Abstract
Background: Cytoreductive nephrectomy (CN) became a standard procedure in metastatic renal cell carcinoma (mRCC) in the immunotherapy era. Historically, median overall survival (OS) of patients treated with interferon alpha (IFN-α) without CN was 7.8 months. Median OS in patients treated with targeted therapy (TT) without CN is unknown.
Patients and methods: We retrospectively reviewed records of patients with mRCC who received TT without CN. Kaplan–Meier methods and Cox regression analysis were used to estimate median OS and identify poor prognostic factors.
Results: One hundred and eighty-eight patients were identified. Most patients had intermediate-risk (54.8%) or poor-risk (44.1%) disease. Median OS for all patients was 10.4 months [95% confidence interval (CI) 8.1–12.5]. By multivariable analysis, elevated baseline lactate dehydrogenase and corrected calcium, performance status of two or more, retroperitoneal nodal metastasis, thrombocytosis, current smoking, two or more metastatic sites, and lymphopenia were independent risk factors for inferior OS. Patients with four or more factors had increased risk of death (hazard ratio 8.83, 95% CI 5.02–15.5, P < 0.001) and 5.5-month median OS. Nineteen patients (10.0%) survived for 2+ years.
Conclusions: These data highlight the improved OS of patients with mRCC treated with TT without CN, compared with historical IFN-α treatment, and may guide the design of trials investigating the role of CN in the TT era.
Keywords: cytoreductive nephrectomy, prognosis, renal cell carcinoma, targeted therapy
introduction
Kidney cancer represents 3%–5% of annual new cancer diagnoses in the United States, with an estimated 57 760 new cases per year, the majority comprised of renal cell carcinoma (RCC). An estimated 12 980 kidney cancer-related deaths occurred in 2009 [1]. Nephrectomy remains the current standard of care for patients with localized disease. Approximately 30% of patients with RCC ultimately require systemic therapy for metastatic disease [2]. The role of cytoreductive nephrectomy (CN) in patients presenting with metastatic disease has been extensively studied in the era of immunotherapy. In two randomized trials with identical design, patients who underwent CN followed by interferon alpha (IFN-α) therapy had improved overall survival (OS) (median 13.6 months) compared with those treated with IFN-α alone (median 7.8 months) [3–5]. The antivascular endothelial growth factor (VEGF) antibody bevacizumab; the multityrosine kinase inhibitors, sorafenib, sunitinib, and pazopanib; and the mammalian target of rapamycin (mTOR) inhibitors, temsirolimus and everolimus, have become the mainstay of therapy for the vast majority of patients with metastatic renal cell carcinoma (mRCC). Large randomized controlled clinical trials have shown improved progression-free survival with these agents and improved OS in selected populations, but the majority of these study patients had prior nephrectomy, conventional (clear cell) histology, and good performance status (PS) [6–12]. Not all patients with mRCC are candidates for CN due to various factors, including poor PS, brain metastasis, comorbid illnesses precluding surgery, or widely disseminated disease in the presence of a small primary tumor [13–15]. In addition, many patients refuse to undergo CN. Prospective randomized trials are currently examining the role of CN in the era of targeted therapy (TT). The outcome of patients treated with TT without CN is unknown.
The aims of this retrospective study were (i) to establish a benchmark for OS in patients with mRCC who are treated with TT and who do not undergo CN and (ii) to evaluate clinical factors that influence patient’s clinical outcome.
patients and methods
This study was approved by the Institutional Review Board for the Protection of Human Subjects at The University of Texas M.D. Anderson Cancer Center (MDACC). We reviewed the medical records of consecutive patients with mRCC and Eastern Cooperative Oncology Group (ECOG) PS of three or less who did not undergo CN but received one or more targeted agents (bevacizumab, sorafenib, sunitinib, temsirolimus, everolimus, and pazopanib) for at least 1 month from 1 January 2003 to 31 December 2009. Patients with any T, any N, and any M1 disease were considered for CN if the primary tumor was felt to be resectable and the patient’s ECOG PS was zero or one. Patients who had chemotherapy or immunotherapy, in addition to TT, were included in this analysis. We excluded patients who underwent embolization or high-energy ablation of the primary tumor at any point during their therapy. Comprehensive clinical, laboratory, and pathological (from fine needle aspiration or core biopsy) data were collected and reviewed to optimize accuracy and completeness. Histology was classified by a dedicated genitourinary pathologist at MDACC as conventional-type versus non-conventional-type RCC, based on pre-treatment biopsy and on the 2004 World Health Organization criteria [16]. Biopsy grade corresponded to the Fuhrman nuclear grading system [17] when available. Number and site(s) of organ metastasis as well as the presence (≥1 cm) of retroperitoneal lymphadenopathy or supradiaphragmatic lymphadenopathy were determined based on radiographic imaging at the time of treatment initiation. The most recent laboratory values before initiation of treatment were used and these were divided into three categories (normal, above normal, and below normal) using reference standards at our institution.
OS time was calculated from the date of treatment initiation to death or last known follow-up, whichever occurred earlier. Kaplan–Meier methods were used to estimate median OS times for the entire cohort, as well as for patients based on whether or not they would have been eligible for CN as established by the European Organization for Research and Treatment of Cancer (EORTC) 30947/Southwest Oncology Group (SWOG) 8949 trials [5]. In order to identify clinical factors prognostic for OS, we carried out univariable and multivariable stepwise Cox proportional hazards regression analyses. Factors remaining significant on multivariable analysis were identified as poor prognostic factors.
For all analyses, Stata 10.1(Stata Corp., College Station, TX) was used. A P value (two sided) of ≤0.05 was considered significant.
results
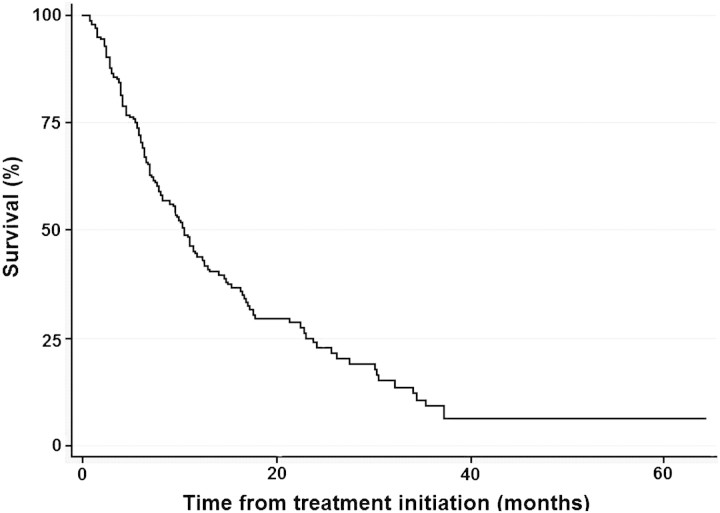
From January 2003 to December 2009, we identified 188 consecutive patients who received TT for mRCC and never underwent CN. A total of 133 (70.7%) patients had died at a median follow-up time of 6.9 months from treatment initiation to death. For patients who were still alive at the time of this analysis, median follow-up time was 13.1 months (range 1.0–64.4 months). Median time from RCC diagnosis to initiation of systemic therapy was 1.2 months (range 0–32.2 months), with median time of total therapy with a systemic targeted agent of 5.6 months (range 1–61.5 months). Baseline patient characteristics for the entire cohort are shown in Table 1. The majority of patients (163 patients or 86.7%) received TT as their first-line treatment regimen. Of all patients, 144 (76.6%) received TT only, while 44 (23.4%) received chemotherapy (gemcitabine plus capecitabine or 5-flourouracil) either concurrently with bevacizumab or sequentially after TT. We considered clinical and pathological factors that differed between patients who received TT only or TT and chemotherapy. More patients with corrected serum calcium ≥10 mmol/l received TT only (n = 38 or 26.4%) than TT and chemotherapy (n = 4 or 9.1%) (P < 0.001). More patients with sarcomatoid dedifferentiation identified from tumor biopsy received chemotherapy and TT (n = 10 or 22.7%) versus TT only (n = 6 or 4.2%) (P < 0.001). However, we observed no statistically significant difference (P = 0.218) in survival between the two groups: TT only, median survival time 10.0 months [95% confidence intervals (CI) 7.5–11.4], versus TT and chemotherapy, median survival time 12.3 months (95% CI 7.8–17.0). Five (2.7%) patients received immunotherapy (IFN-α and/or interleukin-2) as initial treatment followed by TT. Of the 144 patients who received TT only, 81 (56.2%) received only one targeted agent during their entire course of therapy: sunitinib (n = 54), temsirolimus (n = 15), sorafenib (n = 8), bevacizumab (n = 2), everolimus (n = 1), and pazopanib (n = 1). Using Memorial Sloan Kettering Cancer Center (MSKCC) criteria, 2 (1.1%), 103 (54.8%), and 83 (44.1%) patients had favorable-risk, intermediate-risk, and poor-risk disease, respectively [18]. Using the criteria of Heng et al. [19] for mRCC treated with VEGF-TT, 43 (22.9%), 112 (59.6%), and 33 (17.6%) patients had poor-risk, intermediate-risk, and favorable-risk disease, respectively. Ninety-five (50.5%) patients would have been eligible for CN as established by the EORTC 30947/SWOG 8949 trials [5]. Of the 93 (49.5%) patients who would not have been eligible for CN, reasons for ineligibility included compromised ECOG PS or comorbid conditions (two or more) (43 patients or 46.2%), unresectable primary tumor (≥ cT3c or as assessed by urologist) (13 patients or 14.0%), brain metastasis (13 patients or 14.0%), and two of the above features including renal failure (serum creatinine > 3 mg/dl) (24 patients or 25.8%). For the entire cohort, median OS time (Figure 1) was 10.4 months (95% CI 8.1–12.5). For the 95 patients who would have been eligible for CN, median OS time was 12.5 months (95% CI 10.4–17.0). For the 93 patients who would not have been eligible for CN by the SWOG 8949/EORTC 30947 criteria, median OS time was 7.8 months (95% CI 6.0–10.3).
Table 1.
Characteristics of patients treated with targeted therapy without CN at MDACC (2003–2009)
| n (%) | |
| Median age (years) at diagnosis (range) | 60.8 (18.2–83.9) |
| Caucasian race | 134 (71.3) |
| Male gender | 123 (65.4) |
| Biopsy site | |
| Kidney | 71 (38.8) |
| Metastasis | 92 (48.9) |
| Kidney and metastasis | 20 (10.6) |
| Not done/unknown | 5 (2.7) |
| Clear cell histologya | 114 (60.6) |
| Renal cell carcinoma (not otherwise specified) | 30 (16.0) |
| Nonclear cell histology | 25 (13.3) |
| Unclassified | 19 (10.1) |
| Sarcomatoid dedifferentiationa | 16 (8.5) |
| Bilateral tumor | 10 (5.3) |
| ECOG PS | |
| 0 | 16 (8.5) |
| 1 | 106 (56.4) |
| 2 | 40 (21.3) |
| 3 | 26 (13.8) |
| Clinical T stage | |
| T1a | 16 (8.5) |
| T1b | 29 (15.4) |
| T2 | 28 (14.9) |
| T3a | 36 (19.2) |
| T3b | 52 (27.7) |
| T3c | 1 (0.5) |
| T4 | 26 (13.8) |
| Number of visceral/bone metastasis | |
| 1 | 68 (36.2) |
| 2 | 73 (38.8) |
| 3 or more | 47 (25.0) |
| Metastatic site | |
| Lung | 128 (68.1) |
| Bone | 83 (44.2) |
| Liver | 58 (30.9) |
| Adrenal | 44 (23.4) |
| Brain | 22 (11.7) |
| Retroperitoneal lymph node | 85 (45.7) |
| Supradiaphragmatic lymph node | 95 (48.7) |
| Other | 26 (13.8) |
| Symptoms at presentation | |
| None | 15 (10.4) |
| Local | 70 (37.2) |
| Metastatic site | 91 (48.4) |
| Constitutional | 77 (41.0) |
| Tumor thrombus | 67 (35.6) |
Based on biopsy.
CN, cytoreductive nephrectomy; ECOG, Eastern Cooperative Oncology Group; MDACC, M.D. Anderson Cancer Center; PS, performance status.
Figure 1.
Kaplan–Meier survival curve of patients with metastatic renal cell carcinoma and primary tumor in place treated with targeted therapy at M.D. Anderson Cancer Center (2003–2009).
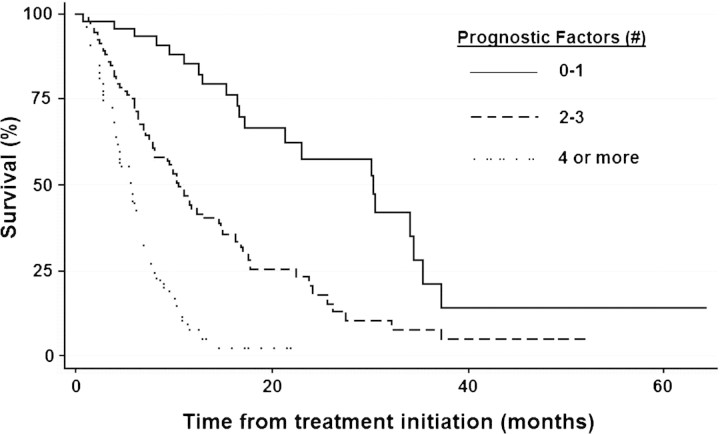
Sixty-four (34.0%) and 19 (10.1%) patients survived for ≥12 and ≥24 months following TT initiation, respectively. Clinical, pathologic, and laboratory variables significant on univariable Cox proportional hazards regression analysis are listed in Table 2. By stepwise multivariable Cox proportional hazards regression analysis, eight factors remained independent for predicting an inferior survival in the entire cohort (Table 3): serum lactate dehydrogenase (LDH) > upper limit of normal (ULN), corrected serum calcium level ≥10.0 mmol/l, ECOG PS two or more, retroperitoneal lymph node metastasis (N2), platelet count > ULN, absolute lymphocyte count < lower limit of normal, two or more visceral/bone metastases, and a current smoker. Patients with two to three of these factors had an increased risk of death [hazard ratio (HR) 2.85, 95% CI 1.73–4.69, P < 0.001] and a shorter median survival time of 10.4 months (95% CI 7.8–14.5) compared with those with zero to one factors who had a median survival time of 30.3 months (95% CI 17.1–34.4). Patients with four or more factors had a much higher risk of death (HR 8.83, 95% CI 5.02–15.5, P < 0.001) and a much shorter median survival time of 5.5 months (95% CI 4.0–6.4) compared with those with zero to one factors. Of the 19 patients surviving ≥24 months, 11 (57.9%) and 8 (42.1%) had zero to one and two to three poor-risk factors, respectively. No patient surviving >24 months after TT initiation had more than three poor prognostic factors; 13 of these patients (68.4%) would have been eligible for CN under the criteria of the EORTC 30947/SWOG 8949 trials.
Table 2.
Univariable Cox proportional hazards regression analysis
| Hazard ratio (95% CI) | P | |
| ECOG PS ≥2 | 1.95 (1.37–2.79) | <0.001 |
| Serum albumin < LLN | 1.64 (1.07–2.51) | 0.022 |
| Serum LDH < LLN | 1.70 (1.28–5.65) | 0.009 |
| Serum LDH > ULN | 2.69 (1.19–2.44) | 0.004 |
| Corrected serum calcium >10 mmol/l | 2.30 (1.55–3.41) | <0.001 |
| Serum alkaline phosphatase > ULN | 1.60 (1.11–2.32) | 0.013 |
| Hemoglobin < LLN | 1.47 (1.01–2.15) | 0.045 |
| Platelet count > ULN | 1.97 (1.31–2.97) | 0.001 |
| Absolute neutrophil count > ULN | 1.66 (1.13–2.42) | 0.010 |
| Absolute lymphocyte count < LLN | 1.59 (1.06–2.39) | 0.025 |
| ≥2 visceral or bone metastases | 1.83 (1.25–2.68) | 0.002 |
| Adrenal metastasis | 1.54 (1.04–2.27) | 0.031 |
| Liver metastasis | 1.55 (1.09–2.20) | 0.014 |
| Retroperitoneal lymph node metastasis (N2) | 2.26 (1.59–3.21) | <0.001 |
| Constitutional symptoms at presentation | 1.83 (1.29–2.60) | 0.001 |
| Clinical evidence of renal vein/caval thrombus | 1.44 (1.00–2.08) | 0.047 |
| Current smoker | 1.96 (1.23–3.13) | 0.005 |
ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase; LLN, lower limit of normal; PS, performance status; ULN, upper limit of normal.
Table 3.
Multivariable Cox proportional hazards regression analysis
| Hazard ratio (95% CI) | P | |
| ECOG PS ≥2 | 1.51 (1.03–2.21) | 0.036 |
| ≥2 visceral or bone metastases | 1.73 (1.14–2.64) | 0.011 |
| Corrected serum calcium >10 mmol/l | 2.07 (1.31–3.27) | 0.002 |
| Serum LDH > ULN | 1.55 (1.06–2.27) | 0.023 |
| Platelet count > ULN | 1.65 (1.03–2.66) | 0.039 |
| Absolute lymphocyte count < LLN | 1.84 (1.18–2.88) | 0.009 |
| Retroperitoneal lymph node metastasis (N2) | 2.15 (1.43–3.23) | <0.001 |
| Current smoker | 1.71 (1.06–2.78) | 0.030 |
ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase; LLN, lower limit of normal; PS, performance status; ULN, upper limit of normal.
discussion
Upfront CN in patients with mRCC became the standard of care after two phase III trials showed improved OS for patients undergoing CN before IFN-α therapy. In the EORTC 30947 trial, Miskich et al. [3] reported a median OS time of 17 months for patients who underwent CN followed by IFN-α versus 7 months for patients treated with IFN-α alone. In the SWOG 8949 trial, Flanigan et al. reported a median survival time of 11.1 months in patients treated with CN followed by IFN-α versus 8.1 months for patients treated with IFN-α alone. Patients were eligible for CN if they had ECOG PS of zero or one, a resectable primary tumor, no prior systemic treatment or radiation, and no tumor thrombus in the inferior vena cava above the hepatic veins [4]. A combined analysis of the two trials yielded a median OS time of 13.6 months for patients treated with CN followed by IFN-α and 7.8 months for patients treated with IFN-α alone [5]. Although targeted agents have led to improved progression-free survival and in some instances OS of patients with mRCC, their impact on survival of patients who are not eligible for or do not undergo CN has not been reported.
In a phase III trial of temsirolimus versus IFN-α versus the combination of these two agents in patients with mRCC having poor-risk features, a subgroup analysis demonstrated improved OS in patients treated with temsirolimus versus IFN-α regardless of nephrectomy status [7]. Results from the expanded access trial of sunitinib, with less stringent inclusion criteria, showed a median OS time for the entire group of 18.4 months. However, patients with nonclear cell histology, ECOG PS two or more, or brain metastases had shorter median OS times of 13.4, 9.2, and 6.7 months, respectively. Eleven percent of the cohort had primary tumor in place at the time of enrollment, but the results of this subgroup were not reported, and it is unclear how many of these patients subsequently underwent CN [20]. Likewise, the Advanced Renal Cell Carcinoma Sorafenib open label trial in North American and in Europe included 11% and 17% of patients treated with primary tumor in place, respectively, but no subgroup analysis was reported [21, 22].
Our retrospective study examined a cohort of patients who did not undergo CN for various reasons and excluded patients who underwent other forms of local therapy to the primary tumor. Ninety-five patients would have been eligible for CN according to Flanigan et al. [4]. Median OS time of these patients was 12.5 months. This compares favorably with the median OS time of 7.8 months in patients historically treated with IFN-α alone without CN.
Motzer et al. [23] identified five prognostic factors, which stratify patients into good, intermediate, or poor risk for survival in the era of immunotherapy. Choueiri et al. [24] reported on 120 patients with mRCC with clear cell histology and ECOG PS of zero or one who were treated with TT following nephrectomy and identified five poor prognostic factors: corrected serum calcium <8.5 mg/dl or >10 mg/dl, absolute neutrophil count >4500/dl, platelets >300 000/dl, time from RCC diagnosis to initiation of TT <2 years, and ECOG PS of more than zero. Heng et al. recently published results of a retrospective study of patients treated with VEGF-TT, validating four of the five MSKCC adverse prognostic factors, and identified neutrophilia and thrombocytosis as two additional prognostic factors. According to these authors, patients were segregated into three risk categories: a favorable-risk group (zero risk factors), an intermediate-risk group (one or two risk factors), and a poor-risk group (three to six risk factors), with median OS times, not reached, 27 months, and 8.8 months, respectively [19].
Our study expands on previous experience but importantly includes only patients with primary tumor in place treated with TT for at least 1 month, regardless of ECOG PS. In addition, this study incorporates patients treated with either VEGF-directed agents or mTOR inhibitors, with or without chemotherapy. Patients received one or more targeted agents sequentially at the clinician’s discretion, as is the case in community practice. Our results confirmed three previously described poor prognostic factors on multivariable analysis: compromised PS, hypercalcemia, and elevated serum LDH level. However, we identified five additional poor prognostic factors, retroperitoneal lymph node involvement, thrombocytosis, smoking, two or more metastatic sites, and lymphopenia. Retroperitoneal lymph node involvement had been previously identified as a stratification variable in selecting patients who may benefit from CN in the immunotherapy era [25]. Patients with four or more of our poor prognostic factors had inferior OS compared with those who had three or less poor prognostic factors (Figure 2). The stratification seems to be validated in that, among the 19 patients from our cohort who survived ≥2 years, none had four or more poor prognostic factors. Furthermore, our data demonstrate that long-term survival of patients with mRCC does occur in the TT era, even without CN. Our data provide readily available and simple prognostic factors that may aid in the identification of patients destined to have poor OS despite therapy, thus sparing them the risk of morbidity and mortality of major surgery. Previous studies have suggested selection criteria for offering CN to patients with mRCC but are yet to be validated in the TT era [26, 27]. While we do not know if upfront CN would have improved the outcome of patients with poor-risk features who subsequently received TT, those demonstrating a durable response or disease stabilization would seem to be ideal candidates for evaluating the role of delayed nephrectomy.
Figure 2.
Kaplan–Meier survival curves of patients with metastatic renal cell carcinoma and primary tumor in place treated with targeted therapy based on the presence zero to one, two to three, or four or more poor prognostic factors.
This is the largest series of patients with mRCC who were treated with TT and followed for OS without CN. Limitations of this study include its single-institution experience and its retrospective nature, both known for inherent selection bias. It is noteworthy to mention that, given the surgical expertise at MDACC, it is possible that a substantial number of patients undergo CN at our institution who would be treated elsewhere with TT, without ever undergoing CN. We did exclude from this current study patients who had undergone embolization or high-energy ablation of their primary tumor, for control of local symptoms, although these modalities have not been shown to prolong survival [28–32]. Therefore, it is possible that excluding these patients could have biased our study. Finally, the selection of a specific TT, the number of agent(s) used, and their sequencing were largely dependent on the availability of these agents on clinical trials and was later dictated by the variable timing of the Food and Drug Administration approval. Nevertheless, our study highlights the benefit of TT in mRCC, when compared with historical controls, even for patients who are unable or unwilling to undergo CN.
In summary, we present survival data on a cohort of patients with mRCC treated with TT, without ever undergoing CN. The patients included in this analysis were stable enough to receive at least 1 month of TT. We identified eight factors associated with inferior survival: serum LDH > ULN, corrected serum calcium level ≥10.0, ECOG PS of two or more, retroperitoneal lymph node involvement (≥1 cm) on imaging, thrombocytosis, smoking, two or more metastatic sites, and lymphopenia. Patients with four or more prognostic factors demonstrated poor survival, and all long-term survivors in this cohort had less than four of these factors. When considering CN in patients with mRCC, the benefits should outweigh the risks of morbidity and mortality of the procedure. However, this risk-benefit ratio has yet to be defined in the context of available systemic therapy options. We believe that our findings provide useful benchmarks for OS in patients with mRCC who are treated with TT only, without CN, and may aid in the design of randomized clinical trials to determine the role of CN in the era of TT.
funding
None.
disclosure
The authors declare no conflict of interest.
References
- 1.American Cancer Society. Cancer Facts and Figures. Atlanta, GA: American Cancer Society 2009; http://www.cancer.org/acs/groups/content/@nho/documents/document/500809webpdf.pdf (8 September 2010, date last accessed) [Google Scholar]
- 2.Lam JS, Shvarts O, Leppert JT, et al. Renal cell carcinoma 2005: new frontiers in staging, prognostication and targeted molecular therapy. J Urol. 2005;173(6):1853–1862. doi: 10.1097/01.ju.0000165693.68449.c3. Review. [DOI] [PubMed] [Google Scholar]
- 3.Mickisch GH, Garin A, van Poppel H, et al. Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: a randomised trial. Lancet. 2001;358(9286):966–970. doi: 10.1016/s0140-6736(01)06103-7. [DOI] [PubMed] [Google Scholar]
- 4.Flanigan RC, Salmon SE, Blumenstein BA, et al. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N Engl J Med. 2001;345(23):1655–1659. doi: 10.1056/NEJMoa003013. [DOI] [PubMed] [Google Scholar]
- 5.Flanigan RC, Mickisch G, Sylvester R, et al. Cytoreductive nephrectomy in patients with metastatic renal cancer: a combined analysis. J Urol. 2004;171(3):1071–1076. doi: 10.1097/01.ju.0000110610.61545.ae. [DOI] [PubMed] [Google Scholar]
- 6.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356(2):125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 7.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356(2):115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 8.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356(22):2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 9.Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet. 2007;370(9605):2103–2111. doi: 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- 10.Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372(9637):449–456. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 11.Rini BI, Halabi S, Rosenberg JE, et al. Bevacizumab plus interferon alfa compared with interferon alfa monotherapy in patients with metastatic renal cell carcinoma: CALGB 90206. J Clin Oncol. 2008;26(33):5422–5428. doi: 10.1200/JCO.2008.16.9847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sternberg CN, Szczylik C, Lee E, et al. A randomized, double-blind phase III study of pazopanib in treatment-naive and cytokine-pretreated patients with advanced renal cell carcinoma (RCC) J Clin Oncol. 2009;27:15s. (Abstr 5021 from 2009 ASCO Annual Meeting) [Google Scholar]
- 13.Culine S, Bekradda M, Kramar A, et al. Prognostic factors for survival in patients with brain metastases from renal cell carcinoma. Cancer. 1998;83(12):2548–2553. [PubMed] [Google Scholar]
- 14.Motzer RJ, Bacik J, Schwartz LH, et al. Prognostic factors for survival in previously treated patients with metastatic renal cell carcinoma. J Clin Oncol. 2004;22(3):454–463. doi: 10.1200/JCO.2004.06.132. [DOI] [PubMed] [Google Scholar]
- 15.Motzer RJ, Bacik J, Mariani T, et al. Treatment outcome and survival associated with metastatic renal cell carcinoma of non-clear-cell histology. J Clin Oncol. 2002;20(9):2376–2381. doi: 10.1200/JCO.2002.11.123. [DOI] [PubMed] [Google Scholar]
- 16.Epstein J, Sauter G. WHO Classification of Tumours: Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs. Lyon, France: IARC Press; 2004. [Google Scholar]
- 17.Greene FL, Page DL, Fleming ID, et al. AJCC Cancer Staging Manual. 6th edition. New York: Springer-Verlag; 2002. [Google Scholar]
- 18.Motzer RJ, Bacik J, Murphy BA, et al. Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol. 2002;20(1):289–296. doi: 10.1200/JCO.2002.20.1.289. [DOI] [PubMed] [Google Scholar]
- 19.Heng DY, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol. 2009;27(34):5794–5799. doi: 10.1200/JCO.2008.21.4809. [DOI] [PubMed] [Google Scholar]
- 20.Gore ME, Szczylik C, Porta C, et al. Safety and efficacy of sunitinib for metastatic renal-cell carcinoma: an expanded-access trial. Lancet Oncol. 2009;10(8):757–763. doi: 10.1016/S1470-2045(09)70162-7. [DOI] [PubMed] [Google Scholar]
- 21.Knox JJ, Figlin RA, Stadler WM, et al. The advanced renal cell carcinoma sorafenib (ARCCS) expanded access trial in North America: safety and efficacy. J Clin Oncol. 2007;25:237s. (Suppl; Abstr 5011) [Google Scholar]
- 22.Beck J, Procopio G, Verzoni E, et al. Large open-label, non-comparative, clinical experience trial of the multi-targeted kinase inhibitor sorafenib in European patients with advanced RCC. J Clin Oncol. 2008;26:16021. (Meeting Abstr) [Google Scholar]
- 23.Motzer RJ, Mazumdar M, Bacik J, et al. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol. 1999;17(8):2530–2540. doi: 10.1200/JCO.1999.17.8.2530. [DOI] [PubMed] [Google Scholar]
- 24.Choueiri TK, Garcia JA, Elson P, et al. Clinical factors associated with outcome in patients with metastatic clear-cell renal cell carcinoma treated with vascular endothelial growth factor-targeted therapy. Cancer. 2007;110(3):543–550. doi: 10.1002/cncr.22827. [DOI] [PubMed] [Google Scholar]
- 25.Pantuck AJ, Zisman A, Dorey F, et al. Renal cell carcinoma with retroperitoneal lymph nodes. Impact on survival and benefits of immunotherapy. Cancer. 2003;97(12):2995–3002. doi: 10.1002/cncr.11422. [DOI] [PubMed] [Google Scholar]
- 26.Leibovich BC, Han KR, Bui MH, et al. Scoring algorithm to predict survival after nephrectomy and immunotherapy in patients with metastatic renal cell carcinoma: a stratification tool for prospective clinical trials. Cancer. 2003;98(12):2566–2575. doi: 10.1002/cncr.11851. [DOI] [PubMed] [Google Scholar]
- 27.Leibovich BC, Cheville JC, Lohse CM, et al. A scoring algorithm to predict survival for patients with metastatic clear cell renal cell carcinoma: a stratification tool for prospective clinical trials. J Urol. 2005;174(5):1759–1763. doi: 10.1097/01.ju.0000177487.64651.3a. discussion 1763. [DOI] [PubMed] [Google Scholar]
- 28.Park JH, Kim SH, Han JK, et al. Transcatheter arterial embolization of unresectable renal cell carcinoma with a mixture of ethanol and iodized oil. Cardiovasc Intervent Radiol. 1994;17(6):323–327. doi: 10.1007/BF00203951. [DOI] [PubMed] [Google Scholar]
- 29.Onishi T, Oishi Y, Suzuki Y, Asano K. Prognostic evaluation of transcatheter arterial embolization for unresectable renal cell carcinoma with distantmetastases. BJU Int. 2001;87:312–315. doi: 10.1046/j.1464-410x.2001.00070.x. [DOI] [PubMed] [Google Scholar]
- 30.Munro NP, Woodhams S, Nawrocki JD, et al. The role of transarterial embolization in the treatment of renal cell carcinoma. BJU Int. 2003;92(3):240–244. doi: 10.1046/j.1464-410x.2003.04314.x. [DOI] [PubMed] [Google Scholar]
- 31.Serafin Z, Karolkiewicz M, Strześniewski P, et al. Palliative percutaneous kidney embolization with enbucrilate in patients with renal cell carcinoma: safety and symptom control. Med Sci Monit. 2007;13(Suppl 1):98–104. [PubMed] [Google Scholar]
- 32.Maxwell NJ, Saleem Amer N, Rogers E, et al. Renal artery embolisation in the palliative treatment of renal carcinoma [erratum appears in Br J Radiol 2007; 80 (958): 853] Br J Radiol. 2007;80(950):96–102. doi: 10.1259/bjr/31311739. [DOI] [PubMed] [Google Scholar]