Abstract
During the last decade we have witnessed an unprecedented outburst of new treatment approaches for the management of metastatic colon cancer. Anti-angiogenic drugs, epidermal growth factor receptor blockers and multi-kinase inhibitors have all resulted in small but consistent improvement in clinical outcomes. However, this progress has paradoxically leaded us into new challenges. In many cases the clinical development was done in parallel and the lack of head-to-head comparison evolved into circumstances where several valid new “standards of care” are available. Even though desirable in essence, the availability of many options as well as different possible combinations frequently leaves the busy clinician in the difficult situation of having to choose between one or the other, sometimes without solid evidence to support each decision. In addition, progress never stops and new agents are continuously tested. For these reason this review will try to summarize all the clinical trials that constitute the theoretical framework that support our daily practice but will also procure the reader with rational answers to common clinical dilemmas by critically appraising the current literature. Lastly, we will provide with a compilation of promising new agents that may soon become our next line of defense against this deadly disease.
Keywords: Colon Cancer, Stage IV, Metastatic, Review, Bevacizumab, Cetuximab, Panitumumab, Aflibercept, Regorafenib
Core tip: This manuscript is a comprehensive review, with the most updated information up to 2014, regarding metastatic colon cancer. It summarizes all those relevant clinical trials that constitute the theoretical framework to support our daily practice and provides rational answers to common clinical dilemmas. Additionally, it gives the reader a compilation of potential new agents that are currently being tested and may soon become the next step in the battle against this disease.
INTRODUCTION
Colon cancer is the second leading cause of cancer-related mortality in the United States and 1.2 millions of new cases are yearly diagnosed worldwide[1]. From the clinical perspective colon cancer could be categorized into the early stages (I-III) and the more advanced and usually lethal metastatic disease. Notably, since the publication of the MOSAIC trial almost ten years ago, no other groundbreaking development in the treatment of resectable colon cancer became available[2]. On the contrary, during the last decade we have witnessed an unprecedented outburst of new treatment approaches for the management of stage IV colon cancer that ultimately evolved into the approval of five new drugs. For simplification purposes, we can subdivide these new drugs into three categories: anti-angiogenic, epidermal growth factor receptor (EGFR) blockers and multi-kinase inhibitors. All of them represent important advances in the fight against this deadly disease. Nonetheless, some issues deserve further attention. First, these new agents were generally combined with at least some of the previously effective chemotherapy regimens (fluoropyrimidines and/or oxaliplatin and/or irinotecan). Also, the clinical development was done in parallel instead of following a rational stepwise approach where each new drug was tested against the new standard of care. This lack of head-to-head comparison resulted in several valid new “standards of care”. Lastly, new combinations are continuously tested making extremely difficult for the busy clinician to keep up with the most updated information.
For the reasons mentioned before, this manuscript will pursue three clear objectives. First summarize all those relevant clinical trials that constitute the theoretical framework to support our daily practice. Second try to provide rational answers to common clinical dilemmas by critically appraising the current literature. Finally, provide the reader with a compilation of potential new agents that are currently being tested and may soon become the next step in the battle against this disease.
ANTI-ANGIOGENESIS AS A TARGET
Angiogenesis consists in a complex multistep process of new vessel formation. The vascular endothelial growth factor (VEGF) and its receptor (VEGFR) play a crucial role in the tumor transition from the “avascular” to the “vascular” phase, acquiring metastatic potential[3,4]. It also stimulates tumor growth, migration and metastasis through mechanisms not entirely related to tumor angiogenesis[5]. Moreover, tissue interstitial pressure is a key factor in chemotherapy delivery and in some tumors this could be up to 15 times higher than the normal counterparts[6]. There is solid evidence that VEGFR inhibition partially restores interstitial fluid pressure and reduces abnormal vasculature with improvement of drug delivery and enhancement of chemotherapy efficacy[7].
Bevacizumab
Bevacizumab (Avastin®, Genentech Inc.), a recombinant humanized monoclonal IgG-1 antibody against soluble VEGF-A, was the first anti-angiogenic drug approved for metastatic colon cancer. It prevents the binding of VEGF-A to the VEGFR and, consequently, inhibits angiogenesis, tumor growth and metastatic development. It was first approved on February 2004 by the FDA as first-line treatment for patients with metastatic colon cancer. Today, almost 10 years later, a substantial body of evidence has accumulated to help clinicians in the judicious use of this molecule. Table 1 summarizes the most relevant clinical trial of the anti-angiogenic drugs.
Table 1.
Selected phase 3 clinical trials involving anti-angiogenic drugs in combination with conventional chemotherapy
| Ref. | Drug and study name | Study description | No. of patients | Comparison | Median OS (mo) | Median TTP/PFS (mo) | ORR | 1-yr survival |
| Bevacizumab (B) | ||||||||
| Hurwitz et al[8] 2004 | AVF2107g trial | RCT, 1st line | 813 (ITT) | IFL + B vs IFL | 20.3 vs 15.6 | 10.6 vs 6.2 | 45% vs 35% | 74% vs 63% |
| Fuchs et al[10] 2007 | BICC-C trial | RCT, 1st line | 117 (2nd period) | FOLFIRI + B vs mIFL + B | 28 vs 19 | 11 vs 8 | 58% vs 53% | 87% vs 61% |
| Giantonio et al[12] 2007 | ECOG 3200 trial | RCT, 2nd line post irinotecan 1st line | 820 (ITT) | FOLFOX-4 + B vs FOLFOX-4 vs B alone | 12.9 vs 10.8 vs 10.2 | 7.3 vs 4.7 vs 2.7 | 23% vs 8.6% vs 3.3% | 56% vs 43% vs 44% |
| Saltz et al[13] 2008 | NO16966 trial | RCT, phase 3, 1st line, factorial 2 x 2 | 1401 | FOLFOX-4 or XELOX + B vs FOLFOX-4 or XELOX | 21.3 vs 19.9 | 9.4 vs 8.0 | 47% vs 49% | Not reported |
| Tebbutt et al[17] 2010 | MAX trial | RCT, open label, 1st line | 471 | Cape alone vs Cape + B vs Cape + B + mitomycin | 18.9 18.9 vs 16.4 | 5.7 vs 8.5 vs 8.4 | 30% vs 38% vs 46% | Not reported |
| Cunningham et al[18] 2013 | AVEX trial | RCT, elder population, 1st line | 280 | Cape alone vs Cape + B | 20.7 vs 16.8 | 9.1 vs 5.1 | 19% vs 10% | 74% vs 44% |
| Falcone et al[21] 2013 | TRIBE trial | RCT, 1st line | 508 | FOLFOXIRI-B vs FOLFIRI-B | 31.0 vs 25.8 | 12.1 vs 9.7 | 65% vs 53% | Not reported |
| Bennouna et al[66] 2013 | ML 18147 | RCT, open label, 2nd line post chemo + B | 409 | 2nd line chemotherapy + B vs 2nd line chemotherapy | 11.2 vs 9.8 | 5.7 vs 4.1 | 5.5% vs 4% | Not reported (approximately 50% vs 40%) |
| Ziv-Aflibercept | ||||||||
| Van Cutsem et al[29] 2012 | VELOUR trial | RCT, 2nd line post oxaliplatin and/or bevacizumab 1st line | 1226 | FOLFIRI + aflibercept vs FOLFIRI + placebo | 13.5 vs 12.0 | 6.9 vs 4.7 | 20% vs 11% | 56% vs 50% |
RCT: Randomized controlled trial; OS: Overall survival; TTP: Time to progression; PFS: Progression free survival; ITT: Intention to treat; ORR: Overall response rate.
The first practice-changing, double blind, randomized phase III trial that was published compared the use of irinotecan, bolus 5-FU and leucovorin (IFL) with or without bevacizumab in metastatic, previously untreated patients[8]. The primary endpoint of the study was overall survival (OS); disease-free survival (DFS) and overall response rate (ORR) were secondary endpoints. OS (20.3 mo vs 15.6 mo; P < 0.001) and PFS (10.6 mo vs 6.2 mo; P < 0.001) and ORR (45% vs 35%) were all significantly improved with bevacizumab. Importantly, patients in the IFL group were not allowed to crossover. Similar results were obtained in the ARTIST trial using a modified version of IFL (5-FU was infused over 6-8 h) plus bevacizumab in metastatic colon cancer, chemotherapy naïve, Chinese patients, confirming that results obtained in Caucasians were also applicable in Asian population[9]. Subsequently, in 2007 results from the BICC-C trial were released showing that bevacizumab combined with the classical bolus and 46-h infusional 5-FU plus leucovorin and irinotecan (FOLFIRI) was superior to a shorter version of IFL as upfront therapy[10]. In the original trial design patients were randomly assigned to receive FOLFIRI, IFL or irinotecan plus capecitabine (CapeIRI) with or without celecoxib. However, after the FDA-approval of bevacizumab the protocol was amended and additional 117 patients were randomized to receive bevacizumab with FOLFIRI (FOLFIRI-B) or IFL (IFL-B); due to excessive toxicity the CapeIRI arm was discontinued. With an updated median follow-up of 34.4 mo, OS was longer in the FOLFIRI-B arm (28.0 mo vs 19.2 mo; P = 0.037)[11]. Thus, infusional 5-FU regimens should be preferred over bolus 5-FU when combined with bevacizumab.
After the initial success with irinotecan combinations, bevacizumab was soon studied in oxaliplatin-based regimens. The first evidence of its synergistic effect came from the ECOG-3200 study that investigated the role of bevacizumab in the second line treatment[12]. In this study patients who had progressed to irinotecan and fluoropyrimidine therapies but who had not received oxaliplatin or bevacizumab were randomized to FOLFOX-4 (control arm), FOLFOX-4 plus bevacizumab (FOLFOX-B) or single agent bevacizumab. With a median follow-up of 28-mo, a modest but statistically significant improvement in OS was shown for the FOLFOX-B arm (12.9 mo vs 10.8 mo, P = 0.0024). Single agent bevacizumab showed virtually no effect. Immediately after the release of this study, and in spite of the lack of evidence in the front line therapy setting, FOLFOX-B was rapidly accepted in the oncology community as a valid front line option for stage IV colon cancer. Evidence to support this practice finally materialized in 2008. The NO16966 study was a non-inferiority trial evaluating the use of XELOX and FOLFOX with or without bevacizumab in a factorial design[13]. The primary analysis demonstrated a statistically significant benefit in terms of progression-free survival (PFS) (9.4 mo vs 8.0 mo; P = 0.002) in patients receiving bevacizumab, irrespectively of the chemotherapy backbone used, but there was no difference in terms of OS and ORR in the final analysis. Moreover, the TREE studies evaluated the use of three different oxaliplatin-based chemotherapies with bevacizumab[14]. A total of 150 patients were randomly assigned to mFOLFOX-6, bFOL (bolus FU and low-dose LV with oxaliplatin) or CapeOx in the TREE-1 cohort and 223 patients were randomized to the same regimens with bevacizumab in the TREE-2 cohort. ORR was superior in each arm with the addition of bevacizumab and, although not statistically significant, it was highest with mFOLFOX-6 and bevacizumab (52%). Additionally, the BEAT study was designed to evaluate the safety and efficacy of several regimens containing bevacizumab used in the daily community practice but outside the formalities of a clinical trial and in a no-comparative fashion[15]. Consistent with previous studies, improved PFS and OS were seen in patients receiving doublet regimens compared to single agent chemotherapy.
A very relevant issue, however, for the daily practice is the fact that many patients with metastatic colon cancer are not suitable (e.g., elder population or poor performance status) to receive multi-agents regimen such as FOLFOX or FOLFIRI. A common practice in these cases is to use single agent fluoropyrimidine (e.g., weekly bolus 5-FU). Even in this situation, there is enough evidence to support the use of bevacizumab. At least one phase II clinical trial proved that the addition of bevacizumab to single agent 5-FU resulted in better PFS (9.2 mo vs 5.5 mo, P < 0.001) when used as first line option[16]. Importantly, the mean age of the participants was more than 70 years old. Further evidence supporting the efficacy of this combination, especially in fragile patients, came from the MAX study where capecitabine and bevacizumab resulted in longer PFS compared to single agent capecitabine (8.5 mo vs 5.7 mo; P < 0.001)[17]. This was confirmed by the AVEX Trial that enrolled elder patients (> 70 years) who were not candidates for treatment with oxaliplatin or irinotecan and randomized them to capecitabine alone or in combination with bevacizumab[18]. With a mean follow up close to 2 years, the median PFS was almost double with bevacizumab (9.1 mo vs 5.1 mo; P < 0.001). ORR was also superior but the study was underpowered to detect a benefit in OS. However, the reader should be aware that the addition of bevacizumab in these three trials resulted in an absolute increment of grade 3-4 toxicity of about 15%-20% with none of them showing a statistically benefit in OS.
A classical paradigm that has been recently called into challenge is the one that discourage the use of multi-agents regimens combining oxaliplatin and irinotecan at the same time. This presumption was based on the results of the N9741 study where the IROX (oxaliplatin + irinotecan) arm showed worse TTP, ORR and OS compare to FOLFOX[19]. However, treatment with the combination of 48-h infusional 5-FU, oxaliplatin and irinotecan (FOLFOXIRI) proved to be superior to FOLFIRI, which is believed to be similar to FOLFOX, in terms of OS, PFS and ORR in patients with mCC[20]. Recently, the results of a phase 3 TRIBE trial that compared FOLFOXIRI and FOLFIRI with the addition of bevacizumab were presented[21]. Both treatments were administered for a maximum of 12 cycles followed by 5-FU + bevacizumab until progression. With a mean follow-up of 26.6 mo, significantly increased PFS was observed in the FOLFOXIRI-B arm (9.7 mo vs 12.2 mo, P = 0.001). As expected, greater neutropenia, diarrhea, stomatitis and neurotoxicity were seen in the FOLFOXIRI arm. Interesting, similar results were obtained in a recent randomized phase II study (OLIVIA) where FOLFOXIRI-B showed better ORR and conversion to R0 resections compared to FOLFOX-B[22]. Data is still immature, but this combination could be a feasible option for fit patients.
To summarize we should emphasize some useful concepts. First, single agent bevacizumab has almost no activity. Second, the best evidence comes from its usage as upfront first line therapy in combination with either FOLFOX or FOLFIRI and perhaps FOLFOXIRI. In all cases, bevacizumab has persistently showed to improve PFS. For second line treatment the ideal scenario would be in patient who did not receive bevacizumab as a first line option. Lastly, continuation beyond progression is also feasible (see below).
Ziv-aflibercept
Ziv-aflibercept (Zaltrap®, Regeneron Pharmaceuticals) is a recombinant fusion protein consisting of the extracellular domains of human VEGFR-1 and 2 fused to the Fc portion of human IgG-1[23]. The decoy protein binds tightly PIGF, VEGF-A and VEGF-B preventing the activation of VEGFR-1 and 2 by these ligands. This is a significant difference with bevacizumab which exclusively blocks the VEGF-A[24]. Pre-clinical studies confirmed that when combined with cytotoxic drugs, ziv-aflibercept exerted considerable inhibition of angiogenesis[25-27]. In 2006, 38 patients were enrolled in a phase I clinical trial were 2, 4, 5 and 6 mg/kg escalating doses of ziv-aflibercept were explored in combination with irinotecan, 5-FU and leucovorin[28]. In the phase 3 VELOUR trial, patients with metastatic colon cancer but previously treated with oxaliplatin-containing regimens were randomly assigned to receive FOLFIRI with or without ziv-aflibercept every 2 wk[29]. Patients could not have received irinotecan before but up to 30% of them received bevacizumab as front line therapy. The ORR (11.1% vs 19.8%, P < 0.001), PFS (6.9 mo vs 4.6 mo, P < 0.001) and OS (13.5 mo vs 12.1 mo, P = 0.003) were all improved in ziv-aflibercept and were not influenced by the prior use of bevacizumab (stratifying variable). However, the absolute benefit was a modest 1.4 mo in OS.
BLOCKING EGFR AND OTHER KINASES
Cetuximab and panitumumab
In addition of blocking the angiogenesis pathway, another line of investigation that lead to practice-changing outcomes was the one advocated to jamming the EGFR. Once activated, the EGFR triggers a series of downstream phenomenon that ultimately result in tumor growth and survival[30]. It is then simple to understand that blocking EGFR could potentially halt tumor progression. Nevertheless, this basic principle is not always applicable. An overwhelming body of evidence confirmed the futility of blocking the EGFR when downstream molecules are anarchically activated. The strongest evidence comes from the presence of KRAS codons 12 and 13 mutations in exon 2 which virtually turns anti-EGFR strategies useless[31]. But, recent investigations have broadened the number of negative predictive mutations found in the RAS genes family to exons 3 and 4 of KRAS and exons 2, 3 and 4 of NRAS genes[32]. In that sense, testing for KRAS/NRAS mutations could exclude 50% of the patients from an ineffective but potentially harmful therapy. BRAF mutations carry a considerable poor prognosis, but its predictive role is somehow controversial. However, and in spite of this obvious limitation, anti-EGFR therapies have found their place in the treatment of stage IV colon cancer. Two compounds, cetuximab (Erbitux®, Bristol-Myers) a chimeric monoclonal IgG-1 antibody against EGFR, and panitumumab (Vertibix®, Amgen) a fully humanized monoclonal IgG-2 antibody also directed against EGFR, have received FDA-approval for this indication. Table 2 summarizes the most relevant clinical trials related to these agents.
Table 2.
Selected clinical trials involving anti-epidermal growth factor receptor, regorafenib or anti-epidermal growth factor receptor vs anti-epidermal growth factor receptor receptor agents
| Ref. | Drug and study name | Study description | No. of patients | Comparison | Median OS (mo) | Median TTP/PFS (mo) | ORR | 1-yr survival |
| Cetuximab (C) | ||||||||
| Cunningham et al[34] 2004 | BOND trial | RCT, phase 2, 2nd line irinotecan-refractory | 329 | Irinotecan + C vs irinotecan | 8.6 vs 6.9 | 4.1 vs 1.5 | 23% vs 11% | 29% vs 32% |
| Van Cutsem et al[37] 2009 | CRYSTAL trial | RCT, 1st line | 1198 | FOLFIRI + C vs FOLFIRI | 20 vs 18.5 and (25 vs 21) | 9 vs 8 and (10 vs 8.7) | 47% vs 39% (59 vs 43%) | Not reported (approximately 35% vs 25%) |
| Maughan et al[59] 2011 | COIN trial | RCT, phase 3, 1st line | 729 (KRAS wild type) | Oxaliplatin-based chemo + C vs chemo alone | 17 vs 17.9 | 8.6 vs 8.6 | 64% vs 57% | Not reported |
| Tveit et al[60] 2011 | NORDIC VII trial | RCT, open label, 1st line | 571 | FLOX + C vs intermittent FLOX + C vs FLOX | 19.7 vs 20.3 vs 20.4 | 8.3 vs 7.3 vs 7.9 | 49% vs 47% vs 41% | Not reported (approximately 70%) |
| Panitumumab (P) | ||||||||
| Douillard et al[39] 2010 | PRIME trial | RCT, phase 3, 1st line | 1183 | FOLFOX-4 + P vs FOLFOX-4 | 24 vs 20 (WT) 15 vs 19 (MT) | 9.6 vs 8 (WT) 7.3 vs 8.8 (MT) | 55 vs 48% (WT) 40 vs 40% (MT) | Approximately 75% both (WT) approximately 60% vs 75% (MT) |
| Regorafenib (R) | ||||||||
| Grothey et al[47] 2013 | CORRECT trial | RCT, phase 3, 3rd line | 760 | Regorafenib vs placebo | 6.4 vs 5.0 | 1.9 vs 1.7 | 1.0% vs 0.4% | 24.3% vs 20.0% |
| Cetuximab (C) vs Bevacizumab (B) | ||||||||
| Stintzing et al[63] 2013 | FIRE-3trial | RCT, phase 3, 1st line | 592 | FOLFIRI + C vs FOLFIRI + B | 28.7 vs 25 | 10 vs 10.3 | 62 % vs 58% | Not reported |
RCT: Randomized controlled trial; OS: Overall survival; TTP: Time to progression; PFS: Progression free survival; ITT: Intention to treat; ORR: Overall response rate.
As part of the pre-clinical investigation, cetuximab was tested in tumor xenografts models and found to have marked synergistic activity with irinotecan, even in previously considered irinotecan-resistant cell lines[33]. This observation was the based for a couple of phase 2 clinical trials which confirmed the clinical utility of cetuximab single agent (approximately 10% ORR) and in combination with irinotecan. However, the first convincing evidence of its clinical utility came from the BOND study where 329 patients with irinotecan-resistant metastatic colon cancer were randomly assigned to either single agent cetuximab (ORR 11%, TTP 1.5 mo) or cetuximab plus irinotecan (ORR 23%, TTP 4.1 mo)[34]. No difference in OS was seen but crossover was allowed. As in the case of cetuximab, single agent panitumumab showed 10% ORR in heavily pretreated patients who formerly received 5-FU, irinotecan and/or oxaliplatin[35,36]. Given the encouraging results as second and third line therapies, it did not take much time until both molecules were tested as first line options. In the CRYSTAL trial, 1217 patients were randomly assigned to FOLFIRI alone or FOLFIRI plus cetuximab as first line treatment[37]. The primary endpoint was PFS and it was statistically prolonged in the cetuximab group, albeit by a modest 1 mo (8.0 mo vs 8.9 mo in the whole population and 8.7 mo vs 9.9 mo in the KRAS wild-type patients). Cetuximab also resulted in an absolute 8% improvement in ORR (all partial responses) but no benefit in OS was observed. Similar results were reported in a randomized, phase 2 study using FOLFOX instead of FOLFIRI[38]. In this case the ORR was improved by 25% in wild-type patients as it was PFS, but only by 15 d (7.2 mo vs 7.7 mo). Interesting, in KRAS mutated patients PFS was actually 3-mo worse in the cetuximab arm. Similarly, in the phase 3 PRIME study, investigators used FOLFOX-4 as the backbone to randomized patients in a 1:1 fashion to panitumumab or placebo[39]. As expected, in the wild-type population ORR (48% vs 55%) and PFS (8.0 mo vs 9.6 mo) was better with anti-EGFR therapy but in KRAS mutated cases the effect was neutral or even worse.
An important point to mention at this moment is in reference to the solid evidence against the presumption that combining both anti-angiogenic and anti-EGFR molecules at the same time would results in a synergistic effect. At least two large, randomized, phase 3 clinical trials consistently showed that combining bevacizumab with EGFR inhibitors is actually deleterious. The first of them (PACCE trial) randomly assigned 1053 patients to either oxaliplatin- or irinotecan-based chemotherapy plus bevacizumab but with and without panitumumab as first line treatment for metastatic colon cancer[40]. The primary objective for the oxaliplatin-based arm was extension of PFS and in the irinotecan group was safety analysis. Secondary end points for both groups were ORR, OS and safety. A planned interim analysis for safety and efficacy was conducted at 50% of the events and panitumumab was removed due to significantly decreased PFS [hazzd ratio (HR), 1.44; P = 0.004] and increase toxicity independently of the KRAS status. Grade 3 or more adverse events were present in 90% of patients treated with panitumumab. The CAIRO-2 trial reported similar detrimental results of adding cetuximab to oxaliplatin, capecitabine and bevacizumab[41]. The addition of cetuximab significantly decreased median PFS (10.7 vs 9.4, P = 0.01). A total of 88% of patients discontinued the study, 45% due to tumor progression and 24.5% due to adverse events. A third study, the CALGB 80405, was initially designed to evaluate the use of FOLFOX or FOLFIRI with bevacizumab, cetuximab, or both agents together. In base of the results of the previous studies, the arm combining cetuximab and bevacizumab was closed (NCT00265850).
Regorafenib
The last drug to receive FDA-approval was regorafenib (Stivarga®, Bayer). The compound is an orally available multi-kinase inhibitor with activity against multiple targets including KIT, PDGFR and VEGFR among others. It is structurally related to sorafenib and the most usual adverse events are hand-foot skin reaction, mucositis, hypertension and diarrhea[42-45]. In an expanded phase I trial with 27 evaluable patients, 74% achieved disease control with 1 patient obtaining partial response and 19 stable disease[46]. Globally, regorafenib was well tolerated and adverse events were clinically manageable leading to a multi-centric phase 3 trial. The CORRECT study enrolled patients who had already received all the approved standard therapies and who had progressed during or within 3 mo after the last therapy[47]. Seven hundred and sixty participants were randomized in a 2:1 ratio to regorafenib or placebo. Median OS was 6.4 mo in the regorafenib group vs 5.0 mo in the placebo group (P = 0.005). The most frequent grade 3 or 4 adverse events were hand-foot skin reaction (17%), fatigue (10%), diarrhea (7%), hypertension (7%), and skin desquamation (6%).
COMMON CLINICAL DILEMMAS
We have witnessed an exponential growth in the number of clinical trials dedicated to metastatic colon cancer which eventually resulted in small but consistent improvement in clinical outcomes (Figure 1). However, this progress has paradoxically leaded us into new challenges. We have arbitrarily chosen 3 topics that in our own opinion are probably the more relevant clinical dilemmas. The reader should be aware, though, that the opinions expressed below come from our own assessment of the literature and they should be considered only as the authors’ point of view.
Figure 1.
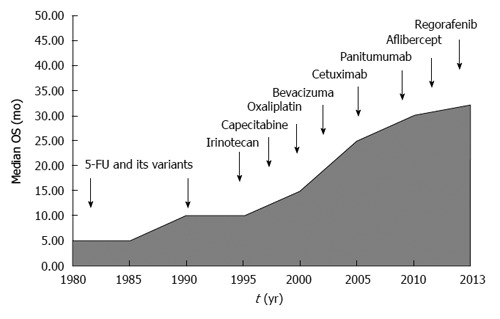
Schematic representation of the recent advances in the treatment of metastatic colon cancer.
Is there any role for peri-operative chemotherapy in potentially resectable liver metastases? Can the new biological agents improve the resectability rate on patients with borderline or unresectable liver metastases? Which regimen to chose?
The first point to consider is whether the patient has upfront resectable disease or not. A set of criteria have been proposed, however in any case this decision require appropriate discussion between the medical and surgical oncologists[48]. For those who are considered resectable common practice is to give them at least 6 mo of chemotherapy. The most solid evidence for this action comes from the EORTC 40983 trial where 364 patients, with one to four resectable liver metastases, were randomly assigned to surgery alone or 6 doses of FOLFOX-4 pre- and post-surgery[49]. The study was positive for its primary endpoint, PFS (20.9 vs 12.5; P = 0.035, per protocol population) and it gained rapid acceptance within the medical community. Oncologist extrapolated these results to the completely neo-adjuvant or adjuvant (stage IV in NED status) setting, albeit with no evidence to support this approach. OS was not improved in the EORTC 40983 but the enrollment of patients was less than originally expected and its statistical power was called into question. Two other studies were reported in the adjuvant setting after complete resection of liver metastases[50]. They were also underpowered and employed outdated chemotherapy (5-FU bolus). The poor accrual in these clinical trials is most likely related to the oncologists’ reluctance to enroll patients in studies that involved a surgery only arm. One single institution, single arm study showed 73% ORR (9% complete pathological response) in 56 patients treated with XELOX + bevacizumab in a peri-operative setting (6 doses pre- and 6 other post-surgery)[51]. The use of biological agents in the post-surgical period, when the patient is NED, is very controversial. Based on the results from adjuvant studies this practice should be discouraged. However, formal studies addressing this issue are missing. Other relevant issue with upfront resectable disease is the fact that chemotherapy could result in liver damage (e.g., steatohepatitis) which could jeopardize patient’s only curative chance.
A different scenario presents when the patient has liver-limited but unresectable metastases. Some of these patients (e.g., low volume but abutting critical structures) have borderline disease, potentially amenable to be converted. In these cases, clinician should choose the best possible regimen to obtain maximal response rate. Before the advent of the anti-EGFR and bevacizumab, conventional chemotherapy agents had already proven to enable surgical resection in a proportion of patients. Regimens such as FOLFOX or FOLFIRI have a conversion rate close to 40% and this could be improved with FOLFOXIRI[20,52,53]. The obvious question then is how much bevacizumab or the anti-EGFR drugs add to this and which one to use. A practical consideration is the fact that bevacizumab, which is the only option in KRAS mutant cases, has to be stopped at least 6-wk before surgery. For wild-type tumors, evidence may be slightly stronger for anti-EGFR drugs.
In the Germanic CELIM phase 2 study, 114 patients were randomly assigned to FOLFOX-6 or FOLFIRI, both regimens with cetuximab[54]. Patients required having technically unresectable liver metastases or more than five lesions. From a 106 evaluable patients, 36 of them (34%) had R0 resection but this proportion reached 60% in the wild-type KRAS population (41/68). Similar results were obtained in retrospective series. Even stronger evidence supporting the use of anti-EGFR in this particular setting came from a recently published Chinese study[55]. This phase 2, randomized study compared the efficacy of conventional chemotherapy (FOLFOX-6 or FOLFIRI) with or without cetuximab. Conversion to resection was the main outcome and after randomizing 138 patients the arm with cetuximab duplicated the proportion of patients deemed eligible for resection (13% vs 29%) and triplicated the R0 rates (7.4% vs 25.7%). Based on these reports chemotherapy plus cetuximab should be strongly considered for patients with wild-type KRAS and liver only metastases. Detractors of this posture may argue, though, that in a fresh head-to-head comparison between cetuximab and bevacizumab, ORR was not different (FIRE-3; see below).
Data supporting the use of bevacizumab in this scenario is somehow controversial. The most vigorous argument against its use comes from the previously mentioned NO16966 study[14]. There was no difference in ORR and there was similar proportion of patients attempted to have curative metastatectomies (8.4% vs 6.0%). However, the study was not designed to test this hypothesis. On the other hand, small phase 2 and retrospective studies brought up to 40% conversion rates and pathological responses when bevacizumab is added to XELOX, representing the fundaments for its use especially in KRAS mutant patients[56,57]. In that regards, the possibility of adding a stronger chemotherapy, such as FOLFOXIRI, should be seriously considered for fit patients.
Which is the ideal chemotherapy mate of the current monoclonal antibodies? And in patients with wild-type KRAS which strategy we should choose? Anti-VEGFR or Anti-EGFR?
Doublet chemotherapy is often used as upfront systemic treatment for advanced CC. It is unclear to these days which doublet is better for each patient and this has to be individualized according to toxicity and comorbidities. FOLFOX, XELOX, and FOLFIRI appear to be similar in efficacy but with different toxicity profile. XELIRI is harder to endure. Most patients tolerate a chemotherapy doublet, but probably not all of them need it as showed by the frequently forgotten Dutch study (CAIRO-1)[58]. The addition of biologics has improved outcomes, but not as much as we hoped. When KRAS is mutated, the chemotherapy chosen must be accompanied with bevacizumab. The dilemma starts with the K-RAS wild type patients. There are clinical trials showing benefit for both approaches: anti-VEGFR and anti-EGFR. The question is which patient would benefit from one or the other schema.
As previously mentioned, in the NO16966 study bevacizumab extended PFS by 1.4 mo, with a more profound effect seen in the XELOX arm[13]. But, why bevacizumab had such a discrete effect on PFS? Was this due to no synergistic or additive effect with FOLFOX/XELOX? The answer is NO, since FOLXOX + bevacizumab is active, even in second line with significant prolongation of OS[12]. Some authors advocate the idea of failure due to the “OPTIMOX” effect, meaning when neurotoxicity occurred oxaliplatin was stopped and fluoropyrimidine plus bevacizumab was continued until progression. This could be the case, since when we observe the difference in PFS of the patients on treatment, this is much more important. It is also feasible that bevacizumab works better with “inferior chemotherapies” such as IFL and have less to offer with “superior chemotherapies” such as XELOX or FOLFOX.
Regarding the anti-EGFR therapies, the earlier cited CRYSTAL and PRIME studies are the foundations for its use in the frontline treatment[40,41]. Nonetheless, in 2011 the COIN study was published[59]. With 2445 KRAS wild-type patients randomized to XELOX or FOLFOX +/- cetuximab, the COIN study represents the biggest trial ever conducted in this population. The results were disappointing. No difference in PFS was seen. Shortly thereafter, the results of the NORDIC VII were released[60]. Patients were randomly assigned to either standard Nordic FLOX or cetuximab + FLOX or cetuximab + intermittent FLOX. The median PFS was 7.9, 8.3, and 7.3 mo respectively and was not significantly different. In patients with KRAS wild-type tumors, cetuximab did not provide any additional benefit but in patients with KRAS mutations a trend toward worsening PFS was observed. The authors concluded that cetuximab did not add significant benefit to the Nordic FLOX regimen as first-line treatment. Additionally, the randomized, phase 2, PEAK study was presented in the 2013 ASCO GI Meeting[61]. This study enrolled 285 patients and evaluated the use of first-line mFOLFOX-6 + panitumumab vs bevacizumab. Again, no difference was observed. It is confusing how to interpret the actual role of anti-EGFR and chemotherapy since COIN, the largest phase 3 randomized trial, was negative. The NORDIC was a negative trial as well, but in the scenario of 5-FU given by bolus, a seldom used strategy nowadays. It is possible that irinotecan-based chemotherapy would be necessary when anti-EGFR is considered in the treatment of metastatic disease. It is also curious that the hazard ratios for PFS with anti-EGFR antibodies tend to become more significant as the number of previously used lines of treatment upsurges. For instance, these agents are useless in the adjuvant setting and grow more active as disease progresses (e.g., 3rd line).
Lastly, the FIRE-3 trial was presented in June 2013[62]. This was a randomized multicenter trial comparing the efficacy of FOLFIRI + cetuximab vs FOLFIRI + bevacizumab in patients with wild-type KRAS metastatic colon cancer. The primary endpoint was ORR and 592 patients were included. The study was negative for its primary end-point, with comparable ORR (62% vs 58%, P = 0.183). Significantly better PFS and OS were seen in the FOLFIRI + cetuximab arm (28.8 mo vs 25.0 mo; P = 0.016) although this was a secondary endpoint. A preplanned analysis of the FIRE-3 was presented at the European Cancer Congress 2013, aimed to investigate the effect of several other mutations beyond the exon 2 as well as BRAF (V600E)[63]. About 15% of patients were found to have these extra mutations. This sub-analysis incorporated 342 KRAS wild-type patients and 178 KRAS mutant patients (113 with exon 2 mutations plus the 65 newly identified patients). The subgroups were compared for ORR, PFS, and OS. Wild-type patients had 33.1 mo OS with FOLFIRI + cetuximab in comparison to 25.6 mo with FOLFIRI + bevacizumab (HR = 0.70; P = 0.011). In KRAS-mutant patients, this difference was not observed. No difference in PFS was seen in the KRAS wild-type group (P = 0.54), but interestingly for KRAS-mutated patients PFS was better in the bevacizumab arm (12.2 mo vs 6.1 mo; P = 0.004). ORR was similar between the arms, irrespective of KRAS status. It is difficult to understand why a treatment that does not improve ORR and PFS could show such an impact on OS.
In conclusion, in 2014 we have only one approach for KRAS mutated tumors which is chemotherapy plus bevacizumab. For KRAS wild type we can use either chemotherapy plus anti-EGFR antibodies OR chemotherapy plus bevacizumab. Going deeply into this last category, at least one clinical trial suggested cetuximab + FOLFIRI as the possible best option. However, head-to-head comparison with FOLFOX+B is lacking and this still represents a valid option. We disfavor oxaliplatin-based chemotherapy with cetuximab based on the MRC COIN study.
Which is the best strategy after progression with bevacizumab-containing regimen? Switch chemotherapy and keep anti-VEGFR or switch to anti-EGFR antibodies?
Preclinical data showed that continuous VEGF inhibition prevents tumor regression[64]. However, risk-benefit ratio associated with continuing bevacizumab use after initial progressive disease was unknown. In 2008, Grothey et al[65] reported a novel observation gathered from the BRiTE study. In this large, observational cohort study patients were classified according to the treatment received once they progressed to first line bevacizumab containing regimens. Three groups were identified; those with no post-progression treatment, those who received no-bevacizumab related treatment and those who continued bevacizumab beyond progression. When adjusted for other variables, bevacizumab beyond progression was associated with longer survival (P < 0.001). Based on the hypothesis generated by the BRiTE investigators, a randomized phase III study-ML18147 trial-was launched[66]. The investigators assessed continuation bevacizumab plus second-line chemotherapy (no anti-EGFR) after standard first-line bevacizumab-based treatment. Bevacizumab lead to a 1.4 mo longer OS (11.2 mo vs 9.8 mo; P = 0.006).
At the present time is unclear how to proceed in patients who are treated with bevacizumab-containing chemotherapy who progress. In the KRAS/NRAS mutated patients the concept is to maintain the anti-angiogenic status in a similar strategy as the one employed in HER-2/Neu positive breast cancers[67]. This could be achieved either by keeping bevacizumab and changing the chemotherapy regimen or by switching to ziv-aflibercept and irinotecan containing regimen. For wild type tumors, the same options applied but anti-EGFR monoclonal antibodies should be strongly considered because it is important to emphasize that independently of the biological agent chosen first, once progressed patients with wild type tumor should be able to receive all agents sequentially[68].
NEW TARGETS
In the previous sections we have focused on the evidence behind what is currently considered the state of the art treatment of metastatic colon cancer. However, since this field is quite dynamic and the frontiers are in continuous expansion, it will be appropriate to discuss some of the new strategies that are currently being investigated. For description purposes, we will subdivide them based on its main mechanism of action.
Intracellular anti-EGFR therapies
Monoclonal antibodies block the extracellular domain of EGFR. Tyrosine kinase inhibitors (e.g., erlotinib or gefitinib) target the intracellular domain of the receptor. Unlike lung cancer, EGFR mutations are rarely found in colon cancer and are usually not associated with response[69]. Moreover, positive EGFR protein expression does not predict response to treatment[70]. Results have been generally disappointing with no objective responses seen with erlotinib and no improvement in OS with the combination of gefitinib and FOLFIRI[71,72]. However, and after many previous unsatisfactory attempts, a positive study was finally published. Tournigand and colleagues recently presented the results of the phase 3 DREAM trial (OPTIMOX III) showing that the addition of erlotinib to bevacizumab maintenance therapy after induction with chemotherapy + bevacizumab resulted in a small, but statistically significant improvement in PFS from 4.6 to 5.8 mo (P = 0.005)[73]. Remarkably, KRAS mutation status was not a determinant of efficacy and patients with KRAS mutated had even better results. Some clinical trials are currently assessing the role of dual EGFR blocking (panitumumab + erlotinib) with or without chemotherapy in patients with progressed KRAS wild type tumors (NCT00940316). This approach is attractive especially in patients with poor performance status. Nonetheless, it will be at least 1 or 2 years before results become available.
BRAF inhibitors
Vemurafenib targets the BRAF V600E mutation and was proved to be effective in advanced melanomas. Unfortunately, results have been elusive in stage IV colon cancer. In a small phase I study in patients with BRAF mutant metastatic disease, only 1 of 19 patients had a partial response with single agent vemurafenib[74]. Apparently, blocking the BRAF pathway causes a reflective hyper-activation of the EGFR pathway. For that reason, there seems to be some rationale in combining BRAF and EGFR inhibitors and in preclinical studies a synergistic effect was found[75]. An ongoing trial is evaluating the combination of vemurafenib and cetuximab (EUDRACT # 2011-004426-10).
Pi3K pathway
PTEN loss has been associated with worse survival outcomes in colon cancer[76]. Some studies have also shown that PIK3CA mutations and PTEN loss are associated with an absence of response to anti-EGFR therapies[77]. Aspirin seems to be able to block the PI3K pathway. In a recent retrospective study only patients with PIK3CA mutant but not wild-type colorectal cancers who took daily aspirin had better cancer-specific and OS than those who did not take aspirin[78]. A phase 2 trial combined capecitabine plus perifosine (an inhibitor of the PI3K/Akt/mTOR pathway) with promising activity; however the phase 3 was negative[79]. Additionally, the combination of MEK and PI3K/mTOR inhibitors is currently being evaluated in a phase 1 trial (NCT 01390818) and Hochster et al[80] recently reported stimulating results with the combination of selumetinib (MEK inhibitor) and irinotecan.
HER-2 pathway
Few studies, with inconsistent results, investigated the role of HER-2 gene amplification as a potential predictive factor for anti-HER2 therapy. Some reported that HER-2 amplification was associated with resistance to cetuximab and worse PFS or OS; others found neither predictive nor prognostic value in HER-2[81-82]. A phase 2 study evaluating the combination of FOLFOX and trastuzumab in patients who have progressed after 5-FU and/or irinotecan-containing therapy was recently concluded; results are pending (NCT00006015).
Antiangiogenics
In addition to bevacizumab and ziv-aflibercept, other anti-angiogenic drugs have been evaluated with mixed results. Cediranib, a VEGFR inhibitor, showed comparable efficacy to bevacizumab but was associated with increased toxicity[83]. A dual EGFR and VEGFR inhibitor, vandetanib, was ineffective[84]. Ramucirumab, an anti-VEGFR-2 monoclonal antibody, is currently under evaluation in a phase 3 (NCT01183780) following promising results in a phase 2 study[85]. Since there is no real validated marker to predict response to anti-angiogenic drugs, it may take some time before any other anti-angiogenic compound make it to the market.
Insulin growth factor axis
The insulin growth factor (IGF) cascade activates a number of intracellular signaling pathways, including the Ras/Raf/MAPK pathway and the PI3K/Akt pathway[86]. Consequently, it is a potential target for a number of drugs. The main drugs developed as IGF inhibitors have been monoclonal antibodies. Dalotuzumab failed at an interim analysis of a phase 2/3 trial but pre-specified biomarker analysis suggested that patients with higher levels of IGF-1 may be a small subgroup who would potentially benefit from this treatment. Consequently, this hypothesis is being evaluated in a phase 2 study (NCT01609231).
Immunotherapy
In spite of the tremendous excitement raised by innovative immune-therapies in other solid tumors the scenario in metastatic colon cancer has been quite frustrating. No responses were seen in early phase trials with ipilimumab[87]. The same occurred with anti-PD-1 antibodies[88]. Currently, some investigators are testing the use of vaccines (NCT01322815). However, colon cancer seems to remain indifferent against this immunological “rush” or “fever” that we are living at this moment.
CONCLUSION
In conclusion we can affirm that over the last couple of years we have made some small but consistent progress against colon cancer. Anti-angiogenic and anti-EGFR strategies have given dividends by prolonging PFS and to a lesser extend prolonging life in patients with metastatic disease. We are still learning how to use them and it may take time before we discover the best sequence and combination. We also expect that in the near future better biomarkers lead us to the deeply desire but still evasive personalized medicine. But beyond these small victories, new horizons are envisioned. For example, half of the patients have KRAS/NRAS mutant tumors, though there are few drugs that target RAS directly. However, bypassing agents such as MEK inhibitors either alone or in combination with PI3K inhibitors may show promising results. It is impossible to predict the future, but it is expectable and even desirable that soon this review will become obsolete. That is human nature. That is progress. And that is why we must force ourselves to keep us continuously updated.
Footnotes
P- Reviewers: de Talamoni NGT, Lee KY, Zhu YL S- Editor: Ji FF L- Editor: A E- Editor: Wang CH
References
- 1.Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010;19:1893–1907. doi: 10.1158/1055-9965.EPI-10-0437. [DOI] [PubMed] [Google Scholar]
- 2.André T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, Topham C, Zaninelli M, Clingan P, Bridgewater J, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343–2351. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]
- 3.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 4.Ellis LM, Hicklin DJ. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer. 2008;8:579–591. doi: 10.1038/nrc2403. [DOI] [PubMed] [Google Scholar]
- 5.Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang AD, Bauer TW, Camp ER, Somcio R, Liu W, Fan F, Ellis LM. Improving delivery of antineoplastic agents with anti-vascular endothelial growth factor therapy. Cancer. 2005;103:1561–1570. doi: 10.1002/cncr.20942. [DOI] [PubMed] [Google Scholar]
- 7.Wildiers H, Guetens G, De Boeck G, Verbeken E, Landuyt B, Landuyt W, de Bruijn EA, van Oosterom AT. Effect of antivascular endothelial growth factor treatment on the intratumoral uptake of CPT-11. Br J Cancer. 2003;88:1979–1986. doi: 10.1038/sj.bjc.6601005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 9.Guan ZZ, Xu JM, Luo RC, Feng FY, Wang LW, Shen L, Yu SY, Ba Y, Liang J, Wang D, et al. Efficacy and safety of bevacizumab plus chemotherapy in Chinese patients with metastatic colorectal cancer: a randomized phase III ARTIST trial. Chin J Cancer. 2011;30:682–689. doi: 10.5732/cjc.011.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuchs CS, Marshall J, Mitchell E, Wierzbicki R, Ganju V, Jeffery M, Schulz J, Richards D, Soufi-Mahjoubi R, Wang B, et al. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: results from the BICC-C Study. J Clin Oncol. 2007;25:4779–4786. doi: 10.1200/JCO.2007.11.3357. [DOI] [PubMed] [Google Scholar]
- 11.Fuchs CS, Marshall J, Barrueco J. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: updated results from the BICC-C study. J Clin Oncol. 2008;26:689–690. doi: 10.1200/JCO.2007.15.5390. [DOI] [PubMed] [Google Scholar]
- 12.Giantonio BJ, Catalano PJ, Meropol NJ, O’Dwyer PJ, Mitchell EP, Alberts SR, Schwartz MA, Benson AB. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539–1544. doi: 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]
- 13.Saltz LB, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26:2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 14.Hochster HS, Hart LL, Ramanathan RK, Childs BH, Hainsworth JD, Cohn AL, Wong L, Fehrenbacher L, Abubakr Y, Saif MW, et al. Safety and efficacy of oxaliplatin and fluoropyrimidine regimens with or without bevacizumab as first-line treatment of metastatic colorectal cancer: results of the TREE Study. J Clin Oncol. 2008;26:3523–3529. doi: 10.1200/JCO.2007.15.4138. [DOI] [PubMed] [Google Scholar]
- 15.Van Cutsem E, Rivera F, Berry S, Kretzschmar A, Michael M, DiBartolomeo M, Mazier MA, Canon JL, Georgoulias V, Peeters M, et al. Safety and efficacy of first-line bevacizumab with FOLFOX, XELOX, FOLFIRI and fluoropyrimidines in metastatic colorectal cancer: the BEAT study. Ann Oncol. 2009;20:1842–1847. doi: 10.1093/annonc/mdp233. [DOI] [PubMed] [Google Scholar]
- 16.Kabbinavar FF, Schulz J, McCleod M, Patel T, Hamm JT, Hecht JR, Mass R, Perrou B, Nelson B, Novotny WF. Addition of bevacizumab to bolus fluorouracil and leucovorin in first-line metastatic colorectal cancer: results of a randomized phase II trial. J Clin Oncol. 2005;23:3697–3705. doi: 10.1200/JCO.2005.05.112. [DOI] [PubMed] [Google Scholar]
- 17.Tebbutt NC, Wilson K, Gebski VJ, Cummins MM, Zannino D, van Hazel GA, Robinson B, Broad A, Ganju V, Ackland SP, et al. Capecitabine, bevacizumab, and mitomycin in first-line treatment of metastatic colorectal cancer: results of the Australasian Gastrointestinal Trials Group Randomized Phase III MAX Study. J Clin Oncol. 2010;28:3191–3198. doi: 10.1200/JCO.2009.27.7723. [DOI] [PubMed] [Google Scholar]
- 18.Cunningham D, Lang I, Marcuello E, Lorusso V, Ocvirk J, Shin DB, Jonker D, Osborne S, Andre N, Waterkamp D, et al. Bevacizumab plus capecitabine versus capecitabine alone in elderly patients with previously untreated metastatic colorectal cancer (AVEX): an open-label, randomised phase 3 trial. Lancet Oncol. 2013;14:1077–1085. doi: 10.1016/S1470-2045(13)70154-2. [DOI] [PubMed] [Google Scholar]
- 19.Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, Findlay BP, Pitot HC, Alberts SR. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004;22:23–30. doi: 10.1200/JCO.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 20.Falcone A, Ricci S, Brunetti I, Pfanner E, Allegrini G, Barbara C, Crinò L, Benedetti G, Evangelista W, Fanchini L, et al. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest. J Clin Oncol. 2007;25:1670–1676. doi: 10.1200/JCO.2006.09.0928. [DOI] [PubMed] [Google Scholar]
- 21.Falcone A, Cremolini C, Masi G, Lonardi S, Zagonel V, Salvatore L, Trenta P, Tomasello G, Ronzoni M, Ciuffreda L, et al. FOLFOXIRI/bevacizumab (bev) versus FOLFIRI/bev as first-line treatment in unresectable metastatic colorectal cancer (mCRC) patients (pts): Results of the phase III TRIBE trial by GONO group. ASCO Meeting Abstracts. 2013;31:3505. [Google Scholar]
- 22.Gruenberger T, Bridgewater JA, Chau I, Garcia Alfonso P, Rivoire M, Lasserre S, Waterkamp D, Adam R. Randomized, phase II study of bevacizumab with mFOLFOX6 or FOLFOXIRI in patients with initially unresectable liver metastases from colorectal cancer: Resectability and safety in OLIVIA. ASCO Meeting Abstracts. 2013;31:3619. [Google Scholar]
- 23.Gaya A, Tse V. A preclinical and clinical review of aflibercept for the management of cancer. Cancer Treat Rev. 2012;38:484–493. doi: 10.1016/j.ctrv.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 24.Holash J, Davis S, Papadopoulos N, Croll SD, Ho L, Russell M, Boland P, Leidich R, Hylton D, Burova E, et al. VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci USA. 2002;99:11393–11398. doi: 10.1073/pnas.172398299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le XF, Mao W, Lu C, Thornton A, Heymach JV, Sood AK, Bast RC. Specific blockade of VEGF and HER2 pathways results in greater growth inhibition of breast cancer xenografts that overexpress HER2. Cell Cycle. 2008;7:3747–3758. doi: 10.4161/cc.7.23.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu L, Hofmann J, Holash J, Yancopoulos GD, Sood AK, Jaffe RB. Vascular endothelial growth factor trap combined with paclitaxel strikingly inhibits tumor and ascites, prolonging survival in a human ovarian cancer model. Clin Cancer Res. 2005;11:6966–6971. doi: 10.1158/1078-0432.CCR-05-0910. [DOI] [PubMed] [Google Scholar]
- 27.Wachsberger PR, Burd R, Cardi C, Thakur M, Daskalakis C, Holash J, Yancopoulos GD, Dicker AP. VEGF trap in combination with radiotherapy improves tumor control in u87 glioblastoma. Int J Radiat Oncol Biol Phys. 2007;67:1526–1537. doi: 10.1016/j.ijrobp.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 28.Van Cutsem E, Khayat D, Verslype C, Billemont B, Tejpar S, Meric JB, Soussan-Lazard K, Assadourian S, Cartot-Cotton S, Rixe O. Phase I dose-escalation study of intravenous aflibercept administered in combination with irinotecan, 5-fluorouracil and leucovorin in patients with advanced solid tumours. Eur J Cancer. 2013;49:17–24. doi: 10.1016/j.ejca.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 29.Van Cutsem E, Tabernero J, Lakomy R, Prenen H, Prausová J, Macarulla T, Ruff P, van Hazel GA, Moiseyenko V, Ferry D, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol. 2012;30:3499–3506. doi: 10.1200/JCO.2012.42.8201. [DOI] [PubMed] [Google Scholar]
- 30.Sasaki T, Hiroki K, Yamashita Y. The role of epidermal growth factor receptor in cancer metastasis and microenvironment. Biomed Res Int. 2013;2013:546318. doi: 10.1155/2013/546318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Cutsem E, Köhne CH, Láng I, Folprecht G, Nowacki MP, Cascinu S, Shchepotin I, Maurel J, Cunningham D, Tejpar S, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29:2011–2019. doi: 10.1200/JCO.2010.33.5091. [DOI] [PubMed] [Google Scholar]
- 32.Douillard JY, Oliner KS, Siena S, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369:1023–1034. doi: 10.1056/NEJMoa1305275. [DOI] [PubMed] [Google Scholar]
- 33.Prewett MC, Hooper AT, Bassi R, Ellis LM, Waksal HW, Hicklin DJ. Enhanced antitumor activity of anti-epidermal growth factor receptor monoclonal antibody IMC-C225 in combination with irinotecan (CPT-11) against human colorectal tumor xenografts. Clin Cancer Res. 2002;8:994–1003. [PubMed] [Google Scholar]
- 34.Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 35.Jonker DJ, O’Callaghan CJ, Karapetis CS, Zalcberg JR, Tu D, Au HJ, Berry SR, Krahn M, Price T, Simes RJ, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med. 2007;357:2040–2048. doi: 10.1056/NEJMoa071834. [DOI] [PubMed] [Google Scholar]
- 36.Van Cutsem E, Peeters M, Siena S, Humblet Y, Hendlisz A, Neyns B, Canon JL, Van Laethem JL, Maurel J, Richardson G, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007;25:1658–1664. doi: 10.1200/JCO.2006.08.1620. [DOI] [PubMed] [Google Scholar]
- 37.Van Cutsem E, Köhne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, D’Haens G, Pintér T, Lim R, Bodoky G, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–1417. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 38.Bokemeyer C, Bondarenko I, Makhson A, Hartmann JT, Aparicio J, de Braud F, Donea S, Ludwig H, Schuch G, Stroh C, et al. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2009;27:663–671. doi: 10.1200/JCO.2008.20.8397. [DOI] [PubMed] [Google Scholar]
- 39.Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol. 2010;28:4697–4705. doi: 10.1200/JCO.2009.27.4860. [DOI] [PubMed] [Google Scholar]
- 40.Hecht JR, Mitchell E, Chidiac T, Scroggin C, Hagenstad C, Spigel D, Marshall J, Cohn A, McCollum D, Stella P, et al. A randomized phase IIIB trial of chemotherapy, bevacizumab, and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. J Clin Oncol. 2009;27:672–680. doi: 10.1200/JCO.2008.19.8135. [DOI] [PubMed] [Google Scholar]
- 41.Tol J, Koopman M, Cats A, Rodenburg CJ, Creemers GJ, Schrama JG, Erdkamp FL, Vos AH, van Groeningen CJ, Sinnige HA, et al. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med. 2009;360:563–572. doi: 10.1056/NEJMoa0808268. [DOI] [PubMed] [Google Scholar]
- 42.Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 43.Fabian MA, Biggs WH, Treiber DK, Atteridge CE, Azimioara MD, Benedetti MG, Carter TA, Ciceri P, Edeen PT, Floyd M, et al. A small molecule-kinase interaction map for clinical kinase inhibitors. Nat Biotechnol. 2005;23:329–336. doi: 10.1038/nbt1068. [DOI] [PubMed] [Google Scholar]
- 44.Wilhelm SM, Dumas J, Adnane L, Lynch M, Carter CA, Schütz G, Thierauch KH, Zopf D. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer. 2011;129:245–255. doi: 10.1002/ijc.25864. [DOI] [PubMed] [Google Scholar]
- 45.Mross K, Frost A, Steinbild S, Hedbom S, Büchert M, Fasol U, Unger C, Krätzschmar J, Heinig R, Boix O, et al. A phase I dose-escalation study of regorafenib (BAY 73-4506), an inhibitor of oncogenic, angiogenic, and stromal kinases, in patients with advanced solid tumors. Clin Cancer Res. 2012;18:2658–2667. doi: 10.1158/1078-0432.CCR-11-1900. [DOI] [PubMed] [Google Scholar]
- 46.Strumberg D, Scheulen ME, Schultheis B, Richly H, Frost A, Büchert M, Christensen O, Jeffers M, Heinig R, Boix O, et al. Regorafenib (BAY 73-4506) in advanced colorectal cancer: a phase I study. Br J Cancer. 2012;106:1722–1727. doi: 10.1038/bjc.2012.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, Humblet Y, Bouché O, Mineur L, Barone C, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303–312. doi: 10.1016/S0140-6736(12)61900-X. [DOI] [PubMed] [Google Scholar]
- 48.Pawlik TM, Schulick RD, Choti MA. Expanding criteria for resectability of colorectal liver metastases. Oncologist. 2008;13:51–64. doi: 10.1634/theoncologist.2007-0142. [DOI] [PubMed] [Google Scholar]
- 49.Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, Bechstein WO, Primrose JN, Walpole ET, Finch-Jones M, et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14:1208–1215. doi: 10.1016/S1470-2045(13)70447-9. [DOI] [PubMed] [Google Scholar]
- 50.Mitry E, Fields AL, Bleiberg H, Labianca R, Portier G, Tu D, Nitti D, Torri V, Elias D, O’Callaghan C, et al. Adjuvant chemotherapy after potentially curative resection of metastases from colorectal cancer: a pooled analysis of two randomized trials. J Clin Oncol. 2008;26:4906–4911. doi: 10.1200/JCO.2008.17.3781. [DOI] [PubMed] [Google Scholar]
- 51.Gruenberger B, Tamandl D, Schueller J, Scheithauer W, Zielinski C, Herbst F, Gruenberger T. Bevacizumab, capecitabine, and oxaliplatin as neoadjuvant therapy for patients with potentially curable metastatic colorectal cancer. J Clin Oncol. 2008;26:1830–1835. doi: 10.1200/JCO.2007.13.7679. [DOI] [PubMed] [Google Scholar]
- 52.Pozzo C, Basso M, Cassano A, Quirino M, Schinzari G, Trigila N, Vellone M, Giuliante F, Nuzzo G, Barone C. Neoadjuvant treatment of unresectable liver disease with irinotecan and 5-fluorouracil plus folinic acid in colorectal cancer patients. Ann Oncol. 2004;15:933–939. doi: 10.1093/annonc/mdh217. [DOI] [PubMed] [Google Scholar]
- 53.Alberts SR, Horvath WL, Sternfeld WC, Goldberg RM, Mahoney MR, Dakhil SR, Levitt R, Rowland K, Nair S, Sargent DJ, et al. Oxaliplatin, fluorouracil, and leucovorin for patients with unresectable liver-only metastases from colorectal cancer: a North Central Cancer Treatment Group phase II study. J Clin Oncol. 2005;23:9243–9249. doi: 10.1200/JCO.2005.07.740. [DOI] [PubMed] [Google Scholar]
- 54.Folprecht G, Gruenberger T, Bechstein WO, Raab HR, Lordick F, Hartmann JT, Lang H, Frilling A, Stoehlmacher J, Weitz J, et al. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: the CELIM randomised phase 2 trial. Lancet Oncol. 2010;11:38–47. doi: 10.1016/S1470-2045(09)70330-4. [DOI] [PubMed] [Google Scholar]
- 55.Ye LC, Liu TS, Ren L, Wei Y, Zhu DX, Zai SY, Ye QH, Yu Y, Xu B, Qin XY, et al. Randomized controlled trial of cetuximab plus chemotherapy for patients with KRAS wild-type unresectable colorectal liver-limited metastases. J Clin Oncol. 2013;31:1931–1938. doi: 10.1200/JCO.2012.44.8308. [DOI] [PubMed] [Google Scholar]
- 56.Wong R, Cunningham D, Barbachano Y, Saffery C, Valle J, Hickish T, Mudan S, Brown G, Khan A, Wotherspoon A, et al. A multicentre study of capecitabine, oxaliplatin plus bevacizumab as perioperative treatment of patients with poor-risk colorectal liver-only metastases not selected for upfront resection. Ann Oncol. 2011;22:2042–2048. doi: 10.1093/annonc/mdq714. [DOI] [PubMed] [Google Scholar]
- 57.Klinger M, Tamandl D, Eipeldauer S, Hacker S, Herberger B, Kaczirek K, Dorfmeister M, Gruenberger B, Gruenberger T. Bevacizumab improves pathological response of colorectal cancer liver metastases treated with XELOX/FOLFOX. Ann Surg Oncol. 2010;17:2059–2065. doi: 10.1245/s10434-010-0972-9. [DOI] [PubMed] [Google Scholar]
- 58.Koopman M, Antonini NF, Douma J, Wals J, Honkoop AH, Erdkamp FL, de Jong RS, Rodenburg CJ, Vreugdenhil G, Loosveld OJ, et al. Sequential versus combination chemotherapy with capecitabine, irinotecan, and oxaliplatin in advanced colorectal cancer (CAIRO): a phase III randomised controlled trial. Lancet. 2007;370:135–142. doi: 10.1016/S0140-6736(07)61086-1. [DOI] [PubMed] [Google Scholar]
- 59.Maughan TS, Adams RA, Smith CG, Meade AM, Seymour MT, Wilson RH, Idziaszczyk S, Harris R, Fisher D, Kenny SL, et al. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet. 2011;377:2103–2114. doi: 10.1016/S0140-6736(11)60613-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tveit KM, Guren T, Glimelius B, Pfeiffer P, Sorbye H, Pyrhonen S, Sigurdsson F, Kure E, Ikdahl T, Skovlund E, et al. Phase III trial of cetuximab with continuous or intermittent fluorouracil, leucovorin, and oxaliplatin (Nordic FLOX) versus FLOX alone in first-line treatment of metastatic colorectal cancer: the NORDIC-VII study. J Clin Oncol. 2012;30:1755–1762. doi: 10.1200/JCO.2011.38.0915. [DOI] [PubMed] [Google Scholar]
- 61.Schwartzberg LS, Rivera F, Karthaus M, Fasola G, Canon J, Yu H, Go WY. PEAK (study 20070509): A randomized phase II study of mFOLFOX6 with either panitumumab (pmab) or bevacizumab (bev) as first-line treatment (tx) in patients (pts) with unresectable wild-type (WT) KRAS metastatic colorectal cancer (mCRC) ASCO Meeting Abstracts. 2013;31:446. [Google Scholar]
- 62.Heinemann V, Fischer von Weikersthal L, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran S, Heintges T, Lerchenmueller J, Kahl C, Seipelt G, et al. Randomized comparison of FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment of KRAS-wildtype metastatic colorectal cancer: German AIO study KRK-0306 (FIRE-3) ASCO Meeting Abstracts. 2013;31:LBA3506. [Google Scholar]
- 63.Stintzing S, Jung A, Rossius L. Analysis of KRAS/NRAS and BRAF mutations in FIRE-3: A randomized phase III study of FOLFIRI plus cetuximab or bevacizumab as first-line treatment for wild-type (WT) KRAS (exon 2) metastatic colorectal cancer (mCRC) patients. See more at: Presented at: Sep 27-Oct 1, 2013. Amsterdam, The Netherlands: European Cancer Congress; 2013. p. Abstract LBA17. [Google Scholar]
- 64.Klement G, Baruchel S, Rak J, Man S, Clark K, Hicklin DJ, Bohlen P, Kerbel RS. Continuous low-dose therapy with vinblastine and VEGF receptor-2 antibody induces sustained tumor regression without overt toxicity. J Clin Invest. 2000;105:R15–R24. doi: 10.1172/JCI8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grothey A, Sugrue MM, Purdie DM, Dong W, Sargent D, Hedrick E, Kozloff M. Bevacizumab beyond first progression is associated with prolonged overall survival in metastatic colorectal cancer: results from a large observational cohort study (BRiTE) J Clin Oncol. 2008;26:5326–5334. doi: 10.1200/JCO.2008.16.3212. [DOI] [PubMed] [Google Scholar]
- 66.Bennouna J, Sastre J, Arnold D, Österlund P, Greil R, Van Cutsem E, von Moos R, Viéitez JM, Bouché O, Borg C, et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol. 2013;14:29–37. doi: 10.1016/S1470-2045(12)70477-1. [DOI] [PubMed] [Google Scholar]
- 67.von Minckwitz G, Schwedler K, Schmidt M, Barinoff J, Mundhenke C, Cufer T, Maartense E, de Jongh FE, Baumann KH, Bischoff J, et al. Trastuzumab beyond progression: overall survival analysis of the GBG 26/BIG 3-05 phase III study in HER2-positive breast cancer. Eur J Cancer. 2011;47:2273–2281. doi: 10.1016/j.ejca.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 68.Grothey A, Sargent D. Overall survival of patients with advanced colorectal cancer correlates with availability of fluorouracil, irinotecan, and oxaliplatin regardless of whether doublet or single-agent therapy is used first line. J Clin Oncol. 2005;23:9441–9442. doi: 10.1200/JCO.2005.04.4792. [DOI] [PubMed] [Google Scholar]
- 69.Barber TD, Vogelstein B, Kinzler KW, Velculescu VE. Somatic mutations of EGFR in colorectal cancers and glioblastomas. N Engl J Med. 2004;351:2883. doi: 10.1056/NEJM200412303512724. [DOI] [PubMed] [Google Scholar]
- 70.Chung KY, Shia J, Kemeny NE, Shah M, Schwartz GK, Tse A, Hamilton A, Pan D, Schrag D, Schwartz L, et al. Cetuximab shows activity in colorectal cancer patients with tumors that do not express the epidermal growth factor receptor by immunohistochemistry. J Clin Oncol. 2005;23:1803–1810. doi: 10.1200/JCO.2005.08.037. [DOI] [PubMed] [Google Scholar]
- 71.Townsley CA, Major P, Siu LL, Dancey J, Chen E, Pond GR, Nicklee T, Ho J, Hedley D, Tsao M, et al. Phase II study of erlotinib (OSI-774) in patients with metastatic colorectal cancer. Br J Cancer. 2006;94:1136–1143. doi: 10.1038/sj.bjc.6603055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Santoro A, Comandone A, Rimassa L, Granetti C, Lorusso V, Oliva C, Ronzoni M, Siena S, Zuradelli M, Mari E, et al. A phase II randomized multicenter trial of gefitinib plus FOLFIRI and FOLFIRI alone in patients with metastatic colorectal cancer. Ann Oncol. 2008;19:1888–1893. doi: 10.1093/annonc/mdn401. [DOI] [PubMed] [Google Scholar]
- 73.Tournigand C, Samson B, Scheithauer W, Lledo G, Viret F, Andre T, Ramee JF, Tubiana-Mathieu N, Dauba J, Dupuis O, et al. Bevacizumab (Bev) with or without erlotinib as maintenance therapy, following induction first-line chemotherapy plus Bev, in patients (pts) with metastatic colorectal cancer (mCRC): Efficacy and safety results of the International GERCOR DREAM phase III trial. ASCO Meeting Abstracts. 2012;30:LBA3500. [Google Scholar]
- 74.Kopetz S, Desai J, Chan E, Hecht JR, O’Dwyer PJ, Lee RJ, Nolop KB, Saltz L. PLX4032 in metastatic colorectal cancer patients with mutant BRAF tumors. ASCO Meeting Abstracts. 2010;28:3534. [Google Scholar]
- 75.Higgins B, Kolinsky KD, Schostack K, Bollag G, Lee RJ, Su F, Packman K. Efficacy of vemurafenib (V), a selective V600EBRAF inhibitor, as monotherapy or in combination with erlotinib (Erl) or erbitux (Erb) and irinotecan (Iri) doublets and triplets in a colorectal cancer (CRC) xenograft model. ASCO Meeting Abstracts. 2012;30:494. [Google Scholar]
- 76.Jang KS, Song YS, Jang SH, Min KW, Na W, Jang SM, Jun YJ, Lee KH, Choi D, Paik SS. Clinicopathological significance of nuclear PTEN expression in colorectal adenocarcinoma. Histopathology. 2010;56:229–239. doi: 10.1111/j.1365-2559.2009.03468.x. [DOI] [PubMed] [Google Scholar]
- 77.Bardelli A, Siena S. Molecular mechanisms of resistance to cetuximab and panitumumab in colorectal cancer. J Clin Oncol. 2010;28:1254–1261. doi: 10.1200/JCO.2009.24.6116. [DOI] [PubMed] [Google Scholar]
- 78.Liao X, Lochhead P, Nishihara R, Morikawa T, Kuchiba A, Yamauchi M, Imamura Y, Qian ZR, Baba Y, Shima K, et al. Aspirin use, tumor PIK3CA mutation, and colorectal-cancer survival. N Engl J Med. 2012;367:1596–1606. doi: 10.1056/NEJMoa1207756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bendell JC, Ervin TJ, Senzer NN, Richards DA, Firdaus I, Lockhart AC, Cohn AL, Saleh MN, Gardner LR, Sportelli P, et al. Results of the X-PECT study: A phase III randomized double-blind, placebo-controlled study of perifosine plus capecitabine (P-CAP) versus placebo plus capecitabine (CAP) in patients (pts) with refractory metastatic colorectal cancer (mCRC) ASCO Meeting Abstracts. 2012;30:LBA3501. [Google Scholar]
- 80.Hochster HS, Messersmith WA, O’Neil BH, Groshen SG, Cohen DJ, Denlinger CS, Gold PJ, Eckhardt SG, Locker GY, Ames P, McKinley M, Leichman LP, Academic GI Cancer Consortium. Second-line therapy of KRAS-mutated (KRASm) metastatic colorectal cancer (CRC) with the MEK inihibitor selumetinib ([SEL], AZ6244, ARRY-142886) in combination with irinotecan (IRI): An AGICC study. ASCO Meeting Abstracts. 2013;31:380. [Google Scholar]
- 81.Barbara C, Martin V, Molinari F, Landi L, Riva A, Saletti P, de Dosso S, Geva R, Tejpar S, Fountzilas G, et al. Use of HER2 gene amplification to identify patients with metastatic colorectal cancer resistant to anti-EGFR monoclonal antibodies. ASCO Meeting Abstracts. 2012;30:474. [Google Scholar]
- 82.Troiani T, Zappavigna S, Martinelli E, Addeo SR, Stiuso P, Ciardiello F, Caraglia M. Optimizing treatment of metastatic colorectal cancer patients with anti-EGFR antibodies: overcoming the mechanisms of cancer cell resistance. Expert Opin Biol Ther. 2013;13:241–255. doi: 10.1517/14712598.2012.756469. [DOI] [PubMed] [Google Scholar]
- 83.Schmoll HJ, Cunningham D, Sobrero A, Karapetis CS, Rougier P, Koski SL, Kocakova I, Bondarenko I, Bodoky G, Mainwaring P, et al. Cediranib with mFOLFOX6 versus bevacizumab with mFOLFOX6 as first-line treatment for patients with advanced colorectal cancer: a double-blind, randomized phase III study (HORIZON III) J Clin Oncol. 2012;30:3588–3595. doi: 10.1200/JCO.2012.42.5355. [DOI] [PubMed] [Google Scholar]
- 84.Morabito A, Piccirillo MC, Costanzo R, Sandomenico C, Carillio G, Daniele G, Giordano P, Bryce J, Carotenuto P, La Rocca A, et al. Vandetanib: An overview of its clinical development in NSCLC and other tumors. Drugs Today (Barc) 2010;46:683–698. doi: 10.1358/dot.2010.46.9.1516989. [DOI] [PubMed] [Google Scholar]
- 85.Garcia-Carbonero R, Rivera F, Maurel J, Ayoub JM, Moore MJ, Cervantes-Ruiperez A, Asmis TR, Schwartz JD, Ballal S, Tabernero J. A phase II, open-label study evaluating the safety and efficacy of ramucirumab combined with mFOLFOX-6 as first-line therapy in patients (pts) with metastatic colorectal cancer (mCRC): CP12-0709/ NCT00862784. ASCO Meeting Abstracts. 2012;30:533. [Google Scholar]
- 86.Scartozzi M, Mandolesi A, Giampieri R, Pierantoni C, Loupakis F, Zaniboni A, Galizia E, Giustini L, Silva RR, Bisonni R, et al. Insulin-like growth factor 1 expression correlates with clinical outcome in K-RAS wild type colorectal cancer patients treated with cetuximab and irinotecan. Int J Cancer. 2010;127:1941–1947. doi: 10.1002/ijc.25193. [DOI] [PubMed] [Google Scholar]
- 87.O’Mahony D, Morris JC, Quinn C, Gao W, Wilson WH, Gause B, Pittaluga S, Neelapu S, Brown M, Fleisher TA, et al. A pilot study of CTLA-4 blockade after cancer vaccine failure in patients with advanced malignancy. Clin Cancer Res. 2007;13:958–964. doi: 10.1158/1078-0432.CCR-06-1974. [DOI] [PubMed] [Google Scholar]
- 88.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]


