Abstract
Specification of the T helper 17 (Th17) cell lineage requires a well defined set of transcription factors, but how these integrate with post-transcriptional and epigenetic programs to regulate gene expression is poorly understood. Here we found defective Th17 cell cytokine expression in miR-155-deficient CD4+ T cells in vitro and in vivo. Mir155 was bound by Th17 cell transcription factors and was highly expressed during Th17 cell differentiation. miR-155-deficient-Th17 and -T regulatory (Treg) cells expressed increased amounts of Jarid2, a DNA-binding protein that recruits the Polycomb Repressive Complex 2 (PRC2) to chromatin. PRC2 binding to chromatin and H3K27 histone methylation was increased in miR-155-deficient cells, coinciding with failure to express Il22, Il10, Il9 and Atf3. Defects in Th17 cell cytokine expression and Treg cell homeostasis in the absence of Mir155 could be partially suppressed by Jarid2 deletion. Thus, miR-155 contributes to Th17 cell function by suppressing the inhibitory effects of Jarid2.
Keywords: regulome, microRNA, gene regulation, T helper cell differentiation, RNA-seq, transcriptome, Polycomb Repressive Complex 2, epigenetics, ChIP-seq
Introduction
The mammalian adaptive immune response is critical for host defense against pathogens, with CD4+ T cells playing important roles in an array of helper, effector and regulatory functions. In response to specific stimuli, CD4+ T cells have the potential to differentiate into several different cell fates that include the T helper 1 (Th1), Th2, Th17 and T regulatory (Treg) cell lineages. Defective differentiation of CD4+ T cells result in human immunodeficiency such as in autosomal dominant hyper-IgE or Job syndrome, where Th17 specification is impaired due to STAT3 loss of function (Milner et al., 2008) and mutations in FOXP3 result in immunodysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome, due to a defect in Treg cells (Bennett et al., 2001; Brunkow et al., 2001; Wildin et al., 2001). Therefore, understanding the molecular basis of CD4+ Th cell differentiation will provide key information into subset specification and facilitate the design of more effective strategies for targeting autoimmunity and augmenting immunizations.
The specification of distinct CD4+ Th cell lineages is orchestrated by a regulatory network that integrates signals from the extracellular cytokine milieu, T cell receptor engagement, and co-stimulatory ligands to achieve a series of programmed gene expression changes (Zhu et al., 2010). Transcriptional and epigenetic programs initiated within CD4+T cells promote changes in gene expression, ultimately resulting in production of their signature cytokines (Ciofani et al., 2012; Kanno et al., 2011; Yosef et al., 2013). In this process, microRNAs (miRNAs) provide a layer of post-transcriptional gene expression control that needs to be considered (Cobb et al., 2006; Muljo et al., 2005).
Recently, a core transcription factor network necessary for Th17 cell differentiation was elucidated (Ciofani et al., 2012). However, the roles of miRNAs and chromatin regulators have not been integrated with this transcriptional network. In Th17 cell responses, miR-155 has been shown to promote the production of interleukin-17 (IL-17) and interferon-γ (IFN-γ) in Helicobacter pylori infection (Oertli et al., 2011) as well as mouse models of inflammatory diseases (Bluml et al., 2011; Escobar et al., 2013; Murugaiyan et al., 2011; O’Connell et al., 2010). However, the mechanisms by which miR-155 acts in Th17 cells are not clear.
Here, we performed unbiased transcriptomic analyses comparing wildtype (WT) and miR-155-deficient Th17 cells and found Jumonji, AT Rich Interactive Domain 2 (Jarid2) to be upregulated in the absence of miR-155. Jarid2 was recently discovered to be essential for recruiting PRC2 to genomic sites in embryonic stem (ES) cells (Landeira et al., 2010; Li et al., 2010; Pasini et al., 2010; Peng et al., 2009; Shen et al., 2009). However, the function of Jarid2 in adult somatic cells such as lymphocytes is not known. Analysis of Jarid2-deficient CD4+ T cells combined with chromatin immunoprecipitation (ChIP) analyses allowed us to identify direct targets of PRC2 in Th17 cells. Furthermore, deletion of Jarid2 in the miR-155-deficient CD4+T cells results in partial rescue of Th17 cell-associated cytokine expression as well as homeostasis of Treg cells. Thus, we demonstrate that miR-155 and Jarid2 form a regulatory circuit that can control lineage specific gene expression in CD4+ T cells through its effect on Polycomb recruitment.
Results
miR-155-deficient CD4+ T cells have cell intrinsic defects
To determine whether miR-155 plays a cell autonomous role in CD4+ T cells in vivo, we generated mixed bone marrow (BM) chimeras and examined miR-155-deficient and WT CD4+ T cells within the same host (Figures S1A–B). As previously reported (Kohlhaas et al., 2009; Lu et al., 2009), we found that miR-155-deficient Treg cells have a competitive disadvantage compared with their WT counterparts (Figures S1C). In contrast, we did not observe differential fitness of congenic WT CD45.1+ and WT CD45.2+ cells in control mixed BM chimeras (data not shown).
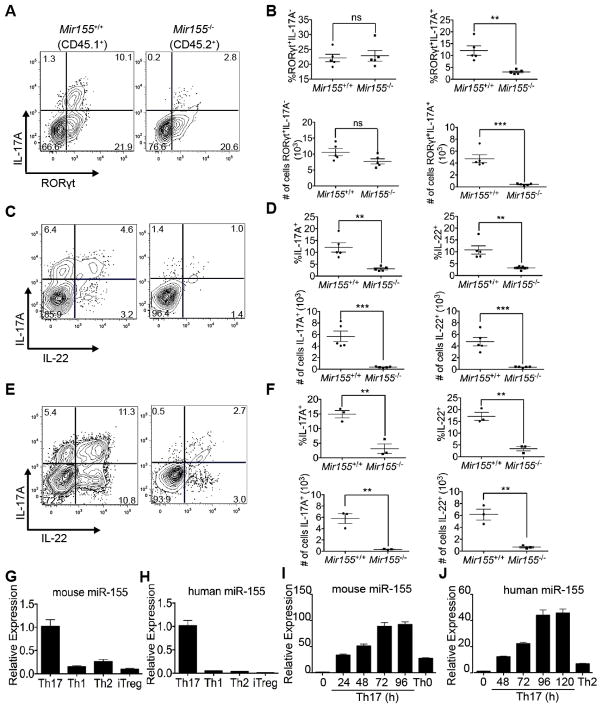
In these mixed BM chimeras, we studied a population of Th17 cells present in the mesenteric lymph nodes (MLN). Flow cytometry of restimulated CD4+TCRβ+CD44+ T cells from the MLN revealed a deficit in RORγt+IL-17A+ cytokine-producing Th17 cells among the miR-155-deficient population compared to WT (Figures 1A–B). No significant change in RORγt+IL-17A− Th cells that have undergone lineage specification was observed. We also found a cell autonomous defect in the production of both IL-17A and IL-22 by miR-155-deficient CD4+ T cells upon restimulation ex vivo (Figures 1C–D). Therefore, CD4+ cells deficient in miR-155 display cell intrinsic defects in Treg homeostasis and Th17 cytokine expression.
Figure 1. miR-155 is expressed by Th17 cells and required for Th17 cell-associated cytokine expression.
(A) FACS analysis, (B) percentages and absolute cell numbers of Mir155+/+ (CD45.1+) or Mir155−/− (CD45.2+) CD4+TCRβ+CD44+ cells in the MLN of mixed BM chimeras to enumerate IL-17A and/or RORγt expressing cells (n=5). (C) FACS analysis, (D) percentages and absolute cell numbers of CD4+TCRβ+CD44+ cells that express IL-17A and/or IL-22 among WT and Mir155−/− cells in the MLN of mixed BM chimeras (n=5). (E) FACS analysis, (F) percentages and absolute cell numbers of CD4+TCRβ+CD44+ cells that express IL-17A and/or IL-22 in the siLP of mixed BM chimeras from Toxoplama infected mice. (G–J) RT-qPCR analysis of mature miR-155 expression normalized to U6 snRNA from the indicated mouse (G) or human (H) CD4+ T cell subsets polarized ex vivo, or during mouse (I) and human (J) Th17 differentiation over a time course of 4 and 5 days, respectively. Statistical significance was determined using unpaired Student’s t test (* p<0.05 and ** p<0.01).
miR-155-deficient CD4+ T cells are Th1 competent upon infection with Toxoplasma
Previously, miR-155-deficient CD4+ T cells were reported to have a defect in IFN-γ and IL-17A expression following H. pylori infection (Oertli et al., 2011). Furthermore, miR-155 is implicated in the development of collagen-induced arthritis, and experimental autoimmune encephalomyelitis and uveitis (Bluml et al., 2011; Escobar et al., 2013; Murugaiyan et al., 2011; O’Connell et al., 2010). As Th1 and Th17 cells can contribute to pathogenesis in these mouse models, it is currently unclear whether miR-155 contributes to development of one or both of these T cell subsets. To address this issue, we employed the murine model of peroral Toxoplasma gondii infection, which is known to induce a highly polarized Th1 effector population as well as a localized Th17 cell response in the small intestine (Liesenfeld, 2002). Analysis of CD4+TCRβ+CD44+ T cells from the MLN at eight days post-oral infection revealed comparable IFN-γ production by both WT and miR-155-deficient cells (Figures S1D–E). Furthermore, there were similar frequencies of T. gondii-specific IFN-γ-producing CD4+ cells among WT and miR-155-deficient populations (Figure S1E). Thus, we found that miR-155 deletion does not significantly impair the Toxoplasma-specific Th1 response. Similar to uninfected chimeras, Foxp3+ Treg cells were reduced among the miR-155-deficient population (Figure S1F). Analysis of CD4+TCRβ+CD44+ T cells in the lamina propria of the small intestine (siLP) revealed a cell intrinsic defect in IL-17A and IL-22 production among the miR-155-deficient population upon restimulation (Figures 1E–F). These data demonstrate that miR-155 is specifically required for Treg cell homeostasis and induction of Th17 cell cytokine expression, but dispensable for the antigen-specific Th1 response upon peroral infection of T. gondii.
miR-155 is highly expressed in mouse and human Th17 cells
Due to the defect in Th17 cells, we hypothesized that miR-155 may be differentially expressed among CD4+ T cell subsets. We found high expression of miR-155 in both the mouse and human Th17 cell cultures relative to Th1, Th2, Th17 or transforming growth factor β (TGFβ)-induced Treg (iTreg) cells (Figures 1G–H). The expression of miR-155 increased over time during polarization of both primary mouse and human Th17 cell cultures (Figures 1I–J). To determine the molecular basis for the high expression in Th17 cells, we examined the genomic localization of Th17 associated transcription factors which include signal transducer and activator of transcription 3 (STAT3), interferon regulatory factor 4 (IRF4), basic leucine zipper transcription factor, ATF-like (BATF), v-maf musculoaponeurotic fibrosarcoma oncogene homolog (c-MAF), and retinoic acid receptor-related orphan receptor gamma t isoform (RORγt) in published ChIP-seq data (Ciofani et al., 2012). We found evidence that the Mir155 locus is directly bound by STAT3, c-MAF, BATF, and IRF4, transcription factors essential during the early phase of Th17 differentiation (Figure S2A). The transcription factor binding profile at the Mir155 locus is similar to the Rorc gene that encodes a Th17-specific master regulator (Fig S2B).
IL-17 but not IL-22 expression in miR-155-deficient Th17 cells can be rescued by IL-1 signaling
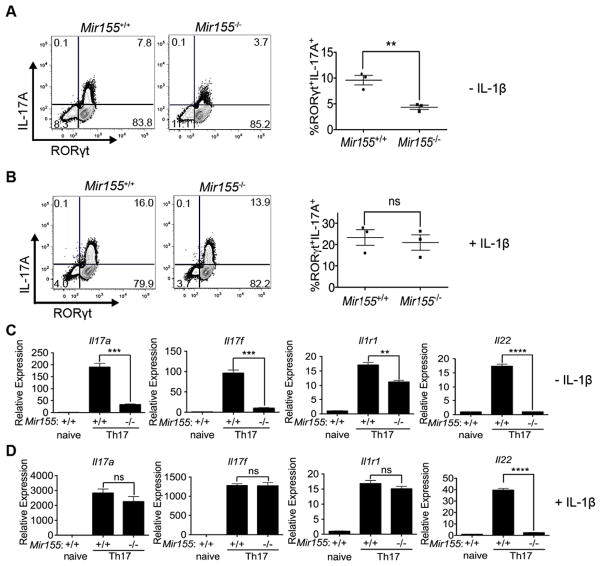
To investigate the mechanism of action for miR-155, we polarized CD4+ T cells from miR-155-deficient mice and littermate controls towards the Th17 cell fate as previously described with IL-6 and TGFβ cytokines (Korn et al., 2007; Nurieva et al., 2007; Veldhoen et al., 2006). As IL-1β promotes the in vivo development of Th17 cells (Ben-Sasson et al., 2009; Chung et al., 2009; Shaw et al., 2012), we also tested the effects of adding or withholding exogenous IL-1β to Th17 cell cultures. Differentiating miR-155-deficient Th17 cell cultures without exogenous IL-1β resulted in reduced IL-17A production (Figure 2A), as reported previously (O’Connell et al., 2010). We found that miR-155-deficient Th17 cell cultures without IL-1β could generate RORγt+ T cells, but they have a defect in producing IL-17A upon restimulation, similar to our results in the in vivo mixed BM chimera study (Figure 2A). This defect can be rescued upon addition of exogenous IL-1β to the differentiation conditions (Figure 2B). IL-1β did not affect cell survival or proliferation (Figures S2C–D) and there was no significant variation in absolute cell numbers in the cultures (data not shown). In addition, we found that transcripts encoding RORγt, BATF and IRF4 remained stable in absence of miR-155 in either condition (Figures S2E–F). However, without exogenous IL-1β, Il17a, Il17f, Il1r1, and Il22 transcripts were decreased in miR-155-deficient Th17 cell cultures compared to WT (Figure 2C). With the exception of Il22, each of these miR-155-dependent gene expression defects were rescued by adding IL-1β to our cultures (Figure 2D). Collectively, these results suggest that IL-1 signaling can partially bypass the need for miR-155 perhaps via an alternate parallel pathway.
Figure 2. IL-1 signaling can partially rescue gene expression defects in miR-155-deficient Th17 cells.
(A,B) FACS analysis and percentages of CD4+CD44+ T cells expressing IL-17A and RORγt in Mir155−/− or WT Th17 cultures polarized (A) without or (B) with exogenous IL-1β. (C,D) RT-qPCR analyses of Il17a, Il17f, Il1r1, and Il22 in Mir155−/− or WT Th17 cell cultures polarized (C) without or (D) with IL-1β. Relative expression was calculated by normalizing to Gapdh. Statistical significance was determined using Student’s t test (* p<0.05, ** p<0.01 and **** p<0.0001).
Il22 transcription is reduced in miR-155-deficient Th17 cell cultures
To systematically determine the consequences of Mir155 deletion on gene expression, we conducted whole transcriptome RNA sequencing (RNA-seq) of Mir155-deficient and WT Th17 cultures with IL-1β. As a result, we found 553 and 551 transcripts that are up- and down-regulated respectively in miR-155-deficient Th17 cultures compared to WT controls (Figure 3A). In the absence of miR-155, most of the known Th17 cell-associated transcription factors and the IL-17 cytokines were unaffected (Figure 3A). However, RNA-seq revealed reduced expression of Il22, Il10 and Il9 in miR-155-deficient Th17 cell cultures (Figures 3A–B), and these findings were validated by RT-qPCR (Figures S3A–B).
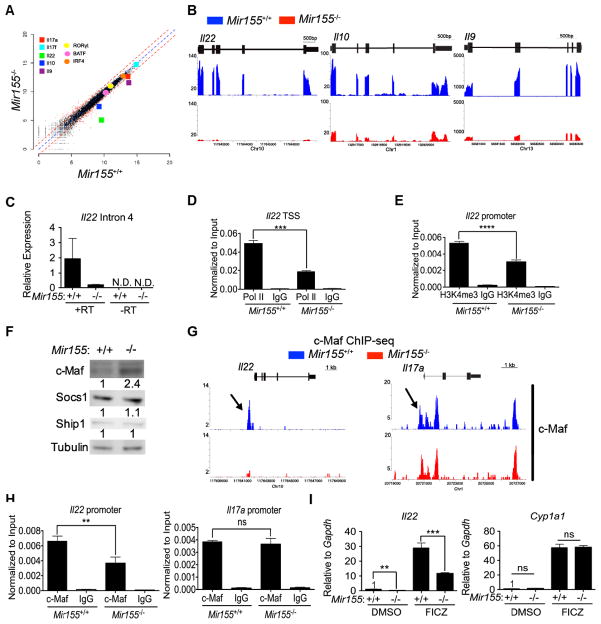
Figure 3. RNA-seq of miR-155-deficient and WT Th17 cells identifies altered cytokine expression and putative miR-155 targets.
(A) Scatter plot of gene expression for 20,096 RefSeq transcripts in Mir155−/− and WT Th17 cultures measured by RNA-seq. Notable transcription factors and cytokines are highlighted. Dashed red lines indicate a fold change of 2. (B) Genome browser screenshots of Il22, Il10 and Il9 loci depict RNA-seq results as normalized coverage tracks in Mir155−/− (red) and WT (blue) Th17 cell cultures. (C) RT -qPCR of Il22 intron 4 ± reverse transcriptase (RT) in Mir155−/− and WT Th17 cultures(+ IL-1β). (D) ChIP-qPCR of RNA polymerase II (Pol II) at Il22 TSS or (E) H3K4me3 atIl22 promoter inMir155−/− and WT Th17 cultures. (F) Western analysis for c-MAF, SOCS1 and SHIP1 in Mir155−/− and WT Th17 cultures with protein expression quantitated using ImageJ and normalized to Tubulin as a control. (G) Genome browser screenshots of c-MAF peaks in the Il22 and Il17a loci in Mir155−/− (red) and WT (blue) Th17 cultures. Arrows mark the regions validated by ChIP-qPCR. (H) Enrichment of Il22 and Il17a promoter was measured by ChIP-qPCR of c-MAF inMir155−/− or WT Th17 cultures. (I) Relative expression of Il22 and Cyp1a1 mRNA normalized to Gapdh was determined by RT-qPCR in Mir155−/− or WT Th17 cultures (+ IL-1β) and treated with DMSO or 0.3 μM of FICZ. Statistical significance was determined using Student’s t test (** p<0.01, *** p<0.001; ns denotes not significant).
We first focused on Il22 gene expression because it was the most differentially expressed transcript between miR-155-deficient and WT Th17 cultures. IL-22 production could be regulated via several mechanisms. To measure recently transcribed Il22, we performed RT-qPCR of unspliced transcripts using primers that amplified an intron of Il22, and found that it was decreased in miR-155-deficient Th17 cultures compared to WT (Figure 3C). Next, we performed chromatin immunoprecipitation (ChIP) of RNA polymerase (pol) II at the Il22 transcriptional start site (TSS) and found decreased occupancy in the absence of miR-155 (Figure 3D). Furthermore, as another indication that the Il22 promoter is less active in miR-155-deficient Th17 cells, we found less histone H3 lysine 4 tri-methylation (H3K4me3) (Figure 3E). Taken together, our data are consistent with a reduction in Il22 transcription in miR-155-deficient Th17 cell cultures.
Given the above results, we hypothesized that miR-155 targets a transcriptional repressor of Il22. We considered c-MAF as a reasonable candidate, since it was previously reported as a target of miR-155 as well as a repressor of Il22 transcription (Rodriguez et al., 2007; Rutz et al., 2011). Indeed, we observed a 1.6 fold up-regulation in c-MAF transcripts in miR-155-deficient Th17 cultures compared to WT by RNA-seq (Figure S3C), and a 2.4 fold up-regulation of c-MAF protein by Western analysis, (Figure 3F). In contrast, Inositol Polyphosphate-5-Phosphatase 1 (SHIP1) and Suppressor Of Cytokine Signaling 1 (SOCS1), other known miR-155 targets (Lu et al., 2009; O’Connell et al., 2009), were not differentially expressed (Figure 3F). Thus, we considered c-MAF as a viable miR-155 target in Th17 cells.
ChIP-seq was conducted to map c-MAF binding to the genome. In total, about 12,554 and 8,815 c-MAF peaks were identified in WT and miR-155-deficient Th17 cells respectively (Figure S3D). Surprisingly, there were fewer peaks of c-MAF binding although c-MAF was overexpressed in absence of miR-155. Importantly, we determined that c-MAF failed to bind to the Il22 locus in miR-155-deficient Th17 cell cultures (Figure 3G). As a control, we did not observe a difference in c-MAF binding to the Il17a locus under these culture conditions (Figure 3G). ChIP findings were validated by qPCR (Figure 3H). Thus, c-MAF is not responsible for silencing Il22 in the absence of miR-155.
The aryl hydrocarbon receptor (AHR) plays a critical role in Il22 transcription (Veldhoen et al., 2008), and thus we asked whether stimulating the activity of this transcription factor could rescue Il22 expression in miR-155-deficient Th17 cells. We found that upon ligation of AHR by an agonist, 6-formylindolo[3,2-b]carbazole (FICZ), the expression of Il22 is increased, but still impaired in miR-155-deficient Th17 cells compared to WT (Figure 3I). In contrast, the canonical AHR target, Cyp1a1, was not differentially regulated in miR-155-deficient and WT Th17 cell cultures with or without FICZ treatment (Figure 3I). These results suggest that AHR activity is specifically inhibited at the Il22 locus and may contribute to the inability of miR-155-deficient Th17 cells to express Il22.
Motifs matching the miR-155 seed sequence are enriched in 3′ UTR of genes up-regulated in miR-155-deficient Th17 cells
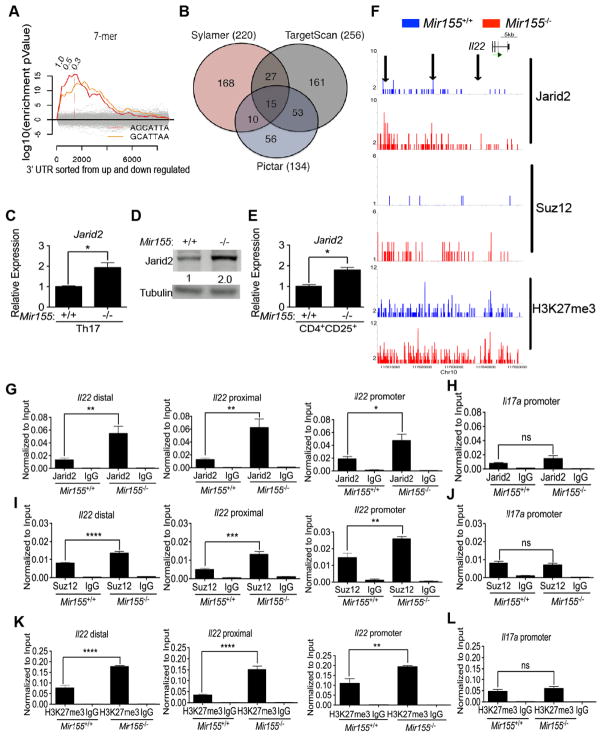
To look for additional potential targets of miR-155, we used the computational tool Sylamer, which searches for correlations between gene expression changes in our RNA-seq dataset and the enrichment of sequence motifs in the 3′ UTR of the corresponding genes (van Dongen et al., 2008). As a testament to the specificity of this analysis, we found that out of the 970 heptameric seed sequences tested, only the two overlapping motifs that match the miR-155 seed sequence (AGCATTA and GCATTAA) were significantly enriched in genes up-regulated in Mir155-deficient compared to WT Th17 cell cultures (Figure 4A). This Sylamer analysis revealed 220 transcripts up-regulated in miR-155-deficient Th17 cell cultures that contain at least one miR-155 seed sequence in their 3′ UTR. To further narrow down the putative miR-155 targets, we compared our gene list with two computational miRNA target prediction methods which invoke evolutionary conservation: TargetScan and PicTar (Krek et al., 2005; Lewis et al., 2003) (Figure 4B). Intersection of the gene lists from Sylamer, TargetScan and PicTar yielded only 15 common putative targets (Figure S4A). In conclusion, we could readily detect a signature of miR-155 mediated tuning within the transcriptome of Th17 cell cultures and identified a short list of candidates for further testing.
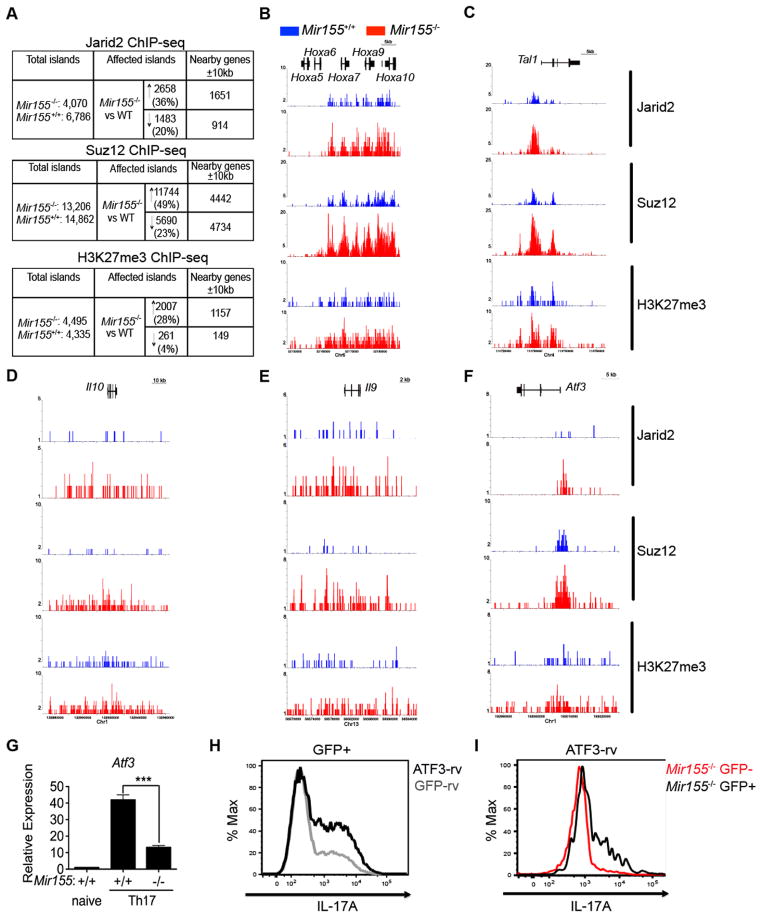
Figure 4. Jarid2 recruits PRC2 to epigenetically silence the Il22 locus in absence of miR-155.
(A) Sylamer analysis of Mir155−/− and WT Th17 RNA-seq is depicted as a landscape plot: hypergeometric significance values for enrichment of heptamers among 3′ UTRs of genes (y-axis) and genes sorted from most up-regulated in Mir155−/− to down-regulated (x-axis). Two heptameric motifs are significantly enriched in genes up-regulated in Mir155−/− Th17 cultures (red and orange lines). The horizontal dotted red line represents an E-value threshold of P < 0.01 (Bonferroni-corrected for multiple testing). Vertical dotted lines indicate log2 fold change cutoffs of differentially expressed transcripts. (B) Comparison of our gene list from Sylamer analysis with predictions from TargetScan and Pictar, shows an overlap of 15 genes with putative miR-155 binding sites. (C) Relative expression of Jarid2 mRNA was determined by RT-qPCR in Mir155−/− or WT Th17 cultures (+ IL-1β). (D) Western blot analysis of Jarid2 protein in miR-Mir155−/− and WT Th17 lysates quantified using ImageJ and Tubulin as a loading control. (E) Relative expression of Jarid2 mRNA in Treg cells was determined by RT-qPCR in Mir155−/− or WT CD4+CD25+ T cells sorted from the spleen. (F) Genome browser screenshots depict Jarid2, Suz12, and H3K27me3 enrichment at the Il22 locus. Arrows indicate promoter, proximal, and distal regions validated by qPCR. (G, H) Enrichment of anti-Jarid2, (I, J) anti-Suz12, and (K, L) anti-H3K27me3 along with control IgG ChIPs of Mir155−/− and WT Th17 cultures at three indicated locations within Il22 locus or the Il17a promoter was determined by qPCR. Validations were normalized to input controls and results are representative of three independent experiments with statistical significance determined using unpaired Student’s t test (* p<0.05, ** p<0.01, *** p<0.001, and **** p<0.0001; ns denotes not significant).
The DNA-binding protein Jarid2 is enriched in miR-155-deficient Th17 cell cultures
Based on our hypothesis that miR-155 is targeting a transcriptional repressor of Il22, we considered four candidate DNA-binding proteins: Bach1, Jarid2, Sp3 and Tle4 (Figure S4A). Jarid2 was investigated further as it is the most highly expressed in Th17 cells of the four candidates (Figure S4B). In mouse ES cells, Jarid2 recruits the histone modifying holoenzyme PRC2 to specific sites in the genome and silences transcription of its target genes through histone 3 lysine 27 (H3K27) trimethylation mediated by the polycomb proteins enhancer of zeste homolog 1 (Ezh1) or Ezh2 (Landeira et al., 2010; Li et al., 2010; Pasini et al., 2010; Peng et al., 2009; Shen et al., 2009). Jarid2 was previously validated to be a miR-155 target in chicken and human cells (Bolisetty et al., 2009). According to the TargetScan and Pictar databases, two heptameric miR-155 seed sequences are conserved in the 3′ UTR of Jarid2 with the upstream predicted site being better conserved (Figure S4C). Furthermore, it was demonstrated by high-throughput sequencing of RNA isolated by crosslinking and immunoprecipitation (HITS-CLIP) that Argonaute2 (Ago2), a key protein of the miRNA-induced silencing complex (miRISC), directly binds to the upstream predicted site in 3′ UTR of Jarid2 in activated WT CD4+ T cells (Figure S4D) (Loeb et al., 2012). The binding of Ago2 to this site is dependent on miR-155 as it is eliminated in miR-155-deficient T cells (Figure S4D). Notably, we found that Jarid2 transcripts and protein levels are both up-regulated in miR-155-deficient Th17 cultures by about two-fold (Figures 4C–D). Jarid2 transcripts are also elevated in miR-155-deficient Treg cells (Figure 4E).
ChIP-seq analyses was conducted to determine whether Jarid2 directly targets Il22 in Th17 cell cultures. We found increased association of Jarid2 with the Il22 locus in miR-155-deficient cells (Figure 4F) and validated enrichment by qPCR at three genomic locations (Figure 4G). Jarid2 did not preferentially bind to the Il17a promoter in miR-155-deficient Th17 cultures consistent with normal expression of IL-17A in these cells (Figure 4H). To determine whether Jarid2 silences Il22 transcription by recruiting PRC2, we conducted ChIP-seq for Suppressor of Zeste 12 (Suz12), a core component of PRC2 (Figure 4F). Analyses at the same genomic locations as Jarid2 confirmed increased association of Suz12 with the Il22 locus in the absence of miR-155 (Figure 4I), but not at the Il17a promoter (Figure 4J). Higher amounts of H3K27 trimethyl (H3K27me3) were detected at the Il22 locus in Mir155-deficient Th17 cultures (Figures 4F and 4K), whereas H3K27me3 at the Il17a promoter was unaffected (Figure 4L).
Jarid2 and PRC2 reprogram the epigenome of miR-155-deficient Th17 cells
As the direct targets of PRC2 in CD4+ T cells have not been systematically examined, we performed ChIP-seq analyses for Jarid2, Suz12, and H3K27me3 in miR-155-deficient Th17 cultures and their WT controls. Our analysis identified 6,786 and 4,070 Jarid2 islands, 13,206 and 14,862 Suz12 islands, and 4,495 and 4,335 H3K27me3 islands in WT and miR-155-deficient Th17 cells, respectively (Figure 5A). Correlating with the increased Jarid2 expression, we found augmented Jarid2 islands as well as Suz12 and H3K27me3 islands when comparing the Mir155-deficient to WT Th17 cells (Figure 5A). To identify genes directly targeted by PRC2, we looked for Jarid2, Suz12, and H3K27me3 islands that increased in miR-155-deficient Th17 cell cultures that were co-localized ± 10 kb from a gene body (Figure 5A). This list included canonical PRC2 target genes, such as the HoxA-D gene clusters (Figures 5B and S5A–C) and embryonic genes including Gata6 and Sox17(S 5D–E). PRC2 also targeted Tal1, a transcription factor gene essential for the specification of hematopoietic stem cells (HSCs) that is silenced upon differentiation (Figure 5C). Jarid2-PRC2 bound to genes such as Eomes and Tbx21 that may mediate Th17 cell fate plasticity (Figure S5F–G). In summary, we found that Jarid2 up-regulation in miR-155-deficient Th17 cells resulted in widespread increased recruitment of the Jarid2-PRC2 holoenzyme that coincided with increased deposition of H3K27me3 at specific sites throughout the genome.
Figure 5. Jarid2 recruits PRC2 to reprogram the epigenome of miR-155-deficient Th17 cells.
(A) Tables summarize Jarid2, Suz12 and H3K27me3 ChIP-seq experiments in Mir155−/− and WT Th17 cultures. First column is total number of peaks in Mir155−/− and WT identified using SICER 3.1. Second column indicates the number of peaks affected (either ↑ or ↓) in Mir155−/− compared to WT controls using SICER 3.3.1. Third column indicates the number of RefSeq genes within 10 kb of the peaks in question from SICER 3.3.1. (B–F) Genome browser screenshots depict Jarid2, Suz12, and H3K27me3 enrichment at the HoxA cluster (B), Tal1 (C), Il10 (D), Il9 (E) and Atf3 (F) loci as a result of ChIP-seq experiments in Mir155−/− (red) and WT (blue) Th17 cells. (G) Relative expression of Atf3 mRNA by RT-qPCR of Mir155−/− and WT Th17 cells. (H) Histogram depicts IL-17A expression from FACS analysis of Th17 cultures transduced with ATF3-rv or empty vector (GFP-rv) and gated on transduced CD4+CD44+GFP+ cells; (I) Mir155−/− Th17 cultures transduced with ATF3-rv and gated on transduced (GFP+) or untransduced (GFP−) CD4+CD44+ cells. Statistical significance was determined using Student’s t test (*** p<0.001).
In addition to Il22, Il10 and Il9 were also directly targeted by the Jarid2-PRC2 holoenzyme (Figures 5D–E). In addition, we found genes not yet known to play a role in Th17 cells, such as Atf3 encoding activating transcription factor 3 (ATF3), a member of the AP-1 superfamily of leucine zipper proteins that includes Fos, Jun and BATF. Atf3 was targeted by PRC2 (Figure 5F) and its expression was reduced in miR-155-deficient Th17 cells as determined by RNA-seq (data not shown) and RT-qPCR (Figure 5G). Atf3 expression was induced in Th17 cells differentiated in the presence of exogenous IL-1β (Figure S5H). To determine the role of Atf3 in Th17 cells, we used a retroviral vector to overexpress Atf3 (ATF3-rv) during Th17 differentiation. Th17 cultures transduced with ATF3-rv expressed more IL-17A compared to the empty virus control (Figure 5H) and untransduced cells in the same cultures (data not shown). Conversely, deletion of Atf3 reduced expression of IL-17A (Figure S5I). Finally ectopic expression of ATF3 rescued IL-17A production in miR-155-deficient Th17 cells (Figure 5I). In summary, Atf3 has the ability to promote IL-17a expression in Th17 cells.
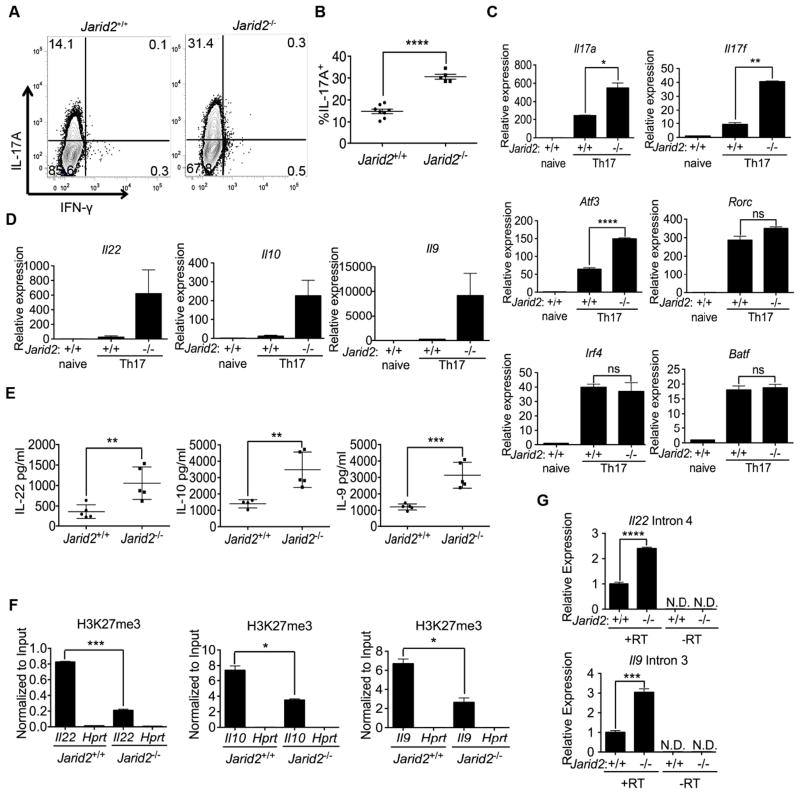
Jarid2 is required for PRC2 recruitment and transcriptional silencing of Th17 cell-associated genes
The miR-155-deficient mouse allowed us to assess Th17 development in the context of Jarid2 gain-of-function. To determine the direct contribution of Jarid2 during Th17 differentiation, we generated Jarid2fl/fl;CD4-cre mice (Figure S6A–C). Mature CD4+ T cells develop in Jarid2 fl/fl;CD4-cre mice, and in contrast to Ezh2-deficient T cells (Su et al., 2005), they do not display a T cell activation defect (data not shown). We performed a variant of ChIP-seq that uses lambda 5′-3′ exonuclease (ChIP-exo) to reduce background and increase resolution of the assay (Rhee and Pugh, 2012). In the absence of Jarid2, genomic recruitment of Suz12 was diminished at the Hoxa and Hoxd clusters, Il22 and Atf3 for example (Figures S6D–G). Furthermore, during in vitro Th17 cell differentiation, IL-17A, but not IFN-γ, expression was increased in cells from Jarid2 fl/fl; CD4-cre mice (Figures 6A–B). In addition, Il17f, Il22, Il10, Il9 and Atf3 transcripts were also increased in Jarid2-deficient Th17 cell cultures (Figures 6C–D), whereas no changes were observed in Rorc, Irf4 and Batfexpression (Figures 6C and S6H–I). As confirmation, increased amounts of IL-22, IL-10 and IL-9 protein were detected in the supernatants of Jarid2-deficient Th17 cell cultures (Figure 6E). Me chanistically, H3K27me3 deposition was decreased at the Il22, Il10, and Il9 promoters upon Jarid2 ablation(Figure 6F). Moreover, we found augmented amounts of unspliced message for Il22 and Il9 in Jarid2-deficient Th17 cultures suggesting increased transcription of these cytokine genes (Figure 6G). Thus, lack of Jarid2 results in enhanced Th17 cytokine gene expression.
Figure 6. Absence of Jarid2 in CD4+ T cells results in enhanced Th17 development.
(A) FACS analysis of IL-17A and IFN-γ expression in CD4+CD44+ cells from Jarid2fl/fl; CD4-cre mice and littermate controls cultured under Th17 polarizing conditions without IL-1β and (B) the corresponding percentages of CD4+CD44+IL-17A+ T cells. (C) Relative expression of Il17a, Il17f, Atf3, Rorc, Irf4, Batf mRNA by RT-qPCR from WT and Jarid2-deficient Th17 cultures. (D) Relative expression of Il22, Il9 and Il10 mRNA in Jarid2-deficient and WT Th17 cultures was determined by RT-qPCR. (E) Cytokine concentrations in supernatant of Th17 cultures were determined by ELISA. (F) H3K27me3 at promoters of Il22, Il10, Il9 and Hprt determined by ChIP-qPCR. (G) RT-qPCR of Il22 intron 4 and Il9 intron 3 of Jarid2-deficient and WT Th17 cultures ± reverse transcriptase (RT).
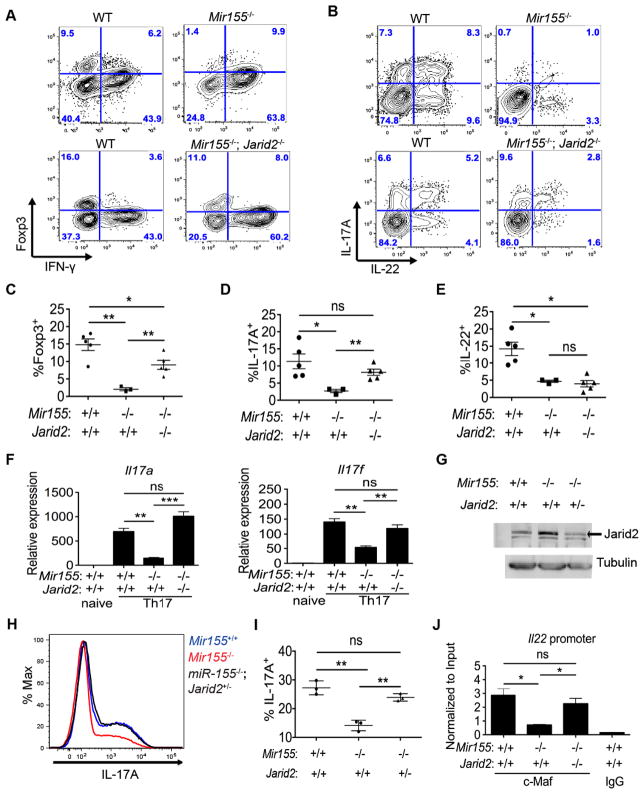
Conditional ablation of Jarid2 partially rescues Th17 and Treg cells in miR-155-deficient mice
To determine whether the Th17 and Treg cell phenotypes in miR-155-deficient mice are caused by increased expression of Jarid2, we generated double deficient mice wherein Jarid2 is conditionally deleted in the T cell lineage of miR-155-deficient mice. We made mixed BM chimeras of WT (CD45.1+) and double deficient (CD45.2+) cells, and infected them with T. gondii as before to assess IL-17A and IL-22 expression as well as Treg homeostasis. Double deficient Treg cells were competitive with WT Treg cells whereas miR-155-deficient Treg cells failed to compete (Figure 7A). In addition, development of CD4+IL-17A+ T cells was restored in the double deficient animals despite IL-22 expression not being rescued (Figure 7B). Thus, Foxp3+ cells and IL-17A expression in vivo could be partially restored upon ablation of Jarid2 in miR-155-deficient T cells (Figures 7C–E). We recapitulated the rescue of Il17a and Il17f expression in vitro with double deficient Th17 cell cultures (Figure 7F). In double deficient Th17 cell cultures, Atf3 expression was increased compared to WT (Figure S7A). Furthermore, we found that conditional deletion of one allele of Jarid2 in miR-155-deficient Th17 cells was sufficient to reduce Jarid2 protein to near WT levels (Figure 7G) and to rescue IL-17A expression (Figures 7H–I).
Figure 7. Epistasis between Jarid2 and miR-155 in Treg and Th17 cells.
(A,B) FACS analysis of WT, Mir155−/− or double deficient Mir155−/−; Jarid2fl/fl; CD4-cre (Mir155−/−; Jarid2−/−) cells from siLP of infected mixed BM chimeras to enumerate CD4+TCRβ+CD44+ cells that are IFN-γ or Foxp3 positive (A), or IL-17A and/or IL-22 positive (B), 8 days after T. gondii infection. (C–E) Indicated percentages of CD4+TCRβ+CD44+ WT, Mir155−/− or Mir155−/−; Jarid2−/− cells from the siLP of infected mixed BM chimeras. (F) Relative expression of Il17a and Il17f mRNA was determined by RT-qPCR in WT, Mir155−/− or Mir155−/−; Jarid2−/− Th17 cultures (− ecogenous IL-1β). (G) Western blot analysis of Jarid2 and tubulin protein in WT, Mir155−/− and Mir155−/−; Jarid2+/− Th17 cells. (H) Histogram depicts intracellular IL-17A expression in WT, Mir155−/− and Mir155−/−; Jarid2+/− Th17 cultures (− IL-1β). (I) The corresponding percentages of CD4+CD44+IL-17A+ T cells. (J) c-MAF and IgG ChIP-qPCR on the Il22 promoter in WT, Mir155−/− and Mir155−/−; Jarid2−/− Th17 cultures normalized to input. Statistical significance determined using unpaired Student’s t test (* p<0.05, ** p<0.01, and *** p<0.001; ns denotes not significant). ns denotes not significant.
As deletion of Jarid2 in miR-155-deficient CD4+ T cells did not rescue Il22 expression, we hypothesized that the chromatin structure at the Il22 promoter is now in a permissive state to allow binding of c-MAF, a known repressor. Indeed, in double deficient Th17 cell cultures, c-MAF bound to the Il22 promoter (Figure 7J). Indeed, Suz12 and H3K27me3 enrichment in double deficient Th17 cell cultures were reduced at the Il22 promoter where c-MAF binds (Figures S7B–C). Furthermore, as Il10 expression is regulated by c-MAF (Xu et al., 2009) and Jarid2-PRC2 (Figure 6C), we checked our c-MAF and Suz12 ChIP-seq dataset and found that c-MAF binding to a previously reported binding site in the Il10 promoter is restricted in Mir155-deficient Th17 cells whereas Suz12 occupancy increases (Figure S7D). At this site, c-MAF binding is increased when both miR-155 and Jarid2 are absent (Figure 7E). Globally, the Jarid2-PRC2 holoenzyme may have the ability to dictate where transcription factors such as c-MAF can bind chromatin by affecting the accessibility of different loci to transcription factors.
In conclusion, we integrated miR-155 and PRC2 with the Th17 transcriptional network reported by Ciofani et al. (2012) (Figure S7F). We find that in Th17 cells, the Jarid2-PRC2 complex targets genes that can contribute to IL-17 expression such as Atf3, cellular plasticity genes such as Tbx21 and Eomes, and immunoregulatory cytokine genes such as Il22, Il10 and Il9. In addition to Jarid2, miR-155 appears to repress c-MAF an important transcription factor in Th17 cells. We revealed that Jarid2 restricted c-MAF binding in at least two cases, Il22 and Il10. Thus, we provide an updated view of the complex regulatory network in Th17 cells to exemplify how a miRNA might interact with a chromatin regulator and transcription factors to orchestrate gene expression programs.
Discussion
We highlight a mechanism by which miR-155 regulates the fate of Th17 and Treg cells. By regulating the expression of Jarid2, miR-155 can indirectly promote chromatin accessibility and gene expression. Using ChIP-seq and RNA-seq approaches, we assessed the genome-wide consequences of Jarid2 up-regulation. We observed increased recruitment of PRC2 to genomic sites accompanied with increased H3K27me3 and silencing of Il22, Il10 and Il9, cytokines involved in mediating tissue repair and homeostasis in response to inflammation (Goswami and Kaplan, 2011; Ouyang et al., 2011; Zenewicz and Flavell, 2011). Although, H3K27me3 is often equated with transcriptional silencing, direct perturbation experiments to address this relationship are lacking in mammalian systems. Here, we ablated Jarid2 in CD4+ T cells to prevent recruitment of PRC2, the holoenzyme that catalyzes trimethylation of H3K27 at its targets. This allowed us to identify Jarid2-dependent PRC2 targets genome-wide and examine the consequences of perturbing this regulatory node on gene expression in Th17 cells. Jarid2-deficient CD4+ T cells undergo enhanced Th17 differentiation in part manifested by up-regulation of Il17a and Atf3. As reduced or excessive H3K27me3 catalysis can affect the efficiency of Th17 differentiation and Treg homeostasis, miR-155 may have evolved to modulate PRC2 recruitment by fine-tuning Jarid2 expression.
We found that Il22, Il10, Il9 and Atf3 are among the direct targets of Jarid2 in Th17 cells, but found additional genes such as Il17a and Il17f that are affected indirectly. We also observed increased Jarid2 and PRC2 occupancy in miR-155-deficient Th17 cultures that did not lead to gene expression changes. Such genes include Tbx21, Eomes, Tal1 and the Hox gene clusters, but since these genes are already silenced in Th17 cells, increased Jarid2 and PRC2 binding had no additional effect. Eomes and Tbx21 could mediate T cell fate plasticity, but since Th17 cells can suppress alternative cell fates via multiple mechanisms (Zhu and Paul, 2010), we did not observe their derepression in Jarid2-deficient Th17 cultures. However, stimulating Jarid2-deficient Th17 cells with IL-12 or IFN-γ, or blocking TGFβ could dramatically change the outcome.
There are several reasons why the consequences of Jarid2 ablation in CD4+ T cells are not more severe. For instance, there are reports that other DNA-binding factors such as adipocyte enhancer-binding protein 2 (Kim et al., 2009), metal response element-binding transcription factor 2 (Li et al., 2011) and additional sex combs-like homolog 1 (Abdel-Wahab et al., 2012) can also serve to recruit PRC2 and may act independently or redundantly with Jarid2. Moreover, there are other mechanisms responsible for epigenetic marks that need to be considered such as methylation of H3K9, H4K20 or DNA, which also mediate transcriptional silencing. Even if the chromatin is poised for transcription, the factors that are required for transcriptional activation may be absent. For example, we found that H3K27me3 is reduced at Tbx21 in the absence of Jarid2, but without the appropriate signal, its transcriptional enhancer is not activated (Vahedi et al., 2012). Finally, we only looked for a relatively local effect (± 10 kb), whereas distal regulatory elements can regulate transcription as far as a Mb away (Bulger and Groudine, 2011).
Our studies show that miR-155 is critical for the in vivo production of IL-17A and IL-22 particularly by CD4+ T cells in the gut. In absence of miR-155, specification of Th17 cells appears intact based on RORγt induction, but expression of the signature cytokine IL-17A is defective. This suggests that miR-155 may exert its regulatory function more towards the latter phases of differentiation as cells are acquiring their effector functions and to a lesser extent during the initial phases of Th17 specification. Additional evidence supporting this idea comes from our time course analysis showing that upon in vitro Th17 polarization, miR-155 expression increased over time with the highest expression after 3–4 days. At this stage of Th17 differentiation, RORγt expression was already induced by the combined action of STAT3, BATF and IRF4 (Ciofani et al., 2012; Yosef et al., 2013). Like in previous studies, we found that IL-1β enhances Th17 development in culture (Chung et al., 2009; Guo et al., 2009; Shaw et al., 2012). IL-1β rescued IL-17 expression in miR-155-deficient Th17 cultures. We believe that IL-1 signaling is promoting IL-17A expression partly through Atf3, since addition of exogenous IL-1β can upregulate expression of Atf3 and overexpression of ATF3 in Th17 cells results in increased IL-17a production. It will be interesting to determine further the role of Atf3in Th17 cells.
Here we have expanded the regulatory network that can modulate cytokine expression in Th17 cells to include post-transcriptional regulation and histone modification, which may inspire novel therapeutic avenues. As IL-22 plays a dual role in Th17-mediated inflammation and tissue repair (Dudakov et al., 2012; Zenewicz and Flavell, 2011; Zheng et al., 2007), understanding the mechanisms behind the regulation of Il22 transcription is critical for modulating the immune response during an inflammatory disease. For instance, inhibition of miR-155 or promotion of PRC2 activity may be useful for controlling inflammation. Our work highlights a miR-155-Jarid2 axis that emanates from the Th17 specification program to regulate cytokine production during the effector phase. Although we focused on elucidating the Th17 network, our in vivo studies revealed epistasis between miR-155 and Jarid2 in Treg cells. Thus, it will be interesting to further elucidate the role of Jarid2 in governing Treg cell fate.
Taken together, our study reveals that miR-155 promotes acquisition of effector functions by regulating a chromatin modifying complex and thus mediating global changes in gene expression. A two-fold change in Jarid2 expression by a miRNA can reprogram the epigenome of a cell via H3K27 trimethylation. How miRNAs, transcription factors and chromatin regulators cooperate to orchestrate a gene expression program has not been systematically analyzed. Using Th17 differentiation as a model system, we demonstrate that miR-155 can have profound effects on the chromatin landscape and links post-transcriptional, epigenetic as well as transcriptional regulation of cellular differentiation.
EXPERIMENTAL PROCEDURES
Mice and Toxoplasma infection
Mir155-deficient mice (Thai et al., 2007) were backcrossed up to 12 generations to C57BL/6 mice (Taconic). Atf3-deficient mice on C57BL/6 background were previously described (Hartman et al., 2004). We obtained a knock-out first conditional allele Jarid2tm1a(KOMP)Wtsi on a C57BL/6 background from the International Knockout Mouse Consortium which we converted into a conditional allele (Jarid2fl/+) by breeding to Flp transgenic mice (Rodriguez et al., 2000). Then, we bred Jarid2fl/+ mice to CD4-cre transgenic mice (Lee et al., 2001). For mixed BM reconstitutions, B6.SJL recipient mice (Taconic) were conditioned with 900 Rads via a Cs137 source prior to injection of 3 million donor BM cells. Mice were then put on Trimethoprim/Sulfamethoxazole antibiotics via drinking water for 5 weeks. T. gondii cysts from the Type II avirulent strain ME-49 were prepared from the brains of infected C57BL/6 mice. For infections, mice were innoculated by oral gavage with 0.5 mL of PBS for an average of 50 cysts per animal (Wilson et al., 2010). All mice experiments were done in compliance with the guidelines of the NIH/NIAID Institutional Animal Care and Use Committee.
Cell isolation and flow cytometry
Single-cell suspensions were prepared from spleens and lymph nodes by mechanical disruption and filtering through 40-μm cell strainers (BD Biosciences). Cells were stimulated with 20 ng/ml of phorbol 12-myristate 13-acetate (PMA) and 1 μg/ml of ionomycin in the presence of BD Golgi Plug containing brefeldin A for 3–5h at 37°C. Cells were subsequently stained with fixable viability dye (eBioscience) and then permeabilized in 500 μl of eBioscience Perm-Fix solution overnight at 4°C. Stained cells were assayed with a BD LSRII flow cytometer or BD LSR Fortessa and results analyzed using FlowJo software. See Supplemental Experimental Procedures for additional details and a list of antibodies used.
T helper cell differentiation
See Supplemental Experimental Procedures for additional details and a list of antibodies used.
Retroviral Cloning and Transductions
See the Supplemental Experimental Procedures for details.
RNA Purification and Quantitative PCR
Total RNA extractions were performed using Applied Biosystems mirVana isolation kit. Applied Biosystems TaqMan Reverse Transcription Reagents and random hexamers were used to prepare cDNAs. For miRNA quantitation, cDNA was prepared using TaqMan microRNA reverse transcription kit (Applied Biosystems). Quantitative PCR was performed on a 7900HT Fast Real Time PCR Systems (Applied Biosystems). A list of primers/probe sets used is included in Supplemental Information.
Protein Quantitation
Cell lysates were prepared by lysis in 420mM NETN buffer containing protease inhibitor and Western performed using standard methods. Image J (http://imagej.nih.gov/ij/) was used to quantify protein expression levels from digital images of Western blots and each assay was normalized to α-Tubulin loading controls. IL-22, IL-9, and IL-10 protein concentrations in Th17 culture supernatants were measured using the corresponding Mouse ELISA Ready-Set-Go! Kit (eBioscience) following the manufacturer’s instructions.
Chromatin Immunoprecipitation
ChIP experiments were performed as described previously (Wei et al., 2009). Briefly, cells were cross-linked with 2% Paraformaldehyde at room temperature for 15 minutes and chromatin sheared to 200 base pairs. For ChIP-exo, sheared chromatin was submitted to Peconic LLC (State College, PA) along with antibodies. List of antibodies and validation primers can be found in Supplemental Information.
RNA-seq
RNA concentration was determined using the Qubit RNA broad range assay in the Qubit Fluorometer (Invitrogen) and RNA integrity was determined using Eukaryote Total RNA Nano Series II ChIP on a 2100 Bioanalyzer (Agilent). Three independent biological replicates were pooled for RNA-seq. RNA-seq libraries were prepared from 4 μg of total RNA using the TruSeq RNA sample prep kit following manufacturer’s protocol (Illumina). Briefly, oligo-dT purified mRNA was fragmented and subjected to first and second strand cDNA synthesis. cDNA fragments were blunt-ended, ligated to Illumina adaptors, and PCR amplified to enrich for the fragments ligated to adaptors. The resulting cDNA libraries were verified and quantified on Agilent Bioanalyzer and single end 96 cycle RNA-seq was conducted using the GAIIx Genome Analyzer (Illumina).
Statistical Significance
GraphPad Prism 5.0 was used for statistical analysis (unpaired, two-tailed, t test with a confidence interval of 95%). A p value ≤ 0.05 was considered statistically significant.
Supplementary Material
Highlights.
miR-155 is highly induced during mouse and human Th17 differentiation
Jarid2 and miR-155 are epistatic in Th17 and Treg cells
Jarid2 is required to recruit PRC2 to genomic sites in Th17 cells
Direct targets of PRC2 in Th17 cells include Il22, Il10, Il9 and Atf3
Acknowledgments
We thank W.E. Paul for his generous support and advice throughout; Y.S. Chang and T. Hai (Ohio State University) for providing Atf3-deficient mice; B.J. Abraham for help with ChIP-seq analyses; E. Shevach for facilitating human T cell studies; B. Kelsall for BM chimera protocol; L. Barron, J. Hu-Li and S. Sharma for technical advice and assistance; J. Edwards for cell sorting; the Biowulf Linux cluster (biowulf.nih.gov) and NIAID Office of Cyber Infrastructure and Computational Biology for providing high performance computing; the NIH Tetramer Core Facility at Emory University for MHC tetramers; W. Ouyang (Genentech) for IL-22 antibody; B. Brady, M. Crank, R. Germain, W. Leonard, A. Oler, J. O’Shea, and anonymous reviewers for constructive criticisms of the manuscript. T.M.E. was in the NIH-Johns Hopkins University Graduate Partnership Program and received valuable guidance from K. Beemon and S. Gottesman. This work was supported by the Intramural Research Program of the NIAID, NIH. The authors declare no competing financial interests.
Footnotes
ACCESSION NUMBERS
RNA-seq and ChIP-seq data are available in the Gene Expression Omnibus (GEO) database (www.ncbi.nlm.nih.gov/geo) under the accession number GSE37228 and GSE47528.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdel-Wahab O, Adli M, LaFave LM, Gao J, Hricik T, Shih AH, Pandey S, Patel JP, Chung YR, Koche R, et al. ASXL1 mutations promote myeloid transformation through loss of PRC2-mediated gene repression. Cancer Cell. 2012;22:180–193. doi: 10.1016/j.ccr.2012.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Sasson SZ, Hu-Li J, Quiel J, Cauchetaux S, Ratner M, Shapira I, Dinarello CA, Paul WE. IL-1 acts directly on CD4 T cells to enhance their antigen-driven expansion and differentiation. Proc Natl Acad Sci U S A. 2009;106:7119–7124. doi: 10.1073/pnas.0902745106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- Bluml S, Bonelli M, Niederreiter B, Puchner A, Mayr G, Hayer S, Koenders MI, van den Berg WB, Smolen J, Redlich K. Essential role of microRNA-155 in the pathogenesis of autoimmune arthritis in mice. Arthritis Rheum. 2011;63:1281–1288. doi: 10.1002/art.30281. [DOI] [PubMed] [Google Scholar]
- Bolisetty MT, Dy G, Tam W, Beemon KL. Reticuloendotheliosis virus strain T induces miR-155, which targets JARID2 and promotes cell survival. J Virol. 2009;83:12009–12017. doi: 10.1128/JVI.01182-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- Bulger M, Groudine M. Functional and mechanistic diversity of distal transcription enhancers. Cell. 2011;144:327–339. doi: 10.1016/j.cell.2011.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS, Ma L, Watowich SS, Jetten AM, Tian Q, Dong C. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30:576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciofani M, Madar A, Galan C, Sellars M, Mace K, Pauli F, Agarwal A, Huang W, Parkurst CN, Muratet M, et al. A validated regulatory network for Th17 cell specification. Cell. 2012;151:289–303. doi: 10.1016/j.cell.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb BS, Hertweck A, Smith J, O’Connor E, Graf D, Cook T, Smale ST, Sakaguchi S, Livesey FJ, Fisher AG, Merkenschlager M. A role for Dicer in immune regulation. J Exp Med. 2006;203:2519–2527. doi: 10.1084/jem.20061692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudakov JA, Hanash AM, Jenq RR, Young LF, Ghosh A, Singer NV, West ML, Smith OM, Holland AM, Tsai JJ, et al. Interleukin-22 Drives Endogenous Thymic Regeneration in Mice. Science. 2012 doi: 10.1126/science.1218004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar T, Yu CR, Muljo SA, Egwuagu CE. STAT3 activates miR-155 in Th17 cells and acts in concert to promote experimental autoimmune uveitis. Invest Ophthalmol Vis Sci. 2013;54:4017–4025. doi: 10.1167/iovs.13-11937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami R, Kaplan MH. A brief history of IL-9. J Immunol. 2011;186:3283–3288. doi: 10.4049/jimmunol.1003049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Wei G, Zhu J, Liao W, Leonard WJ, Zhao K, Paul W. IL-1 family members and STAT activators induce cytokine production by Th2, Th17, and Th1 cells. Proc Natl Acad Sci U S A. 2009;106:13463–13468. doi: 10.1073/pnas.0906988106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman MG, Lu D, Kim ML, Kociba GJ, Shukri T, Buteau J, Wang X, Frankel WL, Guttridge D, Prentki M, et al. Role for activating transcription factor 3 in stress-induced beta-cell apoptosis. Mol Cell Biol. 2004;24:5721–5732. doi: 10.1128/MCB.24.13.5721-5732.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno Y, Vahedi G, Hirahara K, Singleton K, O’Shea JJ. Transcriptional and Epigenetic Control of T Helper Cell Specification: Molecular Mechanisms Underlying Commitment and Plasticity. Annu Rev Immunol. 2011 doi: 10.1146/annurev-immunol-020711-075058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Kang K, Kim J. AEBP2 as a potential targeting protein for Polycomb Repression Complex PRC2. Nucleic Acids Res. 2009;37:2940–2950. doi: 10.1093/nar/gkp149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlhaas S, Garden OA, Scudamore C, Turner M, Okkenhaug K, Vigorito E. Cutting edge: the Foxp3 target miR-155 contributes to the development of regulatory T cells. J Immunol. 2009;182:2578–2582. doi: 10.4049/jimmunol.0803162. [DOI] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krek A, Grun D, Poy MN, Wolf R, Rosenberg L, Epstein EJ, MacMenamin P, da Piedade I, Gunsalus KC, Stoffel M, Rajewsky N. Combinatorial microRNA target predictions. Nat Genet. 2005;37:495–500. doi: 10.1038/ng1536. [DOI] [PubMed] [Google Scholar]
- Landeira D, Sauer S, Poot R, Dvorkina M, Mazzarella L, Jorgensen HF, Pereira CF, Leleu M, Piccolo FM, Spivakov M, et al. Jarid2 is a PRC2 component in embryonic stem cells required for multi-lineage differentiation and recruitment of PRC1 and RNA Polymerase II to developmental regulators. Nature cell biology. 2010;12:618–624. doi: 10.1038/ncb2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PP, Fitzpatrick DR, Beard C, Jessup HK, Lehar S, Makar KW, Perez-Melgosa M, Sweetser MT, Schlissel MS, Nguyen S, et al. A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity. 2001;15:763–774. doi: 10.1016/s1074-7613(01)00227-8. [DOI] [PubMed] [Google Scholar]
- Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell. 2003;115:787–798. doi: 10.1016/s0092-8674(03)01018-3. [DOI] [PubMed] [Google Scholar]
- Li G, Margueron R, Ku M, Chambon P, Bernstein BE, Reinberg D. Jarid2 and PRC2, partners in regulating gene expression. Genes Dev. 2010;24:368–380. doi: 10.1101/gad.1886410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Isono K, Yamada D, Endo TA, Endoh M, Shinga J, Mizutani-Koseki Y, Otte AP, Casanova M, Kitamura H, et al. Mammalian polycomb-like Pcl2/Mtf2 is a novel regulatory component of PRC2 that can differentially modulate polycomb activity both at the Hox gene cluster and at Cdkn2a genes. Mol Cell Biol. 2011;31:351–364. doi: 10.1128/MCB.00259-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesenfeld O. Oral infection of C57BL/6 mice with Toxoplasma gondii: a new model of inflammatory bowel disease? J Infect Dis. 2002;185(Suppl 1):S96–101. doi: 10.1086/338006. [DOI] [PubMed] [Google Scholar]
- Loeb GB, Khan AA, Canner D, Hiatt JB, Shendure J, Darnell RB, Leslie CS, Rudensky AY. Transcriptome-wide miR-155 binding map reveals widespread noncanonical microRNA targeting. Mol Cell. 2012;48:760–770. doi: 10.1016/j.molcel.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu LF, Thai TH, Calado DP, Chaudhry A, Kubo M, Tanaka K, Loeb GB, Lee H, Yoshimura A, Rajewsky K, Rudensky AY. Foxp3-dependent microRNA155 confers competitive fitness to regulatory T cells by targeting SOCS1 protein. Immunity. 2009;30:80–91. doi: 10.1016/j.immuni.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner JD, Brenchley JM, Laurence A, Freeman AF, Hill BJ, Elias KM, Kanno Y, Spalding C, Elloumi HZ, Paulson ML, et al. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 2008;452:773–776. doi: 10.1038/nature06764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muljo SA, Ansel KM, Kanellopoulou C, Livingston DM, Rao A, Rajewsky K. Aberrant T cell differentiation in the absence of Dicer. J Exp Med. 2005;202:261–269. doi: 10.1084/jem.20050678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugaiyan G, Beynon V, Mittal A, Joller N, Weiner HL. Silencing microRNA-155 ameliorates experimental autoimmune encephalomyelitis. J Immunol. 2011;187:2213–2221. doi: 10.4049/jimmunol.1003952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, Tian Q, Watowich SS, Jetten AM, Dong C. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- O’Connell RM, Chaudhuri AA, Rao DS, Baltimore D. Inositol phosphatase SHIP1 is a primary target of miR-155. Proc Natl Acad Sci U S A. 2009;106:7113–7118. doi: 10.1073/pnas.0902636106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell RM, Kahn D, Gibson WS, Round JL, Scholz RL, Chaudhuri AA, Kahn ME, Rao DS, Baltimore D. MicroRNA-155 promotes autoimmune inflammation by enhancing inflammatory T cell development. Immunity. 2010;33:607–619. doi: 10.1016/j.immuni.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertli M, Engler DB, Kohler E, Koch M, Meyer TF, Muller A. MicroRNA-155 is essential for the T cell-mediated control of Helicobacter pylori infection and for the induction of chronic Gastritis and Colitis. J Immunol. 2011;187:3578–3586. doi: 10.4049/jimmunol.1101772. [DOI] [PubMed] [Google Scholar]
- Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol. 2011;29:71–109. doi: 10.1146/annurev-immunol-031210-101312. [DOI] [PubMed] [Google Scholar]
- Pasini D, Cloos PA, Walfridsson J, Olsson L, Bukowski JP, Johansen JV, Bak M, Tommerup N, Rappsilber J, Helin K. JARID2 regulates binding of the Polycomb repressive complex 2 to target genes in ES cells. Nature. 2010;464:306–310. doi: 10.1038/nature08788. [DOI] [PubMed] [Google Scholar]
- Peng JC, Valouev A, Swigut T, Zhang J, Zhao Y, Sidow A, Wysocka J. Jarid2/Jumonji coordinates control of PRC2 enzymatic activity and target gene occupancy in pluripotent cells. Cell. 2009;139:1290–1302. doi: 10.1016/j.cell.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee HS, Pugh BF. ChIP-exo method for identifying genomic location of DNA-binding proteins with near-single-nucleotide accuracy. Curr Protoc Mol Biol. 2012;Chapter 21(Unit 21):24. doi: 10.1002/0471142727.mb2124s100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez A, Vigorito E, Clare S, Warren MV, Couttet P, Soond DR, van Dongen S, Grocock RJ, Das PP, Miska EA, et al. Requirement of bic/microRNA-155 for normal immune function. Science. 2007;316:608–611. doi: 10.1126/science.1139253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez CI, Buchholz F, Galloway J, Sequerra R, Kasper J, Ayala R, Stewart AF, Dymecki SM. High-efficiency deleter mice show that FLPe is an alternative to Cre-loxP. Nat Genet. 2000;25:139–140. doi: 10.1038/75973. [DOI] [PubMed] [Google Scholar]
- Rutz S, Noubade R, Eidenschenk C, Ota N, Zeng W, Zheng Y, Hackney J, Ding J, Singh H, Ouyang W. Transcription factor c-Maf mediates the TGF-beta-dependent suppression of IL-22 production in T(H)17 cells. Nature immunology. 2011;12:1238–1245. doi: 10.1038/ni.2134. [DOI] [PubMed] [Google Scholar]
- Shaw MH, Kamada N, Kim YG, Nunez G. Microbiota-induced IL-1beta, but not IL-6, is critical for the development of steady-state TH17 cells in the intestine. J Exp Med. 2012;209:251–258. doi: 10.1084/jem.20111703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X, Kim W, Fujiwara Y, Simon MD, Liu Y, Mysliwiec MR, Yuan GC, Lee Y, Orkin SH. Jumonji modulates polycomb activity and self-renewal versus differentiation of stem cells. Cell. 2009;139:1303–1314. doi: 10.1016/j.cell.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su IH, Dobenecker MW, Dickinson E, Oser M, Basavaraj A, Marqueron R, Viale A, Reinberg D, Wulfing C, Tarakhovsky A. Polycomb group protein ezh2 controls actin polymerization and cell signaling. Cell. 2005;121:425–436. doi: 10.1016/j.cell.2005.02.029. [DOI] [PubMed] [Google Scholar]
- Thai TH, Calado DP, Casola S, Ansel KM, Xiao C, Xue Y, Murphy A, Frendewey D, Valenzuela D, Kutok JL, et al. Regulation of the germinal center response by microRNA-155. Science. 2007;316:604–608. doi: 10.1126/science.1141229. [DOI] [PubMed] [Google Scholar]
- Vahedi G, Takahashi H, Nakayamada S, Sun HW, Sartorelli V, Kanno Y, O’Shea JJ. STATs shape the active enhancer landscape of T cell populations. Cell. 2012;151:981–993. doi: 10.1016/j.cell.2012.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dongen S, Abreu-Goodger C, Enright AJ. Detecting microRNA binding and siRNA off-target effects from expression data. Nat Methods. 2008;5:1023–1025. doi: 10.1038/nmeth.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld JC, Stockinger B. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Wei G, Wei L, Zhu J, Zang C, Hu-Li J, Yao Z, Cui K, Kanno Y, Roh TY, Watford WT, et al. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity. 2009;30:155–167. doi: 10.1016/j.immuni.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, Levy-Lahad E, Mazzella M, Goulet O, Perroni L, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- Wilson MS, Feng CG, Barber DL, Yarovinsky F, Cheever AW, Sher A, Grigg M, Collins M, Fouser L, Wynn TA. Redundant and pathogenic roles for IL-22 in mycobacterial, protozoan, and helminth infections. J Immunol. 2010;184:4378–4390. doi: 10.4049/jimmunol.0903416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Yang Y, Qiu G, Lal G, Wu Z, Levy DE, Ochando JC, Bromberg JS, Ding Y. c-Maf regulates IL-10 expression during Th17 polarization. J Immunol. 2009;182:6226–6236. doi: 10.4049/jimmunol.0900123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yosef N, Shalek AK, Gaublomme JT, Jin H, Lee Y, Awasthi A, Wu C, Karwacz K, Xiao S, Jorgolli M, et al. Dynamic regulatory network controlling T17 cell differentiation. Nature. 2013 doi: 10.1038/nature11981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenewicz LA, Flavell RA. Recent advances in IL-22 biology. Int Immunol. 2011;23:159–163. doi: 10.1093/intimm/dxr001. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, Ouyang W. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- Zhu J, Paul WE. Peripheral CD4+ T-cell differentiation regulated by networks of cytokines and transcription factors. Immunol Rev. 2010;238:247–262. doi: 10.1111/j.1600-065X.2010.00951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations. Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.