Abstract
Objectives
The primary objective of this study was to determine whether a revision and/or expansion of current audiologic cochlear implant candidacy criteria is warranted.
Design
The study design was a retrospective review of postoperative speech perception performance for 22 adult cochlear implant recipients who demonstrated preoperative Consonant Nucleus Consonant word recognition scores of 30% or higher in the best-aided condition. This criterion was chosen to exceed that specified by the North American clinical trial of the Nucleus Freedom cochlear implant system.
Results
The mean preoperative best-aided monosyllabic word score for the 22 patients was 41% correct. The degree of postoperative benefit for the best postoperative condition (electric only or bimodal) ranged from 10 to 68 percentage points with a mean benefit of 27 percentage points for the electric-only condition and 40 percentage points for the bimodal condition. Statistical analyses revealed highly significant differences between preoperative-aided, implant-only, and bimodal performance on Consonant Nucleus Consonant monosyllabic word recognition performance. That is, both postoperative scores— electric only and bimodal—were significantly different from one another and from the preoperative best-aided performance.
Conclusions
The current results suggest that a large-scale reassessment of manufacturer and Medicare preoperative audiologic candidacy criteria for adults is warranted to allow more hearing-impaired individuals to take advantage of the benefits offered by cochlear implantation.
INTRODUCTION
In 1996, representatives from the American Academy of Otolaryngology—Head and Neck Surgery and the American Academy of Audiology and an experienced group of clinicians/scientists from the cochlear implant manufacturers recommended a minimum speech test battery (MSTB) for determining audiologic cochlear implant candidacy and for longitudinal assessment of postoperative performance (Nilsson et al. 1996). The committee recommended that patient performance should be assessed with both Consonant Nucleus Consonant (CNC) monosyllabic words (Peterson & Lehiste 1962) presented in quiet and the Hearing in Noise Test (HINT) sentences (Nilsson et al. 1994) presented in quiet and in a fixed-level noise background. Although they did not provide a recommended criterion performance for determining implant candidacy, they recommended that “at each preoperative and postoperative evaluation, one 50-word CNC list and two 10-sentence HINT lists should be presented in quiet at 65 dB (A).” The committee also recommended that the HINT sentences be presented in fixed-level noise at +10, +5, or even 0 dB SNR as necessary to avoid ceiling effects (Nilsson et al. 1996). In the most recent version of the MSTB, Luxford et al. (2001) recommended the administration of the HINT in its originally intended format, i.e., administered adaptively in a fixed-level background noise to determine the SNR required for 50% correct performance, so that as technology and performance improved, patient performance could continue to be assessed without ceiling effects. The adaptive HINT, however, was not recommended for determining preoperative candidacy.
Currently, audiologic candidacy for cochlear implantation in the United States is solely based on sentence recognition performance typically using the HINT sentences presented in quiet. Each manufacturer outlines a slightly different set of candidacy indications based on the Food and Drug Administration (FDA)-approved Physician’s Package Insert labeling for the device. To meet labeled criteria for the Nucleus Freedom or Nucleus 5 device (CI24RE and CI512, Cochlear Americas), patients can score up to 50% correct in the ear to be implanted and up to 60% in the best-aided listening condition on recorded tests of open-set sentence recognition, i.e., for HINT sentences or equivalent measures presented in the sound field at 70 dB SPL. To qualify for the Advanced Bionics HR90k device, patients can score up to 50% correct on HINT sentences with “appropriately fit hearing aids.” Advanced Bionics does not specify a recommended presentation level nor a specific testing condition (i.e., monaural versus binaural). To qualify for the MED EL Pulsar and Sonata devices, patients can score up to 40% correct on HINT sentences in the best-aided condition. MED EL also does not specify a recommended presentation level. Current Medicare criteria for cochlear implantation include performance up to 40% correct in the best-aided listening condition (no level recommended) for tape-recorded tests of open-set sentence recognition—the exact tests are not specified (Centers for Medicare and Medicaid Services 2005).
The FDA-approved labeling from the three implant manufacturers has led the majority of clinicians to use the HINT sentences in quiet for determining implant candidacy. The HINT sentences, however, were designed to be administered adaptively in a fixed-level background to determine the SNR required for 50% correct performance (Nilsson et al. 1994). Nilsson et al. (1994) did recommend that the HINT sentences be administered in quiet before administering the adaptive procedure; however, the purpose of this recommendation was to ensure that the listener was able to achieve at least 50% correct in quiet. In 1996, when the MSTB committee recommended the use of the HINT sentences in quiet, it was considered a progressive move away from the much easier Central Institute for the Deaf sentences (Davis & Silverman 1978), which had previously been used for audiologic implant candidacy.
Gifford et al. (2008) examined the appropriateness of different speech recognition materials for preimplant and postimplant assessment of performance. They assessed 156 adult, postlingually deafened, implant recipients and 50 hearing aid users on measures of monosyllabic word recognition (CNC) and sentence recognition in quiet (HINT, Nilsson et al. 1994; and AzBio sentences, Spahr & Dorman 2005) and in noise (Bamford Kowal Bench-Speech in Noise Test, Etymotic Research). For the HINT sentences, 28% of the cochlear implant recipients achieved a perfect score of 100% correct, and 71% of the population achieved scores >85% correct. Moreover, a score of 100% correct for HINT sentences in quiet was associated with a range of performance on CNC words from 20 to 94% correct, which represents virtually the entire range of possible scores. Perhaps more interesting was that 16% of the hearing aid users seen for a cochlear implant evaluation achieved 100% performance on HINT sentences, and 32% of the same hearing aid users achieved scores >85% correct. Gifford et al. concluded that the HINT sentences in quiet suffer from ceiling effects and, as a consequence, are not appropriate for tracking performance over time, nor may they be appropriate for determining implant candidacy. Similar findings have been reported elsewhere with smaller sample sizes (Firszt et al. 2004; Koch et al. 2004; Litovsky et al. 2006).
The other speech perception measures used by Gifford et al. (2008) did not suffer from ceiling effects. None of the 206 subjects achieved 100% performance on CNC words, one subject scored 100% correct on the AzBio sentences, and the pseudoadaptive quality of the (Bamford Kowal Bench-Speech in Noise Test essentially ensures the absence of ceiling effects for cochlear implant users.
Cochlear Implants for the Appropriate Hearing-Impaired Population—Market Penetration
An estimated 28.0 to 31.5 million people in the United States— or roughly 10% of the population—report significant hearing difficulty (Kochkin 2005; NIDCD 2005a). The NIDCD further estimates that up to 750,000 Americans have severe to profound hearing loss (NIDCD 2005a). Wilson and Dorman (2008) reported that there were currently 110,000 cochlear implant recipients worldwide as of 2006, of which approximately 37% are located in the United States (NIDCD 2005b). Thus, with roughly 40,700 cochlear implant recipients in the United States, the market penetration of the 750,000 severe to profound, hearing-impaired population is just 5.4%. Thus, it is reasonable to assume that there are a considerable number of cochlear implant “candidates” not being identified either because of the lack of hearing aid consumer or because of dispenser knowledge, inappropriate preimplant assessment materials, some combination of these as well as other possible factors.
The Search for a Better Test
Candidacy criteria for cochlear implantation in the United States are similar to UK guidelines. The proposed position statement on guidelines for adult cochlear implantation put forth by the British Cochlear Implant Group requires that patients achieve ≤50% correct for recorded BKB sentences presented at 70 dB A in the best-aided condition (British Cochlear Implant Group 2008). A sentence-based criterion for cochlear implantation, however, is not necessarily in place across the world. The use of monosyllables for qualification purposes is already in place in Germany (Aschendorff et al. 2007) where candidacy criteria require that patients achieve <30% correct for Freiberg monosyllables at 70 dB SPL in the best-aided condition. Candidacy criteria used in Spain require that the patients score <50% on disyllabic words with appropriately fitted hearing aids using a presentation level of 70 dB SPL; however, although the ear to be implanted must meet the criteria, the other ear may achieve a disyllabic score of >50% (Manrique, 2008, personal communication). Furthermore, one of the most recently reported North American clinical trials for a new implant system (Nucleus Freedom) required patients to score ≤30% correct on CNC monosyllabic words to meet enrollment candidacy (Balkany et al. 2007). At issue here is whether this criterion qualified a different population of patients than the standard Nucleus criterion, which has been <50% in the ear to be implanted and ≤60% correct in the best-aided condition for HINT sentence recognition in quiet. This does not seem to be the case. Gifford et al. (2008) examined the relationship between CNC word recognition and HINT sentence recognition for 135 cases. Using the ≤30% correct on CNC monosyllabic words for candidacy, 13 of the hearing aid users in that study would have met the criteria for the Freedom clinical trial. Evaluating that same dataset but with the ≤60% correct on HINT sentences in the best-aided condition, the same 13 preimplant patients were identified. Thus, a benchmark of 30% correct on CNC monosyllabic words likely does not identify an entirely new population of implant candidates.
Further evidence that the ≤30% CNC criterion and the ≤60% HINT criterion were identifying similar population of patients stems from the comparison of patients in Nucleus Freedom clinical trial with patients enrolled in previous studies. For the Nucleus Freedom clinical trial, Balkany et al. (2007) reported that the mean preoperative, best-aided CNC performance for the 71 subjects was just 3% correct with a range of 0 to 19% correct. The mean postimplant performance—for 55 of the subjects with at least 6-mo experience—was 57% in the implant-only condition with a range of 3 to 92% correct. Parkinson et al. (2002) reported the results of the multicenter clinical trial for 56 subjects implanted with the N24 series device. The mean preoperative CNC monosyllabic word recognition performance for the ear to be implanted was 4% correct with a range of 0 to 30% correct. The mean postimplant performance for CNC words* in the implant-only condition was 43% at the 6-mo interval with a range of 2 to 80% correct. Zwolan et al. (2001) reported preimplant and postimplant performance data for 56 patients implanted with the Clarion HiFocus electrode with electrode positioner. The mean preoperative CNC word score was just 3% correct (range of 0 to 22%) for the 56 patients enrolled, although it was not clear whether this was for the implanted ear or the binaural-aided condition. They reported that the mean postoperative CNC performance for the implant-only condition was 47% with a range of 0 to 88%. Baumgartner et al. (2007) evaluated performance of 23 German-speaking patients implanted with the MED EL FLEX soft electrode array. They reported a mean preoperative monosyllabic word score of just 2% correct and a mean postoperative word score of 54% correct. They did not report a range of scores nor did they specify whether the preimplant scores were for the implanted ear or binaural-aided condition.
Reviewing the results of the aforementioned studies, the mean preoperative word scores for the four studies differed by just 2 percentage points, and the mean postoperative word scores ranged from just 43 to 57%, a range that would not be considered statistically different using a binomial statistic such as that suggested by Thornton and Raffin (1978). Balkany et al. (2007), however, did demonstrate a statistically significant difference between the N24 contour (Parkinson et al. 2002) and the Nucleus Freedom data. However, a relatively homogenous group of individuals—with respect to preoperative and postoperative word scores— has been implanted from 2001 to 2007 across the United States and Europe.
The Present Study
To determine whether new recommendations for candidacy are warranted, this study evaluated speech perception performance for 22 cochlear implant recipients who demonstrated preoperative CNC word recognition scores ≥30% correct. This criterion was chosen to exceed that outlined by the Nucleus Freedom clinical trial, which required preoperative CNC word recognition <30% correct. The hypothesis was that these nontraditional implant recipients, with higher levels of preoperative speech understanding than traditional patients, would demonstrate significant benefit from cochlear implantation.
SUBJECTS AND METHODS
Subjects
The data reported in this study constitute a retrospective review of data collected across three sites, including Mayo Clinic in Rochester, Mayo Clinic in Scottsdale, and the Cochlear Implant Laboratory at Arizona State University. Speech recognition performance was assessed for 22 subjects (15 men and 7 women). The mean preoperative CNC score for the subjects in the best-aided condition was 41% correct with a range of 30 to 68%. The mean age of the subjects was 64.2 yrs with a range of 32 to 84 yrs. The mean duration of cochlear implant use at the time of evaluation was 17.9 mos with a range of 3 to 66 mos. Table 1 provides details regarding the types of cochlear implant devices, months of experience with electric stimulation, duration of deafness (defined as duration, in years, of severe to profound hearing loss before implantation), etiology, subject age, preoperative sentence recognition score in the best-aided condition, and level used for testing. All subjects were recipients of an implant system that encompasses one of the two most recent generations of devices at the time of manuscript preparation. Fifteen patients were recipients of a Cochlear Americas device (N24 or Freedom 24RE series), five patients were recipients of an Advanced Bionics device (HR90k), and two patients were recipients of a MED EL device (Combi 40+, subject 19 and Sonata, and subject 22).
TABLE 1.
Subject age at testing, device experience, duration of deafness (DOD, in years before implantation), etiology, and speech processor used for testing, the preoperative sentence recognition score in the best-aided condition and associated test measure, and level used for testing both preoperatively and postoperatively
| Subject | Age | Experience (mos) | DOD | Etiology | Speech processor | Preimplant sentence score and test used | Level for testing (DB SPL) |
|---|---|---|---|---|---|---|---|
| S1 | 55 | 5 | 8 | Meniere’s | Freedom | AzBio, 39 | 70 |
| S2 | 77 | 6 | 20 | Noise | Freedom | AzBio, 60 | 70 |
| S3 | 84 | 7 | 12 | Unknown | Auria | AzBio, 38 | 70 |
| S4 | 80 | 5 | 15 | Unknown | Freedom | AzBio, 37 | 70 |
| S5 | 47 | 39 | — | Unknown | 3G | HINT, 50 | 70 |
| S6 | 70 | 28 | 25 | Unknown | 3G | AzBio, 21 | 70 |
| S7 | 69 | 31 | 15 | Familial | Freedom | HINT, 58 | 60 |
| S8 | 50 | 18 | 20 | Autoimmune | Freedom | AzBio, 67 | 60 |
| S9 | 74 | 5 | 5 | Noise | Freedom | AzBio, 39 | 60 |
| S10 | 58 | 66 | 5 | Familial | Auria | AzBio, 53 | 60 |
| S11 | 64 | 9 | 20 | Unknown | Harmony | AzBio, 55 | 60 |
| S12 | 68 | 15 | 15 | Unknown | Harmony | AzBio, 73 | 70 |
| S13 | 83 | 10 | 35 | Unknown | SPrint | AzBio, 21 | 70 |
| S14 | 59 | 18 | — | Usher’s | Freedom | HINT, 55 | 70 |
| S15 | 63 | 22 | 10 | Usher’s | Freedom | AzBio, 35 | 60 |
| S16 | 65 | 8 | 15 | unknown | Harmony | AzBio, 39 | 60 |
| S17 | 53 | 12 | 9 | Meniere’s | Freedom | AzBio, 59 | 60 |
| S18 | 32 | 36 | 10 | Unknown | 3G | HINT, 80 | 60 |
| S19 | 56 | 18 | 11 | Stroke | Tempo + | HINT, 65 | 70 |
| S20 | 62 | 20 | 10 | Noise | Freedom | HINT, 53 | 60 |
| S21 | 84 | 3 | 5 | Unknown | Freedom | AzBio, 28 | 60 |
| S22 | 60 | 12 | 26 | Otosclerosis | Opus 2 | AzBio, 92 | 60 |
| Mean (2 × SE) | 64.2 (5.5) | 17.9 (6.2) | 14.8 (3.3) | N/A | N/A | HINT: 60.2 (9.0) AzBio: 47.3 (9.8) | N/A |
There were five subjects who were found to be “off-label”† cochlear implant recipients as judged by exceeding 60% correct sentence recognition in the best-aided condition. The off-label recipient labels are displayed in bold. Summary statistics including mean and 2 SE measures are provided where appropriate. A horizontal dashed line indicates that this information could not be obtained.
N/A, not applicable; HINT, Hearing in Noise Test.
There were five subjects who, in retrospect, did not meet the manufacturers’ labeled criteria for cochlear implantation (subjects 8, 12, 18, 19, and 22) with respect to sentence recognition performance in the best-aided condition. Three of these subjects, 8, 12, and 19, were seen postoperatively in the Cochlear Implant Laboratory at Arizona State University, which does not have an operating cochlear implant program. One subject, 18, was followed up postoperatively at Mayo Clinic in Rochester but had been implanted at another facility. Subject 22 had lost nearly all hearing in one ear 26 yrs earlier because of a failed stapedectomy procedure; although she achieved high levels of sentence recognition performance in the test booth, her occupational demands were high such that hearing out of just one ear—and having a severe to profound hearing loss in that hearing ear—was causing elevated levels of stress and fatigue. Thus, although all subjects achieved a preoperative, binaural-aided score of 30% correct or higher on CNC words, the majority of subjects (17 of 22) still also met the labeled criterion of <60% correct sentence recognition in quiet for non-Medicare patients and <40% correct on sentences for Medicare patients. The sentences used for determining preoperative candidacy, however, were most generally the AzBio sentences (Spahr and Dorman 2005; Gifford et al. 2008) (see Table 1).
Methods
All subjects received a comprehensive audiologic evaluation preoperatively. This evaluation included air and bone conduction audiometry, immittance measurements, and word recognition testing. Standard pure-tone audiometric procedures using a calibrated audiometer (ANSI S3.6 2004) were followed to determine threshold. All subjects demonstrated hearing loss of a sensorineural nature with no evidence of abnormal tympanometric findings. Preoperative audiometric thresholds were obtained approximately 1 mo before surgery and are shown in Table 2 for the implanted and nonimplanted ears.
TABLE 2.
Preoperative audiometric thresholds for the implanted ear in dB HL
| Frequency (Hz) | 125 | 250 | 500 | 750 | 1000 | 1500 | 2000 | 3000 | 4000 | 6000 | 8000 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | — | 80 (60) | 80 (60) | — | 85 (70) | — | 70 (80) | 75 (80) | 90 (90) | NR (NR) | NR (NR) |
| S2 | 25 (25) | 30 (25) | 55 (50) | 80 (70) | 105 (85) | NR (110) | NR (105) | NR (NR) | NR (110) | NR (110) | NR (NR) |
| S3 | 25 (25) | 30 (35) | 40 (40) | 55 (55) | 90 (80) | 115 (110) | NR (110) | NR (110) | NR (110) | NR (NR) | NR (NR) |
| S4 | 25 (30) | 30 (35) | 45 (45) | 65 (55) | 75 (65) | 85 (80) | 100 (80) | NR (90) | NR (100) | NR (NR) | NR (NR) |
| S5 | — | 20 (25) | 60 (50) | 85 (65) | 100 (65) | 90 (75) | 105 (85) | 110 (95) | 105 (105) | NR (90) | |
| S6 | — | 45 (50) | 55 (70) | 80 (100) | 90 (100) | 95 (110) | 110 (110) | 100 (110) | 110 (110) | 105 (110) | 95 (NR) |
| S7 | — | 40 (45) | 70 (65) | 70 (75) | 70 (75) | 65 (70) | 70 (70) | 75 (70) | 75 (80) | 100 (105) | 100 (95) |
| S8 | 65 (NR) | 85 (NR) | 90 (NR) | 85 (NR) | 90 (NR) | 85 (NR) | 85 (NR) | 85 (NR) | 80 (NR) | 80 (NR) | 70 (NR) |
| S9 | 60 (60) | 65 (60) | 60 (65) | 70 (70) | 85 (75) | 90 (85) | 95 (95) | 120 (100) | 120 (110) | NR (NR) | NR (NR) |
| S10 | — | 40 (55) | 50 (60) | 65 (75) | 85 (85) | NR (85) | NR (90) | NR (75) | NR (80) | NR (65) | NR (75) |
| S11 | 40 (40) | 35 (45) | 50 (60) | — | 60 (70) | — | 70 (90) | NR (NR) | NR (NR) | NR (NR) | NR (NR) |
| S12 | 55 (45) | 65 (45) | 50 (60) | 60 (70) | 80 (70) | 85 (75) | 100 (90) | 120 (110) | 110 (100) | 110 (110) | NR (NR) |
| S13 | — | 100 (105) | 100 (110) | — | 100 (115) | 90 (105) | 85 (105) | 90 (10) | 90 (NR) | 75 (NR) | NR (NR) |
| S14 | — | 55 (50) | 75 (70) | — | 90 (90) | — | 95 (95) | 90 (90) | 90 (90) | NR (NR) | NR (NR) |
| S15 | 35 (35) | 45 (45) | 70 (70) | 75 (85) | 85 (85) | 90 (90) | 90 (95) | NR (NR) | NR (NR) | NR (NR) | NR (NR) |
| S16 | 50 (50) | 55 (55) | 60 (60) | 65 (65) | 70 (70) | 95 (90) | 110 (110) | 120 (115) | 115 (115) | NR (110) | NR (NR) |
| S17 | 75 (NR) | 80 (NR) | 80 (NR) | 80 (NR) | 90 (NR) | 95 (NR) | 100 (NR) | 90 (NR) | 90 (NR) | 85 (NR) | 75 (NR) |
| S18 | — (60) | 85 (60) | NR (75) | NR (80) | NR (90) | NR (100) | NR (95) | NR (85) | NR (95) | NR (85) | NR (90) |
| S19 | — | 80 (60) | 95 (60) | — | 95 (60) | — | 95 (50) | 105 (70) | 110 (70) | 110 (70) | 100 (80) |
| S20 | 20 (25) | 40 (35) | 70 (55) | 95 (80) | 90 (90) | 90 (80) | 85 (75) | 80 (75) | 80 (75) | 90 (80) | 90 (80) |
| S21 | NR (85) | 105 (85) | 110 (75) | 100 (65) | 95 (55) | 75 (75) | 80 (75) | 80 (80) | 90 (85) | 95 (85) | NR (95) |
| S22 | — | 100 (65) | 115 (65) | NR (75) | NR (70) | NR (70) | NR (65) | NR (80) | NR (75) | NR (80) | NR (95) |
Thresholds for the nonimplanted ear are enclosed in parentheses. A dashed line indicates that a threshold was not obtained for that frequency. NR, no response at the limits of the audiometer.
Monosyllabic word recognition performance was assessed using one 50-item list of the CNC test (Peterson & Lehiste 1962). All subjects were assessed in the best-aided condition preoperatively and postoperatively in the electric-only condition. Preoperative-aided CNC word scores for the ear to be implanted were obtained for just 9 of the 22 subjects. For those subjects with residual hearing in the nonimplanted ear, that ear was occluded with a foam earplug for assessment of the electric-only condition. The bimodal condition— or electric plus contralateral acoustic hearing—was tested for the 18 subjects wearing a hearing aid in the nonimplanted ear. For the 18 bimodal subjects, the hearing aid settings for the nonimplanted ear were verified using the speechmap fitting system on the Audioscan Verifit real ear mode to match NAL-NL1 targets for 55-, 60-, and 70-dB SPL speech. Hearing aids that did not initially meet prescribed targets were reprogrammed before testing.
As is standard practice at all three centers, the CNC lists were assigned pseudorandomly for each subject and condition. All testing was completed with recorded stimuli presented via a single loudspeaker at a calibrated level of 60 dB A (Mayo Clinic in Rochester) or 70 dB A (Mayo Clinic in Scottsdale and Arizona State University). The loudspeaker was placed at 0° azimuth at a distance of 1 m from the subject. Given the data reported by Firszt et al. (2004), it is not expected that the 60-and 70-dB SPL levels would yield significantly different results for monosyllabic word recognition for postimplant listeners. A recent study by Alkaf and Firszt (2007), however, reported a significant difference in performance at 60 and 70 dB SPL for CNC word and HINT sentence recognition in hearing aid users who may be considered “borderline” implant candidates. It is not believed that those data are relevant to the current dataset given that all subjects were tested at the same level for both preimplant and postimplant assessments, which ensured within-subject consistency. Thus, there was a within-subject consistency with respect to the stimulus presentation level. However, because the CNC scores were collected at two levels (60 and 70 dB SPL) and because previous reports have found an effect of level (Firzst et al. 2004), we first assessed whether differences in presentation level influenced performance. Analyses of variance indicated no effect of level on raw CNC scores for preimplant, cochlear implant-only, or bimodal performance (F(1,19) = 0.80, p = 0.38) and no effect of level on degree of benefit for either cochlear implant-only (E – preimplant score) or bimodal benefit [(E + A) − E] (F(1,19) = 1.9, p = 0.19, missing data points for patients without bimodal scores were overlooked and not estimated by the statistical program). For that reason, the results from the two presentation levels were combined in all analyses that follow. Table 1 displays the presentation level that was used for preimplant and postimplant testing for each subject.
RESULTS AND DISCUSSION
Speech Perception Performance
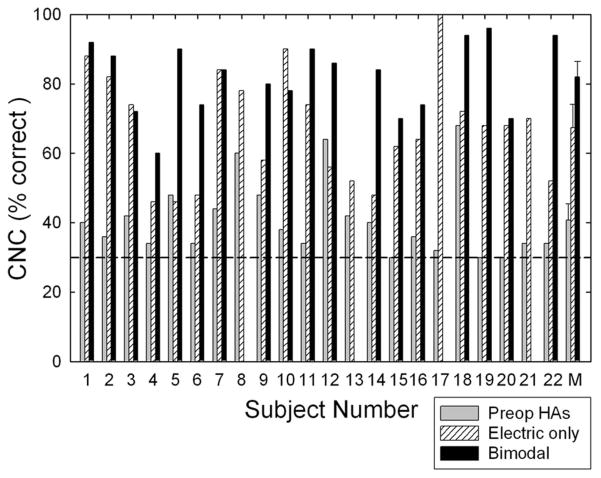
Figure 1 displays the individual and mean CNC word scores for conditions including preoperative binaural hearing aids, cochlear implant only, and bimodal stimulation, i.e., implant plus hearing aid on contralateral ear. Statistical analyses were conducted using a one-way, repeated-measures analysis of variance (ANOVA) with listening condition (preoperative binaural hearing aids, electric only, and bimodal) as the independent variable (missing data points for those patients without bimodal scores were overlooked and not estimated by the statistical program). The analysis revealed a significant effect of condition (F(2,21) = 64.2, p < 0.001). Post hoc tests (Tukey) indicated that scores in the electric-only condition (67% correct) were significantly higher than those in the preoperative binaural-aided condition (41% correct, p < 0.001) and that scores in the bimodal condition (82% correct) were significantly higher than those in the preoperative binaural-aided condition (41% correct, p < 0.001) and in the electric-only condition (67% correct, p < 0.001).
Fig. 1.
Individual and mean performance for Consonant Nucleus Consonant monosyllabic words. The shaded bars represent the best-aided, preoperative score, the hatched bars represent the electric-only score, and the filled bars represent the bimodal score. The horizontal dashed line marks 30% correct performance, which was the binaural-aided preoperative criterion for inclusion in the study. Error bars represent ±2 SE measurements.
The mean preoperative CNC score in the best-aided condition was 41% correct with a range of 30 to 68%. This score was 37 to 39 percentage points higher than the average preimplant scores reported in recent studies (Zwolan et al. 2001; Parkinson et al. 2002; Balkany et al. 2007; Baumgartner et al. 2007). Thus, it is clear that the subjects in this study were functioning at considerably higher preoperative levels than those in the previous studies.
The mean postoperative CNC score for the electric-only condition was 67% correct with a range of 46 to 100%. The mean postoperative CNC scores in the study by Zwolan et al. (2001), Parkinson et al. (2002), Balkany et al. (2007), and Baumgartner et al. (2007) ranged from 43 to 57% correct, with an across-study average of 50% correct. Thus, the mean performance in this study was 11 to 25 percentage points higher than that reported in previous studies.
The mean postoperative CNC score for the bimodal condition was 82% correct with a range of 60 to 96%. A one-way ANOVA on ranks was completed‡ comparing bimodal performance for the subjects in this study with the performance of 36 bimodal subjects in the study by Gifford et al. (2008). The scores for the 18 bimodal subjects in this study were not found to be significantly higher— 82% correct versus 72% correct (H(1) = 3.73, p = 0.053). This analysis suggests that although individuals with higher levels of preoperative speech perception performance are also able to achieve high levels of postoperative performance, they may not necessarily be expected to outperform conventional cochlear implant recipients with poorer preoperative speech perception.
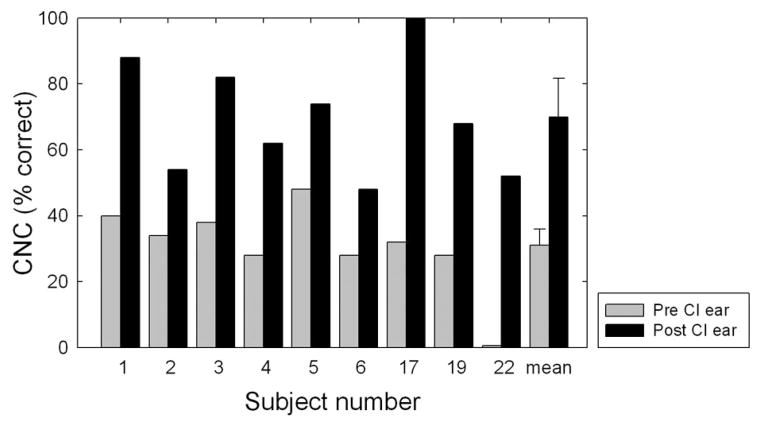
As mentioned in the Methods section, preoperative-aided CNC word recognition was obtained for the ear to be implanted in 9 of the 22 subjects. Individual and mean data for the implanted ear for these 9 subjects are displayed in Figure 2. The shaded bars represent the preoperative monaural-aided score, and the filled bars represent the postoperative, implant-only score. The mean aided preoperative CNC score for the ear to be implanted was 31% correct with a range of 0 to 40% correct. The mean postoperative, implant-only score was 70% correct with a range of 48 to 100% correct. This represented a mean implant-ear benefit of 39 percentage points with a range of 20 to 68 percentage points. A one-way, repeated-measures ANOVA revealed a highly significant effect of cochlear implantation for implanted ear performance in this group of seven subjects (F(1,8) = 54.2, p < 0.001).
Fig. 2.
Individual and mean performance for Consonant Nucleus Consonant monosyllabic words for the nine subjects for whom preimplant and postimplant scores were obtained for the implanted ear. The shaded and filled bars represent the preimplant and postimplant scores, respectively. Error bars represent ±2 SE measurements.
The data were also examined with respect to an individual risk/benefit ratio. Individual subject data analysis was computed using the binomial distribution statistic described by Thornton and Raffin (1978) for 50-item word lists. Using this model, 13 subjects (or 59% of the population) achieved a significantly higher score for the implant-only condition relative to the preoperative binaural-aided score (including subjects 1, 2, 3, 7, 10, 11, 15, 16, 17, 19, 20, 21, and 22). For the nine subjects who did not achieve a significantly higher word score in the implant-only condition, none showed a significant decrement, and 7 of the 9 achieved significantly higher performance in the bimodal condition relative to the preoperative-aided score (including subjects 4, 5, 6, 9, 12, 14, and 18). Thus, there were only 2 subjects who ultimately did not achieve significantly higher word recognition performance postoperatively. These two subjects (8 and 13), however, did not demonstrate a performance decrement in the preimplant to postimplant comparison and in fact demonstrated an improvement of 18 and 10 percentage points, respectively. In addition, these two subjects (8 and 13) did not have hearing in the nonimplanted ear to allow for postoperative bimodal stimulation. Subject 8 ultimately received a second cochlear implant and is now a bilateral user. Although subject 13 had expressed a desire for bilateral implantation, his insurance policy would not authorize it. Overall, 91% of the subject population (20 of 22) demonstrated a statistically significant benefit in a preimplant to postimplant comparison, and none of the subjects experienced a decrement in performance.
Correlations Between Audiometric Threshold, Preoperative Speech Perception, and Postoperative Performance
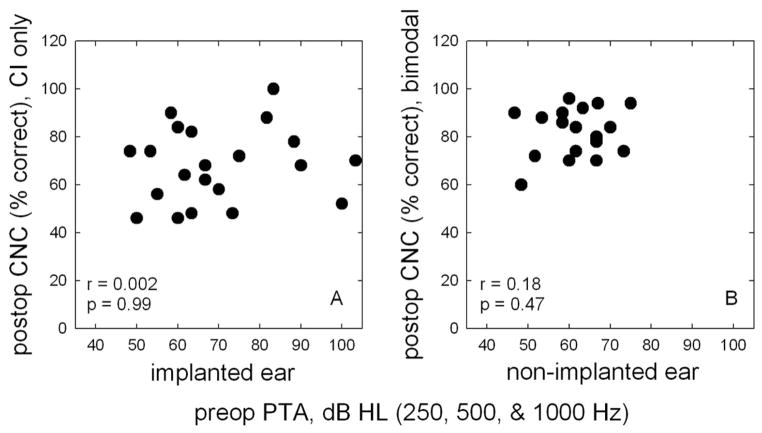
To date, no published study has demonstrated a correlation between preoperative audiometric threshold and postoperative speech perception performance.§ This is true for both the implanted ear (Gantz et al. 1988; Blamey et al. 1992; Gantz et al. 1993; Battmer et al. 1995; Waltzman et al. 1995) and the nonimplanted ear (Ching et al. 2004; Gifford et al. 2007). Figure 3 provides a visual display for the correlation between audiometric threshold and postoperative performance. Panel A in Figure 3 displays CNC word scores for the electric-only condition as a function of the preoperative low-frequency pure-tone average, in dB HL, for 250, 500, and 1000 Hz in the implanted ear. A Pearson product moment analysis revealed no correlation between preoperative implant ear thresholds and postoperative, electric-only word recognition (r = 0.002, p = 0.99).
Fig. 3.
Panel A displays postoperative Consonant Nucleus Consonant word scores (in percent correct) for the electric (E)-only condition as a function of the preoperative, implant ear pure-tone average, in dB HL, for frequencies 250, 500, and 1000 Hz. Panel B displays postoperative Consonant Nucleus Consonant word scores for the bimodal condition as function of the preoperative, nonimplanted ear pure-tone average at 250, 500, and 1000 Hz.
Panel B in Figure 3 displays CNC word scores for the bimodal condition as a function of the low-frequency pure-tone average for 250, 500, and 1000 Hz in the nonimplanted ear. Correlation analysis again revealed no significant correlation between audiometric thresholds for the nonimplanted ear and bimodal word recognition (r = 0.18, p = 0.47).
This finding is contrary to both clinical intuition and the longstanding belief that a higher proportion of surviving spiral ganglion cells most likely yields higher levels of postoperative performance. In fact, there are relatively recent histopathology data from human temporal bones suggesting that there is no correlation between spiral ganglion cell survival and postoperative speech perception performance (Nadol et al. 2001; Gassner et al. 2005; Fayad and Linthicum, 2006). To the extent that audiometric threshold is believed to reflect the integrity of internal auditory structures, it would seem that clinicians must consider variables “beyond the audiogram” because similar ranges of audiometric threshold do not necessarily yield similar levels of postoperative performance.
In addition to the evaluation of audiometric threshold and postoperative performance, similar analyses were conducted to examine the relationship between preoperative and postoperative CNC scores. There was no significant correlation between preoperative word scores and postoperative electric-only word scores (r = −0.04, p = 0.88) nor between preoperative and postoperative bimodal word scores (r = 0.34, p = 0.17). This finding is contrary to previous studies reporting significant correlation between preoperative and postoperative sentence recognition performance (Rubinstein et al. 1999; Gomaa et al. 2003). The reason for this was likely due to relative homogeneity of preoperative performance for the subjects in this study.
The relationship between the duration of deafness— defined as the length of time, in years, that the patient had severe to profound hearing loss before implantation—and postoperative performance was also assessed. There was no significant correlation found between duration of deafness and either electric-only performance (r = −0.42, p = 0.06) or bimodal performance (r = 0.14, p = 0.60), although there was certainly a trend for poorer electric-only performance with longer duration of deafness. There are previous studies with similar findings (Lee et al. 2007; Ruffin et al. 2007) and others that have clearly demonstrated a significant negative correlation between duration of deafness and postoperative performance (Rubinstein et al. 1999; Gomaa et al. 2003; Dowell et al. 2008). The duration of deafness for the subjects in this study encompassed a relatively narrow range such that over two-third of the subjects were in the 5- to 10-yr range. Thus, it is possible that had a broader range of durations been included in this study, a negative correlation would have been observed.
GENERAL DISCUSSION
The results described above are relevant to a consideration of whether FDA and Medicare preoperative candidacy criteria should be expanded to allow more hearing-impaired individuals to take advantage of the benefits offered by cochlear implantation. The mean improvement provided by the cochlear implant alone over the best preoperative-aided score was 27 percentage points. The mean improvement provided by the combination of the implant plus the contralateral hearing aid over the best preoperative score was 41 percentage points. Thus, this group of 22 subjects who would have been excluded from candidacy in the North American clinical trial of the Nucleus Freedom device—and who would likely be excluded from candidacy at a majority of cochlear implant centers—demonstrated significant benefit from cochlear implantation.
The results from this study suggest that the current candidacy criteria are set too low. A low speech recognition criterion ensures not only a high hit rate but also a high miss rate. From an insurance perspective, a conservative criterion is cost effective. However, from a quality-of-life perspective, the outlook is quite different. This is particularly true given the individual risk/benefit assessment determined by the preimplant to postimplant comparison of word recognition performance, which demonstrated that 86% of subject population derived significant individual benefit from cochlear implantation. For elderly individuals with significant hearing loss who do not meet Medicare criteria, a high miss rate may translate to an increased incidence of depression and social withdrawal (The National Council on the Aging 1999; Abrams et al. 2006). This is especially troubling given that adults seeking a cochlear implant evaluation have already been shown to experience significantly higher levels of distress and social discomfort relative to normative population (Knutson et al. 2006). Furthermore, there have been numerous studies documenting the efficacy of cochlear implantation in elderly patients such that (i) benefits associated with speech perception and quality of life are similar to those observed for younger implant recipients (Kelsall et al. 1995; Herzog et al. 2000; Pasanisi et al. 2003; Vermeire et al. 2005; Chatelin et al. 2004); (ii) postoperative benefit outweighs any risks associated with the surgery (Buchmann et al. 1999); and (iii) postoperative improvements in speech perception were correlated with health-related quality of life and emotional benefits (Francis et al. 2002).
The results of this study—as well as many others (Ching et al. 2004; Dunn et al. 2005; Kong et al. 2005; Mok et al. 2006; Gifford et al. 2007)—provide further evidence on the importance of an appropriately fitted hearing aid for use in combination with electric stimulation. There was a significant improvement in bimodal over electric-only performance. This is an important counseling point for clinicians to encourage continued hearing aid use and to ensure that the hearing aid settings are verified with real ear measurements.
SUMMARY AND CONCLUSION
Just over 1 decade ago, Rubinstein et al. (1999) suggested that preoperative candidacy could easily be expanded to include those patients achieving up to 60% correct on Central Institute for the Deaf sentences. The results of this study demonstrate how much the study of cochlear implants has evolved over a relatively short period. The current results conclude that (i) HINT sentences presented in quiet, because of ceiling effects, are not well suited for assessing preoperative candidacy and are not well suited for longitudinal assessment of patient performance; (ii) a criterion of <30% CNC score may not identify a different patient population than that currently identified by a <60% HINT score in the best-aided condition; (iii) patient performance should be assessed with respect to individual ear performance and binaural performance both preimplant and postimplant; and, most importantly, (iv) patients with preimplant CNC scores ranging from 30 to 68% in the best-aided condition—scores that are much higher than scores for the conventional cochlear implant recipient—significantly benefit from a cochlear implant and even more from bimodal listening both at the group level and individually.
On the basis of the current results, we argue that implant criteria should be expanded to include patients achieving preoperative monosyllabic word scores up to at least 40% correct, i.e., approximately the mean preoperative score in this study. We suggest that a large-scale reassessment of cochlear implant candidacy in the United States is warranted.
Acknowledgments
The authors thank Anne Beiter for her thoughtful comments and suggestions on earlier versions of this manuscript. This study was conducted in strict accordance with approved Mayo Clinic and Arizona State University IRB protocols. They also thank the three anonymous reviewers, and Cochlear Implant Section Editor Dr. Jill Firszt for their helpful comments and suggestions.
Footnotes
The CNC performance reported was for the users of the Advanced Combination Encoder strategy.
The term “off-label” refers to any patient implanted who exceeded the FDA labeled criteria indicated in the package insert for a given cochlear implant device. The most liberal labeled criterion is from Cochlear Americas, which states that recipients should not exceed 60% correct on open-set sentence recognition materials such as the HINT or equivalent. There were five subjects who in retrospect were found to be “off-label” recipients. These were subjects 8, 12, 18, 19, and 22. Subject 8 had an autoimmune hearing loss that was also rapidly progressing, and her professional demands were not met with hearing aids. Subject 12 paid for his implant system out of pocket. Subject 18 had a unilateral hearing loss, and her studies were greatly affected by her hearing loss. For her, a petition was made to her insurance company on this basis. Subject 19 was suffering from the side effects of a recent stroke, which resulted in fluctuating hearing loss, speech perception, and debilitating headaches. Although his HINT sentence score exceeded the 60% criterion, his poor word recognition was taken into account for implantation. Subject 22 had a severe to profound hearing loss in one ear and virtually no hearing in the other ear. She experienced high occupational demands for listening to clients and was beginning to experience increasingly higher levels of stress, depression, and fatigue. Thus, although her AzBio sentence recognition score exceeded the 60% criterion, her poor word recognition was considered for implantation.
A nonparametric statistical model was used given that the assumption of equal variance was not met.
A preliminary report out of Australia, however, has suggested that preoperative auditory “skills” (including preoperative speech perception and audiometric thresholds) among other measures were found to be correlated with postoperative performance in 400 patients implanted between 1994 and 2006 (Dowell et al. 2008).
A portion of these data were presented at the 2008 American Academy of Audiology meeting in Charlotte, NC.
References
- Abrams TE, Barnett MJ, Hoth A, et al. The relationship between hearing impairment and depression in older veterans. J Am Geriatr Soc. 2006;54:1475–1477. doi: 10.1111/j.1532-5415.2006.00875.x. [DOI] [PubMed] [Google Scholar]
- Alkaf FM, Firszt JB. Speech recognition in quiet and noise in borderline cochlear implant candidates. J Am Acad Audiol. 2007;18:872–882. doi: 10.3766/jaaa.18.10.6. [DOI] [PubMed] [Google Scholar]
- ANSI S3.6. Specification for Audiometers. New York: American National Standards Institute; 2004. [Google Scholar]
- Aschendorff A, Kromeier J, Klenzer T, et al. Quality control after insertion of the nucleus contour and contour advance electrode in adults. Ear Hear. 2007;28:75S–79S. doi: 10.1097/AUD.0b013e318031542e. [DOI] [PubMed] [Google Scholar]
- Balkany T, Hodges A, Menapace C, et al. Nucleus Freedom North American clinical trial. Otolaryngol Head Neck Surg. 2007;136:757–762. doi: 10.1016/j.otohns.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Battmer RD, Lenarz T, Allum-Mecklenburg DJ, et al. Postoperative results for adults and children implanted with the Clarion device. Ann Otol Rhinol Laryngol Suppl. 1995;166:254–255. [PubMed] [Google Scholar]
- Baumgartner WD, Jappel A, Morera C, et al. Outcomes in adults implanted with the FLEXsoft electrode. Acta Otolaryngol. 2007;127:579–586. doi: 10.1080/00016480600987784. [DOI] [PubMed] [Google Scholar]
- Blamey PJ, Pyman BC, Gordon M, et al. Factors predicting postoperative sentence scores in postlinguistically deaf adult cochlear implant patients. Ann Otol Rhinol Laryngol. 1992;101:342–348. doi: 10.1177/000348949210100410. [DOI] [PubMed] [Google Scholar]
- British Cochlear Implant Group (BCIG) Proposed Position Statement on Guidelines for Adult Cochlear Implantation. 2008 Revision date: May 2008. [Google Scholar]
- Buchman CA, Fucci MJ, Luxford WM. Cochlear implants in the geriatric population: Benefits outweigh risks. Ear Noise Throat J. 1999;78:489–494. [PubMed] [Google Scholar]
- Centers for Medicare and Medicaid Services (CMS) CMS Manual System, Pub 100 – 03, Medicare National Coverage Determination, Subject: Cochlear Implantation Transmittal 42. Baltimore, MD: Department of Health & Human Services, Center for Medicare and Medicaid Services; 2005. [Google Scholar]
- Chatelin V, Kim EJ, Driscoll C, et al. Cochlear implant outcomes in the elderly. Otol Neurotol. 2004;25:298–301. doi: 10.1097/00129492-200405000-00017. [DOI] [PubMed] [Google Scholar]
- Ching TY, Incerti P, Hill M. Binaural benefits for adults who use hearing aids and cochlear implants in opposite ears. Ear Hear. 2004;25:9–21. doi: 10.1097/01.AUD.0000111261.84611.C8. [DOI] [PubMed] [Google Scholar]
- Davis H, Silverman SR. Hearing and Deafness. 4. New York: Holt, Rinehard and Winston; 1978. [Google Scholar]
- Dowell RC, Fellows J, Hollow R. Prediction of clinical outcomes in adults using cochlear implants. 10th International Conference on Cochlear Implants and other implantable auditory technologies; April 10–12, 2008; San Diego, CA.. 2008. [Google Scholar]
- Dunn CC, Tyler RS, Witt SA. Benefit of wearing a hearing aid on the unimplanted ear in adult users of a cochlear implant. J Speech Lang Hear Res. 2005;48:668–680. doi: 10.1044/1092-4388(2005/046). [DOI] [PubMed] [Google Scholar]
- Fayad JN, Linthicum FH., Jr Multichannel cochlear implants: relation of histopathology to performance. Laryngoscope. 2006;116:1310–1320. doi: 10.1097/01.mlg.0000227176.09500.28. [DOI] [PubMed] [Google Scholar]
- Firszt JB, Holden LK, Skinner MW, et al. Recognition of speech presented at soft to loud levels by adult cochlear implant recipients of three cochlear implant systems. Ear Hear. 2004;25:375–387. doi: 10.1097/01.aud.0000134552.22205.ee. [DOI] [PubMed] [Google Scholar]
- Francis HW, Chee N, Yeagle J, et al. Impact of cochlear implants on the functional health status of older adults. Laryngoscope. 2002;112:1482–1488. doi: 10.1097/00005537-200208000-00028. [DOI] [PubMed] [Google Scholar]
- Gantz BJ, Tyler RS, Knutson JF, et al. Evaluation of five different cochlear implant designs: audiologic assessment and predictors of performance. Laryngoscope. 1988;98:1100–1106. doi: 10.1288/00005537-198810000-00013. [DOI] [PubMed] [Google Scholar]
- Gantz BJ, Woodworth GG, Knutson JF, et al. Multivariate predictors of audiological success with multichannel cochlear implants. Ann Otol Rhinol Laryngol. 1993;102:909–916. doi: 10.1177/000348949310201201. [DOI] [PubMed] [Google Scholar]
- Gassner HG, Shallop JK, Driscoll CL. Long-term clinical course and temporal bone histology after cochlear implantation. Cochlear Implants Int. 2005;6:67–76. doi: 10.1179/cim.2005.6.2.67. [DOI] [PubMed] [Google Scholar]
- Gifford RH, Dorman MF, McKarns SA, et al. Combined electric and contralateral acoustic hearing: Word and sentence recognition with bimodal hearing. J Speech Lang Hear Res. 2007;50:835–843. doi: 10.1044/1092-4388(2007/058). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford RH, Shallop JK, Peterson A. Speech recognition materials and ceiling effects: Considerations for cochlear implant programs. Audiol Neurootol. 2008;13:193–205. doi: 10.1159/000113510. [DOI] [PubMed] [Google Scholar]
- Gomaa NA, Rubinstein JT, Lowder MW, et al. Residual speech perception and cochlear implant performance in postlingually deafened adults. Ear Hear. 2003;24:539–544. doi: 10.1097/01.AUD.0000100208.26628.2D. [DOI] [PubMed] [Google Scholar]
- Herzog M, Muller J, Milewski C, et al. Cochlear implantation in the elderly. Adv Otorhinolaryngol. 2000;57:393–396. doi: 10.1159/000059189. [DOI] [PubMed] [Google Scholar]
- Kelsall DC, Shallop JK, Burnelli T. Cochlear implantation in the elderly. Am J Otolaryngol. 1995;16:609–615. [PubMed] [Google Scholar]
- Knutson JF, Johnson A, Murray KT. Social and emotional characteristics of adults seeking a cochlear implant and their spouses. Br J Health Psychol. 2006;11:279–292. doi: 10.1348/135910705X52273. [DOI] [PubMed] [Google Scholar]
- Koch DB, Osberger MJ, Segel P, et al. HiResolution and conventional sound processing in the HiResolution bionic ear: Using appropriate outcome measures to assess speech recognition ability. Audiol Neurootol. 2004;9:214–223. doi: 10.1159/000078391. [DOI] [PubMed] [Google Scholar]
- Kochkin SG. MarkeTrak VII: Hearing loss population tops 31 million. Hear Rev. 2005;12:16–29. [Google Scholar]
- Kong YY, Stickney GS, Zeng FG. Speech and melody recognition in binaurally combined acoustic and electric hearing. J Acoust Soc Am. 2005;117:1351–1361. doi: 10.1121/1.1857526. [DOI] [PubMed] [Google Scholar]
- Lee JC, Yoo MH, Ahn JH, et al. Value of the promontory stimulation test in predicting speech perception after cochlear implantation. Laryngoscope. 2007;117:1988–1992. doi: 10.1097/MLG.0b013e31813437e6. [DOI] [PubMed] [Google Scholar]
- Litovsky R, Parkinson A, Arcaroli J, et al. Simultaneous bilateral cochlear implantation in adults: A multicenter clinical study. Ear Hear. 2006;27:714–731. doi: 10.1097/01.aud.0000246816.50820.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luxford WM, Allum D, Balkany T, et al. Minimum speech test battery for postlingually deafened adult cochlear implant patients. Otolaryngol Head Neck Surg. 2001;124:125–126. doi: 10.1067/mhn.2001.113035. [DOI] [PubMed] [Google Scholar]
- Mok M, Grayden D, Dowell RC, et al. Speech perception for adults who use hearing aids in conjunction with cochlear implants in opposite ears. J Speech Lang Hear Res. 2006;49:338–351. doi: 10.1044/1092-4388(2006/027). [DOI] [PubMed] [Google Scholar]
- Nadol JB, Jr, Shiao JY, Burgess BJ, et al. Histopathology of cochlear implants in humans. Ann Otol Rhinol Laryngol. 2001;110:883–891. doi: 10.1177/000348940111000914. [DOI] [PubMed] [Google Scholar]
- NIDCD. Healthy Hearing 2010: Where Are We Now. DA-TA2010, the Healthy People 2010 Database 2005a [Google Scholar]
- NIDCD. Cochlear Implants. 2005b NIH Publication No. 00 – 4798. [Google Scholar]
- Nilsson MJ, McCaw VM, Soli SD. Minimum Speech Test Battery for Adult Cochlear Implant Users: User Manual. Los Angeles, CA: House Ear Institute; 1996. [Google Scholar]
- Nilsson MJ, Soli SD, Sullivan J. Development of the hearing in noise test for the measurement of speech reception thresholds in quiet and in noise. J Acoust Soc Am. 1994;95:1085–1099. doi: 10.1121/1.408469. [DOI] [PubMed] [Google Scholar]
- Parkinson AJ, Arcaroli J, Staller SJ, et al. The Nucleus 24 contour cochlear implant system: Adult clinical trial results. Ear Hear. 2002;23:41S– 48S. doi: 10.1097/00003446-200202001-00005. [DOI] [PubMed] [Google Scholar]
- Pasanisi E, Bacciu A, Vincenti V, et al. Speech recognition in elderly cochlear implant recipients. Clin Otolaryngol. 2003;28:154–157. doi: 10.1046/j.1365-2273.2003.00681.x. [DOI] [PubMed] [Google Scholar]
- Peterson GE, Lehiste I. Revised CNC lists for auditory tests. J Speech Hear Disord. 1962;27:62–70. doi: 10.1044/jshd.2701.62. [DOI] [PubMed] [Google Scholar]
- Rubinstein JT, Parkinson WS, Tyler RS, et al. Residual speech recognition and cochlear implant performance: Effects of implantation criteria. Am J Otolaryngol. 1999;20:440–452. [PubMed] [Google Scholar]
- Ruffin CV, Tyler RS, Witt SA, et al. Long-term performance of Clarion 1.0 cochlear implant users. Laryngoscope. 2007;117:1183–1189. doi: 10.1097/MLG.0b013e318058191a. [DOI] [PubMed] [Google Scholar]
- Spahr AJ, Dorman MF. Effects of minimum stimulation setting for the Med El Tempo+ speech processor on speech understanding. Ear Hear. 2005;26:2S– 6S. doi: 10.1097/00003446-200508001-00002. [DOI] [PubMed] [Google Scholar]
- The National Council on the Aging. The Consequences of Untreated Hearing Loss in Older Persons. Seniors Research Group—an alliance between The National Council on the Aging and Market Strategies Inc; 1999. Retrieved April 15 2008, from http://www.ncoa.org/ [Google Scholar]
- Thornton AR, Raffin MJ. Speech-discrimination scores modeled as a binomial variable. J Speech Hear Res. 1978;21:507–518. doi: 10.1044/jshr.2103.507. [DOI] [PubMed] [Google Scholar]
- Vermeire K, Brokx JP, Wuyts FL, et al. Quality-of-life benefit from cochlear implantation in the elderly. Otol Neurotol. 2005;26:188–195. doi: 10.1097/00129492-200503000-00010. [DOI] [PubMed] [Google Scholar]
- Waltzman SB, Fisher SG, Niparko JK, et al. Predictors of postoperative performance with cochlear implants. Ann Otol Rhinol Laryngol Suppl. 1995;165:15–18. [PubMed] [Google Scholar]
- Wilson BS, Dorman MF. Interfacing sensors with the nervous system: Lessons from the development and success of the cochlear implant. IEEE Sensors J. 2008;8:131–147. [Google Scholar]
- Zwolan T, Kileny PR, Smith S, et al. Adult cochlear implant patient performance with evolving electrode technology. Otol Neurotol. 2001;22:844–849. doi: 10.1097/00129492-200111000-00022. [DOI] [PubMed] [Google Scholar]