Abstract
In Arabidopsis, axillary buds may become dormant in response to a far-red rich light or to increased apical dominance. BRANCHED1 (BRC1) is required for this response. Transcriptional profiling studies of wild-type and brc1 mutant buds allowed the identification of sets of BRC1-dependent genes including a group of ABA-related genes upregulated in dormant buds. By using BRC1 inducible lines we demonstrate that 2 of these ABA response factors, ABF3 and ABI5, are positively regulated in axillary buds by BRC1 after induction. To get further insight into the genetic control of the growth-to-dormancy transition in buds we have also compared this transcriptomic data with 2 additional “active vs dormant bud” transcriptomic data sets and found “core” co-regulated gene networks tightly associated to each condition.
Keywords: axillary bud, bud dormancy, shoot branching, ABA, cell cycle, BRANCHED1, Arabidopsis, shade avoidance
Axillary buds are small structures localized at the base of leaves. They are branch primordia that contain, preformed, all the elements of an adult branch: a shoot apical meristem, several leaf primordia, a compressed shoot and, sometimes, inflorescence and flower meristems. Axillary buds may continue to grow until they elongate to give a branch or they may become quiescent until endogenous and environmental conditions are optimal for their development. One environmental signal controlling bud activity is a change in light quality, in particular, in the red (R) to far-red (FR) light ratio (R:FR). During photosynthesis, leaves absorb R and reflect FR, therefore plants interpret a decrease in R:FR as a signal of impending shading by neighboring vegetation. In low R:FR, plants trigger a group of developmental responses collectively known as shade avoidance syndrome (SAS) one of which is the promotion of axillary bud arrest and suppression of lateral shoot outgrowth.
In Arabidopsis, this response requires the BRANCHED1 (BRC1) gene function. BRC1, expressed in axillary buds and encoding a class II TCP transcription factor,1-3 delays bud growth and development, and promotes bud dormancy. In a recent work, published in Plant Cell4 we showed that under a FR-rich light, axillary buds have 2 to 3 times more BRC1 mRNA levels than in high R:FR. Moreover, brc1 mutants can develop many branches in the shade, unlike wild-type plants. To further investigate BRC1 function in the SAS, we looked for genes downstream of BRC1 in low R:FR. For that, we treated Arabidopsis wild-type and brc1 plants (whose axillary buds were beginning to develop) with 8 h of low R:FR and studied gene expression in axillary buds and subtending tissue (Fig. 1A). In wild type, bud dormancy markers were upregulated after the treatment whereas their levels were unchanged in brc1 mutants. This confirmed that wild-type but not brc1 buds were becoming dormant after the treatment. We then expanded this analysis by performing microarray hybridizations with the same mRNAs. We hypothesized that genes whose expression changed in low R:FR in wild type but not in brc1 required BRC1 function for their regulation and termed them BRC1-dependent genes. We identified 2 BRC1-dependent networks of cell cycle- and ribosome-related genes significantly downregulated in wild-type.4 Genes in these networks have promoters enriched in TCP binding sites, indicating that they could be directly controlled by TCP factors. A tight relationship between BRC1 function and the regulation of some of the cell cycle-related genes was further supported by their downregulation in estradiol-inducible BRC1 seedlings grown in high R:FR.
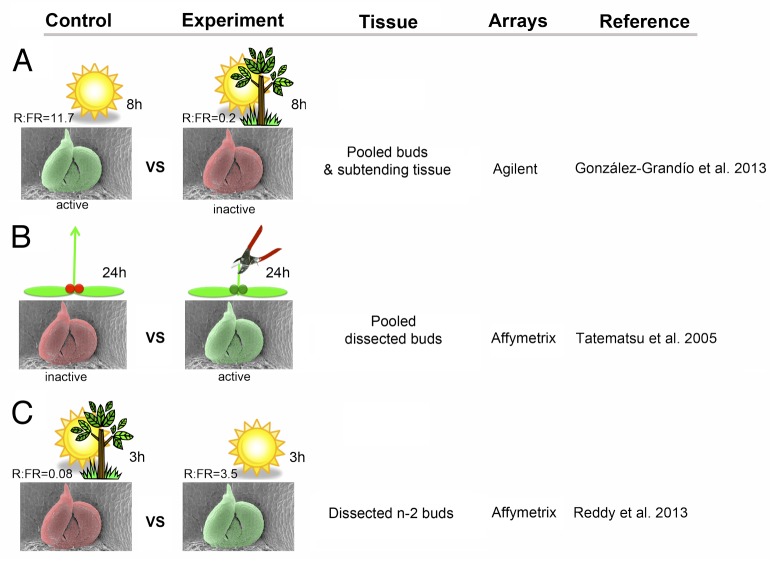
Figure 1. Transcriptomic profiling experiments comparing active vs dormant buds in Arabidopsis. (A) axillary bud dormancy was induced by an 8-hour treatment of low R:FR = 0.2. (B) bud activation was induced by decapitation of the main apex. Buds of intact or decapitated plants were pool-collected and transcriptional profiles compared after 24 h. (C) bud activation was induced by exposing plants grown in shade (R:FR = 0.08) to high R:FR = 3.5 for 3 hours. Bud n-2 (n being the uppermost rosette bud) was analyzed. Red, dormant buds. Green, active buds.
In addition, a significant proportion of ABA-responding genes5 were induced in low R:FR in wild type, indicating high ABA signaling. In contrast, ABA response was significantly reduced in brc1 mutants. Global analysis of upregulated gene promoters showed a significant overrepresentation of the aCACGTGt motif, which contains the ABA Responsive Element (ABRE, ACGT).6 All these results agreed with an activation of ABA signaling in buds entering dormancy in low R:FR, and with a role of BRC1 in maintaining this response. ABA has been classically associated to bud dormancy in many species, however in Arabidopsis this association remained elusive. In a recent study,7 Reddy et al. quantified ABA levels in axillary buds with different growth capabilities (due either to their node position or to exposure to different light conditions) and confirmed that ABA abundance directly correlated with the degree of bud dormancy also in Arabidopsis. Moreover, they showed that ABA biosynthesis mutants nced3–2 and aba2–1 had enhanced branching in low R:FR. Finally, transcriptomic analyses of inactive vs active axillary buds were also consistent with a high ABA signaling in inactive buds. All these results support our findings. One possibility to explain the observed relationship between BRC1 and ABA signaling could be that BRC1 promotes the expression of key ABA transcription factors such as ABF3 and ABI5. These genes are BRC1-dependent and contain TCP binding sites in their promoters. To test this, we have studied their transcriptional response in BRC1 estradiol-inducible lines. Our results confirm that both genes are quickly upregulated in axillary buds by the sole induction of BRC1, even in high R:FR (Fig. 2). These results indicate that, although the molecular mechanisms are still unclear, ABA signaling probably plays an important role in the control of axillary bud arrest in Arabidopsis and that BRC1, required to maintain ABA signaling, could be regulating the transcription of essential ABA-related factors.
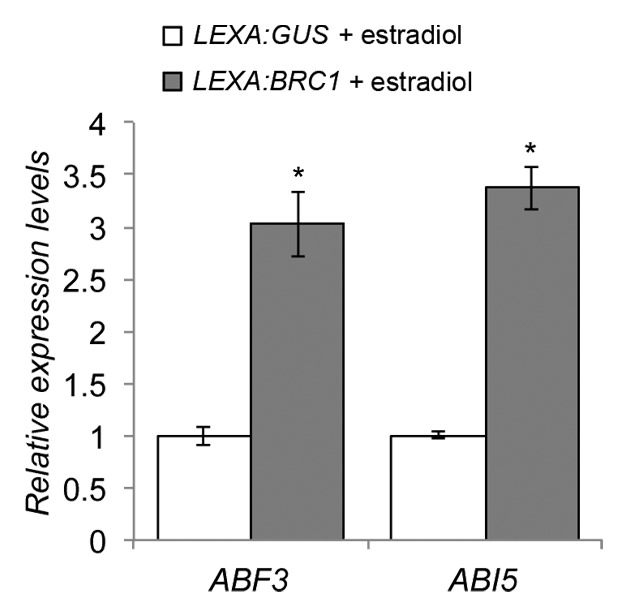
Figure 2. Response of ABA related factors to BRC1 induction. mRNA levels of ABF3 and ABI5 were measured by qPCR as described in González-Grandío et al. (2013). Estradiol-inducible BRC1 plants and estradiol-inducible GUS plants (control) were treated with 20 µM estradiol for 8 h. Error bars are SEM of 3 biological replicates of 8 plants each. Asterisks are significant differences (Student t-test, P < 0.05). Primers used were CCAAAGAGCG CCCTGGAT and TTTTCTCACT CGCCCAAACA T for ABF3, and CACCAGTTCA GGCAGGTGTT T and TGTCCACCCG CTCCAAA for ABI5. SAND was used as a reference gene.16
To identify general gene functions controlling bud activity in Arabidopsis irrespective of the inducing signals involved, we compared our transcriptomic analyses with 2 additional ones in which mRNA of active vs inactive buds were analyzed. In these studies bud activation was triggered either by decapitation of the main apex,8 (Fig. 1B) or by exposure of plants grown in low R:FR to a short period of high R:FR7 (Fig. 1C). Thus, the 3 experiments used different treatments, bud material as mRNA source, microarrays platforms (Affymetrix or Agilent), and sampling time points (Fig. 1). Genes changing similarly in all active or dormant bud samples should be tightly associated to bud growth activity. As the experiments displayed different transcriptomic profiles, we selected the 1000 genes most significantly (lowest FDR) up- and downregulated for each data set, regardless their fold change (provided it was >1.1 or <-1.1, Table S1) and we looked for common elements using Venny (http://bioinfogp.cnb.csic.es/tools/venny/). Twenty-five genes (termed bud activation genes) were upregulated in all 3 active bud samples and downregulated in all 3 dormant bud samples. Likewise, 78 genes (bud dormancy genes) were up and downregulated in dormant and active buds, respectively, in the 3 data sets.
Bud activation genes fell in 3 groups of co-regulated genes (ATTED-II9) related to DNA replication, S phase and mitosis (7 genes), flavonoid synthesis (5 genes), and cytokinin signaling (11 genes) (Figure S1). The cytokinin-related network comprised genes encoding proteins involved in cell wall synthesis, sucrose, fatty acid, and phenylpropanoid metabolism, several of them targeted to plastids, mitochondria, and vacuole (Table S1). Two additional identified genes were related to ribosome biogenesis (At5g61220) and RNA processing (At5g63120), respectively.
Bud dormancy genes fell into 4 groups of co-regulated genes. The largest group comprised 26 genes related to ABA, including the ABA synthesis gene NCED3, HIS1–3, and the transcription factors ABI1, ABF3, AFP1, AFP3, NAC019, NAC092, and NAP, further confirming the implication of ABA in bud dormancy (Figure S2). A group of 10 co-regulated genes included ethylene and auxin-related genes such as WES1, involved in auxin synthesis, ethylene receptor ERS2, the transcription factors TEMPRANILLO, HAT4, and SHINE3 and the F-box EBF2 (Figure S3). A group of 20 genes was highly upregulated in dark and night-extension treatments10 (according to Genevestigator11) and strongly downregulated by glucose or sucrose treatments.12,13 Members of this group were related to sucrose metabolism and signaling (COR414-TM1, AKINBETA1), senescence (SAG21), protein degradation (RING and F box proteins), and abiotic stress (DNAJ11, DNAJ20) (Figure S4). Another group of 19 genes comprised proteins involved in autophagy (NUDIX HYDROLASE HOMOLOG 15), protein and aminoacid degradation (BTB, FBP7, HGO) vesicular transport (MEMBRIN11), and genes encoding proteins targeted to vacuoles and endoplasmic reticulum (Figure S5).
Genes identified as bud dormancy and bud activation genes constitute very early markers (they respond after 3 hours) of eco and paradormancy (they respond to changes in light quality and decapitation) in Arabidopsis. Moreover, these genes must have a sustained response as they are still differentially expressed 24 h after beginning of the treatment.8 In addition, their regulation must be positively and negatively controlled as they display opposite behaviors during bud activation7,8 and bud dormancy induction.4 Some of them could play key roles in the coordinated regulation of gene expression leading to changes in bud growth status. The identification of these central factors will help us understand this process in Arabidopsis. Comparison of these profiles with those of more distantly related species in which bud responses have also been carefully analyzed (e.g., Vitis,14 populus15) should help us find even more common themes in the control of bud dormancy and bud release.
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The BRC1-inducible line was generated in the TRANSPLANTA consortium. This work was supported by the Ministerio de Educación y Ciencia (BIO2008–00581 and CSD2007–00057) and Ministerio de Ciencia y Tecnología (BIO2011–25687), EG-G was a predoctoral fellow of Fundación Ramón Areces and now is a CSIC JAE-Predoc fellow.
Supplemental Materials
Supplemental materials may be found here: www.landesbioscience.com/journals/psb/article/27994
References
- 1.Aguilar-Martínez JA, Poza-Carrión C, Cubas P. Arabidopsis BRANCHED1 acts as an integrator of branching signals within axillary buds. Plant Cell. 2007;19:458–72. doi: 10.1105/tpc.106.048934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finlayson SA. Arabidopsis Teosinte Branched1-like 1 regulates axillary bud outgrowth and is homologous to monocot Teosinte Branched1. Plant Cell Physiol. 2007;48:667–77. doi: 10.1093/pcp/pcm044. [DOI] [PubMed] [Google Scholar]
- 3.Martín-Trillo M, Cubas P. TCP genes: a family snapshot ten years later. Trends Plant Sci. 2010;15:31–9. doi: 10.1016/j.tplants.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 4.González-Grandío E, Poza-Carrión C, Sorzano CO, Cubas P. BRANCHED1 promotes axillary bud dormancy in response to shade in Arabidopsis. Plant Cell. 2013;25:834–50. doi: 10.1105/tpc.112.108480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nemhauser JL, Hong F, Chory J. Different plant hormones regulate similar processes through largely nonoverlapping transcriptional responses. Cell. 2006;126:467–75. doi: 10.1016/j.cell.2006.05.050. [DOI] [PubMed] [Google Scholar]
- 6.Simpson SD, Nakashima K, Narusaka Y, Seki M, Shinozaki K, Yamaguchi-Shinozaki K. Two different novel cis-acting elements of erd1, a clpA homologous Arabidopsis gene function in induction by dehydration stress and dark-induced senescence. Plant J. 2003;33:259–70. doi: 10.1046/j.1365-313X.2003.01624.x. [DOI] [PubMed] [Google Scholar]
- 7.Reddy SK, Holalu SV, Casal JJ, Finlayson SA. Abscisic acid regulates axillary bud outgrowth responses to the ratio of red to far-red light. Plant Physiol. 2013;163:1047–58. doi: 10.1104/pp.113.221895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tatematsu K, Ward S, Leyser O, Kamiya Y, Nambara E. Identification of cis-elements that regulate gene expression during initiation of axillary bud outgrowth in Arabidopsis. Plant Physiol. 2005;138:757–66. doi: 10.1104/pp.104.057984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Obayashi T, Kinoshita K, Nakai K, Shibaoka M, Hayashi S, Saeki M, Shibata D, Saito K, Ohta H. ATTED-II: a database of co-expressed genes and cis elements for identifying co-regulated gene groups in Arabidopsis. Nucleic Acids Res. 2007;35:D863–9. doi: 10.1093/nar/gkl783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Usadel B, Bläsing OE, Gibon Y, Retzlaff K, Höhne M, Günther M, Stitt M. Global transcript levels respond to small changes of the carbon status during progressive exhaustion of carbohydrates in Arabidopsis rosettes. Plant Physiol. 2008;146:1834–61. doi: 10.1104/pp.107.115592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hruz T, Laule O, Szabo G, Wessendorp F, Bleuler S, Oertle L, Widmayer P, Gruissem W, Zimmermann P. Genevestigator v3: a reference expression database for the meta-analysis of transcriptomes. Adv Bioinformatics. 2008;2008:420747. doi: 10.1155/2008/420747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y, Lee KK, Walsh S, Smith C, Hadingham S, Sorefan K, Cawley G, Bevan MW. Establishing glucose- and ABA-regulated transcription networks in Arabidopsis by microarray analysis and promoter classification using a Relevance Vector Machine. Genome Res. 2006;16:414–27. doi: 10.1101/gr.4237406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thum KE, Shin MJ, Gutiérrez RA, Mukherjee I, Katari MS, Nero D, Shasha D, Coruzzi GM. An integrated genetic, genomic and systems approach defines gene networks regulated by the interaction of light and carbon signaling pathways in Arabidopsis. BMC Syst Biol. 2008;2:31. doi: 10.1186/1752-0509-2-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Díaz-Riquelme J, Grimplet J, Martínez-Zapater JM, Carmona MJ. Transcriptome variation along bud development in grapevine (Vitis vinifera L.) BMC Plant Biol. 2012;12:181. doi: 10.1186/1471-2229-12-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruttink T, Arend M, Morreel K, Storme V, Rombauts S, Fromm J, Bhalerao RP, Boerjan W, Rohde A. A molecular timetable for apical bud formation and dormancy induction in poplar. Plant Cell. 2007;19:2370–90. doi: 10.1105/tpc.107.052811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Czechowski T, Stitt M, Altmann T, Udvardi MK, Scheible WR. Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol. 2005;139:5–17. doi: 10.1104/pp.105.063743. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.