Abstract
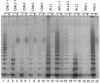
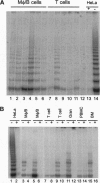
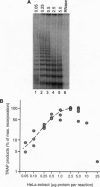
Bone marrow and peripheral blood leukocytes from 19 leukemia patients were found to contain telomerase activity detectable by a PCR-based assay. Telomerase was also detectable in nonmalignant bone marrow and peripheral blood leukocytes from normal donors, including fractions enriched for granulocytes, T lymphocytes, and monocytes/B cells. Semiquantitative comparison revealed considerable overlap between telomerase activities in samples from normal subjects and leukemia patients, confounding evaluation of the role of telomerase in this disease. These data indicate that human telomerase is not restricted to immortal cells and suggest that the somatic expression of this enzyme may be more widespread than was previously inferred from the decline of human telomeres.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allshire R. C., Gosden J. R., Cross S. H., Cranston G., Rout D., Sugawara N., Szostak J. W., Fantes P. A., Hastie N. D. Telomeric repeat from T. thermophila cross hybridizes with human telomeres. Nature. 1988 Apr 14;332(6165):656–659. doi: 10.1038/332656a0. [DOI] [PubMed] [Google Scholar]
- Allsopp R. C., Vaziri H., Patterson C., Goldstein S., Younglai E. V., Futcher A. B., Greider C. W., Harley C. B. Telomere length predicts replicative capacity of human fibroblasts. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10114–10118. doi: 10.1073/pnas.89.21.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke H. J., Smith B. A. Variability at the telomeres of the human X/Y pseudoautosomal region. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 1):213–219. doi: 10.1101/sqb.1986.051.01.026. [DOI] [PubMed] [Google Scholar]
- Counter C. M., Avilion A. A., LeFeuvre C. E., Stewart N. G., Greider C. W., Harley C. B., Bacchetti S. Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. EMBO J. 1992 May;11(5):1921–1929. doi: 10.1002/j.1460-2075.1992.tb05245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counter C. M., Botelho F. M., Wang P., Harley C. B., Bacchetti S. Stabilization of short telomeres and telomerase activity accompany immortalization of Epstein-Barr virus-transformed human B lymphocytes. J Virol. 1994 May;68(5):3410–3414. doi: 10.1128/jvi.68.5.3410-3414.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counter C. M., Gupta J., Harley C. B., Leber B., Bacchetti S. Telomerase activity in normal leukocytes and in hematologic malignancies. Blood. 1995 May 1;85(9):2315–2320. [PubMed] [Google Scholar]
- Counter C. M., Hirte H. W., Bacchetti S., Harley C. B. Telomerase activity in human ovarian carcinoma. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):2900–2904. doi: 10.1073/pnas.91.8.2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greider C. W. Mammalian telomere dynamics: healing, fragmentation shortening and stabilization. Curr Opin Genet Dev. 1994 Apr;4(2):203–211. doi: 10.1016/s0959-437x(05)80046-2. [DOI] [PubMed] [Google Scholar]
- Harley C. B., Futcher A. B., Greider C. W. Telomeres shorten during ageing of human fibroblasts. Nature. 1990 May 31;345(6274):458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- Harley C. B. Telomere loss: mitotic clock or genetic time bomb? Mutat Res. 1991 Mar-Nov;256(2-6):271–282. doi: 10.1016/0921-8734(91)90018-7. [DOI] [PubMed] [Google Scholar]
- Hastie N. D., Dempster M., Dunlop M. G., Thompson A. M., Green D. K., Allshire R. C. Telomere reduction in human colorectal carcinoma and with ageing. Nature. 1990 Aug 30;346(6287):866–868. doi: 10.1038/346866a0. [DOI] [PubMed] [Google Scholar]
- Ishikawa F., Matunis M. J., Dreyfuss G., Cech T. R. Nuclear proteins that bind the pre-mRNA 3' splice site sequence r(UUAG/G) and the human telomeric DNA sequence d(TTAGGG)n. Mol Cell Biol. 1993 Jul;13(7):4301–4310. doi: 10.1128/mcb.13.7.4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim N. W., Piatyszek M. A., Prowse K. R., Harley C. B., West M. D., Ho P. L., Coviello G. M., Wright W. E., Weinrich S. L., Shay J. W. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994 Dec 23;266(5193):2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- Klingelhutz A. J., Barber S. A., Smith P. P., Dyer K., McDougall J. K. Restoration of telomeres in human papillomavirus-immortalized human anogenital epithelial cells. Mol Cell Biol. 1994 Feb;14(2):961–969. doi: 10.1128/mcb.14.2.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin G. B. Recognition of a chromosome truncation site associated with alpha-thalassaemia by human telomerase. Nature. 1991 Oct 3;353(6343):454–456. doi: 10.1038/353454a0. [DOI] [PubMed] [Google Scholar]
- Morin G. B. The human telomere terminal transferase enzyme is a ribonucleoprotein that synthesizes TTAGGG repeats. Cell. 1989 Nov 3;59(3):521–529. doi: 10.1016/0092-8674(89)90035-4. [DOI] [PubMed] [Google Scholar]
- Shay J. W., Wright W. E., Brasiskyte D., Van der Haegen B. A. E6 of human papillomavirus type 16 can overcome the M1 stage of immortalization in human mammary epithelial cells but not in human fibroblasts. Oncogene. 1993 Jun;8(6):1407–1413. [PubMed] [Google Scholar]
- Vaziri H., Dragowska W., Allsopp R. C., Thomas T. E., Harley C. B., Lansdorp P. M. Evidence for a mitotic clock in human hematopoietic stem cells: loss of telomeric DNA with age. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):9857–9860. doi: 10.1073/pnas.91.21.9857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaziri H., Schächter F., Uchida I., Wei L., Zhu X., Effros R., Cohen D., Harley C. B. Loss of telomeric DNA during aging of normal and trisomy 21 human lymphocytes. Am J Hum Genet. 1993 Apr;52(4):661–667. [PMC free article] [PubMed] [Google Scholar]
- Yudkin J. Sugar and disease. Nature. 1972 Sep 22;239(5369):197–199. doi: 10.1038/239197a0. [DOI] [PubMed] [Google Scholar]
- de Lange T. Activation of telomerase in a human tumor. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):2882–2885. doi: 10.1073/pnas.91.8.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lange T., Shiue L., Myers R. M., Cox D. R., Naylor S. L., Killery A. M., Varmus H. E. Structure and variability of human chromosome ends. Mol Cell Biol. 1990 Feb;10(2):518–527. doi: 10.1128/mcb.10.2.518. [DOI] [PMC free article] [PubMed] [Google Scholar]