Summary
Background
Airway pressure release ventilation (APRV) and high frequency oscillatory ventilation (HFOV) are frequently used in acute lung injury (ALI) refractory to conventional ventilation. Our aim was to describe our experience with APRV and HFOV in refractory pediatric ALI, and to identify factors associated with survival.
Methods
We analyzed 104 patients with hypoxemia refractory to conventional ventilation transitioned to either APRV or HFOV. Demographics, oxygenation index (OI), and PaO2/FiO2 (PF ratio) were recorded before transition to either mode of nonconventional ventilation (NCV) and for every 12 hr after transition.
Results
Relative to APRV, patients on HFOV were younger and had more significant lung disease evidenced by higher OI (28.5 [18.6, 36.2] vs. 21.0 [15.5, 30.0], P = 0.008), lower PF ratios (73 [59,94] vs. 99 [76,131], P = 0.002), and more frequent use of inhaled nitric oxide. In univariate analysis, HFOV was associated with more frequent neuromuscular blockade. Forty-one of 104 patients died on NCV (39.4%). Survivors demonstrated improvement in OI 24 hr after transition to NCV, whereas non-survivors did not (12.9 [8.9, 20.9] vs. 28.1 [17.6, 37.1], P < 0.001). After controlling for immunocompromised status, number of vasopressors, and OI before transition, mode of NCV was not associated with mortality.
Conclusions
In a heterogeneous PICU population with hypoxemia refractory to conventional ventilation transitioned to NCV, improvement in oxygenation at 24 hr was associated with survival. Immunocompromised status, number of vasopressor infusions, and the OI before transition to NCV were independently associated with survival.
Keywords: mechanical ventilation, high frequency oscillatory ventilation, airway pressure release ventilation, acute respiratory distress syndrome, acute lung injury, pediatric
INTRODUCTION
Mechanical ventilation remains the cornerstone of therapy for acute lung injury (ALI) and acute respiratory distress syndrome (ARDS). Since publication of the landmark ARDS Network trial demonstrating improved outcomes utilizing lower tidal volumes and plateau pressures,1 pediatric intensive care units (PICUs) have largely adopted this practice.2 Efforts to limit ventilator-induced lung injury focus on avoiding alveolar hyperinflation and cyclic collapse and re-expansion, while maintaining alveolar recruitment. Concerns for injury from excess pressures, hyperinflation, and inadequate recruitment have prompted the development of alternative “nonconventional” modes of ventilation.
High frequency oscillatory ventilation (HFOV) achieves gas exchange using very high respiratory rates oscillating around a fixed mean airway pressure (mPaw) delivering sub-dead space volumes while simultaneously maintaining alveolar recruitment. Its use has been described extensively in neonatal and pediatric respiratory failure, demonstrating improvement of gas exchange, with no consistent benefits on clinical outcomes including mortality or duration of mechanical ventilation.3–7 In the absence of evidence demonstrating superiority over conventional mechanical ventilation in children or adults,4,8–10 HFOV is used predominantly as a rescue mode for patients with oxygenation failure.
More recently, airway pressure release ventilation (APRV) has been described as an alternative mode of nonconventional ventilation (NCV). APRV applies a prolonged continuous positive airway pressure to maintain adequate lung volumes and recruit available lung units with varying time constants while using lower peak pressures and inspiratory flow rates than conventional, with periodic time-cycled releases to facilitate carbon dioxide clearance.11,12 In contrast to HFOV, APRV has the benefit of not requiring a change in the ventilator circuit. Pediatric experience with APRV is limited, but initial reports suggest potential advantages, including improved cardiac output, decreased vasopressor requirement, and cardiorespiratory benefits of spontaneous respiration throughout the ventilator cycle.13–15 APRV, like HFOV, is typically described as a rescue mode for hypoxemic patients.
Despite the availability of these modes of NCV, their utilization in the management of pediatric ALI is not well described. The purpose of the current study was twofold: to describe our single center experience with APRV and HFOV in pediatric ALI refractory to conventional ventilation, and to identify factors associated with mortality in this population. We hypothesized that less severe oxygenation impairment prior to NCV and improved oxygenation while on NCV would be associated with survival.
METHODS
Design
We conducted a retrospective cohort study in pediatric patients receiving either APRV or HFOV at the Children’s Hospital of Philadelphia (CHOP), a tertiary care PICU. The study was approved by the hospital Institutional Review Board with waiver of the requirement for informed consent.
Patient Selection
Patients were identified by query of a prospectively collected admission database. All patients between ages of 1 month and 21 years who transitioned from conventional ventilation to either APRV or HFOV between January 1, 2004 and June 30, 2009, and remained on that mode of NCV for at least 24 hr in the PICU were eligible. Patients were excluded if they were exposed to either APRV or HFOV prior to admission to the CHOP PICU.
Conventional Ventilation Strategy
Determination of failure of conventional ventilation and decision to employ alternate modes was left to the discretion of the attending physician. Our institutional practice for respiratory failure is to initiate conventional ventilation with a minimum of 5 cm H2O of positive end expiratory pressure (PEEP), and to attempt to wean FiO2 to ≤0.60. Inability to wean FiO2 prompts escalation of PEEP and subsequent repeat efforts to wean FiO2, with the goal to maintain peak inspiratory pressures ≤35 cm H2O. Persistently elevated peak pressures (≥35 cm H2O), ongoing hypercarbia (PaCO2 ≥80 or pH < 7.30), or oxygenation difficulties (inability to wean FIO2 ≤0.60 despite increasing PEEP) prompted consideration for a change in the mode of ventilation.
APRV Strategy
APRV was initiated selecting peak pressure (Phigh) and inspiratory time (Thigh) to match the mPaw being delivered on conventional, with stepwise increases in mPaw by adjusting Phigh or Thigh if unable to wean FiO2 to ≤0.60. Our institutional practice is to set the low pressure on APRV (Plow) to 0 to facilitate rapid emptying, and to adjust expiratory time (Tlow) to terminate at 50–75% of peak expiratory flow.11
HFOV Strategy
HFOV was initiated by setting mPaw to match that being delivered on conventional ventilation, with escalation of mPaw until FiO2 could be weaned to ≤0.60. Amplitude was increased to achieve oscillations visible to the pelvis, and initial frequency set based on patient weight. For both APRV and HFOV, the oxygenation goal was an O2 saturation of ≥88% on FiO2 ≤0.60, and the ventilation goal was PaCO2 ≤60 with pH ≥ 7.3. Use of inhaled nitric oxide, exogenous surfactant, neuromuscular blockade, or conversion to a different mode of NCV or to extracorporeal support, was left to the discretion of the attending physician.
Data Collection
Data abstracted from eligible patients’ medical records included admission diagnoses, demographics, severity of illness scores at PICU admission (Pediatric Risk of Mortality III [PRISM III] at 24 hr), co-morbidities, indication for mechanical ventilation, length of mechanical ventilation, and survival to hospital discharge. We recorded ventilator settings, vasopressor use in the first 72 hr after transition, evidence of pneumomediastinum or pneumothorax at any time on NCV, and the use of adjunctive therapies for ALI (inhaled nitric oxide, surfactant, and neuromuscular blockade in the first 72 hr after transition to NCV). Oxygenation index was calculated every 12 hr for the first 7 days of NCV if arterial blood gases from indwelling catheters were available and if the patient had not expired or transitioned back to conventional.
Equations and Definitions
Measures of oxygenation calculated were the PF ratio (PaO2/FiO2) and the oxygenation index (OI, [mPaw × FiO2 × 100]/PaO2).
Statistical Analysis
Continuous data were all non-normally distributed and are reported as median [25th and 75th percentiles]. Categorical data are reported as frequencies and percentages. Continuous variables were compared using Wilcoxon rank sum test, and categorical variables compared using the Fisher exact or chi-square test. Categorical variables with potential effect modification were tested using the Mantel–Haenszel test. Multilevel mixed effects linear regression was used to model the change in OI over time after transition to NCV. Cox proportional hazard modeling was used to determine the relationship between mode of NCV and mortality after adjustment for confounding variables. Potential confounding variables affecting mortality were identified by selecting factors associated with mortality in univariate analysis with P < 0.2, and including them in a Cox proportional hazard model. Variables were checked for multicollinearity prior to modeling: corticosteroid use was found to be collinear with vasopressor infusions, and PF ratio before NCV was found to be collinear with OIpre; therefore, only vasopressor infusion and OIpre were modeled. Goodness of fit of the Cox proportional hazard model was assessed by calculating a Harrell’s C concordance statistic. Calculations were performed in Stata 10.0 (StataCorp, LP, College Station, TX).
RESULTS
Patient Characteristics
One hundred sixteen patients transitioned from conventional ventilation to either APRV or HFOV for >24 hr during the study period, of which 12 were excluded: 3 for age >21 years, and 9 for exposure to NCV outside of CHOP PICU. Of the remaining 104 patients, 49 patients received APRV, and 55 HFOV (Table 1). All patients met radiographic criteria for ALI with PF ratio ≤300; 100 patients had PF ratio ≤200 at the time of transition to NCV.
TABLE 1.
Characteristics of Patients Exposed to nonconventional Ventilation
| Variable1 | Total (n = 104) | APRV (n = 49) | HFOV (n = 55) | P-value2 |
|---|---|---|---|---|
| Age (years) | 3.9 [1.1, 12.1] | 6.8 [2.4, 13.9] | 2.3 [0.7, 7.2] | <0.001 |
| PRISM III at 24 hr | 16 [7, 24] | 16 [9, 23] | 16.5 [6.5, 27] | 0.797 |
| Length of conventional ventilation before switch to NCV (days) | 1 [0, 3] | 1 [0, 3] | 1 [0, 3] | 1.0 |
| mPaw before switch to NCV (cm H2O) | 21 [18, 24] | 20 [16, 24] | 22 [19, 24] | 0.163 |
| PaO2/FiO2 before switch to NCV | 83.2 [63.8, 116.1] | 99 [76, 131] | 73 [59, 94] | 0.002 |
| OI before switch to NCV (OIpre) | 23.8 [17.1, 33.0] | 21.0 [15.5, 30.0] | 28.5 [18.6, 36.2] | 0.008 |
| Immunocompromised | 31 (30%) | 14 (29%) | 17 (31%) | 0.795 |
| Vasopressor infusions on NCV | ||||
| 0 | 14 (13%) | 11 (22%) | 3 (5%) | |
| 1 | 29 (28%) | 14 (29%) | 15 (27%) | 0.062 |
| 2 | 33 (32%) | 14 (29%) | 19 (35%) | |
| 3 or more | 28 (27%) | 10 (20%) | 18 (33%) | |
| Inhaled nitric oxide | 81 (78%) | 32 (65%) | 49 (89%) | 0.004 |
| Surfactant | 10 (10%) | 2 (4%) | 8 (15%) | 0.071 |
| Continuous neuromuscular blockade on NCV | 65 (62.5%) | 13 (27%) | 52 (95%) | <0.001 |
| New air leak on NCV | 13 (12.5%) | 4 (8%) | 9 (16%) | 0.207 |
| Failure of NCV | ||||
| Switch modes of NCV | 6 (6%) | 4 (8%) | 2 (2%) | 0.501 |
| ECMO | 6 (6%) | 2 (4%) | 4 (7%) | |
| mPaw 24 hr after switch to NCV (cmH2O) | 31 [26.5, 35] | 28 [24, 31.3] | 33 [29, 35.8] | <0.001 |
| OI 24 hr after initiation of NCV (OI24) | 16.1 [10.2, 28.2] | 13 [8.3, 17.4] | 22.8 [12.7, 32.5] | <0.001 |
| OI24/OIpre | 0.75 [0.48, 1.16] | 0.64 [0.36, 1.12] | 0.79 [0.58, 1.25] | 0.105 |
| Length of NCV (days) | 6 [3, 11] | 6 [4, 10.3] | 6 [3, 12] | 0.650 |
| Ventilator free days at 28 days | 0 [0, 12] | 1 [0, 15.8] | 0 [0, 9] | 0.028 |
| Mortality (non-survivor) | 41 (39.4%) | 16 (33%) | 25 (45%) | 0.182 |
APRV, airway pressure release ventilation; HFOV, high frequency oscillatory ventilation; NCV, nonconventional ventilation; PRISM III, Pediatric Risk of Mortality III; mPaw, mean airway pressure; OI, oxygenation index; ECMO, extracorporeal membrane oxygenation; OI24/OIpre, OI 24 hr after initiation of NCV as a fraction of OI before switch to NCV.
Continuous data are in the form of median [25th and 75th percentile], and categorical data are in the form of n (%).
Medians are compared using a Wilcoxon rank sum test for unpaired data. Categorical variables are compared using a Fisher exact or chi-square test.
Characteristics Associated With Mode of Nonconventional Ventilation
Patients treated with HFOV were younger (P < 0.001) and had worse oxygenation when transitioned to NCV, as measured by PF ratio (P = 0.002) and OI on conventional ventilation before switch (OIpre, P = 0.008). HFOV patients had more frequent exposure to inhaled nitric oxide (P = 0.004) and continuous neuromuscular blockade (P < 0.001) in the first 72 hr after transition to NCV, and a trend towards more vasopressor infusions (P = 0.062) and surfactant utilization (P = 0.071) compared to APRV, despite similar PRISM III scores, diagnoses, and proportion of immunocompromised patients (Table 1). Four HFOV patients required extracorporeal membrane oxygenation, whereas only 2 APRV patients required escalation to extracorporeal support. Conversely, 4 APRV patients were transitioned to HFOV for refractory hypoxemia, whereas only 2 patients switched from HFOV to APRV. Mortality was not associated with mode of NCV. Ventilator free days at 28 days was higher in the APRV group (P = 0.028) in univariate analysis, but not when adjusted for OIpre.
Variables Associated With Mortality
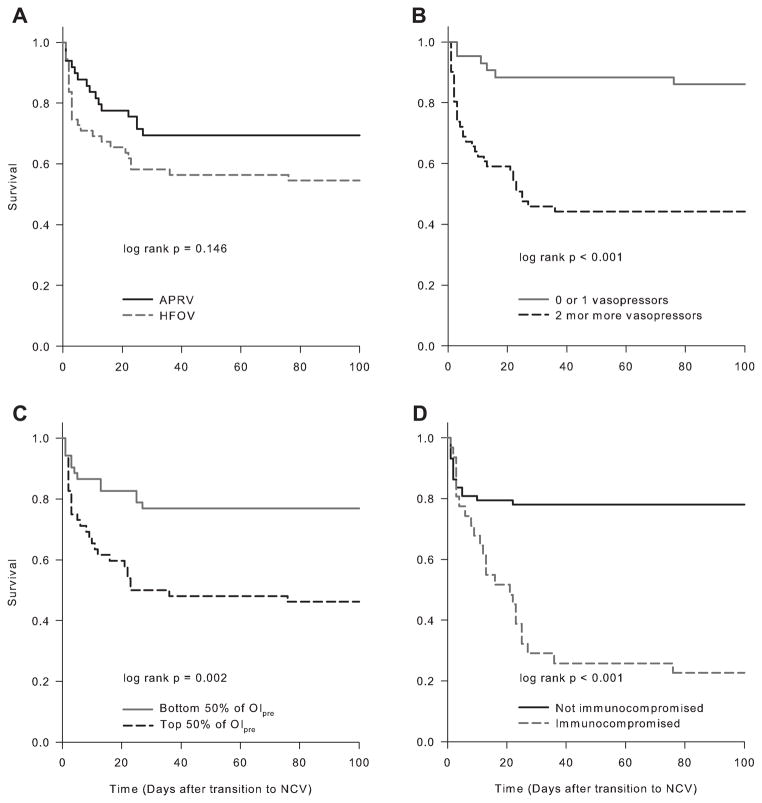
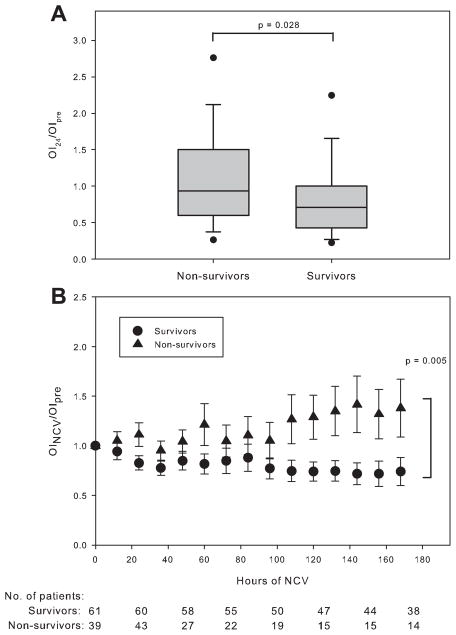
Forty-one of 104 patients died (39.4%): 16 of 49 (33%) of patients receiving APRV, and 25 of 55 (45%) patients on HFOV. In univariate analysis, the PF ratio (P = 0.019) and OIpre (P = 0.002) on conventional ventilation immediately prior to switching to NCV were worse amongst non-survivors than survivors (Table 2). Mortality was associated with presence of an immunocompromised condition (P < 0.001), more vasopressor infusions (P < 0.001), and more frequent corticosteroid exposure (P = 0.01). In Cox proportional hazard modeling, survival time was not associated with NCV mode, but was associated with the presence of an immunocompromised condition, the use of ≥2 vasopressor medication infusions, and the OIpre (Table 3 and Fig. 1). Survivors demonstrated a larger fractional reduction in OI at 24 hr (OI24/OIpre,) and a larger fractional increase in PF ratio (both P = 0.028) compared to non-survivors (Fig. 2A and Table 2). Multilevel mixed effects linear regression tested the relationship between survival and the change in OI over time, with a significant effect (P = 0.005) of survival status over time on OI after transition to NCV (Fig. 2B).
TABLE 2.
Univariate Testing of Characteristics Associated With Mortality
| Variable1 | Survivors (n = 63) | Non-survivors (n = 41) | P-value2 |
|---|---|---|---|
| Age (years) | 4.2 [1.2, 12] | 3.5 [1, 12] | 0.852 |
| PRISM III at 24 hrs | 15 [5.8, 23.2] | 18 [9, 24.8] | 0.252 |
| Sex | |||
| Male | 40 (63%) | 21 (51%) | 0.214 |
| Female | 23 (37%) | 20 (49%) | |
| Race | |||
| White | 30 (48%) | 17 (41%) | |
| Black | 22 (35%) | 15 (37%) | |
| Hispanic | 4 (6%) | 4 (10%) | 0.400 |
| Asian | 0 (0%) | 2 (5%) | |
| Other | 7 (11%) | 3 (7%) | |
| Length of conventional ventilation before switch to NCV (days) | 1 [0, 3] | 1 [0, 3] | 0.846 |
| PaO2/FiO2 before switch to NCV | 85 [69, 126] | 80 [58, 100] | 0.019 |
| OI before switch to NCV (OIpre) | 21.2 [14.1, 31.9] | 28.9 [22.4, 36.6] | 0.002 |
| Mode of NCV | |||
| APRV | 33 (52%) | 16 (39%) | 0.182 |
| HFOV | 30 (48%) | 25 (61%) | |
| Diagnosis | |||
| Sepsis | 5 (8%) | 6 (15%) | |
| Trauma | 2 (3%) | 2 (5%) | |
| Drowning | 4 (6%) | 1 (2%) | 0.207 |
| Cardiac arrest | 4 (6%) | 2 (5%) | |
| Surgery | 4 (6%) | 2 (5%) | |
| Pneumonia | 41 (65%) | 20 (49%) | |
| Other | 3 (5%) | 8 (19%) | |
| Immunocompromised | 7 (11%) | 24 (59%) | <0.001 |
| Vasopressor infusions on NCV | |||
| 0 | 13 (21%) | 1 (2%) | |
| 1 | 24 (38%) | 5 (12%) | <0.001 |
| 2 | 15 (24%) | 18 (44%) | |
| 3 or more | 11 (17%) | 17 (41%) | |
| Corticosteroids | 39 (62%) | 35 (85%) | 0.010 |
| Length of NCV (days) | 6 [4, 11] | 4 [2, 11.3] | 0.116 |
| OI 24 hr after initiation of NCV (OI24) | 12.9 [8.9, 20.9] | 28.1 [17.6, 37.1] | <0.001 |
| OI24/OIpre | 0.71 [0.43, 1.00] | 0.94 [0.61, 1.47] | 0.028 |
| PF ratio24/PF ratiopre | 1.98 [1.39, 3.55] | 1.51 [1.11, 2.29] | 0.028 |
APRV, airway pressure release ventilation; HFOV, high frequency oscillatory ventilation; NCV, nonconventional ventilation; PRISM III, Pediatric Risk of Mortality III; OI, oxygenation index; OI24/OIpre, OI 24 hr after initiation of NCV as a fraction of OI before switch to NCV; PF ratio24/PF ratiopre, PaO2/FiO2 24 hr after initiation of NCV as a fraction of PaO2/FiO2 before switch.
Continuous data are in the form of median [25th and 75th percentile], and categorical data are in the form of n (%).
Medians are compared using a Wilcoxon rank sum test for unpaired data. Categorical variables are compared using a Fisher exact or chi-square test.
TABLE 3.
Summary of Cox Proportional Hazard Model for Mortality
| Variable1 | Adjusted hazard ratio for non-survival | 95% Confidence interval | P-value |
|---|---|---|---|
| Mode of NCV | 0.51–1.96 | 0.992 | |
| APRV | 1.00 | ||
| HFOV (reference) | 1 | ||
| Immunocompromised condition | 1.71–6.40 | <0.001 | |
| Yes | 3.31 | ||
| No (reference) | 1 | ||
| Number of vasopressors | 1.78–12.12 | 0.002 | |
| ≥2 vasopressors | 4.64 | ||
| ≤1 vasopressor (reference) | 1 | ||
| OIpre (per every 1 unit increase) | 1.04 | 1.01–1.06 | 0.003 |
NCV, nonconventional ventilation; APRV, airway pressure release ventilation; HFOV, high frequency oscillatory ventilation; OIpre, oxygenation index before switch to nonconventional ventilation.
Cox proportional hazard modeling was used to determine the relationship between mode of NCV and mortality after adjustment for immunocompromised status, vasopressor score, and OIpre. This model produces a Harrell’s C concordance statistic of 0.780.
Fig. 1.
Survival curves for patients stratified by (A) mode of NCV, (B) number of vasopressor infusions, (C) top or bottom half of OIpre, and (D) presence or absence of an immunocompromised condition. Unadjested log-rank P values are shown.
Fig. 2.
A: Oxygenation index at 24 hr (OI24) of nonconventional ventilation (NCV) as a fraction of OI before transition to NCV (OIpre). Bars indicate median, boxes interquartile range, error bars 10th and 90th percentiles, and dots 5th and 95th percentiles. OI24/OIpre was higher for non-survivors (Wilcoxon rank sum P = 0.028). B: Change in fractional OI over hours of NCV between survivors and non-survivors. The OI before transition to nonconventional ventilation (OIpre) is the OI at hour 0 and is set to 1. OINCV was calculated every 12 hr for the first 7 days after transition, and is reported as a fraction of OIpre. Values of OINCV/OIpre are expressed as mean (±SEM). Sample sizes for each grouping, representing patients with arterial access still alive on NCV, are given at hour 0, 24, 48, 72, 96, 120, 144, and 168. Patients were dropped from data collection if they lost arterial access, changed modes of ventilation, transitioned to extracorporeal support, or died. Multilevel mixed effects linear regression was used to test the relationship between survival and the change in OI over time, with a significant effect (P = 0.005) of survival status over time on OINCV/OIpre.
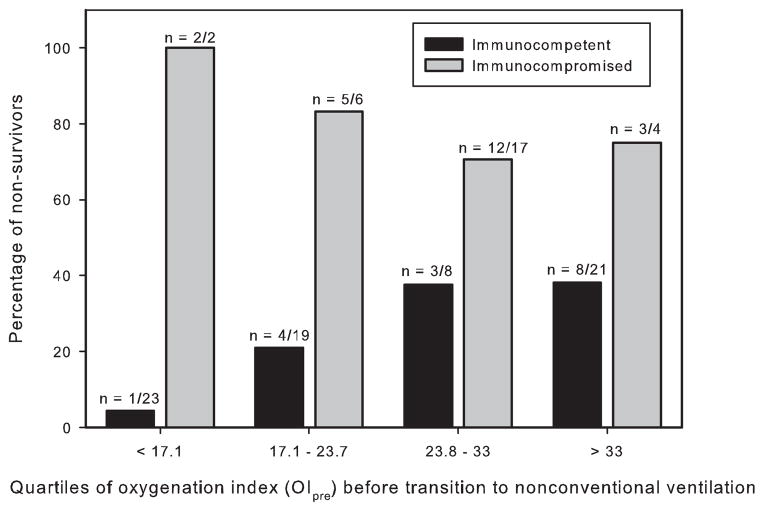
Given the substantial contribution of immunocompromised status to mortality (24 of 31 immunocompromised patients died), we examined the relationship between OIpre and mortality stratified by immunocompromised status (Fig. 3). Mortality was determined in the four quartiles of OIpre in both immunocompetent and immunocompromised patients. Among immunocompetent patients, mortality increased with increasing OIpre quartile. In every quartile, mortality in immunocompromised patients exceeded that of immunocompetent (Mantel–Haenszel odds ratio 9.3, 95% confidence interval 2.7 to 32.3, P < 0.0001).
Fig. 3.
Mortality stratified by quartile of oxygenation index before transition to nonconventional ventilation (OIpre) for immunocompetent (black bars) and immunocompromised (gray bars) patients. Sample size for each grouping is given above the respective bar. Relationship between mortality and immuno-compromised status was tested across all four quartiles using the Mantel–Haenszel test, which demonstrated a significant odds ratio for non-survival in immunocompromised patients referenced to immunocompetent (Mantel–Haenszel P < 0.0001).
DISCUSSION
We describe the characteristics of a modestly large cohort of pediatric ALI refractory to conventional ventilation transitioned to APRV and HFOV. Improved oxygenation after 24 hr on either mode of NCV (OI24/OIpre) was associated with survival. Mode of NCV was not associated with mortality after adjusting for immunocompromised status, number of vasopressor infusions, and severity of lung disease (measured by OIpre).
Younger and sicker patients were preferentially transitioned to HFOV, which may reflect the greater experience with HFOV, especially in neonatal lung disease. Recently, 20 children (mean age ± SD of 14 ± 14.6 months) undergoing congenital heart disease surgery were exposed to APRV and conventional mechanical ventilation in a crossover physiologic study, and demonstrated improved pulmonary blood flow with spontaneous breathing on APRV.15 This suggests safe and efficacious application of APRV in a young population, albeit one without significant lung disease. Future investigations may not reveal as significant of a bias against APRV in younger patients.
Mortality was 39.4% in our study, consistent with reported mortality in NCV used for respiratory failure refractory to conventional ventilation.16,17 Survival was associated with improved oxygenation after 24 hr on NCV (OI24/OIpre), consistent with other reports of NCV.3–5,7,17 Improved oxygenation may reflect alveolar recruitment by NCV, better matching of ventilation and perfusion, or in the case of HFOV, non-bulk flow mechanisms of gas exchange. Interestingly, the PF ratio at 24 hr of NCV also improved in non-survivors, but not as much as it did in survivors, where it was nearly double. As the PF ratio does not directly account for mPaw, OI at 24 hr may be a more informative predictor for outcome than PF ratio. The lack of improvement in oxygenation on NCV in non-survivors suggests that an unimproved OI after 24 hr of NCV may serve as an early indicator prompting reassessment of the ventilator strategy. Potential alternatives for these patients may be transitioning to another mode of ventilation or escalation to extracorporeal support earlier. It is also possible that non-survivors had more significant lung disease, as evidenced by their higher OIpre prior to transitioning to NCV. This higher OIpre suggests that increased mortality was due to the underlying severity of disease and etiology of ALI, and may be independent of mode of ventilation.
Recent trials in adult patients with ARDS suggested increased mortality9 or no effect10 of HFOV compared to conventional. These data, where by design HFOV was applied relatively early in the course of ARDS, call into question the use of early oscillatory ventilation. The applicability to pediatrics is limited, as the protocols utilized do not reflect the way HFOV is typically applied in children at our institution. In our study, children were transitioned to both HFOV and APRV with higher OI and lower PF ratios than in either adult HFOV trial, and both modes of NCV reflected “rescue” from hypoxemia refractory to conventional ventilation. Our comparable results despite worse oxygenation defects may reflect differences in the pathophysiology of ARDS between adult and pediatric patients, differences in co-morbidities, different patient selection, or more experience with HFOV in pediatric ICUs.
Underlying severity of lung disease, as reflected by the PF ratio and OIpre before transition to NCV, were good predictors of mortality in both univariate and multivariate analysis. In adults, re-analysis of the pre-enrollment ventilator parameters of 2451 patients enrolled between 1996 and 2005 in 6 ARDS Network trials demonstrated a linear relationship between pre-enrollment plateau pressures (adjusted by tidal volume) and hospital mortality.18 The authors concluded that pre-enrollment plateau pressure was a marker of lung disease severity which affected mortality independent of the effects of tidal volume. OIpre in our study may be a similar patient-specific value that reflects lung disease severity and has strong prognostic significance. Prior studies of pediatric ALI have also demonstrated that the severity of hypoxemia as measured by initial PF ratio independently correlates with mortality,2,19 whereas the initial oxygenation defect has not consistently been found to be an independent predictor of mortality in adult ARDS.20
Immunocompromised patients represent a severely ill population, with higher mortality and worse lung injury.3,21,22 Twenty-four of 31 immunocompromised patients (77%) died in our study, consistent with other reports on mechanically ventilated immunocompromised children.3 Mortality was worse in every quartile of OIpre for immunocompromised patients relative to immunocompetent. Presence of an immunocompromising condition has been shown to affect mortality in pediatric ALI.3,21,22 In a prospective study of exogenous surfactant in pediatric ALI, mortality in the immunocompromised group was four times that of the immunocompetent (56% vs. 13%), and adjustment for immunocompromised status attenuated the observed mortality benefit of surfactant treatment.21,22 Arnold et al., examined the records of 232 children receiving HFOV at 10 academic PICUs and demonstrated significant effects of immunocompromised status on predicted mortality, with immunocompromised patients having a higher risk of mortality at lower OI.3
The hemodynamic consequences of HFOV have been well documented, with investigators reporting increased central venous and pulmonary capillary wedge pressures,23,24 worsening of right ventricular dysfunction,25 and an increased need for fluid resuscitation or inotropic support.9,16 APRV, in contrast, is associated with fewer adverse hemodynamic effects.11,13,15 Recently, APRV was shown to improve pulmonary blood flow in a subset of pediatric patients requiring mechanical ventilation after repair or palliation of congenital cardiac defects.15 Our patients demonstrated similar reductions of OI over time on both APRV and HFOV, suggesting that a prospective trial investigating the benefits of one mode of NCV over the other for refractory hypoxemia may be warranted.
Our study has several limitations. The retrospective nature precludes us from making firm conclusions about the benefits or risks of either APRV or HFOV. Clinician preference, for instance, likely influenced the assignment of patients with more significant lung disease to HFOV, which introduces the possibility of unmeasured confounders when directly comparing these modes. We attempted to account for this bias with multivariate analysis. Cumulative fluid balance, one of the factors demonstrated to be associated with mortality in other studies on pediatric26 and adult27 ALI, was not studied or reported because of the inability to accurately collect this information retrospectively. The single center design limits its generalizability, although the patient population represented here is heterogeneous. These limitations are best addressed by a prospective comparison between APRV and HFOV, with clearly defined indications for transitioning onto and off of either mode of NCV.
One of the strengths of our study is the larger sample size for infrequently utilized modes of ventilation. This represents the largest reported experience with pediatric APRV. We also used OI over 7 days to assess oxygenation outcomes thus accounting for the impact of mPaw. Several prior adult studies of HFOV report efficacy as an improved PF ratio; however, this ignores contribution of increased mPaw to oxygenation. Our patients’ gas exchange measurements were followed for up to 7 days, longer than prior studies, which generally assess gas exchange within the first 24–48 hr. Finally, these data suggest that an improved OI at 24 hr may be a useful predictor of survival on NCV.
In conclusion, an improved OI 24 hr after transition to NCV was associated with survival. NCV mode was not associated with mortality when adjusted for immunocompromised status, number of vasopressors, and severity of existing lung disease.
Acknowledgments
Funding source: Russell C. Raphaely Endowed Chair in Critical Care Medicine, Department of Anesthesiology and Critical Care, Children’s Hospital of Philadelphia
Footnotes
Conflict of interest: None.
References
- 1.The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 2.Lopez-Fernandez Y, Azagra AM, de la Oliva P, Modesto V, Sanchez JI, Parrilla J, Arroyo MJ, Reyes SB, Pons-Odena M, Lopez-Herce J, Fernandez RL, Kacmarek RM, Villar J Pediatric Acute Lung Injury Epidemiology and Natural History (PED-ALIEN) Network. Pediatric Acute Lung Injury Epidemiology and Natural History Study: Incidence and outcome of the acute respiratory distress syndrome in children. Crit Care Med. 2012;40:3238–3245. doi: 10.1097/CCM.0b013e318260caa3. [DOI] [PubMed] [Google Scholar]
- 3.Arnold JH, Anas NG, Luckett P, Cheifetz IM, Reyes G, Newth CJ, Kocis KC, Heidemann SM, Hanson JH, Brogan TV, Bohn DJ. High-frequency oscillatory ventilation in pediatric respiratory failure: a multicenter experience. Crit Care Med. 2000;28:3913–3919. doi: 10.1097/00003246-200012000-00031. [DOI] [PubMed] [Google Scholar]
- 4.Arnold JH, Hanson JH, Toro-Figuero LO, Gutierrez J, Berens RJ, Anglin DL. Prospective, randomized comparison of high-frequency oscillatory ventilation and conventional mechanical ventilation in pediatric respiratory failure. Crit Care Med. 1994;22:1530–1539. [PubMed] [Google Scholar]
- 5.Ben Jaballah N, Khaldi A, Mnif K, Bouziri A, Belhadj S, Hamdi A, Kchaou W. High-frequency oscillatory ventilation in pediatric patients with acute respiratory failure. Pediatr Crit Care Med. 2006;7:362–367. doi: 10.1097/01.PCC.0000227108.38119.2E. [DOI] [PubMed] [Google Scholar]
- 6.Bojan M, Gioanni S, Mauriat P, Pouard P. High-frequency oscillatory ventilation and short-term outcome in neonates and infants undergoing cardiac surgery: a propensity score analysis. Crit Care. 2011;15:R259. doi: 10.1186/cc10521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sarnaik AP, Meert KL, Pappas MD, Simpson PM, Lieh-Lai MW, Heidemann SM. Predicting outcome in children with severe acute respiratory failure treated with high-frequency ventilation. Crit Care Med. 1996;24:1396–1402. doi: 10.1097/00003246-199608000-00020. [DOI] [PubMed] [Google Scholar]
- 8.Sud S, Sud M, Friedrich JO, Meade MO, Ferguson ND, Wunsch H, Adhikari NK. High frequency oscillation in patients with acute lung injury and acute respiratory distress syndrome (ARDS): systematic review and meta-analysis. BMJ. 2010;340:c2327. doi: 10.1136/bmj.c2327. [DOI] [PubMed] [Google Scholar]
- 9.Ferguson ND, Cook DJ, Guyatt GH, Mehta S, Hand L, Austin P, Zhou Q, Matte A, Walter SD, Lamontagne F, Granton JT, Arabi YM, Arroliga AC, Stewart TE, Slutsky AS, Meade MO, The OTI The Canadian Critical Care Trials G. High-frequency oscillation in early acute respiratory distress syndrome. N Engl J Med. 2013;368:795–805. doi: 10.1056/NEJMoa1215554. [DOI] [PubMed] [Google Scholar]
- 10.Young D, Lamb S, Shah S, Mackenzie I, Tunnicliffe W, Lall R, Rowan K, Cuthbertson BH, The OSG. High-frequency oscillation for acute respiratory distress syndrome. N Engl J Med. 2013;368:806–813. doi: 10.1056/NEJMoa1215716. [DOI] [PubMed] [Google Scholar]
- 11.Habashi NM. Other approaches to open-lung ventilation: airway pressure release ventilation. Crit Care Med. 2005;33:S228–S240. doi: 10.1097/01.ccm.0000155920.11893.37. [DOI] [PubMed] [Google Scholar]
- 12.Varpula T, Valta P, Niemi R, Takkunen O, Hynynen M, Pettila VV. Airway pressure release ventilation as a primary ventilatory mode in acute respiratory distress syndrome. Acta Anaesthesiol Scand. 2004;48:722–731. doi: 10.1111/j.0001-5172.2004.00411.x. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan LJ, Bailey H, Formosa V. Airway pressure release ventilation increases cardiac performance in patients with acute lung injury/adult respiratory distress syndrome. Crit Care. 2001;5:221–226. doi: 10.1186/cc1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schultz TR, Costarino AJA, Durning SM, Napoli LA, Schears G, Godinez RI, Priestley M, Dominguez T, Lin R, Helfaer M. Airway pressure release ventilation in pediatrics. Pediatr Crit Care Med. 2001;2:243–246. doi: 10.1097/00130478-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Walsh MA, Merat M, La Rotta G, Joshi P, Joshi V, Tran T, Jarvis S, Caldarone CA, Van Arsdell GS, Redington AN, Kavanagh BP. Airway pressure release ventilation improves pulmonary blood flow in infants after cardiac surgery. Crit Care Med. 2011;39:2599–2604. doi: 10.1097/CCM.0b013e318228297a. [DOI] [PubMed] [Google Scholar]
- 16.Adhikari NK, Bashir A, Lamontagne F, Mehta S, Ferguson ND, Zhou Q, Hand L, Czarnecka K, Cook DJ, Granton JT, Friedrich JO, Freitag A, Watpool I, Meade MO. High-frequency oscillation in adults: a utilization review. Crit Care Med. 2011;39:2631–2644. doi: 10.1097/CCM.0b013e318226675e. [DOI] [PubMed] [Google Scholar]
- 17.Babbitt CJ, Cooper MC, Nussbaum E, Liao E, Levine GK, Randhawa IS. High-frequency oscillatory ventilation in pediatric acute hypoxemic respiratory failure: disease-specific morbidity survival analysis. Lung. 2012;190:685–690. doi: 10.1007/s00408-012-9417-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Checkley W, Brower R, Korpak A, Thompson BT. Effects of a clinical trial on mechanical ventilation practices in patients with acute lung injury. Am J Respir Crit Care Med. 2008;177:1215–1222. doi: 10.1164/rccm.200709-1424OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flori HR, Glidden DV, Rutherford GW, Matthay MA. Pediatric acute lung injury: prospective evaluation of risk factors associated with mortality. Am J Respir Crit Care Med. 2005;171:995–1001. doi: 10.1164/rccm.200404-544OC. [DOI] [PubMed] [Google Scholar]
- 20.Nuckton TJ, Alonso JA, Kallet RH, Daniel BM, Pittet JF, Eisner MD, Matthay MA. Pulmonary dead-space fraction as a risk factor for death in the acute respiratory distress syndrome. N Engl J Med. 2002;346:1281–1286. doi: 10.1056/NEJMoa012835. [DOI] [PubMed] [Google Scholar]
- 21.Tamburro RF, Thomas NJ, Pon S, Jacobs BR, Dicarlo JV, Markovitz BP, Jefferson LS, Willson DF. Post hoc analysis of calfactant use in immunocompromised children with acute lung injury: Impact and feasibility of further clinical trials. Pediatr Crit Care Med. 2008;9:459–464. doi: 10.1097/PCC.0b013e3181849bec. [DOI] [PubMed] [Google Scholar]
- 22.Willson DF, Thomas NJ, Markovitz BP, Bauman LA, DiCarlo JV, Pon S, Jacobs BR, Jefferson LS, Conaway MR, Egan EA. Effect of exogenous surfactant (calfactant) in pediatric acute lung injury: a randomized controlled trial. JAMA. 2005;293:470–476. doi: 10.1001/jama.293.4.470. [DOI] [PubMed] [Google Scholar]
- 23.Derdak S, Mehta S, Stewart TE, Smith T, Rogers M, Buchman TG, Carlin B, Lowson S, Granton J. High-frequency oscillatory ventilation for acute respiratory distress syndrome in adults: a randomized, controlled trial. Am J Respir Crit Care Med. 2002;166:801–808. doi: 10.1164/rccm.2108052. [DOI] [PubMed] [Google Scholar]
- 24.Mehta S, Granton J, MacDonald RJ, Bowman D, Matte-Martyn A, Bachman T, Smith T, Stewart TE. High-frequency oscillatory ventilation in adults: the Toronto experience. Chest. 2004;126:518–527. doi: 10.1378/chest.126.2.518. [DOI] [PubMed] [Google Scholar]
- 25.Guervilly C, Forel JM, Hraiech S, Demory D, Allardet-Servent J, Adda M, Barreau-Baumstark K, Castanier M, Papazian L, Roch A. Right ventricular function during high-frequency oscillatory ventilation in adults with acute respiratory distress syndrome. Crit Care Med. 2012;40:1539–1545. doi: 10.1097/CCM.0b013e3182451b4a. [DOI] [PubMed] [Google Scholar]
- 26.Valentine SL, Sapru A, Higgerson RA, Spinella PC, Flori HR, Graham DA, Brett M, Convery M, Christie LM, Karamessinis L, Randolph AG Pediatric Acute Lung Injury and Sepsis Investigator’s (PALISI) Network, Acute Respiratory Distress Syndrome Clinical Research Network (ARDSNet) . Fluid balance in critically ill children with acute lung injury. Crit Care Med. 2012;40:2883–2889. doi: 10.1097/CCM.0b013e31825bc54d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murphy CV, Schramm GE, Doherty JA, Reichley RM, Gajic O, Afessa B, Micek ST, Kollef MH. The importance of fluid management in acute lung injury secondary to septic shock. Chest. 2009;136:102–109. doi: 10.1378/chest.08-2706. [DOI] [PubMed] [Google Scholar]