Abstract
New strategies to treat antibiotic-resistant infections are urgently needed. We serendipitously discovered that stem cell conditioned media possessed broad antimicrobial properties. Biochemical, functional, and genetic assays confirmed that the antimicrobial effect was mediated by supra-physiological concentrations of transferrin. Human transferrin inhibited growth of gram-positive (Staphylococcus aureus), gram-negative (Acinetobacter baumannii), and fungal (Candida albicans) pathogens by sequestering iron and disrupting membrane potential. Serial passage in subtherapeutic transferrin concentrations resulted in no emergence of resistance. Infected mice treated with intravenous human transferrin had improved survival and reduced microbial burden. Finally, adjunctive transferrin reduced the emergence of rifampin-resistant mutants of S. aureus in infected mice treated with rifampin. Transferrin is a promising, novel antimicrobial agent that merits clinical investigation. These results provide proof of principle that bacterial infections can be treated in vivo by attacking host targets (ie, trace metal availability) rather than microbial targets.
Keywords: transferrin, iron, Staphylococcus aureus, Acinetobacter baumannii, Candida albicans, in vivo treatment
The ongoing crisis of antibiotic resistance demands new methods to prevent and treat infections caused by highly resistant organisms [1, 2]. Although new antibiotics are critically needed, ultimately resistance will develop to any new antibiotic developed. Thus, policy advocates and thought leaders have called for increased exploration of novel anti-infective strategies that are less likely to induce resistance, such as by stimulation of host defenses rather than directly attacking microbial targets [1, 3].
Virtually all microbial pathogens require exogenous sources of iron in order to survive and grow [4–13]. Numerous investigators over many years have described small molecule and biological iron sequestering agents that can inhibit the growth of microbes in vitro [5, 6, 14–19]. In particular, transferrin has long been known to restrict microbial growth in vitro due to its iron sequestration capacity [20–25]. However, to date, despite these in vitro findings, no viable iron-blockade option has been successfully deployed clinically for the treatment of infections, and a recent randomized, controlled trial of patients with mucormycosis suggested that small molecule-mediated iron chelation may not be as effective as previously hoped [26].
While exploring stem cell cultures, we incidentally noticed that conditioned media had cross-kingdom (bacterial and fungal) antimicrobial properties, reversible with iron, that were not present in control media. Detailed biochemical, genetic, microbiological, and in vivo efficacy studies were undertaken to define the mechanism of effect and potential therapeutic impact of the identified antimicrobial effector.
MATERIALS AND METHODS
Stem Cell Culture and Culture Supernatant Collection
In total, 129/SVEV mouse embryonic stem cells (MES, Millipore) and NGFP2 primary induced pluripotent stem (iPS, Stemgent) were grown without antibiotics in Knockout DMEM medium (Invitrogen) supplemented with fetal bovine serum (FBS) and 5 × 102 U/mL mouse leukemia inhibitory factor (LIF; Stemgent).
Microbial Strains and Culture Conditions
Candida albicans SC5314, Staphylococcus aureus LAC (MRSA, USA 300), and Acinetobacter baumannii HUMC1 (carbapenem-resistant) are clinical bloodstream isolates that are highly virulent in murine models of infection [27, 28]. Microbes were passaged to log phase at 37°C, washed, and 107 were incubated for 1 hour at 37°C in stem cell conditioned or control media. Tubes containing C. albicans but not the bacteria were sonicated.
Apo-transferrin (iron-depleted), used for all experiments and heretofore referred to as transferrin, was generated by resuspending transferrin powder (Sigma) in saline at pH 6.55, rinsing 3 times in a 30 kD centrifugal filter spin column (Millipore) with saline at pH 5.8, followed by 3 rinses in phosphate-buffered saline (PBS). Iron levels were then tested with the QuantiChromTM Iron Assay Kit (BioAssay systems) to ensure that the levels were below the limit of detection (27 µg/dL = 4.8 µM). Mouse and human transferrin was added to the microbes with or without deferoxamine (225 µM; Sigma-Aldrich) or enterobactin (112.5 µM; Sigma-Aldrich), ferric chloride (FeCl3, 100 µM, Sigma-Aldrich), or anti-transferrin antibody (10 µg/mL, Sigma-Aldrich).
The minimum inhibitory concentration (MIC) of transferrin and time-kill assays were conducted by passaging S. aureus LAC and A. baumannii HUMC1 in Mueller Hinton II Broth (MH-II) to an optical density of 0.5 at 37°C with shaking. C. albicans was grown overnight in yeast peptone dextrose (YPD) at room temperature in a rotor. Bacteria or C. albicans were diluted to106 or 2 × 105 cells/mL, respectively, in Roswell Park Memorial Institute medium (RPMI) 1640 medium (MOPS was added for C. albicans), consistent with standard CLSI methodologies. Transferrin was serially diluted across the plate, which was incubated at 37°C (or 35°C for C. albicans) for 20 hours. The minimum inhibitory concentration (MIC) value was read as the lowest concentration that prevented visible growth of bacteria, or 90% inhibition of visible growth for C. albicans. The minimum bactericidal concentration (MBC) was defined as the concentration of transferrin at which a 1000-fold reduction in bacterial concentration was achieved at 24 hours. Transferrin was considered static if it did not achieve an MBC against a target pathogen or if the MBC was >4-fold above the MIC for the target pathogen [29, 30].
HPLC and MALDI-TOF Analysis
Five-day stem cell conditioned media or unconditioned control media were analyzed by HPLC at the UCLA Department of Pathology HT Clinical Proteomics Core. Fractions were separated by HPLC using a Mono Q 5/50 GL strong anion exchange column (GE Healthcare). A gradient was run from 50 mM NaCl to 1M NaCl (in 50 mM Tris pH 8.0) over 40 minutes at 0.5 mL/minutes at RT while collecting 0.4 mL fractions.
A polyacrylamide gel was run with the fraction 79 from control and 5-day conditioned media and stained with SilverSNAP Stain (Pierce). Protein slices of interest were excised and sent to the UCLA W. M. Keck Proteomic Center for identification on a Thermo LTQ-Orbitrap XL mass spectrometer (San Jose, CA) equipped with an Eksigent (Dublin, CA) NanoLiquid chromatography-1D plus system and an Eksigent autosampler.
Transferrin ELISA and siRNA
Stem cells culture supernatants were analyzed for transferrin content using a quantitative mouse enzyme-linked immunosorbent assay (ELISA) kit (KAMIYA Biomedical Company) according to the manufacturer's instructions. The plate was read using a spectrophotometer (BioTek Instruments, Inc) at 450 nm.
Transferrin target siRNA and nontarget a scrambled control siRNA were purchased from Sigma-Aldrich. The siRNA fragment sequence used was A 5″-GGAAUAUAAUGGUUACA CA-3′. The designed scrambled control siRNA did not target any known gene in the cells. The siRNA transfection was performed with siRNA transfection reagents (Santa Cruz Biotechnology) according to the manufacturer's instructions.
RNA Isolation and Reverse Transcription Polymerase Chain Reaction
Total RNA was prepared from stem cells with an RNA isolation kit (Ambion). The RNA was reverse-transcribed with oligo(dT) primer using the SuperScript First-Strand Synthesis System (Invitrogen) to generate first-strand cDNA. RT-PCR was used to detect the expression of transferrin and G3PDH (glyceraldehyde-3-phosphate dehydrogenase). The PCR primers were:
Forward primer: 5′-ATGAGGCTCACCGTGGGTGC-3′ and Reverse primer 5′-TTAATGTTTGTGGAAAGTGC-3′. The PCR products were separated on a 1% agarose gel containing 0.1 µg/mL ethidium bromide.
Membrane Potential and Microbial Iron Content
To quantify iron content in the microbes, 5 × 105 cells/mL of S. aureus, A. baumannii, and 1 × 105 cells/mL C. albicans were prepared as for the growth inhibitory assays and incubated with various concentrations of human transferrin with or without 100 µM ferric chloride (1.5 molar ratio to transferrin). In some experiments, microbes were separated from transferrin by a 10 kD size exclusion membrane (Thermo scientific). At serial time points, microbial membrane potential was analyzed using the BacLight Bacterial Membrane Potential kit (Molecular Probes, Eugene, OR).
Iron content in the microbes was quantified by a modification of a previously published method [31], using HR-ICPMS (Thermo Fisher Scientific, Bremen, Germany). In brief, bacterial pellets were dried by incubation at 50°C overnight and then digested by boiling in nitric acid for 6 hours at 130°C. Elemental quantification was performed on the Thermo Element 2 HR-ICPMS (Thermo Fisher Scientific, Bremen, Germany) coupled with ESI auto sampler (Elemental Scientific, Omaha, NE). The HR-ICPMS is equipped with a PFA microflow nebulizer (Elemental Scientific, Omaha, NE), a double channel spray chamber (at room temperature), a magnetic sector followed by an electric sector, and a second electron multiplier.
In vivo Infection Models
Male BALB/c mice (7–8 weeks) were infected via the tail-vein with C. albicans SC5314 or S. aureus LAC. C3H/FeJ mice were infected via the tail-vein with A. baumannii HUMC1. Transferrin therapy was administered intravenously within 1 hour after infection and then once daily for 3 additional days (total of 4 days). Some mice were also treated with rifampin (20 mg/kg ip once daily for 4 days) or with ferric chloride (0.4 mg/kg intraperitoneal). All animal experiments were approved by the Institutional Committee on the Use and Care of Animals at the Los Angeles Biomedical Research Institute, following the National Institutes of Health guidelines for animal housing and care.
To determine frequency of rifampin-escape mutants, kidney homogenates (in PBS) were passaged in tryptic soy broth (TSB) overnight (without rifampin selective-pressure) and then plated at a density of 108 per plate with or without 8 µg/mL of rifampin to detect escape mutant colonies.
Statistics
Continuous variables were compared using the Mann–Whitney U test for nonparametric, unpaired comparisons. Survival curves were compared using the nonparametric log rank test. P values of < .05 were considered significant.
Results
Murine Transferrin Inhibits Microbial Growth
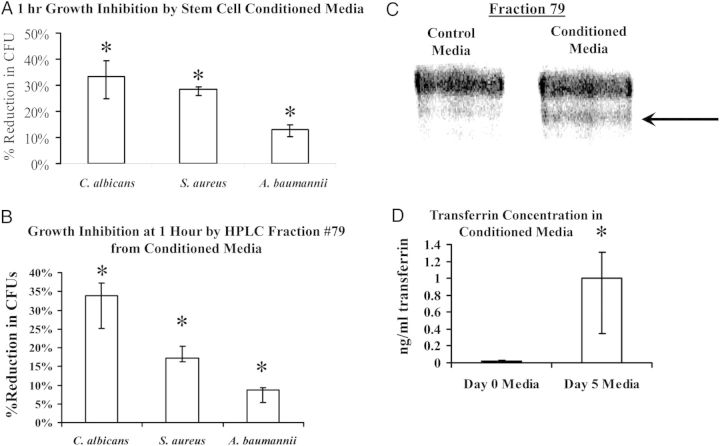
During routine culturing of mouse embryonic stem (MES) cells in media containing no antibiotics, we incidentally noted that exposure of S. aureus to conditioned media inhibited bacterial growth. We subsequently confirmed that 5-day MES conditioned media significantly reduced viable colony forming units (CFUs) of S. aureus, A. baumannii, and C. albicans at 1 hour (Figure 1A). Conditioned or control media were separated into 88 fractions by HPLC, and 12 were collected for analysis that differed between conditioned and control media. Of the 12 fractions, only number 79 possessed 1-hour antimicrobial effects similar to that of 5-day conditioned media (Figure 1B).
Figure 1.
Stem cell conditioned media had broad antimicrobial effects. A, Growth inhibition of bacteria or fungi after 1 hour in cell culture media harvested after 5 days of MES culture. *P < .05 vs control culture without transferrin. B, HPLC-purified fraction 79 recapitulated conditioned media growth inhibitory effects. C, Silver-stained PAGE gel of Fraction 79 revealed a single band distinguishing conditioned from control media. D, After identification by MALDI-TOF of transferrin in the Fraction 79 band from conditioned media, ELISA confirmed the presence of higher quantities of transferrin in conditioned than control media). *P < .05 vs control. Abbreviations: ELISA, enzyme-linked immunosorbent assay; MES, mouse embryonic stem cell.
Polyacrylamide gels of fraction 79 from control and 5-day conditioned media stained with SilverSNAP Stain (Pierce) revealed a band with substantially higher density in the conditioned than the control media (Figure 1C). We excised the band and used MALDI-TOF to identify it as mouse transferrin. To confirm that stem cells secreted transferrin, we quantified transferrin levels in conditioned vs control media by quantitative ELISA. The concentration of mouse transferrin in day 5 conditioned media was >50-fold greater than in control media (Figure 1D).
Transferrin Mediates Effects by Iron Starvation
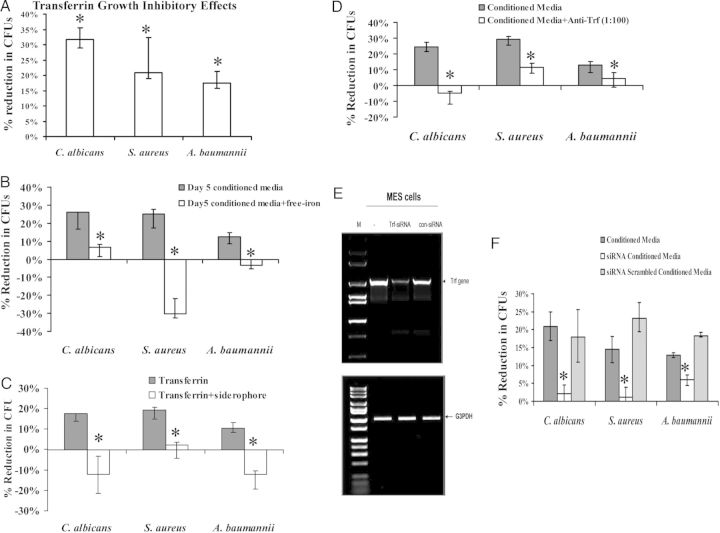
Addition of mouse transferrin at 1 ng/mL (comparable to levels in conditioned media) resulted in 1-hour growth inhibition of all 3 organisms similar to that achieved with 5-day conditioned media (Figure 2A). Growth inhibition was reversed by addition of free iron or exogenous iron-loaded siderophores (Figure 2B and 2C). Furthermore, anti-transferrin antibody substantially reduced antimicrobial effects of conditioned media against S. aureus, A. baumannii, and C. albicans (Figure 2D). Finally, we sought to corroborate these results by using siRNA to knockdown expression of transferrin in stem cells during culture. siRNA successfully reduced transferrin mRNA (Figure 2E) and reduced the ability of 5-day conditioned media to inhibit the growth of S. aureus, A. baumannii, and C. albicans. However, the scrambled negative control siRNA sequence had no effect on microbial growth inhibition (Figure 2F).
Figure 2.
Transferrin mediated broad growth inhibitory effects by restricting access to free iron. A, Reduction in microbial CFUs at 1 hour by mouse transferrin. B, Free iron (FeCl3) was added into conditioned media which was able to reverse microbial growth inhibition. C, Reversal of growth inhibition by addition of exogenous iron siderophores (deferoxamine for Staphylococcus aureus and Candida albicans, and enterobactin for Acinetobacter baumannii). D–F, Growth inhibition assays with conditioned media were repeated in the presence of anti-transferrin monoclonal antibody (D) or after transfection of stem cells with anti-transferrin siRNA constructs that suppressed Trf gene expression (E) and blocked growth inhibitory effects (F). *P < .05 vs control. Abbreviation: CFU, colony-forming unit.
Human Transferrin Had Broad Antimicrobial Effects
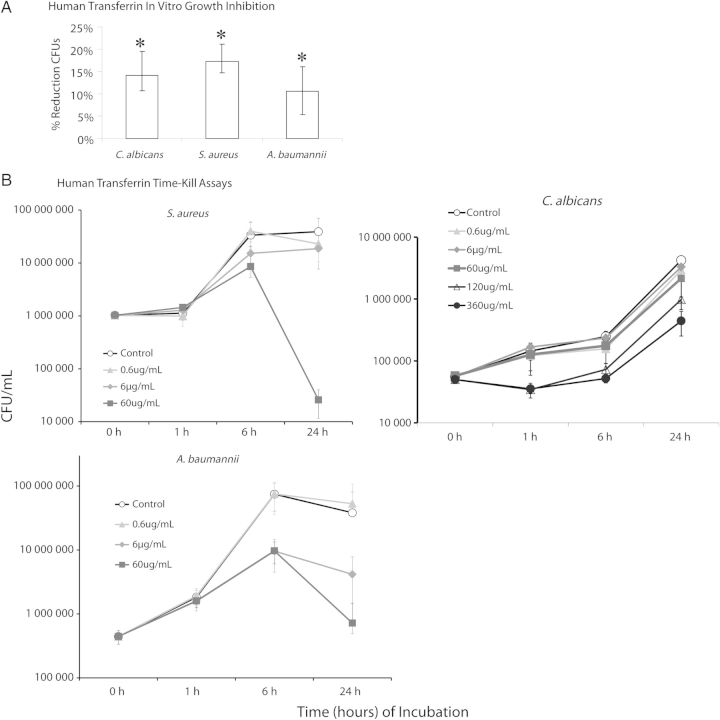
To focus on translational relevance, we repeated the growth inhibitory assays with human transferrin. Like murine transferrin, exposure to 1 ng/mL of human transferrin for 1 hour significantly reduced viable S. aureus, A. baumanii, and C. albicans CFUs (Figure 3A). We then performed a dose response experiment to determine the MIC of human transferrin. The human transferrin MIC was 6 µg/mL for S. aureus and A. baumannii, and 60 µg/mL for C. albicans.
Figure 3.
Human recombinant transferrin had similar broad spectrum antimicrobial effects, mediated by iron starvation. A, One-hour growth inhibition assays were repeated using human transferrin (instead of murine transferrin). B, Time-kill assay using human transferrin for all 3 pathogens. *P < .05 vs control.
To determine potential for selection of resistant mutants of the microbes, we serially passaged all 3 organisms 20 times in the presence of half-MIC concentrations of human transferrin (3 µg/mL for S. aureus and A. baumannii and 30 µg/mL for C. albicans). Afterward we remeasured the transferrin MICs and found them to be identical (6 µg/mL for S. aureus and A. baumannii and 60 µg/mL for C. albicans).
In dose response, time-kill assays we found that human transferrin exhibited static activity against all 3 pathogens (Figure 3B). For all 3 organisms, growth curves diverged substantially by 6 hours. At the 60 µg/mL concentration, transferrin mediated a >1000-fold reduction in S. aureus CFUs at 24 hours compared to growth control. For A. baumannii, both the 6 and 60 µg/mL concentration mediated 10–100-fold reductions in CFUs/mL at 24 hours compared to growth control. The effect for C. albicans was more complex, possibly reflective of the higher MIC of the organism and the need to use a different temperature for the prolonged incubation to avoid substantial hyphal formation that would make CFU quantification impossible. We therefore tested 2 higher concentrations against C. albicans. The 120 and 360 µg/mL concentrations exhibited substantial static inhibition of growth at all time points, whereas the 60 µg/mL dose resulted in a small divergence in growth at 6 hours that extended to 24 hours.
Transferrin and Cellular Physiology
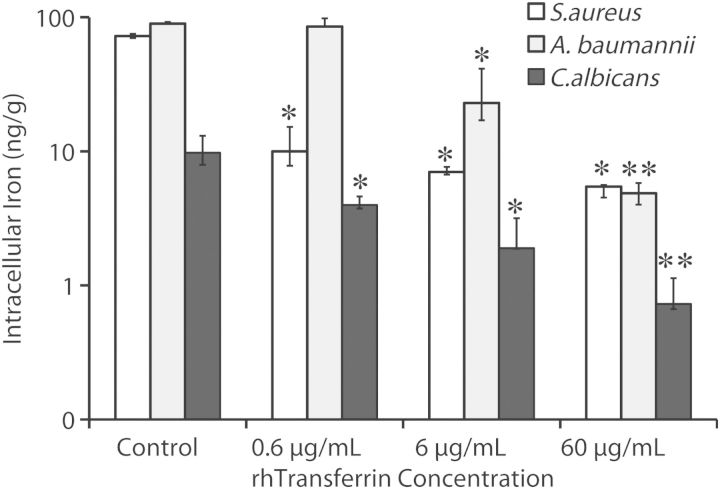
We sought to define the impact of transferrin exposure on intracellular iron levels in the microbes. Exposure to human transferrin resulted in marked reductions in intracellular iron content of all 3 organisms in a dose-dependent manner (Figure 4).
Figure 4.
Human transferrin reduced intracellular iron content. Staphylococcus aureus LAC, Acinetobacter baumannii HUMC1, or Candida albicans SC5314 were cultured in media for 6 hours in the presence or absence of escalating concentrations of human transferrin prior to measurement of trace metal concentrations.
Iron is a critical electron acceptor in the oxidative phosphorylation cascade that leads to ATP generation in both prokaryotes and eukaryotes, and maintenance of transmembrane potential critically depends on ATP generation [32]. We therefore hypothesized that transferrin exposure might disrupt transmembrane potential by sequestration of iron from the organisms. We conducted a flow cytometric-based assay to measure transmembrane potential in the 3 pathogens in the presence of varying concentrations of transferrin. Transferrin dose-dependently disrupted membrane potential as early as 1 hour, with increased effect at 6 hours for all 3 pathogens (Figure 5). In order to determine if the transferrin had any direct effects on the microbes that required direct contact of the protein, we repeated the assay but with separation of the transferrin from the bacteria by a 10 kD size exclusion membrane (which prevents transferrin diffusion). Despite being physically separated from the microbes, transferrin mediated substantial loss of membrane potential, which was totally reversed by supplementation with iron.
Figure 5.
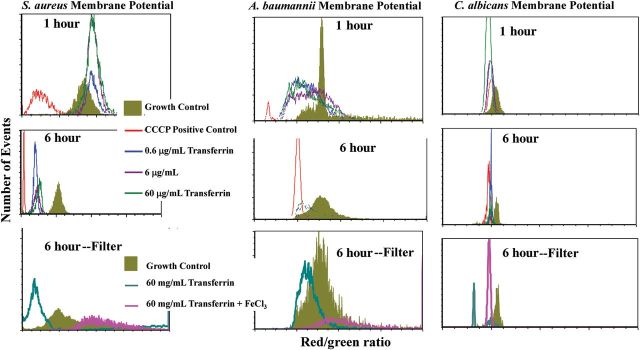
Transferrin disrupted transmembrane potential of the microbes, which was reversible with exogenous iron. Staphylococcus aureus LAC, Acinetobacter baumannii HUMC1, or Candida albicans SC5314 were cultured in media for 6 hours in the presence or absence of escalating concentrations of human transferrin prior to measurement of trace metal concentrations. Membrane potential of the microbes after exposure to human transferrin for 1 or 6 hours. Loss of membrane potential was seen as early as 1 hour for A. baumannii and C. albicans but first became apparent at 6 hours for S. aureus. The 6-hour time point was repeated with use of a 10 kD size-exclusion membrane filter to physically separate the transferrin from the microbes. The loss of membrane potential caused by the transferrin under these conditions demonstrated that transferrin did not need to physically contact the microbes to mediate the effect, and the effect was reversed by addition of saturating concentrations of iron. CCC P = carbonyl cyanide m-chlorophenyl hydrazine, a positive control that uncouples the proton gradient driving oxidative phosphorylation. Medians and interquartile ranges are shown. *P < .05 vs control, **P < .05 vs all other groups.
In vivo Effects of Human Transferrin
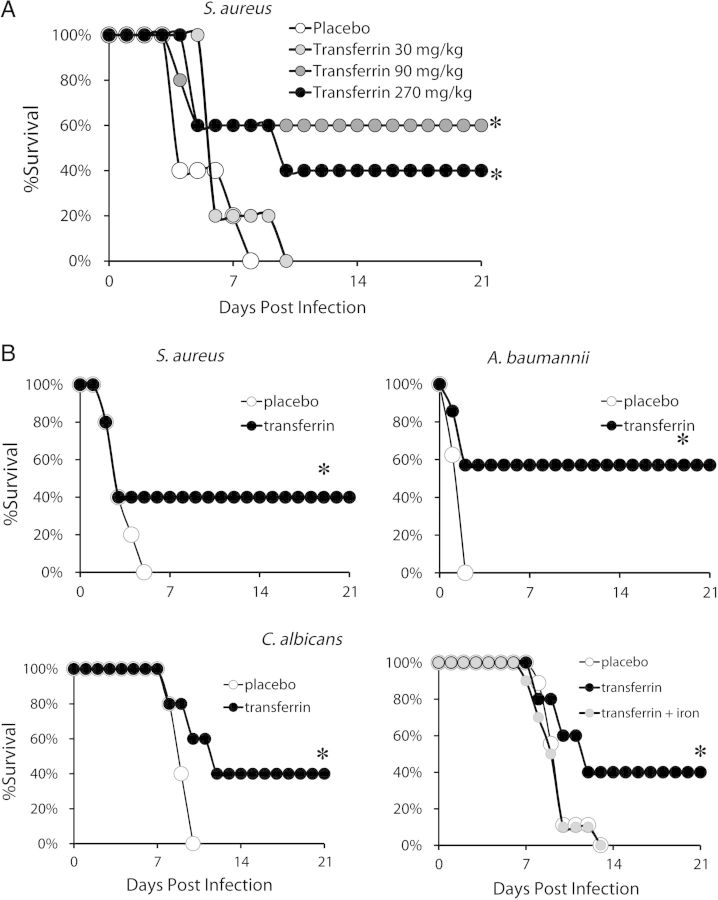
To define appropriate in vivo dosing, we conducted an initial dose response in vivo in mice during S. aureus infection. When we gave mice a lethal inoculum of intravenous methicillin-resistant S. aureus (MRSA) and treated with 3-fold dose escalation of human transferrin, the 2 higher doses substantially improved survival (Figure 6A).
Figure 6.
In vivo effects of human transferrin during lethal infection in mice. A, Mice (n = 5 per group) were treated with 30, 90, and 270 mg/kg intravenously of human transferrin once daily for total 3 days. B, Human transferrin (90 mg/kg/d × 4 d) administered to BALB/c mice (N = 10 per group) and infected with 5 × 107 Staphylococcus aureus LAC or 105 Candida albicans SC5314, or administered to C3H/FeJ mice (n = 10 per group) and infected with 2 × 107 Acinetobacter baumannii HUMC1. The experiments were terminated at 21 days with all remaining mice appearing clinically well. Some mice were also treated with 0.4 mg/kg of FeCl3 to saturate transferrin with free iron. *P < .05 vs control.
Because the 90 and 270 mg/kg doses of transferrin appeared similarly effective, we tested the 90 mg/kg dose against all 3 pathogens. We administered lethal intravenous challenges of S. aureus, A. baumannii, and C. albicans and treated with human transferrin at 90 mg/kg/d. Four once daily doses of intravenous human transferrin markedly improved murine survival against all 3 infections (Figure 6B). To confirm the mechanism of in vivo efficacy, we infected mice with C. albicans and treated with placebo, transferrin, or transferrin plus free iron (ferric chloride). Transferrin was effective, but addition of ferric chloride reversed the efficacy of the transferrin (Figure 6B).
To determine the impact of transferrin on blood and tissue bacterial burden during infection, we infected and treated mice with 4 doses of transferrin as above. We also sought to determine if transferrin mediated additive benefit with background antibacterial therapy. We selected rifampin as the antibiotic to test because emergence of resistance occurs rapidly during monotherapy due to the single step mutation that results in high level resistance [33]. Rifampin was only tested against S. aureus, because it is not typically used to treat the other pathogens. In in vitro testing, a sub-MIC concentration of transferrin (3 µg/mL) synergized with rifampin, decreasing the S. aureus LAC MIC of rifampin from 0.15 µg/mL to 0.019 µg/mL (8-fold decrease).
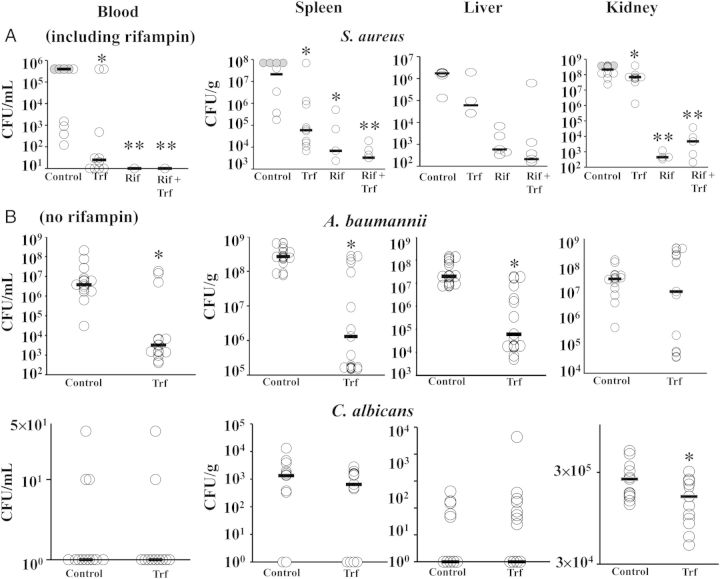
During infection in mice, transferrin significantly reduced blood, spleen, and liver CFUs for S. aureus (Figure 7A) and A. baumannii (Figure 7B). Transferrin reduced kidney CFUs for S. aureus and C. albicans but not A. baumannii. C. albicans CFUs in blood, liver, and spleen were low and not significantly different for both treatment groups (Figure 7B).
Figure 7.
Human transferrin reduced in vivo microbial density. A, Mice (N = 10 per group from 2 experiments) were infected with Staphylococcus aureus and treated with placebo, rhTransferrin, rifampin, or combination therapy and euthanized after 4 doses (4th day post-infection). Gray circles reflect S. aureus infected control mice that died shortly before collection of tissues. B, Mice (N = 13–15 per group from 2 experiments) were infected with Acinetobacter baumannii or Candida albicans infection and treated with rhTransferrin or placebo. CFUs were quantified after 2 doses for A. baumannii (due to more rapid onset of death in the control mice) or 4 doses for C. albicans. Bars show median values, and the lower bound of the Y axis is set at the limit of detection based on dilutions used. *P < .05 vs control; **P < .05 vs control and transferrin alone. Trf = human transferrin; Rif = rifampin. Abbreviation: CFU, colony-forming unit.
Rifampin alone was highly effective at reducing CFUs of S. aureus, as was combination therapy (Figure 7A). The low number of organisms in the rifampin and combination therapy-treated mice also made it difficult to detect resistant mutants directly from organ homogenates. Therefore, organ homogenates were subcultured in liquid broth (without rifampin) overnight to expand the number of organisms. The expanded organisms were then plated on agar with or without 8 µg/mL of rifampin to detect the frequency of rifampin-resistant escape mutants. Due to variance in the escape mutant frequency across organs (spleen vs liver vs kidney) and across experiments, the escape mutant frequency was normalized to the frequency in the control (placebo-treated) organs within each experiment. Organs from mice treated with rifampin alone had a median (IQ range) 83% (49%–102%) increase in the frequency of rifampin-resistant S. aureus escape mutants compared to organs from placebo-treated mice (P = .004), which was more than twice the increase in frequency seen in organs from mice treated with rifampin plus transferrin (34% [19%–70%], P = .003 vs rifampin alone).
DISCUSSION
The current crisis of antibiotic resistance, combined with the inadequate pipeline of small molecule antibiotics, has created an increasingly urgent need for novel antimicrobial strategies, particularly strategies that are less likely to drive resistance [1, 3]. Iron has long been known to be essential for the growth and viability of virtually all microbes [4–12, 34]. However, in vivo testing of iron chelation strategies has focused on eukaryotic pathogens (eg, malaria and molds) [35–37]. One reason may be the production by bacteria of siderophores (eg, S. aureus staphyloferrin A and B, and A. baumannii acinetobactin) that possess iron affinities that are 1010- to 1020-fold higher than small molecule or biological iron chelators [17, 38–40]. Similarly, Candida species also produces high affinity iron siderophores, and both Candida and Acinetobacter can also uptake high affinity xeno-siderophores that are produced by other bacteria (eg, desferoxamine) [15, 41–45]. Thus there has been a perception that iron acquisition by high affinity siderophores cannot be overcome in vivo by chelation-based therapy. Our results underscore that high concentrations of a biological agent that has lower affinity than bacterial siderophores can overcome the affinity gap by mass action and improve survival of the infected host.
We found no evidence of increase in MIC to transferrin when bacterial and fungal pathogens were serially passaged in the presence of subinhibitory concentrations of transferrin. Furthermore, transferrin's ability to reduce the frequency of emergence of resistance to rifampin, which is widely known to have perhaps the lowest barrier to emergence of resistance of any systemic antimicrobial agent on the market [46–49], suggests its potential as an adjunctive agent to reduce the frequency of emergence of resistance to antibacterial therapy.
Small molecule chelators alter metabolic disposition of iron in ways that may be unfavorable for host defenses. For example, chelators reduce iron availability to myeloid cells, which are normally the predominant recyclers of iron in the host, and increase its excretion into renal tubules where iron is not normally found. Thus, serious toxicity to bone marrow, kidneys, and other organ systems can occur during small molecular iron chelator therapy [50–52]. In contrast to small molecule approaches, transferrin is a biological approach to mediating iron starvation that takes advantage of the normal iron sequestering mechanism in mammals. Furthermore, transferrin has been studied in clinical trials for patients with iron overload, and although it is not yet FDA-approved, no substantial toxicity signals have been seen in these trials [25].
We initially sought to determine MICs of transferrin in the presence of serum and found much lower values (ng/mL). However, there was substantial variability between assays using serum from different batches due to variability in the concentration of iron and iron binding proteins in the serum. Thus, in order to enable reproducibility in the MIC testing, it was necessary to conduct the assay in media without serum. When tested in the absence of serum, human apo-transferrin MICs were higher but still substantially lower than the physiological concentration of transferrin (a mixture of apo- and holo-transferrin) in human blood (1–5 mg/mL [53]). The amount of apo-transferrin that is required to be added into biological matrices to inhibit microbial growth will be difficult to predict given the complex dynamics of free vs bound iron in such matrices. Thus, although we suggest that in vitro reproducibility of MIC testing is likely to require assays run in the absence of serum, clinical investigation is going to be required to define how breakpoints set by such assays predict in vivo efficacy.
Caution is warranted in interpreting the relative potencies between bacteria and C. albicans. The 24-hour time kill curves and MIC tests for C. albicans had to be run at a different temperature than the bacteria to prevent filamentation of the fungus from occurring. Furthermore, it is not known how much growth inhibition is required to alter in vivo virulence of bacteria vs fungi. Slower fungal replication and the need for fungi to filament in order to cause virulence may mean that important biological effects are mediated by transferrin even at concentrations below the in vitro MIC. Furthermore, transferrin may interact with other important iron regulatory molecules, such as hepcidin [54], and as such may mediate important in vivo biological effects that do not correlate well with in vitro microbial growth inhibition. The in vivo efficacy we found of transferrin, which was similar across all three pathogens, underscores the complexity of translating MICs to activity for this iron sequestering strategy.
In summary, recombinant human transferrin is an effective, broad-spectrum antimicrobial agent with substantial efficacy in lethal challenge models in mice. GMP-compliant recombinant human transferrin is already available and has been studied in human patients with iron disorders [25]. Thus, the current results lay the groundwork for rapid translational development of recombinant human transferrin as a therapy for infections in patients. Finally, transferrin provides proof of principle that blocking nutrient (ie, trace metal) availability can effectively treat bacterial infections in vivo while reducing the emergence of resistance to traditional antibacterial therapy. Thus, this strategy represents a different paradigm than traditional antimicrobial therapy, focused on passively starving microbes of host nutrients rather than directly attacking a microbial target.
Notes
Financial support. This work was supported by NIH/NIAID R01 AI081719, R21 AI101750, and R21 AI101492 to B. S., R21 AI107233 and S10RR026742 to E. P. S., and Los Angeles Biomedical Research Institute Seed Grant to L. L. The authors thank Dr Eric P. Brass for experimental advice and critical review of the article.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Spellberg B, Blaser M, Guidos R, et al. for the Infectious Diseases Society of America. Combating antimicrobial resistance: policy recommendations to save lives. Clin Infect Dis. 2011;52(S5):S397–428. doi: 10.1093/cid/cir153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choffnes ER, Relman DA, Mack A for the Forum on Microbial Threats, Institute of Medicine of the National Academies. Antibiotic resistance: implications for global health and novel intervention strategies. Washington, DC: The National Academies Press; 2010. [PubMed] [Google Scholar]
- 3.Spellberg B, Bartlett JG, Gilbert DN. The future of antibiotics and resistance. N Engl J Med. 2013;368:299–302. doi: 10.1056/NEJMp1215093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goel VK, Kapil A. Monoclonal antibodies against the iron regulated outer membrane proteins of Acinetobacter baumannii are bactericidal. BMC Microbiol. 2001;1:16. doi: 10.1186/1471-2180-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Actis LA, Tolmasky ME, Crosa LM, Crosa JH. Effect of iron-limiting conditions on growth of clinical isolates of Acinetobacter baumannii. J Clin Microbiol. 1993;31:2812–5. doi: 10.1128/jcm.31.10.2812-2815.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aguila A, Herrera AG, Morrison D, et al. Bacteriostatic activity of human lactoferrin against Staphylococcus aureus is a function of its iron-binding properties and is not influenced by antibiotic resistance. FEMS Immunol Med Microbiol. 2001;31:145–52. doi: 10.1111/j.1574-695X.2001.tb00511.x. [DOI] [PubMed] [Google Scholar]
- 7.Goel VK, Kapil A, Das B, Rao DN. Influence of iron on growth and extracellular products of Acinetobacter baumannii. Jpn J Med Sci Biol. 1998;51:25–33. doi: 10.7883/yoken1952.51.25. [DOI] [PubMed] [Google Scholar]
- 8.Kuklin NA, Clark DJ, Secore S, et al. A novel Staphylococcus aureus vaccine: iron surface determinant B induces rapid antibody responses in rhesus macaques and specific increased survival in a murine S. aureus sepsis model. Infect Immun. 2006;74:2215–23. doi: 10.1128/IAI.74.4.2215-2223.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skaar EP, Humayun M, Bae T, DeBord KL, Schneewind O. Iron-source preference of Staphylococcus aureus infections. Science. 2004;305:1626–8. doi: 10.1126/science.1099930. [DOI] [PubMed] [Google Scholar]
- 10.Skaar EP, Schneewind O. Iron-regulated surface determinants (Isd) of Staphylococcus aureus: stealing iron from heme. Microbes Infect. 2004;6:390–7. doi: 10.1016/j.micinf.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 11.Sutak R, Lesuisse E, Tachezy J, Richardson DR. Crusade for iron: iron uptake in unicellular eukaryotes and its significance for virulence. Trends Microbiol. 2008;16:261–8. doi: 10.1016/j.tim.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 12.Skaar EP. The battle for iron between bacterial pathogens and their vertebrate hosts. PLoS Pathog. 2010;6:e1000949. doi: 10.1371/journal.ppat.1000949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crusio R, Rao S, Changawala N, et al. Epidemiology and outcome of infections with carbapenem-resistant Gram-negative bacteria treated with polymyxin B-based combination therapy. Scand J Infect Dis. 2013;46:1–8. doi: 10.3109/00365548.2013.844350. [DOI] [PubMed] [Google Scholar]
- 14.Bezkorovainy A, Topouzian N. The effect of metal chelators and other metabolic inhibitors on the growth of Bifidobacterium bifidus var. Pennsylvanicus. Clin Biochem. 1981;14:135–41. doi: 10.1016/s0009-9120(81)90281-2. [DOI] [PubMed] [Google Scholar]
- 15.Dorsey CW, Tomaras AP, Connerly PL, Tolmasky ME, Crosa JH, Actis LA. The siderophore-mediated iron acquisition systems of Acinetobacter baumannii ATCC 19606 and Vibrio anguillarum 775 are structurally and functionally related. Microbiology. 2004;150(Pt 11):3657–67. doi: 10.1099/mic.0.27371-0. [DOI] [PubMed] [Google Scholar]
- 16.Ibrahim AS, Gebermariam T, Fu Y, et al. The iron chelator deferasirox protects mice from mucormycosis through iron starvation. J Clin Invest. 2007;117:2649–57. doi: 10.1172/JCI32338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miethke M, Marahiel MA. Siderophore-based iron acquisition and pathogen control. Microbiol Mol Biol Rev. 2007;71:413–51. doi: 10.1128/MMBR.00012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.von Bonsdorff L, Sahlstedt L, Ebeling F, Ruutu T, Parkkinen J. Apotransferrin administration prevents growth of Staphylococcus epidermidis in serum of stem cell transplant patients by binding of free iron. FEMS Immunol Med Microbiol. 2003;37:45–51. doi: 10.1016/S0928-8244(03)00109-3. [DOI] [PubMed] [Google Scholar]
- 19.Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev. 2008;21:538–82. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forsberg CM, Bullen JJ. The effect of passage and iron on the virulence of Pseudomonas aeruginosa. J Clin Pathol. 1972;25:65–8. doi: 10.1136/jcp.25.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bezkorovainy A. Antimicrobial properties of iron-binding proteins. Adv Exp Med Biol. 1981;135:139–54. doi: 10.1007/978-1-4615-9200-6_8. [DOI] [PubMed] [Google Scholar]
- 22.Ellison RT, III, LaForce FM, Giehl TJ, Boose DS, Dunn BE. Lactoferrin and transferrin damage of the gram-negative outer membrane is modulated by Ca2+ and Mg2+ J Gen Microbiol. 1990;136:1437–46. doi: 10.1099/00221287-136-7-1437. [DOI] [PubMed] [Google Scholar]
- 23.Bullen JJ, Rogers HJ, Spalding PB, Ward CG. Natural resistance, iron and infection: a challenge for clinical medicine. J Med Microbiol. 2006;55(Pt 3):251–8. doi: 10.1099/jmm.0.46386-0. [DOI] [PubMed] [Google Scholar]
- 24.Rooijakkers SH, Rasmussen SL, McGillivray SM, et al. Human transferrin confers serum resistance against Bacillus anthracis. J Biol Chem. 2010;285:27609–13. doi: 10.1074/jbc.M110.154930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brandsma ME, Jevnikar AM, Ma S. Recombinant human transferrin: beyond iron binding and transport. Biotechnol Adv. 2011;29:230–8. doi: 10.1016/j.biotechadv.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 26.Spellberg B, Ibrahim AS, Chin-Hong PV, et al. The Deferasirox-AmBisome Therapy for Mucormycosis (DEFEAT Mucor) study: a randomized, double-blinded, placebo-controlled trial. J Antimicrob Chemother. 2012;67:715–22. doi: 10.1093/jac/dkr375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin L, Ibrahim AS, Xu X, et al. Th1-Th17 cells mediate protective adaptive immunity against Staphylococcus aureus and Candida albicans infection in mice. PLoS Pathog. 2009;5:e1000703. doi: 10.1371/journal.ppat.1000703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo G, Lin L, Ibrahim AS, et al. Active and passive immunization protects against lethal, extreme drug resistant-Acinetobacter baumannii infection. PLoS One. 2012;7:e29446. doi: 10.1371/journal.pone.0029446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barry AL, Craig WA, Nadler H, Reller LB, Sanders CC, Swenson JM. Methods for determining bactericidal activity of antimicrobial agents. 1999. M26-A. Vol. 19: Clinical and Laboratory Standards Institute (formerly NCCLS)
- 30.Pankey GA, Sabath LD. Clinical relevance of bacteriostatic versus bactericidal mechanisms of action in the treatment of gram-positive bacterial infections. Clin Infect Dis. 2004;38:864–70. doi: 10.1086/381972. [DOI] [PubMed] [Google Scholar]
- 31.Hood MI, Mortensen BL, Moore JL, et al. Identification of an Acinetobacter baumannii zinc acquisition system that facilitates resistance to calprotectin-mediated zinc sequestration. PLoS Pathog. 2012;8:e1003068. doi: 10.1371/journal.ppat.1003068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bird LJ, Bonnefoy V, Newman DK. Bioenergetic challenges of microbial iron metabolisms. Trends Microbiol. 2011;19:330–40. doi: 10.1016/j.tim.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 33.Spratt BG. Resistance to antibiotics mediated by target alterations. Science. 1994;264:388–93. doi: 10.1126/science.8153626. [DOI] [PubMed] [Google Scholar]
- 34.Schaible UE, Kaufmann SH. Iron and microbial infection. Nat Rev Microbiol. 2004;2:946–53. doi: 10.1038/nrmicro1046. [DOI] [PubMed] [Google Scholar]
- 35.Hershko C. Oral iron chelating drugs: coming but not yet ready for clinical use. Br Med J (Clin Res Ed) 1988;296:1081–2. doi: 10.1136/bmj.296.6629.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gordeuk VR, Thuma PE, Brittenham GM, et al. Iron chelation as a chemotherapeutic strategy for falciparum malaria. Am J Trop Med Hyg. 1993;48:193–7. doi: 10.4269/ajtmh.1993.48.193. [DOI] [PubMed] [Google Scholar]
- 37.Santos AL, Sodre CL, Valle RS, et al. Antimicrobial action of chelating agents: repercussions on the microorganism development, virulence and pathogenesis. Curr Med Chem. 2012;19:2715–37. doi: 10.2174/092986712800609788. [DOI] [PubMed] [Google Scholar]
- 38.Wandersman C, Delepelaire P. Bacterial iron sources: from siderophores to hemophores. Ann Rev Microbiol. 2004;58:611–47. doi: 10.1146/annurev.micro.58.030603.123811. [DOI] [PubMed] [Google Scholar]
- 39.Fischbach MA, Lin H, Liu DR, Walsh CT. How pathogenic bacteria evade mammalian sabotage in the battle for iron. Nat Chem Biol. 2006;2:132–8. doi: 10.1038/nchembio771. [DOI] [PubMed] [Google Scholar]
- 40.Baker HM, Baker EN. Lactoferrin and iron: structural and dynamic aspects of binding and release. Biometals. 2004;17:209–16. doi: 10.1023/b:biom.0000027694.40260.70. [DOI] [PubMed] [Google Scholar]
- 41.Funahashi T, Tanabe T, Mihara K, Miyamoto K, Tsujibo H, Yamamoto S. Identification and Characterization of an Outer Membrane Receptor Gene in Acinetobacter baumannii required for utilization of desferricoprogen, rhodotorulic acid, and desferrioxamine B as xenosiderophores. Biol Pharm Bull. 2012;35:753–60. doi: 10.1248/bpb.35.753. [DOI] [PubMed] [Google Scholar]
- 42.Heymann P, Gerads M, Schaller M, Dromer F, Winkelmann G, Ernst JF. The siderophore iron transporter of Candida albicans (Sit1p/Arn1p) mediates uptake of ferrichrome-type siderophores and is required for epithelial invasion. Infect Immun. 2002;70:5246–55. doi: 10.1128/IAI.70.9.5246-5255.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haas H. Molecular genetics of fungal siderophore biosynthesis and uptake: the role of siderophores in iron uptake and storage. Appl Microbiol Biotechnol. 2003;62:316–30. doi: 10.1007/s00253-003-1335-2. [DOI] [PubMed] [Google Scholar]
- 44.Bernier G, Girijavallabhan V, Murray A, et al. Desketoneoenactin-siderophore conjugates for Candida: evidence of iron transport-dependent species selectivity. Antimicrob Agents Chemother. 2005;49:241–8. doi: 10.1128/AAC.49.1.241-248.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee JH, Han Y. Candida albicans can utilize siderophore during candidastasis caused by apotransferrin. Arch Pharm Res. 2006;29:249–55. doi: 10.1007/BF02969401. [DOI] [PubMed] [Google Scholar]
- 46.Sande MA, Mandell GL. Effect of rifampin on nasal carriage of Staphylococcus aureus. Antimicrob Agents Chemother. 1975;7:294–7. doi: 10.1128/aac.7.3.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sande MA. The use of rifampin in the treatment of nontuberculous infections: an overview. Rev Infect Dis. 1983;5(Suppl 3):S399–401. doi: 10.1093/clinids/5.supplement_3.s399. [DOI] [PubMed] [Google Scholar]
- 48.Simon GL, Smith RH, Sande MA. Emergence of rifampin-resistant strains of Staphylococcus aureus during combination therapy with vancomycin and rifampin: a report of two cases. Rev Infect Dis. 1983;5(Suppl 3):S507–8. doi: 10.1093/clinids/5.supplement_3.s507. [DOI] [PubMed] [Google Scholar]
- 49.Zavasky DM, Sande MA. Reconsideration of rifampin: a unique drug for a unique infection. JAMA. 1998;279:1575–7. doi: 10.1001/jama.279.19.1575. [DOI] [PubMed] [Google Scholar]
- 50.Cappellini MD, Cohen A, Piga A, et al. A phase 3 study of deferasirox (ICL670), a once-daily oral iron chelator, in patients with beta-thalassemia. Blood. 2006;107:3455–62. doi: 10.1182/blood-2005-08-3430. [DOI] [PubMed] [Google Scholar]
- 51.Cappellini MD, Giardina P, Porter J, et al. Long-term safety and tolerability of the once-daily, oral iron chelator deferasirox (Exjade, ICL670) in patients with transfusional iron overload. Abstract 896. American Society of Hematology; Orlando, FL. 2006. [Google Scholar]
- 52.Piga A, Bejaoui M, Kilinc Y, et al. Long-term treatment with the once-daily oral iron chelator deferasirox (Exjade, ICL1670) is effective and generally well tolerated in pediatric patients. Abstract 909. American Society of Hematology; Orlando, FL. 2006. [Google Scholar]
- 53.Gambino R, Desvarieux E, Orth M, et al. The relation between chemically measured total iron-binding capacity concentrations and immunologically measured transferrin concentrations in human serum. Clin Chem. 1997;43:2408–12. [PubMed] [Google Scholar]
- 54.Drakesmith H, Prentice AM. Hepcidin and the iron-infection axis. Science. 2012;338:768–72. doi: 10.1126/science.1224577. [DOI] [PubMed] [Google Scholar]