988;4(2):11–22. doi: 10.1177/074823378800400102. [DOI] [PubMed] [Google Scholar]
- 52.Mehrotra NK, Saxena AK. Evaluation of carcinogenic effect of mineral oil used in the processing of jute fibres. Br. J. Exp. Pathol. 1979;60(5):518–525. [PMC free article] [PubMed] [Google Scholar]
- 53.Lu YP, Lou YR, Xie JG. Tumorigenic Effect of Some Commonly Used Moisturizing Creams when Applied Topically to UVB-Pretreated High-Risk Mice. J. Invest Dermatol. 2009;129(2):468–475. doi: 10.1038/jid.2008.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Islam S, Harnarayan P, Cawich SO, et al. Secondary Prevention of Diabetic Foot Infections in a Caribbean Nation: A Call for Improved Patient Education. International J. Lower Ext. Wounds. 2013;12(3):232–236. doi: 10.1177/1534734613486151. [DOI] [PubMed] [Google Scholar]
- 55.Fujiwara Y, Kishida K, Terao M, et al. Patient education for preventing diabetic foot ulceration: a systematic review. Endocrinol Metab. Clin. North Am. 2002;31:633–658. doi: 10.1016/s0889-8529(02)00021-x. [DOI] [PubMed] [Google Scholar]


