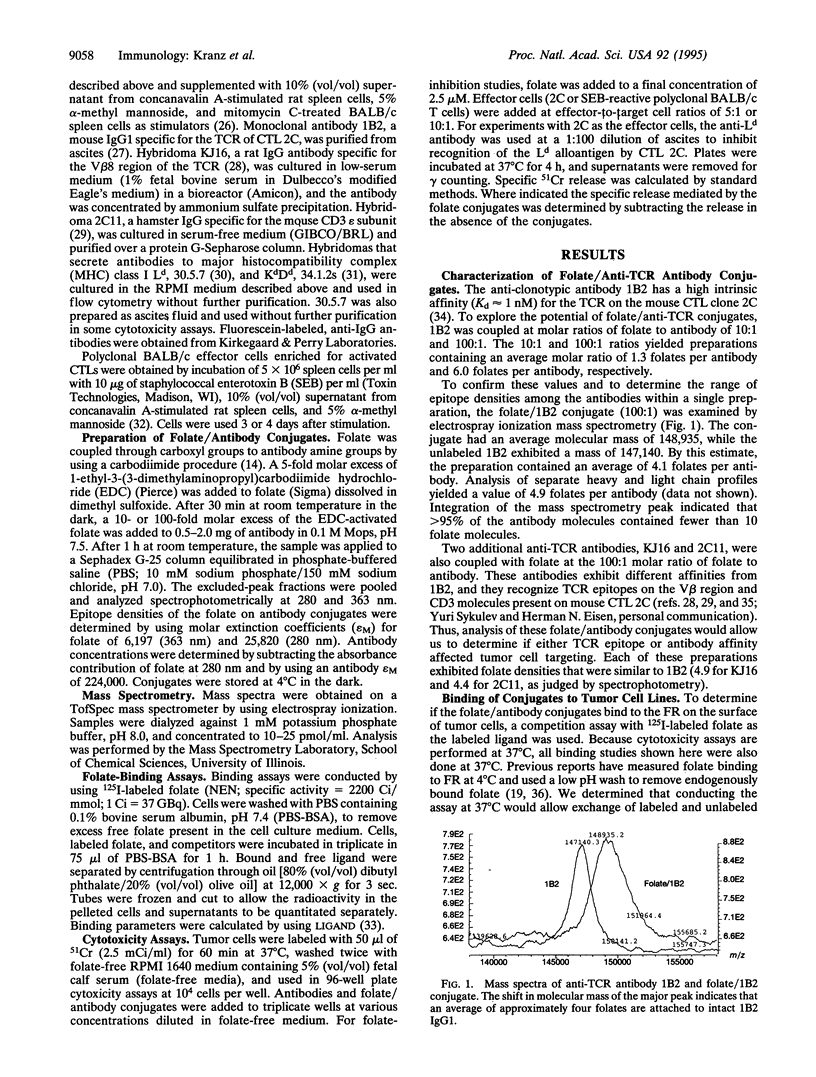
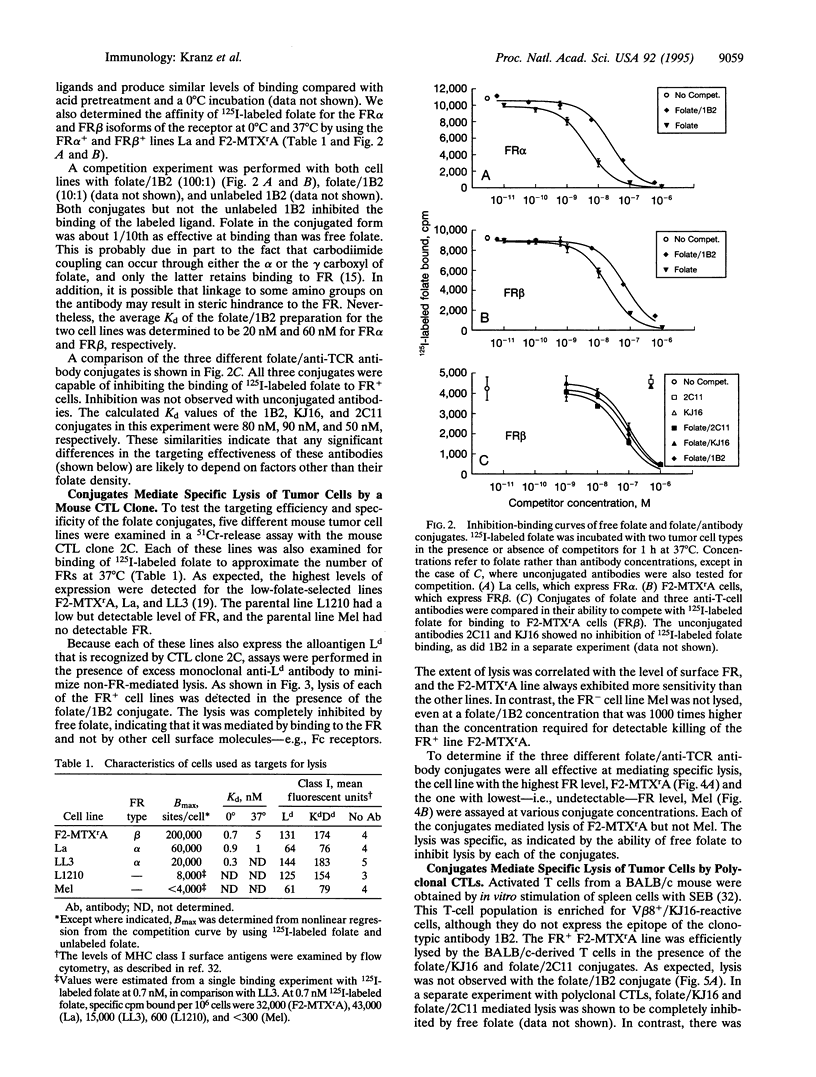
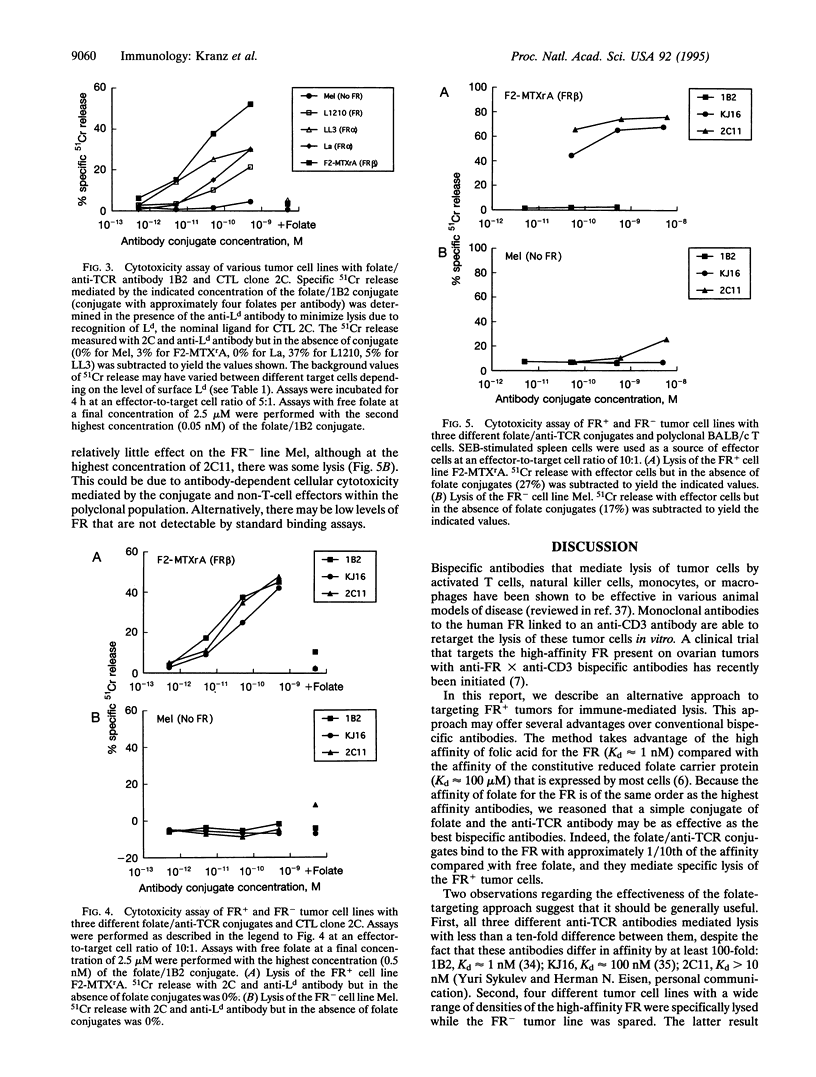
Abstract
High-affinity folate receptors (FRs) are expressed at elevated levels on many human tumors. Bispecific antibodies that bind the FR and the T-cell receptor (TCR) mediate lysis of these tumor cells by cytotoxic T lymphocytes. In this report, conjugates that consist of folate covalently linked to anti-TCR antibodies are shown to be potent in mediating lysis of tumor cells that express either the alpha or beta isoform of the FR. Intact antibodies with an average of five folate per molecule exhibited high affinity for FR+ tumor cells but did not bind to FR- tumor cells. Lysis of FR+ cell lines could be detected at concentrations as low as 1 pM (approximately 0.1 ng/ml), which was 1/1000th the concentration required to detect binding to the FR+ cells. Various FR+ mouse tumor cell lines could be targeted with each of three different anti-TCR antibodies that were tested as conjugates. The antibodies included 1B2, a clonotypic antibody specific for the cytotoxic T cell clone 2C; KJ16, an anti-V beta 8 antibody; and 2C11, an anti-CD3 antibody. These antibodies differ in affinities by up to 100-fold, yet the cytolytic capabilities of the folate/antibody conjugates differed by no more than 10-fold. The reduced size (in comparison with bispecific antibodies) and high affinity of folate conjugates suggest that they may be useful as immunotherapeutic agents in targeting tumors that express folate receptors.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolhuis R. L., Lamers C. H., Goey S. H., Eggermont A. M., Trimbos J. B., Stoter G., Lanzavecchia A., di Re E., Miotti S., Raspagliesi F. Adoptive immunotherapy of ovarian carcinoma with bs-MAb-targeted lymphocytes: a multicenter study. Int J Cancer Suppl. 1992;7:78–81. [PubMed] [Google Scholar]
- Brigle K. E., Seither R. L., Westin E. H., Goldman I. D. Increased expression and genomic organization of a folate-binding protein homologous to the human placental isoform in L1210 murine leukemia cell lines with a defective reduced folate carrier. J Biol Chem. 1994 Feb 11;269(6):4267–4272. [PubMed] [Google Scholar]
- Brigle K. E., Spinella M. J., Westin E. H., Goldman I. D. Increased expression and characterization of two distinct folate binding proteins in murine erythroleukemia cells. Biochem Pharmacol. 1994 Jan 20;47(2):337–345. doi: 10.1016/0006-2952(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Brigle K. E., Westin E. H., Houghton M. T., Goldman I. D. Characterization of two cDNAs encoding folate-binding proteins from L1210 murine leukemia cells. Increased expression associated with a genomic rearrangement. J Biol Chem. 1991 Sep 15;266(26):17243–17249. [PubMed] [Google Scholar]
- Canevari S., Miotti S., Bottero F., Valota O., Colnaghi M. I. Ovarian carcinoma therapy with monoclonal antibodies. Hybridoma. 1993 Oct;12(5):501–507. doi: 10.1089/hyb.1993.12.501. [DOI] [PubMed] [Google Scholar]
- Coney L. R., Mezzanzanica D., Sanborn D., Casalini P., Colnaghi M. I., Zurawski V. R., Jr Chimeric murine-human antibodies directed against folate binding receptor are efficient mediators of ovarian carcinoma cell killing. Cancer Res. 1994 May 1;54(9):2448–2455. [PubMed] [Google Scholar]
- Coney L. R., Tomassetti A., Carayannopoulos L., Frasca V., Kamen B. A., Colnaghi M. I., Zurawski V. R., Jr Cloning of a tumor-associated antigen: MOv18 and MOv19 antibodies recognize a folate-binding protein. Cancer Res. 1991 Nov 15;51(22):6125–6132. [PubMed] [Google Scholar]
- Fanger M. W., Morganelli P. M., Guyre P. M. Bispecific antibodies. Crit Rev Immunol. 1992;12(3-4):101–124. [PubMed] [Google Scholar]
- Ferrini S., Cambiaggi A., Cantoni C., Canevari S., Mezzanzanica D., Colnaghi M. I., Moretta L. Targeting of T or NK lymphocytes against tumor cells by bispecific monoclonal antibodies: role of different triggering molecules. Int J Cancer Suppl. 1992;7:15–18. [PubMed] [Google Scholar]
- Friend C., Patuleia M. C., De Harven E. Erythrocytic maturation in vitro of murine (Friend) virus-induced leukemic cells. Natl Cancer Inst Monogr. 1966 Sep;22:505–522. [PubMed] [Google Scholar]
- Goldman I. D. The characteristics of the membrane transport of amethopterin and the naturally occurring folates. Ann N Y Acad Sci. 1971 Nov 30;186:400–422. doi: 10.1111/j.1749-6632.1971.tb46996.x. [DOI] [PubMed] [Google Scholar]
- Gruber M., Schodin B. A., Wilson E. R., Kranz D. M. Efficient tumor cell lysis mediated by a bispecific single chain antibody expressed in Escherichia coli. J Immunol. 1994 Jun 1;152(11):5368–5374. [PubMed] [Google Scholar]
- Haskins K., Hannum C., White J., Roehm N., Kubo R., Kappler J., Marrack P. The antigen-specific, major histocompatibility complex-restricted receptor on T cells. VI. An antibody to a receptor allotype. J Exp Med. 1984 Aug 1;160(2):452–471. doi: 10.1084/jem.160.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm J., Hansen S. I., Høier-Madsen M., Bostad L. High-affinity folate binding in human choroid plexus. Characterization of radioligand binding, immunoreactivity, molecular heterogeneity and hydrophobic domain of the binding protein. Biochem J. 1991 Nov 15;280(Pt 1):267–271. doi: 10.1042/bj2800267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoo W. S., Kranz D. M. Role of CD8 in staphylococcal enterotoxin B-mediated lysis by cytotoxic T lymphocytes. J Immunol. 1993 May 15;150(10):4331–4337. [PubMed] [Google Scholar]
- Jansen G., Schornagel J. H., Westerhof G. R., Rijksen G., Newell D. R., Jackman A. L. Multiple membrane transport systems for the uptake of folate-based thymidylate synthase inhibitors. Cancer Res. 1990 Dec 1;50(23):7544–7548. [PubMed] [Google Scholar]
- Jost C. R., Kurucz I., Jacobus C. M., Titus J. A., George A. J., Segal D. M. Mammalian expression and secretion of functional single-chain Fv molecules. J Biol Chem. 1994 Oct 21;269(42):26267–26273. [PubMed] [Google Scholar]
- Kageyama S., Tsomides T. J., Sykulev Y., Eisen H. N. Variations in the number of peptide-MHC class I complexes required to activate cytotoxic T cell responses. J Immunol. 1995 Jan 15;154(2):567–576. [PubMed] [Google Scholar]
- Kranz D. M., Sherman D. H., Sitkovsky M. V., Pasternack M. S., Eisen H. N. Immunoprecipitation of cell surface structures of cloned cytotoxic T lymphocytes by clone-specific antisera. Proc Natl Acad Sci U S A. 1984 Jan;81(2):573–577. doi: 10.1073/pnas.81.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranz D. M., Tonegawa S., Eisen H. N. Attachment of an anti-receptor antibody to non-target cells renders them susceptible to lysis by a clone of cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7922–7926. doi: 10.1073/pnas.81.24.7922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers C. H., Gratama J. W., Warnaar S. O., Stoter G., Bolhuis R. L. Inhibition of bispecific monoclonal antibody (bsAb)-targeted cytolysis by human anti-mouse antibodies in ovarian carcinoma patients treated with bsAb-targeted activated T-lymphocytes. Int J Cancer. 1995 Feb 8;60(4):450–457. doi: 10.1002/ijc.2910600405. [DOI] [PubMed] [Google Scholar]
- Leamon C. P., Low P. S. Cytotoxicity of momordin-folate conjugates in cultured human cells. J Biol Chem. 1992 Dec 15;267(35):24966–24971. [PubMed] [Google Scholar]
- Leamon C. P., Pastan I., Low P. S. Cytotoxicity of folate-Pseudomonas exotoxin conjugates toward tumor cells. Contribution of translocation domain. J Biol Chem. 1993 Nov 25;268(33):24847–24854. [PubMed] [Google Scholar]
- Lee R. J., Low P. S. Folate-mediated tumor cell targeting of liposome-entrapped doxorubicin in vitro. Biochim Biophys Acta. 1995 Feb 15;1233(2):134–144. doi: 10.1016/0005-2736(94)00235-h. [DOI] [PubMed] [Google Scholar]
- Leo O., Foo M., Sachs D. H., Samelson L. E., Bluestone J. A. Identification of a monoclonal antibody specific for a murine T3 polypeptide. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1374–1378. doi: 10.1073/pnas.84.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M. A., Nussbaum S. R., Eisen H. N. Hormone conjugated with antibody to CD3 mediates cytotoxic T cell lysis of human melanoma cells. Science. 1988 Jan 22;239(4838):395–398. doi: 10.1126/science.3257303. [DOI] [PubMed] [Google Scholar]
- Mezzanzanica D., Garrido M. A., Neblock D. S., Daddona P. E., Andrew S. M., Zurawski V. R., Jr, Segal D. M., Wunderlich J. R. Human T-lymphocytes targeted against an established human ovarian carcinoma with a bispecific F(ab')2 antibody prolong host survival in a murine xenograft model. Cancer Res. 1991 Oct 15;51(20):5716–5721. [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Ozato K., Hansen T. H., Sachs D. H. Monoclonal antibodies to mouse MHC antigens. II. Antibodies to the H-2Ld antigen, the products of a third polymorphic locus of the mouse major histocompatibility complex. J Immunol. 1980 Dec;125(6):2473–2477. [PubMed] [Google Scholar]
- Ozato K., Mayer N. M., Sachs D. H. Monoclonal antibodies to mouse major histocompatibility complex antigens. Transplantation. 1982 Sep;34(3):113–120. doi: 10.1097/00007890-198209000-00001. [DOI] [PubMed] [Google Scholar]
- Rojo J. M., Janeway C. A., Jr The biologic activity of anti-T cell receptor V region monoclonal antibodies is determined by the epitope recognized. J Immunol. 1988 Feb 15;140(4):1081–1088. [PubMed] [Google Scholar]
- Ross J. F., Chaudhuri P. K., Ratnam M. Differential regulation of folate receptor isoforms in normal and malignant tissues in vivo and in established cell lines. Physiologic and clinical implications. Cancer. 1994 May 1;73(9):2432–2443. doi: 10.1002/1097-0142(19940501)73:9<2432::aid-cncr2820730929>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Schodin B. A., Kranz D. M. Binding affinity and inhibitory properties of a single-chain anti-T cell receptor antibody. J Biol Chem. 1993 Dec 5;268(34):25722–25727. [PubMed] [Google Scholar]
- Van Dyke T. A., Finlay C., Miller D., Marks J., Lozano G., Levine A. J. Relationship between simian virus 40 large tumor antigen expression and tumor formation in transgenic mice. J Virol. 1987 Jun;61(6):2029–2032. doi: 10.1128/jvi.61.6.2029-2032.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitman S. D., Frazier K. M., Kamen B. A. The folate receptor in central nervous system malignancies of childhood. J Neurooncol. 1994;21(2):107–112. doi: 10.1007/BF01052894. [DOI] [PubMed] [Google Scholar]
- Weitman S. D., Lark R. H., Coney L. R., Fort D. W., Frasca V., Zurawski V. R., Jr, Kamen B. A. Distribution of the folate receptor GP38 in normal and malignant cell lines and tissues. Cancer Res. 1992 Jun 15;52(12):3396–3401. [PubMed] [Google Scholar]
- Westerhof G. R., Jansen G., van Emmerik N., Kathmann I., Rijksen G., Jackman A. L., Schornagel J. H. Membrane transport of natural folates and antifolate compounds in murine L1210 leukemia cells: role of carrier- and receptor-mediated transport systems. Cancer Res. 1991 Oct 15;51(20):5507–5513. [PubMed] [Google Scholar]
- Yokota T., Milenic D. E., Whitlow M., Schlom J. Rapid tumor penetration of a single-chain Fv and comparison with other immunoglobulin forms. Cancer Res. 1992 Jun 15;52(12):3402–3408. [PubMed] [Google Scholar]
- van Ravenswaay Claasen H. H., van de Griend R. J., Mezzanzanica D., Bolhuis R. L., Warnaar S. O., Fleuren G. J. Analysis of production, purification, and cytolytic potential of bi-specific antibodies reactive with ovarian-carcinoma-associated antigens and the T-cell antigen CD3. Int J Cancer. 1993 Aug 19;55(1):128–136. doi: 10.1002/ijc.2910550123. [DOI] [PubMed] [Google Scholar]



