Abstract
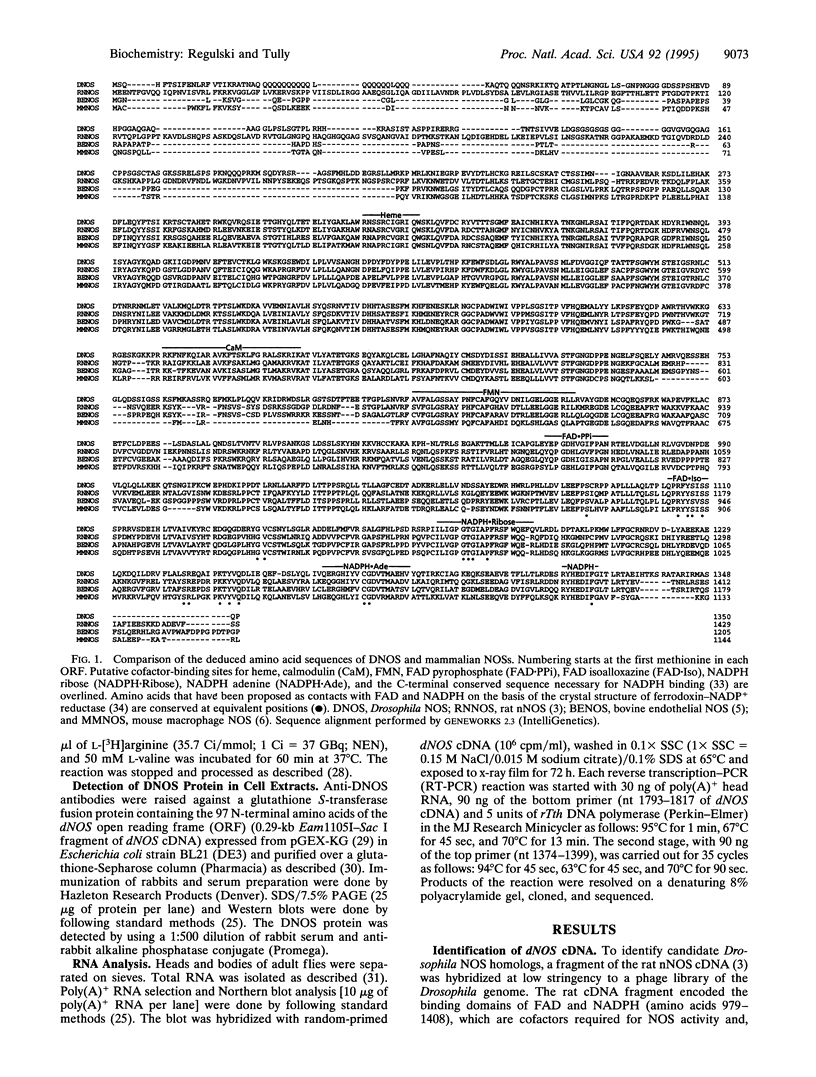
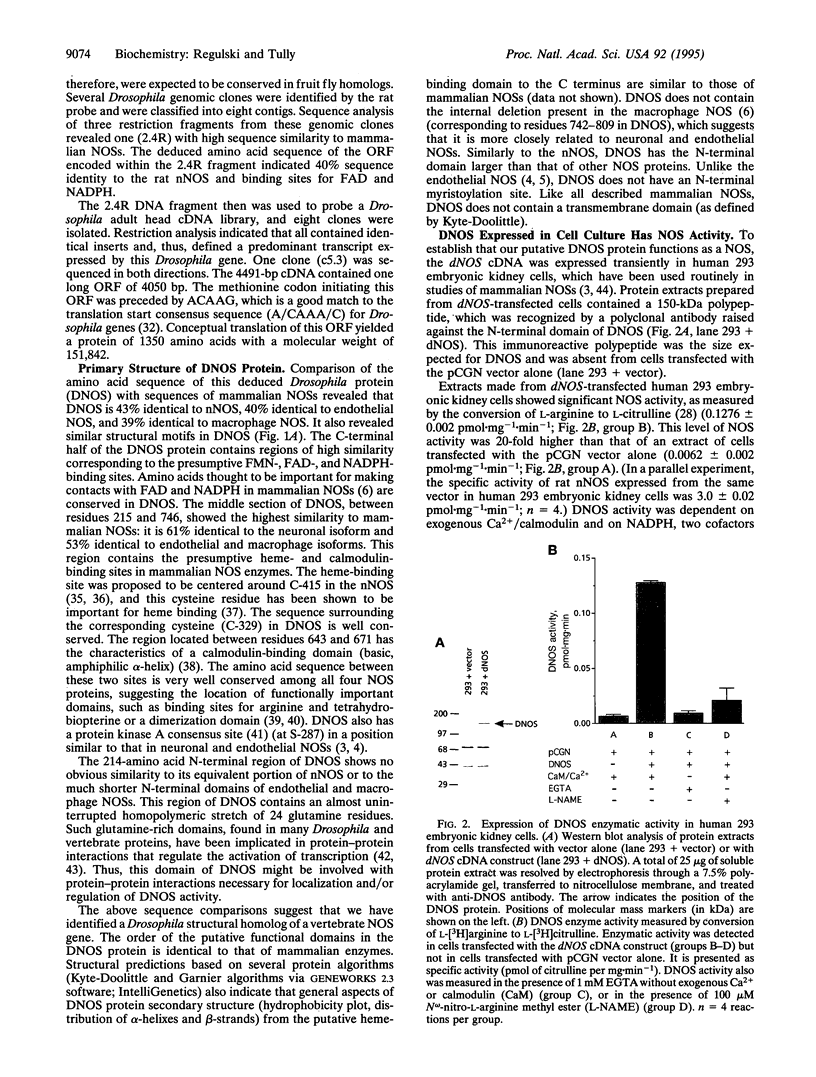
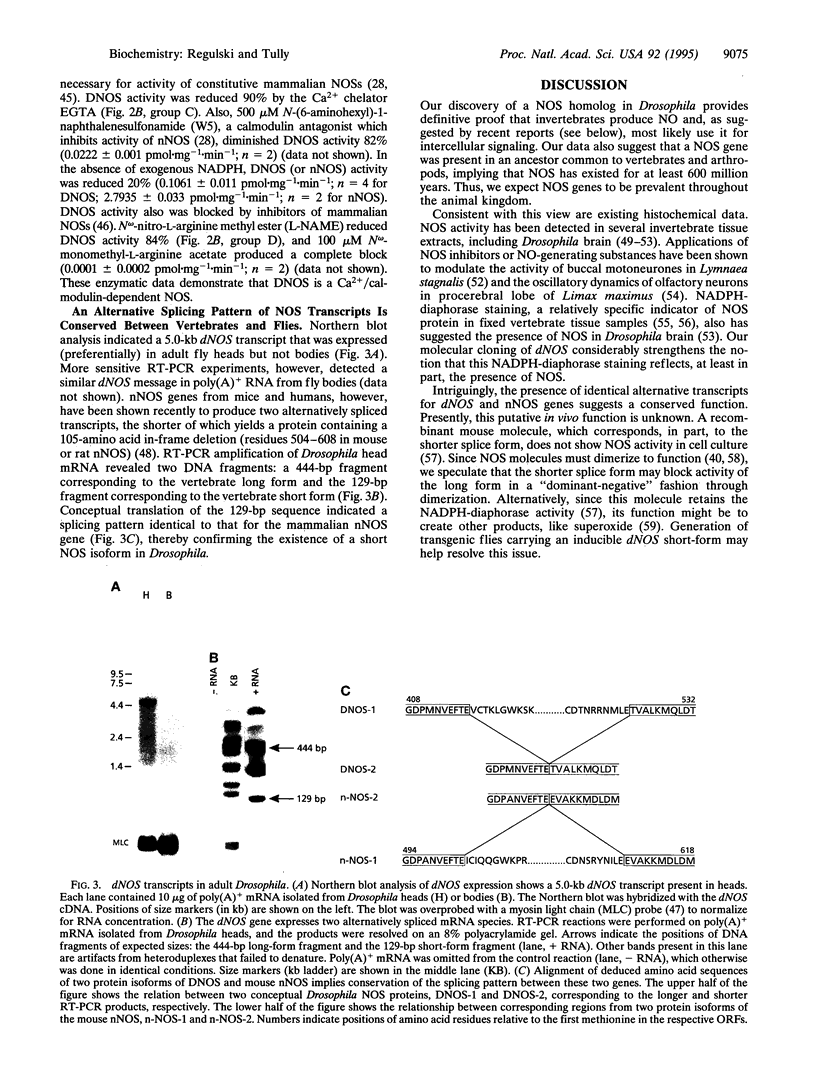
Nitric oxide (NO) is an intercellular messenger involved with various aspects of mammalian physiology ranging from vasodilation and macrophage cytotoxicity to neuronal transmission. NO is synthesized from L-arginine by NO synthase (NOS). Here, we report the cloning of a Drosophila NOS gene, dNOS, located at cytological position 32B. The dNOS cDNA encodes a protein of 152 kDa, with 43% amino acid sequence identity to rat neuronal NOS. Like mammalian NOSs, DNOS protein contains putative binding sites for calmodulin, FMN, FAD, and NADPH. DNOS activity is Ca2+/calmodulin dependent when expressed in cell culture. An alternative RNA splicing pattern also exists for dNOS, which is identical to that for vertebrate neuronal NOS. These structural and functional observations demonstrate remarkable conservation of NOS between vertebrates and invertebrates.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberini C. M., Ghirardi M., Metz R., Kandel E. R. C/EBP is an immediate-early gene required for the consolidation of long-term facilitation in Aplysia. Cell. 1994 Mar 25;76(6):1099–1114. doi: 10.1016/0092-8674(94)90386-7. [DOI] [PubMed] [Google Scholar]
- Baek K. J., Thiel B. A., Lucas S., Stuehr D. J. Macrophage nitric oxide synthase subunits. Purification, characterization, and role of prosthetic groups and substrate in regulating their association into a dimeric enzyme. J Biol Chem. 1993 Oct 5;268(28):21120–21129. [PubMed] [Google Scholar]
- Bannerman D. M., Chapman P. F., Kelly P. A., Butcher S. P., Morris R. G. Inhibition of nitric oxide synthase does not impair spatial learning. J Neurosci. 1994 Dec;14(12):7404–7414. doi: 10.1523/JNEUROSCI.14-12-07404.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannerman D. M., Chapman P. F., Kelly P. A., Butcher S. P., Morris R. G. Inhibition of nitric oxide synthase does not prevent the induction of long-term potentiation in vivo. J Neurosci. 1994 Dec;14(12):7415–7425. doi: 10.1523/JNEUROSCI.14-12-07415.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourtchuladze R., Frenguelli B., Blendy J., Cioffi D., Schutz G., Silva A. J. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell. 1994 Oct 7;79(1):59–68. doi: 10.1016/0092-8674(94)90400-6. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Hwang P. M., Glatt C. E., Lowenstein C., Reed R. R., Snyder S. H. Cloned and expressed nitric oxide synthase structurally resembles cytochrome P-450 reductase. Nature. 1991 Jun 27;351(6329):714–718. doi: 10.1038/351714a0. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Snyder S. H. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci U S A. 1990 Jan;87(2):682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredt D. S., Snyder S. H. Transient nitric oxide synthase neurons in embryonic cerebral cortical plate, sensory ganglia, and olfactory epithelium. Neuron. 1994 Aug;13(2):301–313. doi: 10.1016/0896-6273(94)90348-4. [DOI] [PubMed] [Google Scholar]
- Böhme G. A., Bon C., Lemaire M., Reibaud M., Piot O., Stutzmann J. M., Doble A., Blanchard J. C. Altered synaptic plasticity and memory formation in nitric oxide synthase inhibitor-treated rats. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):9191–9194. doi: 10.1073/pnas.90.19.9191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhme G. A., Bon C., Stutzmann J. M., Doble A., Blanchard J. C. Possible involvement of nitric oxide in long-term potentiation. Eur J Pharmacol. 1991 Jul 9;199(3):379–381. doi: 10.1016/0014-2999(91)90505-k. [DOI] [PubMed] [Google Scholar]
- Cavener D. R. Comparison of the consensus sequence flanking translational start sites in Drosophila and vertebrates. Nucleic Acids Res. 1987 Feb 25;15(4):1353–1361. doi: 10.1093/nar/15.4.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman P. F., Atkins C. M., Allen M. T., Haley J. E., Steinmetz J. E. Inhibition of nitric oxide synthesis impairs two different forms of learning. Neuroreport. 1992 Jul;3(7):567–570. doi: 10.1097/00001756-199207000-00005. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dawson T. M., Bredt D. S., Fotuhi M., Hwang P. M., Snyder S. H. Nitric oxide synthase and neuronal NADPH diaphorase are identical in brain and peripheral tissues. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7797–7801. doi: 10.1073/pnas.88.17.7797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeZazzo J., Tully T. Dissection of memory formation: from behavioral pharmacology to molecular genetics. Trends Neurosci. 1995 May;18(5):212–218. doi: 10.1016/0166-2236(95)93905-d. [DOI] [PubMed] [Google Scholar]
- Dinerman J. L., Dawson T. M., Schell M. J., Snowman A., Snyder S. H. Endothelial nitric oxide synthase localized to hippocampal pyramidal cells: implications for synaptic plasticity. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4214–4218. doi: 10.1073/pnas.91.10.4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elofsson R., Carlberg M., Moroz L., Nezlin L., Sakharov D. Is nitric oxide (NO) produced by invertebrate neurones? Neuroreport. 1993 Mar;4(3):279–282. doi: 10.1097/00001756-199303000-00013. [DOI] [PubMed] [Google Scholar]
- Elphick M. R., Green I. C., O'Shea M. Nitric oxide synthesis and action in an invertebrate brain. Brain Res. 1993 Aug 13;619(1-2):344–346. doi: 10.1016/0006-8993(93)91632-3. [DOI] [PubMed] [Google Scholar]
- Franks R. G., Crews S. T. Transcriptional activation domains of the single-minded bHLH protein are required for CNS midline cell development. Mech Dev. 1994 Mar;45(3):269–277. doi: 10.1016/0925-4773(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Geller D. A., Lowenstein C. J., Shapiro R. A., Nussler A. K., Di Silvio M., Wang S. C., Nakayama D. K., Simmons R. L., Snyder S. H., Billiar T. R. Molecular cloning and expression of inducible nitric oxide synthase from human hepatocytes. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3491–3495. doi: 10.1073/pnas.90.8.3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelperin A. Nitric oxide mediates network oscillations of olfactory interneurons in a terrestrial mollusc. Nature. 1994 May 5;369(6475):61–63. doi: 10.1038/369061a0. [DOI] [PubMed] [Google Scholar]
- Gerber H. P., Seipel K., Georgiev O., Höfferer M., Hug M., Rusconi S., Schaffner W. Transcriptional activation modulated by homopolymeric glutamine and proline stretches. Science. 1994 Feb 11;263(5148):808–811. doi: 10.1126/science.8303297. [DOI] [PubMed] [Google Scholar]
- Ghosh D. K., Stuehr D. J. Macrophage NO synthase: characterization of isolated oxygenase and reductase domains reveals a head-to-head subunit interaction. Biochemistry. 1995 Jan 24;34(3):801–807. doi: 10.1021/bi00003a013. [DOI] [PubMed] [Google Scholar]
- Guan K. L., Dixon J. E. Eukaryotic proteins expressed in Escherichia coli: an improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal Biochem. 1991 Feb 1;192(2):262–267. doi: 10.1016/0003-2697(91)90534-z. [DOI] [PubMed] [Google Scholar]
- Haley J. E., Wilcox G. L., Chapman P. F. The role of nitric oxide in hippocampal long-term potentiation. Neuron. 1992 Feb;8(2):211–216. doi: 10.1016/0896-6273(92)90288-o. [DOI] [PubMed] [Google Scholar]
- Hannon G. J., Demetrick D., Beach D. Isolation of the Rb-related p130 through its interaction with CDK2 and cyclins. Genes Dev. 1993 Dec;7(12A):2378–2391. doi: 10.1101/gad.7.12a.2378. [DOI] [PubMed] [Google Scholar]
- Heisenberg M., Heusipp M., Wanke C. Structural plasticity in the Drosophila brain. J Neurosci. 1995 Mar;15(3 Pt 1):1951–1960. doi: 10.1523/JNEUROSCI.15-03-01951.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope B. T., Michael G. J., Knigge K. M., Vincent S. R. Neuronal NADPH diaphorase is a nitric oxide synthase. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2811–2814. doi: 10.1073/pnas.88.7.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P. L., Dawson T. M., Bredt D. S., Snyder S. H., Fishman M. C. Targeted disruption of the neuronal nitric oxide synthase gene. Cell. 1993 Dec 31;75(7):1273–1286. doi: 10.1016/0092-8674(93)90615-w. [DOI] [PubMed] [Google Scholar]
- Hölscher C., Rose S. P. An inhibitor of nitric oxide synthesis prevents memory formation in the chick. Neurosci Lett. 1992 Oct 12;145(2):165–167. doi: 10.1016/0304-3940(92)90012-v. [DOI] [PubMed] [Google Scholar]
- Imperiale M. J., Feldman L. T., Nevins J. R. Activation of gene expression by adenovirus and herpesvirus regulatory genes acting in trans and by a cis-acting adenovirus enhancer element. Cell. 1983 Nov;35(1):127–136. doi: 10.1016/0092-8674(83)90215-5. [DOI] [PubMed] [Google Scholar]
- Iyengar R., Stuehr D. J., Marletta M. A. Macrophage synthesis of nitrite, nitrate, and N-nitrosamines: precursors and role of the respiratory burst. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6369–6373. doi: 10.1073/pnas.84.18.6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karplus P. A., Daniels M. J., Herriott J. R. Atomic structure of ferredoxin-NADP+ reductase: prototype for a structurally novel flavoenzyme family. Science. 1991 Jan 4;251(4989):60–66. [PubMed] [Google Scholar]
- Klatt P., Schmid M., Leopold E., Schmidt K., Werner E. R., Mayer B. The pteridine binding site of brain nitric oxide synthase. Tetrahydrobiopterin binding kinetics, specificity, and allosteric interaction with the substrate domain. J Biol Chem. 1994 May 13;269(19):13861–13866. [PubMed] [Google Scholar]
- Lamas S., Marsden P. A., Li G. K., Tempst P., Michel T. Endothelial nitric oxide synthase: molecular cloning and characterization of a distinct constitutive enzyme isoform. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6348–6352. doi: 10.1073/pnas.89.14.6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marletta M. A. Nitric oxide synthase: aspects concerning structure and catalysis. Cell. 1994 Sep 23;78(6):927–930. doi: 10.1016/0092-8674(94)90268-2. [DOI] [PubMed] [Google Scholar]
- McGinnis W., Levine M. S., Hafen E., Kuroiwa A., Gehring W. J. A conserved DNA sequence in homoeotic genes of the Drosophila Antennapedia and bithorax complexes. 1984 Mar 29-Apr 4Nature. 308(5958):428–433. doi: 10.1038/308428a0. [DOI] [PubMed] [Google Scholar]
- McMillan K., Bredt D. S., Hirsch D. J., Snyder S. H., Clark J. E., Masters B. S. Cloned, expressed rat cerebellar nitric oxide synthase contains stoichiometric amounts of heme, which binds carbon monoxide. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11141–11145. doi: 10.1073/pnas.89.23.11141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller U. Ca2+/calmodulin-dependent nitric oxide synthase in Apis mellifera and Drosophila melanogaster. Eur J Neurosci. 1994 Aug 1;6(8):1362–1370. doi: 10.1111/j.1460-9568.1994.tb00327.x. [DOI] [PubMed] [Google Scholar]
- Nathan C., Xie Q. W. Nitric oxide synthases: roles, tolls, and controls. Cell. 1994 Sep 23;78(6):915–918. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- Nathan C., Xie Q. W. Regulation of biosynthesis of nitric oxide. J Biol Chem. 1994 May 13;269(19):13725–13728. [PubMed] [Google Scholar]
- O'Dell T. J., Hawkins R. D., Kandel E. R., Arancio O. Tests of the roles of two diffusible substances in long-term potentiation: evidence for nitric oxide as a possible early retrograde messenger. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11285–11289. doi: 10.1073/pnas.88.24.11285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dell T. J., Huang P. L., Dawson T. M., Dinerman J. L., Snyder S. H., Kandel E. R., Fishman M. C. Endothelial NOS and the blockade of LTP by NOS inhibitors in mice lacking neuronal NOS. Science. 1994 Jul 22;265(5171):542–546. doi: 10.1126/science.7518615. [DOI] [PubMed] [Google Scholar]
- O'Neil K. T., DeGrado W. F. How calmodulin binds its targets: sequence independent recognition of amphiphilic alpha-helices. Trends Biochem Sci. 1990 Feb;15(2):59–64. doi: 10.1016/0968-0004(90)90177-d. [DOI] [PubMed] [Google Scholar]
- Ogura T., Yokoyama T., Fujisawa H., Kurashima Y., Esumi H. Structural diversity of neuronal nitric oxide synthase mRNA in the nervous system. Biochem Biophys Res Commun. 1993 Jun 30;193(3):1014–1022. doi: 10.1006/bbrc.1993.1726. [DOI] [PubMed] [Google Scholar]
- Parker V. P., Falkenthal S., Davidson N. Characterization of the myosin light-chain-2 gene of Drosophila melanogaster. Mol Cell Biol. 1985 Nov;5(11):3058–3068. doi: 10.1128/mcb.5.11.3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson R. B., Kemp B. E. Protein kinase phosphorylation site sequences and consensus specificity motifs: tabulations. Methods Enzymol. 1991;200:62–81. doi: 10.1016/0076-6879(91)00127-i. [DOI] [PubMed] [Google Scholar]
- Peunova N., Enikolopov G. Nitric oxide triggers a switch to growth arrest during differentiation of neuronal cells. Nature. 1995 May 4;375(6526):68–73. doi: 10.1038/375068a0. [DOI] [PubMed] [Google Scholar]
- Pyza E., Meinertzhagen I. A. Monopolar cell axons in the first optic neuropil of the housefly, Musca domestica L., undergo daily fluctuations in diameter that have a circadian basis. J Neurosci. 1995 Jan;15(1 Pt 1):407–418. doi: 10.1523/JNEUROSCI.15-01-00407.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Schulz R., Hodson H. F., Moncada S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br J Pharmacol. 1990 Nov;101(3):746–752. doi: 10.1111/j.1476-5381.1990.tb14151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaud J. P., Boucher J. L., Vadon S., Delaforge M., Mansuy D. Particular ability of liver P450s3A to catalyze the oxidation of N omega-hydroxyarginine to citrulline and nitrogen oxides and occurrence in no synthases of a sequence very similar to the heme-binding sequence in P450s. Biochem Biophys Res Commun. 1993 Apr 15;192(1):53–60. doi: 10.1006/bbrc.1993.1380. [DOI] [PubMed] [Google Scholar]
- Ribeiro J. M., Nussenzveig R. H. Nitric oxide synthase activity from a hematophagous insect salivary gland. FEBS Lett. 1993 Sep 13;330(2):165–168. doi: 10.1016/0014-5793(93)80265-v. [DOI] [PubMed] [Google Scholar]
- Richards M. K., Marletta M. A. Characterization of neuronal nitric oxide synthase and a C415H mutant, purified from a baculovirus overexpression system. Biochemistry. 1994 Dec 13;33(49):14723–14732. doi: 10.1021/bi00253a010. [DOI] [PubMed] [Google Scholar]
- Rickard N. S., Ng K. T., Gibbs M. E. A nitric oxide agonist stimulates consolidation of long-term memory in the 1-day-old chick. Behav Neurosci. 1994 Jun;108(3):640–644. doi: 10.1037//0735-7044.108.3.640. [DOI] [PubMed] [Google Scholar]
- Schmidt H. H., Walter U. NO at work. Cell. 1994 Sep 23;78(6):919–925. doi: 10.1016/0092-8674(94)90267-4. [DOI] [PubMed] [Google Scholar]
- Schuman E. M., Madison D. V. A requirement for the intercellular messenger nitric oxide in long-term potentiation. Science. 1991 Dec 6;254(5037):1503–1506. doi: 10.1126/science.1720572. [DOI] [PubMed] [Google Scholar]
- Sessa W. C., Harrison J. K., Barber C. M., Zeng D., Durieux M. E., D'Angelo D. D., Lynch K. R., Peach M. J. Molecular cloning and expression of a cDNA encoding endothelial cell nitric oxide synthase. J Biol Chem. 1992 Aug 5;267(22):15274–15276. [PubMed] [Google Scholar]
- Shibuki K., Okada D. Endogenous nitric oxide release required for long-term synaptic depression in the cerebellum. Nature. 1991 Jan 24;349(6307):326–328. doi: 10.1038/349326a0. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Herr W. Differential transcriptional activation by Oct-1 and Oct-2: interdependent activation domains induce Oct-2 phosphorylation. Cell. 1990 Feb 9;60(3):375–386. doi: 10.1016/0092-8674(90)90589-7. [DOI] [PubMed] [Google Scholar]
- Tully T., Preat T., Boynton S. C., Del Vecchio M. Genetic dissection of consolidated memory in Drosophila. Cell. 1994 Oct 7;79(1):35–47. doi: 10.1016/0092-8674(94)90398-0. [DOI] [PubMed] [Google Scholar]
- Wolf R., Heisenberg M. Basic organization of operant behavior as revealed in Drosophila flight orientation. J Comp Physiol A. 1991 Dec;169(6):699–705. doi: 10.1007/BF00194898. [DOI] [PubMed] [Google Scholar]
- Xie Q. W., Cho H. J., Calaycay J., Mumford R. A., Swiderek K. M., Lee T. D., Ding A., Troso T., Nathan C. Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science. 1992 Apr 10;256(5054):225–228. doi: 10.1126/science.1373522. [DOI] [PubMed] [Google Scholar]
- Xie Q. W., Cho H., Kashiwabara Y., Baum M., Weidner J. R., Elliston K., Mumford R., Nathan C. Carboxyl terminus of inducible nitric oxide synthase. Contribution to NADPH binding and enzymatic activity. J Biol Chem. 1994 Nov 11;269(45):28500–28505. [PubMed] [Google Scholar]
- Yin J. C., Del Vecchio M., Zhou H., Tully T. CREB as a memory modulator: induced expression of a dCREB2 activator isoform enhances long-term memory in Drosophila. Cell. 1995 Apr 7;81(1):107–115. doi: 10.1016/0092-8674(95)90375-5. [DOI] [PubMed] [Google Scholar]
- Yin J. C., Wallach J. S., Del Vecchio M., Wilder E. L., Zhou H., Quinn W. G., Tully T. Induction of a dominant negative CREB transgene specifically blocks long-term memory in Drosophila. Cell. 1994 Oct 7;79(1):49–58. doi: 10.1016/0092-8674(94)90399-9. [DOI] [PubMed] [Google Scholar]
- Zhong Y., Wu C. F. Altered synaptic plasticity in Drosophila memory mutants with a defective cyclic AMP cascade. Science. 1991 Jan 11;251(4990):198–201. doi: 10.1126/science.1670967. [DOI] [PubMed] [Google Scholar]





