Abstract
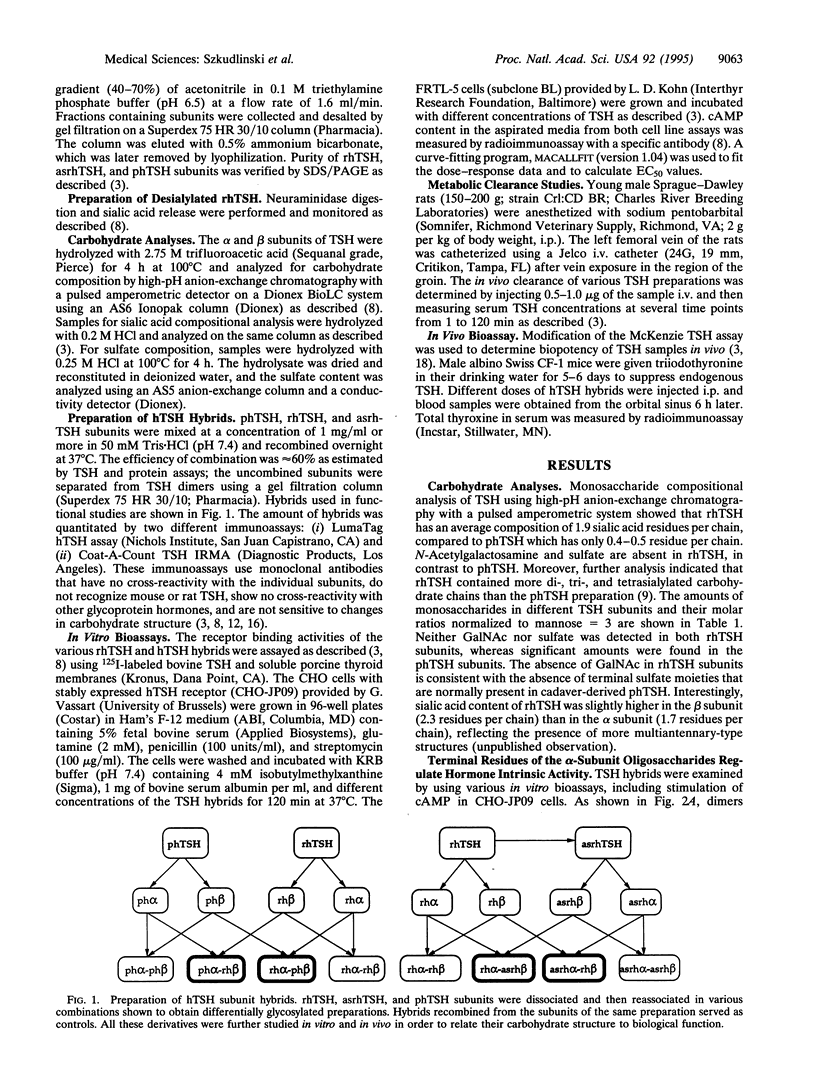
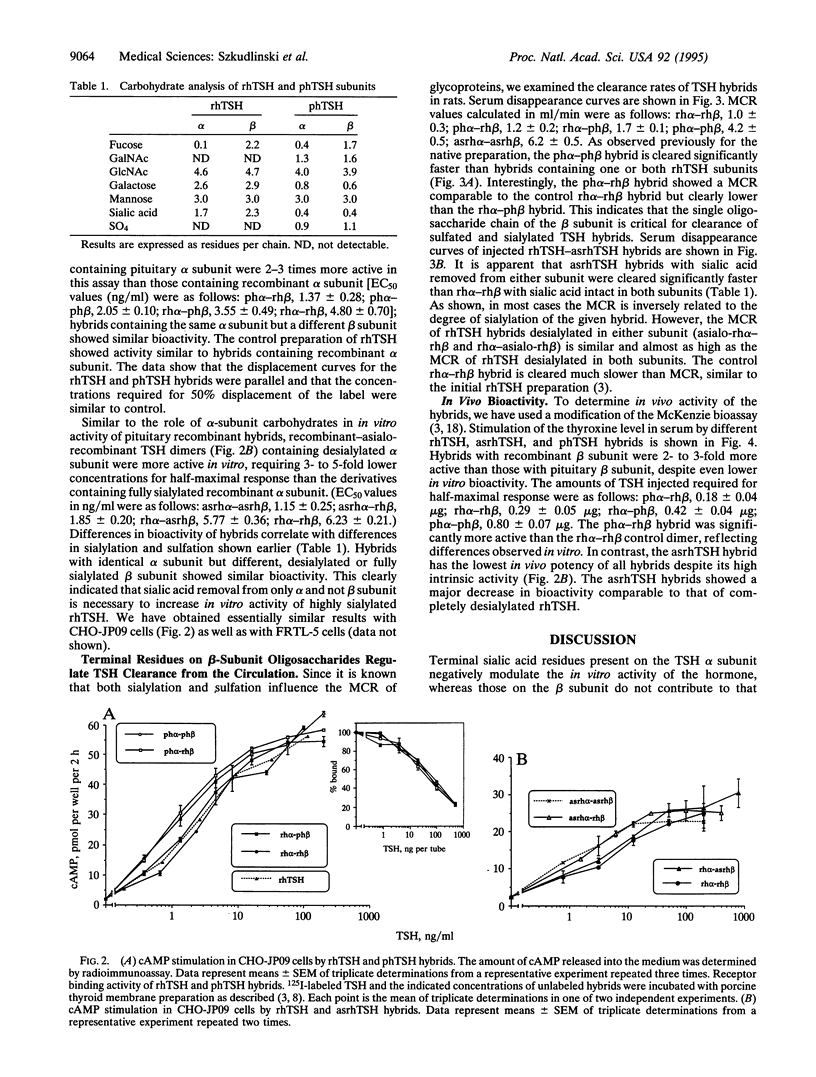
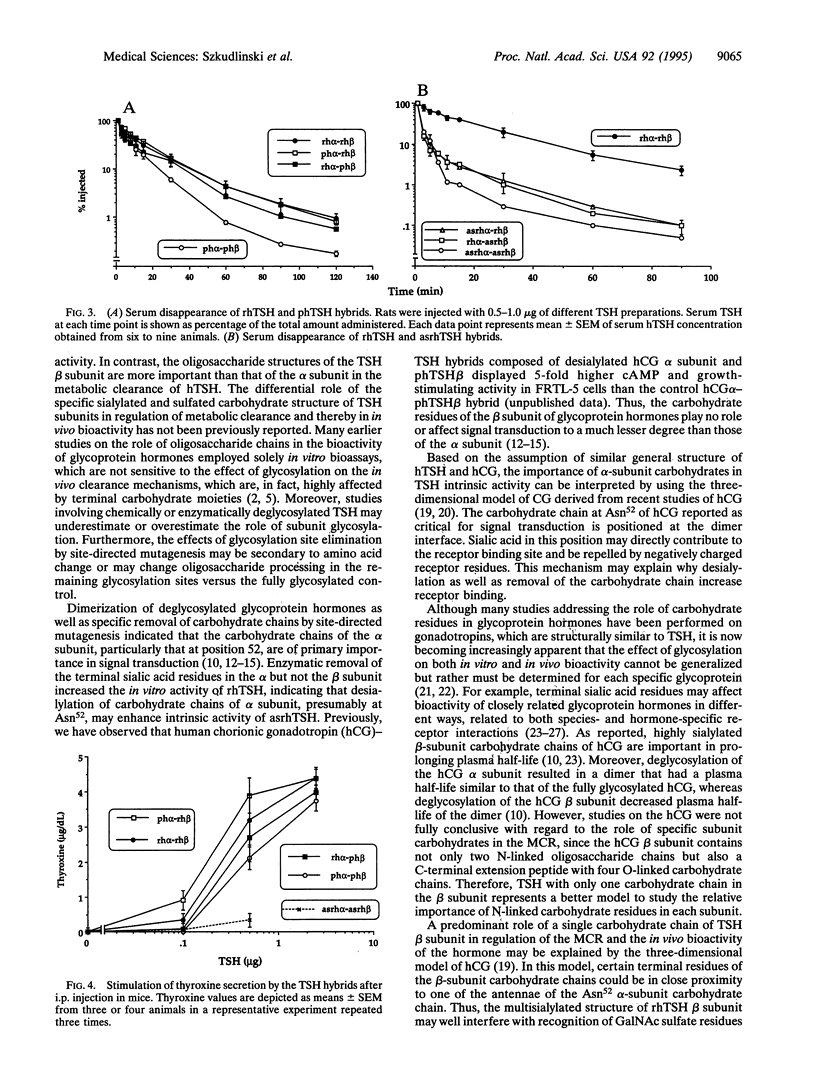
The recombinant human thyroid stimulating hormone (rhTSH) containing oligosaccharides terminated with NeuAc(alpha 2-3)Gal(beta 1-4)GlcNAc beta 1 showed higher in vivo activity and lower metabolic clearance rate (MCR) than pituitary human TSH (phTSH), which contains oligosaccharides terminating predominantly in SO(4)4GalNAc(beta 1-4)GlcNAc beta 1. To elucidate the relative contribution of the sulfated and sialylated carbohydrate chains of each subunit in the MCR and bioactivity of the hormone, the alpha and beta subunits of phTSH, rhTSH, and enzymatically desialylated rhTSH (asialo-rhTSH; asrhTSH) were isolated, their oligosaccharides were analyzed, and the respective subunits were dimerized in various combinations. The hybrids containing alpha subunit from phTSH or asrhTSH showed higher in vitro activity than those with alpha subunit from rhTSH, indicating that sialylation of alpha but not beta subunit attenuates the intrinsic activity of TSH. In contrast, hybrids with beta subunit from rhTSH displayed lower MCR compared to those with beta subunit from phTSH. The phTSH alpha-rhTSH beta hybrid had the highest in vivo bioactivity followed by rhTSH alpha-rhTSH beta, rhTSH alpha-phTSH beta, phTSH alpha-phTSH beta, and asrhTSH dimers. These differences indicated that hybrids with beta subunit from rhTSH displayed the highest in vivo activity and relatively low MCR, probably due to higher sialylation, more multiantennary structure, and/or the unique location of the beta-subunit oligosaccharide chain in the molecule. Thus, the N-linked oligosaccharides of the beta subunit of glycoprotein hormones have a more pronounced role than those from the alpha subunit in the metabolic clearance and thereby in the in vivo bioactivity. In contrast, the terminal residues of alpha-subunit oligosaccharides have a major impact on TSH intrinsic potency.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amano J., Sato S., Nishimura R., Mochizuki M., Kobata A. Sialic acids, but not their linkage to galactose residues, are required for full expression of the biological activity of human chorionic gonadotropin. J Biochem. 1989 Mar;105(3):339–340. doi: 10.1093/oxfordjournals.jbchem.a122664. [DOI] [PubMed] [Google Scholar]
- Amir S. M., Kubota K., Tramontano D., Ingbar S. H., Keutmann H. T. The carbohydrate moiety of bovine thyrotropin is essential for full bioactivity but not for receptor recognition. Endocrinology. 1987 Jan;120(1):345–352. doi: 10.1210/endo-120-1-345. [DOI] [PubMed] [Google Scholar]
- Baenziger J. U., Kumar S., Brodbeck R. M., Smith P. L., Beranek M. C. Circulatory half-life but not interaction with the lutropin/chorionic gonadotropin receptor is modulated by sulfation of bovine lutropin oligosaccharides. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):334–338. doi: 10.1073/pnas.89.1.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop L. A., Robertson D. M., Cahir N., Schofield P. R. Specific roles for the asparagine-linked carbohydrate residues of recombinant human follicle stimulating hormone in receptor binding and signal transduction. Mol Endocrinol. 1994 Jun;8(6):722–731. doi: 10.1210/mend.8.6.7935488. [DOI] [PubMed] [Google Scholar]
- Cole E. S., Lee K., Lauziere K., Kelton C., Chappel S., Weintraub B., Ferrara D., Peterson P., Bernasconi R., Edmunds T. Recombinant human thyroid stimulating hormone: development of a biotechnology product for detection of metastatic lesions of thyroid carcinoma. Biotechnology (N Y) 1993 Sep;11(9):1014–1024. doi: 10.1038/nbt0993-1014. [DOI] [PubMed] [Google Scholar]
- Corless C. L., Matzuk M. M., Ramabhadran T. V., Krichevsky A., Boime I. Gonadotropin beta subunits determine the rate of assembly and the oligosaccharide processing of hormone dimer in transfected cells. J Cell Biol. 1987 May;104(5):1173–1181. doi: 10.1083/jcb.104.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwek R. A., Edge C. J., Harvey D. J., Wormald M. R., Parekh R. B. Analysis of glycoprotein-associated oligosaccharides. Annu Rev Biochem. 1993;62:65–100. doi: 10.1146/annurev.bi.62.070193.000433. [DOI] [PubMed] [Google Scholar]
- East-Palmer J., Szkudlinski M. W., Lee J., Thotakura N. R., Weintraub B. D. A novel, nonradioactive in vivo bioassay of thyrotropin (TSH). Thyroid. 1995 Feb;5(1):55–59. doi: 10.1089/thy.1995.5.55. [DOI] [PubMed] [Google Scholar]
- Flack M. R., Froehlich J., Bennet A. P., Anasti J., Nisula B. C. Site-directed mutagenesis defines the individual roles of the glycosylation sites on follicle-stimulating hormone. J Biol Chem. 1994 May 13;269(19):14015–14020. [PubMed] [Google Scholar]
- Gesundheit N., Weintraub B. D. Mechanisms and regulation of TSH glycosylation. Adv Exp Med Biol. 1986;205:87–105. doi: 10.1007/978-1-4684-5209-9_3. [DOI] [PubMed] [Google Scholar]
- Green E. D., Baenziger J. U. Asparagine-linked oligosaccharides on lutropin, follitropin, and thyrotropin. I. Structural elucidation of the sulfated and sialylated oligosaccharides on bovine, ovine, and human pituitary glycoprotein hormones. J Biol Chem. 1988 Jan 5;263(1):25–35. [PubMed] [Google Scholar]
- Gyves P. W., Gesundheit N., Thotakura N. R., Stannard B. S., DeCherney G. S., Weintraub B. D. Changes in the sialylation and sulfation of secreted thyrotropin in congenital hypothyroidism. Proc Natl Acad Sci U S A. 1990 May;87(10):3792–3796. doi: 10.1073/pnas.87.10.3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiyama J., Weisshaar G., Renwick A. G. The asparagine-linked oligosaccharides at individual glycosylation sites in human thyrotrophin. Glycobiology. 1992 Oct;2(5):401–409. doi: 10.1093/glycob/2.5.401. [DOI] [PubMed] [Google Scholar]
- Hoermann R., Kubota K., Amir S. M. Role of subunit sialic acid in hepatic binding, plasma survival rate, and in vivo thyrotropic activity of human chorionic gonadotropin. Thyroid. 1993 Spring;3(1):41–47. doi: 10.1089/thy.1993.3.41. [DOI] [PubMed] [Google Scholar]
- Huth J. R., Norton S. E., Lockridge O., Shikone T., Hsueh A. J., Ruddon R. W. Bacterial expression and in vitro folding of the beta-subunit of human chorionic gonadotropin (hCG beta) and functional assembly of recombinant hCG beta with hCG alpha. Endocrinology. 1994 Sep;135(3):911–918. doi: 10.1210/endo.135.3.8070386. [DOI] [PubMed] [Google Scholar]
- Kalyan N. K., Bahl O. P. Role of carbohydrate in human chorionic gonadotropin. Effect of deglycosylation on the subunit interaction and on its in vitro and in vivo biological properties. J Biol Chem. 1983 Jan 10;258(1):67–74. [PubMed] [Google Scholar]
- Lapthorn A. J., Harris D. C., Littlejohn A., Lustbader J. W., Canfield R. E., Machin K. J., Morgan F. J., Isaacs N. W. Crystal structure of human chorionic gonadotropin. Nature. 1994 Jun 9;369(6480):455–461. doi: 10.1038/369455a0. [DOI] [PubMed] [Google Scholar]
- Magner J. A. Thyroid-stimulating hormone: biosynthesis, cell biology, and bioactivity. Endocr Rev. 1990 May;11(2):354–385. doi: 10.1210/edrv-11-2-354. [DOI] [PubMed] [Google Scholar]
- Matzuk M. M., Keene J. L., Boime I. Site specificity of the chorionic gonadotropin N-linked oligosaccharides in signal transduction. J Biol Chem. 1989 Feb 15;264(5):2409–2414. [PubMed] [Google Scholar]
- Meier C. A., Braverman L. E., Ebner S. A., Veronikis I., Daniels G. H., Ross D. S., Deraska D. J., Davies T. F., Valentine M., DeGroot L. J. Diagnostic use of recombinant human thyrotropin in patients with thyroid carcinoma (phase I/II study). J Clin Endocrinol Metab. 1994 Jan;78(1):188–196. doi: 10.1210/jcem.78.1.8288703. [DOI] [PubMed] [Google Scholar]
- Morell A. G., Gregoriadis G., Scheinberg I. H., Hickman J., Ashwell G. The role of sialic acid in determining the survival of glycoproteins in the circulation. J Biol Chem. 1971 Mar 10;246(5):1461–1467. [PubMed] [Google Scholar]
- Pierce J. G., Parsons T. F. Glycoprotein hormones: structure and function. Annu Rev Biochem. 1981;50:465–495. doi: 10.1146/annurev.bi.50.070181.002341. [DOI] [PubMed] [Google Scholar]
- Sairam M. R. Role of carbohydrates in glycoprotein hormone signal transduction. FASEB J. 1989 Jun;3(8):1915–1926. doi: 10.1096/fasebj.3.8.2542111. [DOI] [PubMed] [Google Scholar]
- Stockell Hartree A., Renwick A. G. Molecular structures of glycoprotein hormones and functions of their carbohydrate components. Biochem J. 1992 Nov 1;287(Pt 3):665–679. doi: 10.1042/bj2870665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szkudlinski M. W., Thotakura N. R., Bucci I., Joshi L. R., Tsai A., East-Palmer J., Shiloach J., Weintraub B. D. Purification and characterization of recombinant human thyrotropin (TSH) isoforms produced by Chinese hamster ovary cells: the role of sialylation and sulfation in TSH bioactivity. Endocrinology. 1993 Oct;133(4):1490–1503. doi: 10.1210/endo.133.4.8404588. [DOI] [PubMed] [Google Scholar]
- Szkudlinski M. W., Thotakura N. R., Tropea J. E., Grossmann M., Weintraub B. D. Asparagine-linked oligosaccharide structures determine clearance and organ distribution of pituitary and recombinant thyrotropin. Endocrinology. 1995 Aug;136(8):3325–3330. doi: 10.1210/endo.136.8.7628367. [DOI] [PubMed] [Google Scholar]
- Thotakura N. R., Desai R. K., Bates L. G., Cole E. S., Pratt B. M., Weintraub B. D. Biological activity and metabolic clearance of a recombinant human thyrotropin produced in Chinese hamster ovary cells. Endocrinology. 1991 Jan;128(1):341–348. doi: 10.1210/endo-128-1-341. [DOI] [PubMed] [Google Scholar]
- Thotakura N. R., Desai R. K., Szkudlinski M. W., Weintraub B. D. The role of the oligosaccharide chains of thyrotropin alpha- and beta-subunits in hormone action. Endocrinology. 1992 Jul;131(1):82–88. doi: 10.1210/endo.131.1.1377127. [DOI] [PubMed] [Google Scholar]
- Thotakura N. R., Szkudlinski M. W., Weintraub B. D. Structure-function studies of oligosaccharides of recombinant human thyrotrophin by sequential deglycosylation and resialylation. Glycobiology. 1994 Aug;4(4):525–533. doi: 10.1093/glycob/4.4.525. [DOI] [PubMed] [Google Scholar]
- Varki A. Biological roles of oligosaccharides: all of the theories are correct. Glycobiology. 1993 Apr;3(2):97–130. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H., Lustbader J. W., Liu Y., Canfield R. E., Hendrickson W. A. Structure of human chorionic gonadotropin at 2.6 A resolution from MAD analysis of the selenomethionyl protein. Structure. 1994 Jun 15;2(6):545–558. doi: 10.1016/s0969-2126(00)00054-x. [DOI] [PubMed] [Google Scholar]



