Abstract
Objective
To describe the association of lactate levels within the first 12 hours after successful resuscitation from pediatric cardiopulmonary arrest with hospital mortality.
Design
Retrospective cohort study.
Setting
Fifteen children’s hospital associated with the Pediatric Emergency Care Applied Research Network.
Patients
Patients between 1 day and 18 years old who had a cardiopulmonary arrest, received chest compressions more than 1 minute, had a return of spontaneous circulation more than 20 minutes, and had lactate measurements within 6 hours of arrest.
Interventions
None.
Measurements and Main Results
Two hundred sixty-four patients had a lactate sampled between 0 and 6 hours (lactate0–6) and were evaluable. Of those, 153 patients had a lactate sampled between 7 and 12 hours (lactate7–12). One hundred thirty-eight patients (52%) died. After controlling for arrest location, total number of epinephrine doses, initial rhythm, and other potential confounders, the odds of death per 1 mmol/L increase in lactate 0–6 was 1.14 (1.08, 1.19) (p < 0.001) and the odds of death per 1 mmol/L increase in lactate7–12 was 1.20 (1.11, 1.30) (p < 0.0001). Area under the curve for in-hospital arrest mortality for lactate0–6 was 0.72 and for lactate7–12 was 0.76. Area under the curve for out-of-hospital arrest mortality for lactate0–6 was 0.8 and for lactate7–12 was 0.75.
Conclusions
Elevated lactate levels in the first 12 hours after successful resuscitation from pediatric cardiac arrest are associated with increased mortality. Lactate levels alone are not able to predict outcomes accurately enough for definitive prognostication but may approximate mortality observed in this large cohort of children’s hospitals.
Keywords: children, heart arrest, lactate, resuscitation
Pediatric cardiac arrest (CA) results in high mortality and in poor functional outcome for many who survive (1, 2). Multiple arrest and postarrest characteristics have been evaluated to estimate severity of injury to prognosticate survival and functional outcome, including initial rhythm, arrest location, duration of cardiopulmonary resuscitation (CPR), number of epinephrine and atropine doses, temperature, pH, bystander CPR status, and sodium bicarbonate administration (2–5). Although these characteristics are associated with outcomes to varying degrees, none directly measures the magnitude of an individual patient’s postarrest ischemic injury. Biomarkers for brain injury assessment following pediatric CA have been evaluated (neuron-specific enolase, S100Beta, glial fibrillary acidic protein), but most are not yet available for real-time point-of-care use (6, 7). These markers also fail to assess nonbrain or more global organ injury. It may be informative to have less specific biomarkers that assess multisystem post-CA injury severity early after the return of spontaneous circulation (ROSC). Such measures might better prognosticate outcome or stratify injury severity with the goal of titrating postresuscitative interventions to improve survival.
Post-CA excess lactate production is believed to be largely due to tissue hypoxia with associated anaerobic metabolism. CA results in one of the most extreme cases of tissue hypoxia with “no flow” leading to greatly reduced oxygen delivery and increased anaerobic conditions. When CPR is initiated, a “low flow” state continues with variable oxygen delivery to tissues (organs) depending on the quality of CPR and other factors. Following adult out-of-hospital (OH) CA, lower lactate levels at admission are associated with increased hospital survival (8, 9). However, it is unclear what lactate levels after CA and early resuscitation mean. We sought to investigate the association of lactate levels as markers of global ischemic injury with mortality following pediatric CA. We hypothesized that higher lactate measurements would be associated with higher mortality.
MATERIALS AND METHODS
This study was conducted using an existing Pediatric Emergency Care Applied Research Network (PECARN) database, which had been created with support from the National Institute of Child Health and Human Development to the current National Heart, Lung and Blood Institute-funded Therapeutic Hypothermia for Pediatric Cardiac Arrest Trials (NCT00880087 and NCT00878644). The original database was designed as a retrospective cohort study of inxhospital (IH) and OH CA. It was conducted between July 1, 2003, and December 31, 2004, at 15 children’s hospitals associated with the PECARN. Patients from 1 day (24 hr) to 18 years old (inclusive) who experienced CA requiring at least 1 minute of chest compressions and who had ROSC for a minimum of 20 minutes were eligible for inclusion. Case classification as OH was assigned if chest compressions were initiated prior to hospital arrival. IH classification was assigned when chest compressions were initiated in the emergency department or other hospital setting. Patients cared for in a neonatal ICU or who had planned CA in the operating room as part of congenital heart disease surgical repair were excluded. Identification of patients and database management for this database has been previously described (4, 5, 10).
Variables collected as part of the original database included 1) patient characteristics including age, weight, gender, race, ethnicity, insurance type, and chronic preexisting conditions; 2) event characteristics including location and timing of CA, first and subsequent monitored cardiac rhythms, presence and types of vascular access, endotracheal tube, monitoring devices and other interventions prior to arrest, use of defibrillation, and drugs administered during the arrest; 3) etiology of CA; 4) hospital course including use of extracorporeal membrane oxygenation (ECMO), therapeutic hypothermia, other intensive care monitoring devices and interventions, drug therapies, and subsequent arrests and seizures; 5) physiologic and laboratory data, such as pupillary reflexes, body temperature, blood pH, glucose, and lactate concentrations, in the first 12 hours post arrest; 6) Pediatric Cerebral Performance Category scores prior to CA and at hospital discharge; and 7) survival to hospital discharge. Hypotension was defined as a systolic blood pressure less than 5th percentile for age (11). Dates and times of important clinical events were recorded, and related time intervals were determined. Utstein-style definitions were used for variables where such definitions exist (12, 13).
The study was approved and a waiver of informed consent was granted by the institutional review board at each site. All patients entered in the database were screened for evaluation. Patients without a documented lactate in the 0–6 hours following arrest were excluded. Time 0 represents the time chest compressions were initiated. Both physiologic and laboratory data were collected as minimum and maximum values obtained from 0 to 6 hours and 7 to 12 hours. These intervals were selected for analysis because they were most likely to reflect the impact of arrest and early postresuscitation management. If there was only one value provided for a time interval, it was assigned to both the minimum and maximum. If there was no documented value within a given time period, it was considered missing and left blank.
The associations between maximum lactate level at 0–6 hours (lactate0–6) or maximum lactate level at 7–12 hours (lactate 7–12) and hospital mortality were assessed. All relationships were adjusted for arrest location (IH vs OH), total epinephrine doses, and first rhythm described. Additional covariates eligible for inclusion (p < 0.25 in univariate analysis) in stepwise mortality models were genetic condition, lung/airway disease, heart disease, heme/onc, endocrine, renal condition, age in months, CPR to ROSC (min), minimum temperature 0–6, maximum temperature 0–6, minimum glucose 0–6, minimum systolic blood pressure (BP) value 0–6, minimum diastolic BP value 0–6, and number of vasopressors 0–6.
Statistical Analyses
Summary statistics are reported as medians and interquartile ranges (25th–75th percentiles) for continuous variables and proportions depicted as percentages for categorical variables. The association of each variable with hospital mortality was examined using chi-square or Fisher exact tests for categorical variables and the Wilcoxon rank-sum test or Kruskal-Wallis test for continuous variables.
Logistic regression was used to test associations between exposures and outcome. The association of maximum interval lactate levels with outcomes was assessed, adjusting in all cases for a priori potential confounders including arrest location (IH vs OH), total epinephrine doses received, and initial rhythm. Additional covariates were eligible for inclusion in the model if they had a univariate analysis p value of less than 0.25. Receiver-operating curves (ROC) were calculated to evaluate the predictive value of lactate levels for mortality at different time intervals. A significance level of 0.05 was used for all analyses. All analyses were performed using SAS 9.2 for Windows (SAS, Cary, NC).
RESULTS
Four hundred ninety-one patients were in the dataset. Two hundred sixty-four patients had a lactate0–6 and were evaluable (Fig. 1). One hundred eleven patients were missing a lactate level at 7–12 hours, leaving 153 patients who were evaluable for lactate7–12. The 227 patients who did not have a lactate sampled and were ineligible for this analysis had similar hospital discharge mortality as those who had lactates sampled at the 0–6 hours and were analyzed (56% vs 52%).
Figure 1.
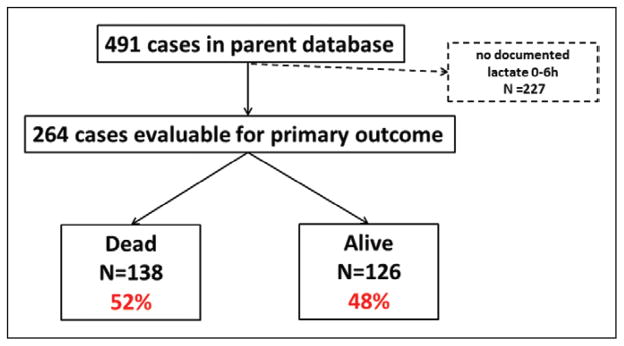
The distribution of patients from the Pediatric Emergency Care Applied Research Network with a lactate 0–6 by discharge mortality groupings.
Seventy-eight percent (206 of 264) of arrests occurred in an IH setting and 22% (58 of 264) occurred in an OH setting. The median age was 13 months (2, 96) and 61% of patients were male. Forty-three percent of patients had preexisting congenital heart disease. The most common initial rhythm was bradycardia (42%) (Table 1). The median duration of CPR was 14 minutes (5, 35). Fifty-three percent of patients experienced systolic hypotension within the first 6 hours and 13% (34 of 264) were treated with ECMO.
Table 1.
Description of Cohort Demographics and Arrest Characteristics and the Association of Cardiac Arrest Variables With Mortality
| Survival Outcome (n = 264)
|
p | |||
|---|---|---|---|---|
| Total (n = 264), n (%) or Median (Interquartile Range) | Nonsurvivors (n = 138), n (%) | Survivors (n = 126), n (%) | ||
| Age (mo) | 13 (2, 96) | 16 (2, 130) | 9 (2, 73) | 0.09 |
|
| ||||
| Gender | 0.75 | |||
| Male | 161 (61) | 82 (51) | 79 (49) | |
| Female | 100 (38) | 53 (53) | 47 (47) | |
| Unknown | 3 (1) | 3 (100) | 0 (0) | |
|
| ||||
| Race | 0.82 | |||
| Black | 124 (47) | 32 (49) | 33 (51) | |
| White | 65 (25) | 67 (54) | 57 (46) | |
| Other/unknown | 75 (28) | 39 (52) | 36 (48) | |
|
| ||||
| Preexisting condition | ||||
| Prenatal | 31 (12) | 16 (52) | 15 (48) | 0.94 |
| Lung or airway | 67 (25) | 28 (42) | 39 (58) | 0.047 |
| Congenital heart disease | 113 (43) | 53 (47) | 60 (53) | 0.13 |
| Acquired heart disease | 27 (10) | 14 (52) | 13 (48) | 0.96 |
| Hematology/oncology/immunology | 30 (11) | 20 (67) | 10 (33) | 0.09 |
| Gastrointestinal | 39 (15) | 21 (54) | 18 (46) | 0.83 |
| Genetic/metabolic | 31 (12) | 21 (68) | 10 (32) | 0.07 |
| Endocrine | 6 (2) | 5 (83) | 1 (17) | 0.22 |
| Renal | 24 (9) | 17 (71) | 7 (29) | 0.056 |
| Neurologic | 62 (24) | 30 (48) | 32 (52) | 0.48 |
|
| ||||
| Initial rhythm | 0.013 | |||
| Asystole/pulseless electrical activity | 76 (29) | 49 (64) | 27 (36) | |
| Ventricular fibrillation/pulseless ventricular tachycardia | 30 (11) | 15 (50) | 15 (50) | |
| Bradycardia | 111 (42) | 46 (41) | 65 (59) | |
| Other/unknown | 47 (18) | 28 (60) | 19 (40) | |
|
| ||||
| Cardiopulmonary resuscitation (min) | 14 (5, 35) | 19 (6, 47) | 8 (3, 20) | < 0.001 |
|
| ||||
| Epinephrine doses | < 0.001 | |||
| 0 | 36 (14) | 14 (39) | 22 (61) | |
| 1 | 61 (23) | 24 (39) | 37 (61) | |
| 2 | 44 (17) | 19 (43) | 25 (57) | |
| 3 | 45 (17) | 26 (58) | 19 (42) | |
| ≥ 4 | 68 (26) | 49 (72) | 19 (28) | |
| Unknown | 10 (4) | 6 (60) | 4 (40) | |
|
| ||||
| Witnessed | 0.37 | |||
| Yes | 25 | 14 (56) | 11 (44) | |
| No | 31 | 21 (68) | 10 (32) | |
|
| ||||
| Location | 0.047 | |||
| In hospital | 206 (78) | 101 (49) | 105 (51) | |
| Out of hospital | 58 (22) | 37 (64) | 21 (36) | |
|
| ||||
| Number of vasoactives 0–6 hr | 0.29 | |||
| 0 | 47 (18) | 21 (15) | 26 (21) | |
| 1 | 66 (25) | 31 (22) | 35 (28) | |
| 2 | 80 (30) | 48 (35) | 32 (25) | |
| ≥ 3 | 71 (27) | 38 (28) | 33 (26) | |
|
| ||||
| Minimum pH 0–6 | 7.20 (7.00, 7.33) | 7.09 (6.88, 7.28) | 7.28 (7.14, 7.37) | < 0.001 |
|
| ||||
| Minimum pH 7–12 | 7.37 (7.27, 7.45) | 7.31 (7.20, 7.42) | 7.40 (7.35, 7.46) | < 0.001 |
|
| ||||
| Maximum lactate 0–6 | 8.2 (3.3, 14.1) | 11.9 (6.4, 18.1) | 5.3 (2.2, 10) | < 0.001 |
|
| ||||
| Maximum lactate 7–12a | 4.7 (2, 10.7) | 8.8 (3.8, 15.9) | 2.7 (1.6, 5.7) | < 0.001 |
Age and lactate variables are represented as median (interquartile range) Each patient can have more than one preexisting condition.
n = 153.
The median lactate0–6 level was 8.2 (3.3, 14.1) mmol/L, and median lactate7–12 level was 4.7 (2, 10.7) mmol/L. Lactate0–6 levels were significantly higher in patients who received more doses of epinephrine (p < 0.001) or had trauma (p = 0.03) or a neurologic condition (p = 0.049) as the etiology of their arrest (Table 2). There was no significant association between lactate0–6 and initial cardiac rhythm, but patients with a cardiovascular etiology of their arrest had a lower lactate0–6. Furthermore, there were no significant associations between arrest location (IH vs OH) and lactate0–6 (8.1 [3.2, 13.7] vs 10 [4.9, 14.6] mmol/L; p = 0.28) or lactate7–12 (3.9 [1.8, 11.1] vs 5.4 [3.5, 10.7] mmol/L; p = 0.35).
Table 2.
Maximum Lactate Levels at 0–6 Hours for Cardiac Arrest Characteristics
| n | Median (Interquartile Range) | p | ||
|---|---|---|---|---|
| Initial rhythm | 264 | 0.08 | ||
| Asystole/pulseless electrical activity | 10.1 (6.5, 14.5) | |||
| Ventricular fibrillation/pulseless ventricular tachycardia | 7.1 (3.3, 17) | |||
| Bradycardia | 6.5 (3, 13.3) | |||
| Other/unknown | 9.3 (4.4, 17.7) | |||
|
| ||||
| Epinephrine doses | 254 | < 0.001 | ||
| 0 | 4.9 (2.7, 11.6) | |||
| 1 | 6.2 (2, 10.7) | |||
| 2 | 6.6 (3.2, 9.8) | |||
| 3 | 11.3 (4.8, 15) | |||
| ≥ 4 | 12.1 (7.2, 18.7) | |||
|
| ||||
| Location | 264 | 0.28 | ||
| In hospital | 8.1 (3.2, 13.7) | |||
| Out of hospital | 10 (4.9, 14.6) | |||
|
| ||||
| Etiology of arrest | ||||
| Cardiovascular | 93 | 6.5 (2.3, 12.3) | 0.009 | |
| Neurologic | 6 | 19.1 (7.5, 22.3) | 0.049 | |
| Congenital heart disease | 89 | 8.8 (4.8, 16.2) | 0.26 | |
| Respiratory | 121 | 8.3 (3.1. 14.8) | 0.91 | |
| Electrolyte imbalance | 20 | 9 (3.9, 12.9) | 0.58 | |
| Trauma | 13 | 11.8 (9, 16.3) | 0.032 | |
On univariate analysis, nonsurvivors had higher lactate levels than survivors at 0–6 hours (11.9 [6.4, 18.1] vs 5.3 [2.2, 10] mmol/L; p < 0.001) and 7–12 hours (8.8 [3.8, 15.9] vs 2.7 [1.6, 5.7] mmol/L; p < 0.001) (Table 1).
Of the 206 IH CA patients, 33 patients (16%) were treated with ECMO. Fifty-two percent of those treated with ECMO died. The use of ECMO was not associated with outcome. ECMO patients had a significantly higher lactate0–6 than non-ECMO patients (17 [9.2, 22.7] vs 7.5 [3.1, 12] mmol/L; p ≤ 0.001). ECMO patients were younger (3 [0, 25] vs 10 [2, 75] mo; p = 0.052), more likely to receive more than or equal to four doses of epinephrine (39% vs 19%; p = 0.032), and more likely to have preexisting congenital heart disease (79% vs 46%; p < 0.001).
As previously reported, nonsurvivors had a lower pH and lower body temperature recorded within the first 6 hours of arrest than survivors (34.8°C [33.1, 36.1] vs 35.5°C [34.6, 36.3]; p = 0.006) (10). There was no difference in maximum blood glucose in the first 6 hours (163 [92, 268] vs 142 [96, 204]; p = 0.19), nor was there a difference in the number of postarrest vasopressor/inotropes administered between survival groups (2 [1, 3] vs 2 [1, 3]; p = 0.21).
After controlling for a priori covariates and potential confounders, lactate0–6 levels and lactate7–12 levels were associated with higher odds of mortality. The odds of death per 1 mmol/L increase in lactate0–6 (n = 254) was 1.14 (1.08, 1.19) (p < 0.001). The odds of death per 1 mmol/L increase in lactate 7–12 (n = 148) was 1.2 (1.11, 1.30) (p < 0.001). The relationship between increase in lactate and odds of death was linear.
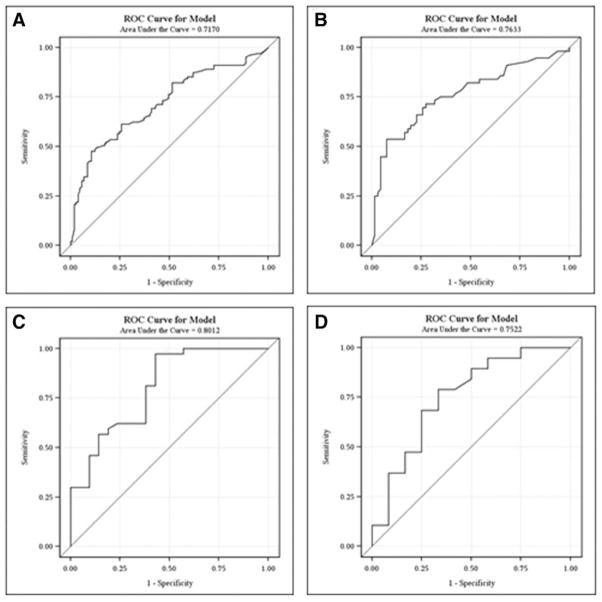
ROCs were generated by arrest location: IH and OH. Areas under the curve (AUCs) for IH arrests were 0.72 for lactate0–6 and 0.76 for lactate7–12. AUCs for OH arrests were 0.8 for lactate 0–6 and 0.75 for lactate7–12 (Fig. 2).
Figure 2.
Receiver-operating characteristic (ROC) curves for maximum lactate levels at 0–6 hr for in-hospital arrest mortality (n = 206) (A), 7–12 hr for in-hospital arrest mortality (n = 122) (B), 0–6 hr for out-of-hospital arrest mortality (n = 58) (C), and 7–12 hr for out-of-hospital arrest mortality (n = 31) (D).
For each arrest location, we estimated the probability of death by lactate level based on the corresponding univariate logistic regression model. For IH CA, lactate0–6 less than 0.9 would have less than 25% risk of death, lactate0–6 greater than 18.6 would have greater than 75% risk of death, and lactate0–6 greater than 27.5 would have a greater than 90% risk of death (Table 3). For OH CA, lactate0–6 less than 0.6 would have less than 25% risk of death, lactate0–6 greater than 11.9 would have a greater than 75% risk of death, and lactate0–6 greater than 17.5 would have a risk of greater than 90%.
Table 3.
Mortality by Arrest Location and Maximum Lactate Level During First 12 Hours Postarrest
| Chance of Death, % | In Hospital
|
Out of Hospital
|
||
|---|---|---|---|---|
| Lactate, 0–6 | Lactate, 7–12 | Lactate, 0–6 | Lactate, 7–12 | |
| 25 | 0.9 | 1.6 | 0.6 | |
|
| ||||
| 50 | 9.7 | 7.9 | 6.2 | 3.8 |
|
| ||||
| 75 | 18.6 | 14.2 | 11.9 | 10.5 |
|
| ||||
| 90 | 27.5 | 20.5 | 17.5 | 17.1 |
|
| ||||
| 95 | 33.5 | 24.8 | 21.4 | 21.7 |
Estimates based on univariate logistic regression models.
DISCUSSION
To our knowledge this is the largest study evaluating the association of serum lactate levels and outcomes following pediatric CA. As expected, children with higher serum lactate levels early after successful resuscitation from CA are more likely to die. Although higher levels of lactate are associated with a higher likelihood of death, serum lactate levels alone do not uniformly predict poor outcomes.
Initial work from this combined IH and OH CA patient cohort showed that there was no difference between lactate levels over 12 hours for all patients when comparing IH and OH arrest location (10). However, when looking at lactate levels and mortality, in both IH and OH settings, higher lactate levels over the first 12 hours were associated with mortality (4, 5). Prodhan et al (14) evaluated the impact of ECMO and mortality mostly in a heart disease-associated CA population and reported lactate levels 24 hours following ROSC were not significantly different between survivors and nonsurvivors. This may be attributed to ECMO providing adequate oxygen delivery for a long enough period to clear lactate. Our population differs because most of our patients were not treated with ECMO.
In our study, we found lower lactate levels at 0–6 hours in subjects who had a cardiovascular cause of arrest, such as dehydration, sepsis, or arrhythmia. It is possible that these patients had lower lactate levels because they did not have a prolonged hypoxic event prior to requiring CPR or had CPR initiated when they were bradycardic. In contrast, patients who had an IH CA and received ECMO had significantly higher lactate0–6 than those who did not receive ECMO. Notably, a significantly larger proportion of ECMO patients received more than or equal to four doses of epinephrine. This likely speaks to the prolonged arrest prior to ECMO cannulation which may explain these patients’ higher lactate levels. However, despite these higher early lactate levels, there was no difference in mortality between patients who did and did not receive ECMO. It may be that ECMO support provides more optimal postarrest end-organ perfusion thus mitigating some secondary injury. Clinicians should keep in mind that patients treated with ECMO may require different interpretation of their lactate levels for decision making.
We found that initial lactate levels following ROSC were associated with mortality, even after controlling for potential confounders such as arrest location: IH versus OH, initial rhythm, and number of doses of epinephrine administered. Forty-nine patients had a lactate sampled within the 2 hours preceding their arrest, and not surprisingly, nonsurvivors were more likely to have a higher prearrest lactate. The rise of serum lactate is the end result of tissue hypoxemia and the patient’s transition to anaerobic metabolism. Multiple studies have evaluated the association between initial elevations in lactate and outcomes. Following adult CA, absolute lactate levels on hospital admission were significantly higher in nonsurvivors than survivors, but the differences were small (9, 15–18). Patients resuscitated from CA have increasing levels of lactate as doses of epinephrine increase. In patients with status asthmaticus, severe lactic acidosis occurs with excessive use of β-adrenergic medication despite adequate oxygen delivery (19). This is likely due to both the severity of the arrest and the numerous doses of epinephrine administered. Although initial lactate levels reflect “severity of injury,” using a single lactate level for determination of outcome may be challenging for several reasons. For example, interventions that alter outcomes may not have been implemented, the cause of CA may in some cases be a progressive untreatable process, or the patient may have been terminally ill with do-not-resuscitate status being addressed only after the patient progressed to a CA event.
Despite the clear difference in lactate levels between survivors and nonsurvivors, when a clinician is posed with a child post-CA, statistics aid only in determining that a biomarker may be associated with an outcome in a population but not necessarily how to interpret it for an individual patient. Therefore, we specifically looked at ROC curves. IH and OH CA differ significantly both in etiology and outcome (3, 20, 21); we analyzed lactate ROC curves for location of arrest separately. AUC ranges from 0.72 to 0.8 depending on arrest location and timing of lactate sampling. Alone these tests are not robust enough to confidently prognosticate outcome for the individual patient. The test used for decision making such as withdrawing life-sustaining technologic support must be 100% sensitive for death. Importantly, to prevent continuing aggressive care on patients who will die, a test would have to be 100% specific for death. The AUCs were not high enough to fulfill these criteria; therefore, we also evaluated the simple risk of death by maximum lactate level during the 0- to 6-hour and 7- to 12-hour intervals for the IH and OH CA cohorts. Crude mortality risk for several cut-points is reported, which may be of value to clinicians. Notably, in this specific population, lactate levels associated with a 75% risk of death were lower for OH CA patients than IH CA patients. For OH CA, lactate levels greater than 17 and greater than 21 at 0–6 and 6–12 hours were associated with 90% and 95% mortality rates, respectively. For IH CA, a lactate level of 27.5 during the 0- to 6-hour interval and 20.5 during the 7- to 12-hour interval was associated with a 90% mortality rate. Although very high lactate levels were associated with near 100% mortality, moderately elevated lactates were not able to distinguish outcomes. Therefore, lactate levels should be used in conjunction with other diagnostic tools to make decisions about limitation of care.
Our study has several limitations. A large number of cases from our original dataset did not have lactates during the 0- to 6-hour time frame for inclusion in this report; this may be because patients were too well and rapidly improved or were moribund. This was a retrospective study, and lactate levels were sampled at variable times in relation to ROSC. It is possible that one sample was obtained shortly after a brief arrest and another was sampled hours after a prolonged arrest. These results may have different meanings for these different patients despite being sampled within the first 6 hours following ROSC. We only captured data regarding the first CA per patient, and therefore, we did not account for repeat arrests and withdrawal of technologic support for non-CA-associated reasons. Estimates of lactate levels that correspond to specific probability of death cutoffs were based on univariate logistic regression models. Such estimates use a simplified relationship between lactate and mortality and do not take into account other patient characteristics and potential confounders. These data represent our dataset and may not be able to be extrapolated to other cohorts. Despite these limitations, this is one of the largest pediatric CA patient cohorts specifically analyzing lactate levels association with outcome.
CONCLUSION
Elevated lactate levels in the first 12 hours after successful resuscitation from pediatric CA are associated with increased mortality. Lactate levels alone are not able to predict outcomes accurately enough for definitive prognostication but may approximate mortality observed in this large cohort of children’s hospitals. Future investigations in larger homogenous cohorts with standardized lactate measurement times and in combination with other markers may result in improved prognostic utility.
Acknowledgments
Dr. Topjian is funded by a National Institutes of Health (NIH) career development award K23NS075363 and U01HL094345. Dr. Dean is funded by NIH awards K12HD047349, U01HD049934, U01HL094339, HRSA award U03MC00008, and the HA and Edna Benning Presidential Endowment. Dr. Moler was supported by federal grants NIH NICHD R21 HD044955 and R34 HD 050531. The Pediatric Emergency Care Applied Research Network is supported by cooperative agreements U03MC00001, U03MC00003, U03MC00006, U03MC00007, and U03MC00008 from the Emergency Medical Services for Children program of the Maternal and Child Health Bureau, Health Resources and Services Administration, and U.S. Department of Health and Human Services. Dr. Topjian is employed by CHOP and received grant support from NIH. Dr. Clark received grant support from NIH. Dr. Casper disclosed that he does not have any potential conflicts of interest. Dr. Berger received grant support from NIH. Dr. Schelein served as a board member for Out2Play, provided expert testimony for multiple law firms, received royalties from Kluwers for two textbooks, has stock options with PingMD (equity position in a startup of a smart phone-based product linking parents to pediatricians), and received funding from NIH. Dr. Dean received grant support from NIH (multiple) and EMSC. Dr. Moler received grant and support for travel from NICHD/NIH, NHLBI (THAPCA), NHLBI (HIP), NICHD (CPC-CRN), and HRSA/PECARN.
Participating children’s hospital, university affiliation, and site investigators are listed in alphabetical order: Children’s Hospital Medical Center, University of Cincinnati, Cincinnati, OH (R. Brilli); Children’s Hospital of Buffalo, SUNY-Buffalo, Buffalo, NY (B. Fuhrman); Children’s Hospital of Michigan, Wayne State University, Detroit, MI (K. Meert); Children’s Hospital of New York, Columbia University, New York, NY (C. Schleien); Children’s Hospital of Philadelphia, University of Pennsylvania, Philadelphia, PA (V. Nadkarni). Children’s Hospital of Pittsburgh, University of Pittsburgh, Pittsburgh, PA (R. Clark); Children’s Hospital of Wisconsin, Medical College of Wisconsin, Milwaukee, WI (K. Tieves); Children’s National Medical Center, George Washington University, Washington, DC (H. Dalton); C.S. Mott Children’s Hospital, University of Michigan, Ann Arbor, MI (F. Moler); Helen DeVos Children’s Hospital, Michigan State University, Grand Rapids, MI (R. Hackbarth); Primary Children’s Medical Center, University of Utah, Salt Lake City, UT (K. Statler Bennett); St. Louis Children’s Hospital, Washington University St. Louis, MO (F. Levy/D. Jaffe); Golisano Children’s Hospital, University of Rochester, Rochester, NY (E. van der Jagt); The Johns Hopkins Hospital, Johns Hopkins University, Baltimore, MD (H. Shaffner); University of California at Davis, Sacramento, CA (R. Pretzlaff).
We acknowledge the efforts of the following individuals participating in Pediatric Emergency Care Applied Research Network (PECARN) at the time this study was initiated. PECARN Steering Committee: N. Kuppermann (Chair), E. Alpern, J. Chamberlain, J. M. Dean, M. Gerardi, J. Goepp, M. Gorelick, J. Hoyle, D. Jaffe, C. Johns, N. Levick, P. Mahajan, R. Maio, K. Melville, S. Miller (deceased), D. Monroe, R. Ruddy, R. Stanley, D. Treloar, M. Tunik, A. Walker. MCHB/EMSC liaisons: D. Kavanaugh, H. Park. Central Data Management and Coordinating Center: M. Dean, R. Holubkov, S. Knight, A. Clark. Data Analysis and Management Subcommittee: J. Chamberlain (Chair), M. Brown, H. Corneli, J. Goepp, R. Holubkov, P. Mahajan, K. Melville, E. Stremski, M. Tunik. Grants and Publications Subcommittee: M. Gorelick (Chair), E. Alpern, J. M. Dean, G. Foltin, J. Joseph, S. Miller (deceased), F. Moler, R. Stanley, S. Teach. Protocol Concept Review and Development Subcommittee: D. Jaffe (Chair), K. Brown, A. Cooper, J. M. Dean, C. Johns, R. Maio, N. C. Mann, D. Monroe, K. Shaw, D. Teitelbaum, D. Treloar. Quality Assurance Subcommittee: R. Stanley (Chair), D. Alexander, J. Brown, M. Gerardi, M. Gregor, R. Holubkov, K. Lillis, B. Nordberg, R. Ruddy, M. Shults, A. Walker. Safety and Regulatory Affairs Subcommittee: N. Levick (Chair), J. Brennan, J. Brown, J. M. Dean, J. Hoyle, R. Maio, R. Ruddy, W. Schalick, T. Singh, J. Wright.
References
- 1.Atkins DL, Everson-Stewart S, Sears GK, et al. Resuscitation Outcomes Consortium Investigators: Epidemiology and outcomes from out-of-hospital cardiac arrest in children: The resuscitation outcomes consortium epistry-cardiac arrest. Circulation. 2009;119:1484–1491. doi: 10.1161/CIRCULATIONAHA.108.802678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nadkarni VM, Larkin GL, Peberdy MA, et al. National Registry of Cardiopulmonary Resuscitation Investigators: First documented rhythm and clinical outcome from in-hospital cardiac arrest among children and adults. JAMA. 2006;295:50–57. doi: 10.1001/jama.295.1.50. [DOI] [PubMed] [Google Scholar]
- 3.Holmberg M, Holmberg S, Herlitz J. Effect of bystander cardiopulmonary resuscitation in out-of-hospital cardiac arrest patients in Sweden. Resuscitation. 2000;47:59–70. doi: 10.1016/s0300-9572(00)00199-4. [DOI] [PubMed] [Google Scholar]
- 4.Moler FW, Donaldson AE, Meert K, et al. Pediatric Emergency Care Applied Research Network: Multicenter cohort study of out-of-hospital pediatric cardiac arrest. Crit Care Med. 2011;39:141–149. doi: 10.1097/CCM.0b013e3181fa3c17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meert KL, Donaldson A, Nadkarni V, et al. Pediatric Emergency Care Applied Research Network: Multicenter cohort study of in-hospital pediatric cardiac arrest. Pediatr Crit Care Med. 2009;10:544–553. doi: 10.1097/PCC.0b013e3181a7045c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaneko T, Kasaoka S, Miyauchi T, et al. Serum glial fibrillary acidic protein as a predictive biomarker of neurological outcome after cardiac arrest. Resuscitation. 2009;80:790–794. doi: 10.1016/j.resuscitation.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Topjian AA, Lin R, Morris MC, et al. Neuron-specific enolase and S-100B are associated with neurologic outcome after pediatric cardiac arrest. Pediatr Crit Care Med. 2009;10:479–490. doi: 10.1097/PCC.0b013e318198bdb5. [DOI] [PubMed] [Google Scholar]
- 8.Donnino MW, Miller J, Goyal N, et al. Effective lactate clearance is associated with improved outcome in post-cardiac arrest patients. Resuscitation. 2007;75:229–234. doi: 10.1016/j.resuscitation.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 9.Shinozaki K, Oda S, Sadahiro T, et al. Blood ammonia and lactate levels on hospital arrival as a predictive biomarker in patients with out-of-hospital cardiac arrest. Resuscitation. 2011;82:404–409. doi: 10.1016/j.resuscitation.2010.10.026. [DOI] [PubMed] [Google Scholar]
- 10.Moler FW, Meert K, Donaldson AE, et al. Pediatric Emergency Care Applied Research Network: In-hospital versus out-of-hospital pediatric cardiac arrest: A multicenter cohort study. Crit Care Med. 2009;37:2259–2267. doi: 10.1097/CCM.0b013e3181a00a6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kleinman ME, Chameides L, Schexnayder SM, et al. Part 14: Pediatric advanced life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18 Suppl 3):S876–S908. doi: 10.1161/CIRCULATIONAHA.110.971101. [DOI] [PubMed] [Google Scholar]
- 12.Zaritsky A, Nadkarni V, Hazinski MF, et al. Recommended guidelines for uniform reporting of pediatric advanced life support: The pediatric Utstein style. A statement for healthcare professionals from a task force of the American Academy of Pediatrics, the American Heart Association, and the European Resuscitation Council. Writing Group. Circulation. 1995;92:2006–2020. doi: 10.1161/01.cir.92.7.2006. [DOI] [PubMed] [Google Scholar]
- 13.Jacobs I, Nadkarni V, Bahr J, et al. International Liaison Committee on Resuscitation; American Heart Association; European Resuscitation Council; Australian Resuscitation Council; New Zealand Resuscitation Council; Heart and Stroke Foundation of Canada; Inter American Heart Foundation; Resuscitation Councils of Southern Africa; ILCOR Task Force on Cardiac Arrest and Cardiopulmonary Resuscitation Outcomes: Cardiac arrest and cardiopulmonary resuscitation outcome reports: Update and simplification of the Utstein templates for resuscitation registries: A statement for healthcare professionals from a task force of the International Liaison Committee on Resuscitation (American Heart Association, European Resuscitation Council, Australian Resuscitation Council, New Zealand Resuscitation Council, Heart and Stroke Foundation of Canada, Inter American Heart Foundation, Resuscitation Councils of Southern Africa) Circulation. 2004;110:3385–3397. doi: 10.1161/01.CIR.0000147236.85306.15. [DOI] [PubMed] [Google Scholar]
- 14.Prodhan P, Fiser RT, Dyamenahalli U, et al. Outcomes after extracorporeal cardiopulmonary resuscitation (ECPR) following refractory pediatric cardiac arrest in the intensive care unit. Resuscitation. 2009;80:1124–1129. doi: 10.1016/j.resuscitation.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kliegel A, Losert H, Sterz F, et al. Serial lactate determinations for prediction of outcome after cardiac arrest. Medicine (Baltimore) 2004;83:274–279. doi: 10.1097/01.md.0000141098.46118.4c. [DOI] [PubMed] [Google Scholar]
- 16.Cocchi MN, Miller J, Hunziker S, et al. The association of lactate and vasopressor need for mortality prediction in survivors of cardiac arrest. Minerva Anestesiol. 2011;77:1063–1071. [PubMed] [Google Scholar]
- 17.Müllner M, Sterz F, Domanovits H, et al. The association between blood lactate concentration on admission, duration of cardiac arrest, and functional neurological recovery in patients resuscitated from ventricular fibrillation. Intensive Care Med. 1997;23:1138–1143. doi: 10.1007/s001340050470. [DOI] [PubMed] [Google Scholar]
- 18.Adrie C, Adib-Conquy M, Laurent I, et al. Successful cardiopulmonary resuscitation after cardiac arrest as a “sepsis-like” syndrome. Circulation. 2002;106:562–568. doi: 10.1161/01.cir.0000023891.80661.ad. [DOI] [PubMed] [Google Scholar]
- 19.Meert KL, McCaulley L, Sarnaik AP. Mechanism of lactic acidosis in children with acute severe asthma. Pediatr Crit Care Med. 2012;13:28–31. doi: 10.1097/PCC.0b013e3182196aa2. [DOI] [PubMed] [Google Scholar]
- 20.Donoghue AJ, Nadkarni V, Berg RA, et al. CanAm Pediatric Cardiac Arrest Investigators: Out-of-hospital pediatric cardiac arrest: An epidemiologic review and assessment of current knowledge. Ann Emerg Med. 2005;46:512–522. doi: 10.1016/j.annemergmed.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 21.López-Herce J, García C, Domínguez P, et al. Spanish Study Group of Cardiopulmonary Arrest in Children: Characteristics and outcome of cardiorespiratory arrest in children. Resuscitation. 2004;63:311–320. doi: 10.1016/j.resuscitation.2004.06.008. [DOI] [PubMed] [Google Scholar]