Abstract
Two experiments were conducted to investigate the potential for Forsythia suspensa extract (FSE) to substitute for antibiotic in broiler chicken. First, a well-diffusion assay procedure and a 2-fold dilution method were used to determine the bacteriostatic activity of FSE on Escherichia coli K88, staphylococcus aureus, and salmonella was assayed. An inhibitory effect of FSE was observed on the growth of these bacteria. This effect seems to be dose depended, which disappeared after 25.00, 12.50, 1.56 mg/ml. Second, a 42-d trial with 252 broiler chickens (d 1, 38.7±1.1 g BW) was conducted to evaluate the effect of dietary supplementation of FSE in broiler chicken. The feeding program consisted of a starter diet from d 1 to 21 and a finisher diet from d 22 to 42. Dietary treatments included were: i) NC: negative control fed a corn-soybean meal based diet; ii) PC: positive control group fed based diet with chlortetracycline; and iii) FC: a test group fed with 100 mg FSE/kg diet. In this study growth performance did not differ among treatments during the starter period. However, dietary supplemental chlortetracycline and FSE increased (p<0.05) average daily gain (ADG), average daily feed intake (ADFI) compared with NC during the finisher and overall phase. Apparent digestibility of calcium on d 21, digestibility of energy and calcium on d 42 of FC was greater (p<0.05) than NC. Moreover, cecal Escherichia coli counts for birds from FC were lower (p<0.05) than NC. Dietary FSE supplementation also improved (p<0.05) villus height and villus height to crypt depth ratios in both duodenum and ileum and decreased (p<0.05) crypt depth in the duodenum. Duodenum villus height and villus height to crypt depth ratio in both duodenum and ileum from the FC group were also greater (p<0.05). Serum growth hormone and IGF-1 were not influenced by different treatments. Apparently, FSE has the potential to substitute for antibiotic in broiler chicken.
Keywords: Antibiotic, Broiler, Forsythia Suspensa, Performance
INTRODUCTION
Subtherapeutic use of antibiotics has widely been applied to maintain livestock health and improve growth performance (Lien et al., 2007). However, issues with the development of bacterial resistance make the subtherapeutic use of antibiotics seem to be a double edged sword (Cassel, 1995; Bach Knudsen, 2001; Smith et al., 2002). Their use for therapeutic and growth improvement purposes at least in Europe have been forbidden. Therefore, substitutes for antibiotic with high efficiency and low toxicity are required.
Many Chinese herbal medicines such as Coptidis rhizome and Phelodendri cortex have been used to treat patients with gastroenteritis, inflammation, diarrhea, gout, and hyperuricemia which are related to the effect of antibiotics (Zhou and Mineshita., 2000; Kong et al., 2004; Shen et al., 2010).
Forsythia suspensa Vahl (Oleaceae) (FSE) is a climbing plant widely distributed in China, Japan and Korea. The extracts of the dried fruits have been used for a long time as traditional Asian medicines to treat gonorrhea, erysipedas, inflammation and pharyngitis (Piao et al., 2008a). The major active components of F. suspensa extract are identified as phenethyl alcohol glycoside, lignan, pentacyclic triterpenoids and volatile oil (Zhang, 2000). FSE was reported to exhibit potential antibacterial (Niu et al., 2002; Li et al., 2007; Liu et al., 2007), antiviral (Liu et al., 2004), and anti-inflammatory (Hu et al., 2007) properties. In the recent years, its antioxidant activity has been also intensively investigated (Schinella et al., 2002; Qu et al., 2008; Piao et al., 2008a; Wang et al., 2008; Piao et al., 2009). New monoepoxylignans forsythialan A, forsythialan B and another two known components (phillygenin and 8-hydroxypinoresinol), extracted from F. suspensa fruit, also showed their protective effects against peroxynitrite-induced oxidative stress in LLC-PK1 cells (Piao et al., 2008a). In addition, FSE could reduce oxidative stress for broiler chickens and rats (Wang et al., 2008; Lu et al., 2010). In a word, it is reasonable to conclude that FSE has the potential to be a substitute for antibiotics to maintain livestock health and improve their growth performance.
Under farming conditions, Escherichia coli, salmonella, and Staphylococcus aureus are three of the most harmful bacteria for broiler chickens (Chinivasagam et al., 2009; Revolledo and Ferreira, 2010). Therefore, we evaluated the bacteriostatic activities of F. suspensa extract on Escherichia coli K88, staphylococcus aureus, and salmonella in vitro, an animal experiment was also conducted to evaluate the effect of FSE as a substitute for antibiotic.
MATERIALS AND METHODS
Extracts preparation from Forsythia suspensa
Forsythia suspensa extract was prepared using the method described by Wang et al. (2008). Dried fruits of Forsythia suspensa were purchased from Tong Ren Tang (Beijing, China; collected from Shanxi province, August). In brief, dried fruits of Forsythia suspensa were ground to powder (100 g), extracted with 500 ml of 80% methanol, sonicated for 3 h, filtered, and extracted twice (500 ml each time). The filtrates were combined, and dried by rotary vaporization (Büchi, Rotavapor R-124, Flawil, Switzerland).
Bacteriostatic activities of Forsythia suspense extract in vitro
Escherichia coli K88, Staphylococcus aureus, and Salmonella enteric 34R99 were purchased from the China Veterinary Culture Collection Center (Beijing, China). The strains were kept at −70°C in LB agar, activated by transferring into nutritive agar, and incubating at 37±1.0°C for 18 h.
A well-diffusion assay procedure and a 2-fold dilution method were used to assay the bacteriostatic activity of FSE (Dimov, 2007; Lima et al., 2008). Briefly, Forsythia suspensa extract from each serial dilution was placed into 5-mm wells of the plates seeded with the bioassay strain. After 18 h of incubation at 37°C, clear zones of inhibition appeared where the strain was sensitive. The diameter of these zones was then measured using a vernier caliper. Diameter beyond 8.0 mm was considered as sensitive inhibition (Yang et al., 2009). Media for Escherichia coli K88 and Staphylococcus aureus were extract broth (beef extract, 3 g; peptone, 10 g; NaCl, 5 g; agar, 15 to 18 g; distilled water, 1,000 ml; pH 7.0), for Salmonella enteric BPY agar media (beef extract, 5 g; peptone, 10 g; yeast extract, 5 g; glucose, 5 g; NaCl, 5 g; agar, 15 g; distilled water, 1,000 ml; pH 7.0) was used.
Experimental animals
A total of 252 male broiler chickens (38.7±1.1 g, Arbor Acres, 1 d old) were used in this study. There were 14 cages per treatment for replication and 6 birds per cage. All birds were housed in wire-floored cages in an environmentally controlled room with continuous light. The lighting regimen and ventilation were continuously monitored from d 1 to 42. The birds had access to feed and water ad libitum. During the experimental period, relative humidity was 44±6%. The room temperature was maintained at 33°C for the first 3 d, after which the temperature was gradually reduced to 24°C; which was maintained during the 42 d experiment period. All birds were inoculated with inactivated infectious bursa disease vaccine on d 14 and 21 and with Newcastle disease vaccine on d 7 and 28. The trial was conducted in 2 phases consisting of a starter phase from d 1 to 21 and a finisher phase from d 22 to 42. The animal care protocol in this experiment was approved by the Animal Welfare Committee of China Agricultural University.
Experimental design and diets
The broilers were randomly allotted to 3 dietary treatments (Table 1) including i) NC: a negative control group fed with a corn-soybean meal based diet without chlortetracycline and FSE; ii) PC: a positive control group fed a diet with 80 mg chlortetracycline/kg diet during 1 to 21 d, and 50 mg/kg during 22 to 42 d; iii) FC: a test group fed with 100 mg FSE/kg diet. There were 14 cages per treatment with 6 birds per cage. All essential nutrients contained in the basal diet (Table 1) met or exceeded nutrient requirements suggestion from NRC (2004). All diets were fed in a mash form.
Table 1.
Composition of basal diets and nutrient levels1
| Items | Starter phase (1–21 d) | Finisher phase (22–42 d) |
|---|---|---|
| Ingredients (%) | ||
| Corn | 55.62 | 60.99 |
| Soybean meal, 44% crude protein | 32.20 | 26.83 |
| Fish meal, 64% crude protein | 4.00 | 4.00 |
| Soybean oil | 3.86 | 4.33 |
| Limestone | 1.27 | 1.18 |
| Dicalcium phosphate | 1.31 | 1.08 |
| Salt | 0.35 | 0.35 |
| Premix2 | 1.00 | 1.00 |
| L-lysine·HCl, 78% | 0.11 | 0.09 |
| DL-methionine, 98% | 0.28 | 0.15 |
| Total | 100.00 | 100.00 |
| Nutrient levels | ||
| Metabolic energy (kcal/g) | 3.00 | 3.10 |
| Crude protein (%) | 21.50 | 19.50 |
| Calcium (%) | 1.00 | 0.90 |
| Available phosphorus (%) | 0.45 | 0.40 |
| Lysine (%) | 1.30 | 1.15 |
| Methionine (%) | 0.65 | 0.50 |
| Analyzed composition | ||
| Crude protein (%) | 21.56 | 19.51 |
| Calcium (%) | 1.01 | 0.88 |
| Phosphorus (%) | 0.68 | 0.58 |
| Lysine (%) | 1.33 | 1.14 |
| Methionine (%) | 0.66 | 0.52 |
The basal pretreatment diet and treatment diets were the same. Treatment diets were supplemented with 100 mg/kg Forsythia suspensa extract, or chlortetracycline (80 mg/kg for the starter birds and 50 mg/kg for the finisher birds) to the basal diet as part of the premix.
The premix provided the following per kilogram of compound feed: zinc, 60 mg; iron, 95 mg; manganese, 80 mg; copper, 10 mg; iodine, 0.35 mg; selenium, 0.3 mg; vitamin A, 10,000 IU; vitamin D3, 2,750 IU; vitamin E, 30 IU; vitamin K3, 2 mg; vitamin B12, 12 μg; riboflavin, 6 mg; nicotinic acid, 40 mg; pantothenic acid, 12 mg; pyridoxine, 3 mg; biotin, 0.2 mg; choline chloride, 800 mg.
Sampling and sample processing procedure
The excreta from each cage were collected from d 19 to 21 and from d 40 to 42, weighed, and dried at 60°C for 72 h. The feed and dried excreta samples were ground to pass through a 40-mesh screen and mixed thoroughly before analysis. The DM, CP, calcium, and phosphorus contents were determined according to AOAC (2000), and gross energy content was measured by an adiabatic bomb calorimeter (Model 1281, Parr, Moline, IL, USA) to calculate CP retention and apparent nutrient digestibility.
On d 21 and 42, body weight (BW) and feed intake (FI) were measured after 12 h fasting to determine average daily gain (ADG), average daily feed intake (ADFI), and feed conversion ratio (FCR). One bird per cage was randomly selected and euthanized for sampling. Blood was collected (5 ml) by cardiac puncture using a 10-ml anticoagulant-free vacutainer tube (Greiner Bio-One GmbH, Kremsmunster, Austria), centrifuged at 3,000×g for 10 min to obtain the serum, and stored at −80°C until analysis for growth hormone (GH) and insulin-like growth factor 1 (IGF-1). Samples of digesta (5 ml) from the cecum were obtained, and stored at −80°C until microbial counting. On d 42, about 5 cm segments of duodenum, jejunum, and ileum were removed and immediately flushed with ice-cold 0.9% saline solution to remove any excess blood and digesta. Tissues were then fixed with 10% formaldehyde-phosphate buffer, and kept at 4°C until microscopic assessments of mucosa morphology.
Assay of serum GH and IGF-1
Serum GH level was determined using a radioimmunoassay kit (Beijing Siano-uk Institute of Biological Technology, Beijing) (Li et al., 2007). Variation between or within groups was less than 13% and 9.0%, respectively. Recovery was from 95% to 104%. Serum IGF-1 level was also determined using the radioimmunoassay kit (Diagnostic System Laboratories Inc., America). Variation between or within groups was less than 8.2% and 3.4%, respectively. Recovery was from 89% to 122%.
Microbial counting
Microbial analysis was carried out according to the procedure introduced by Shen et al. (2009). Escherichia coli were cultured in MacConkey agar (Beijing Haidian Microbiological Culture Factory, Beijing, China). Lactobacillus was determined using MRS agar (Beijing Haidian Microbiological Culture Factory, Beijing, China). Data between 30 to 300 were available. Results were expressed as log10 cfu per gram.
Statistical analysis
Data were subjected to ANOVA using the GLM procedure of SAS (SAS Institute, 1996). Cage was the experimental unit. Differences among treatments were separated by Duncan’s multiple range tests. Probability values less than 0.05 were considered significant.
RESULTS
Bacteriostatic activities of Forsythia suspense extract in vitro
Bacteriostatic activities of FSE on all three bacteria were observed (Figure 1). The inhibition effect on the growth of these bacteria seems to be dose depended. In this serial dilution method based experiment, the inhibitory effect of FSE on Escherichia coli K88, Staphylococcus aureus, and Salmonella 34R99 disappeared after 25.00, 12.50, 1.56 mg/ml, respectively (Table 2).
Figure 1.
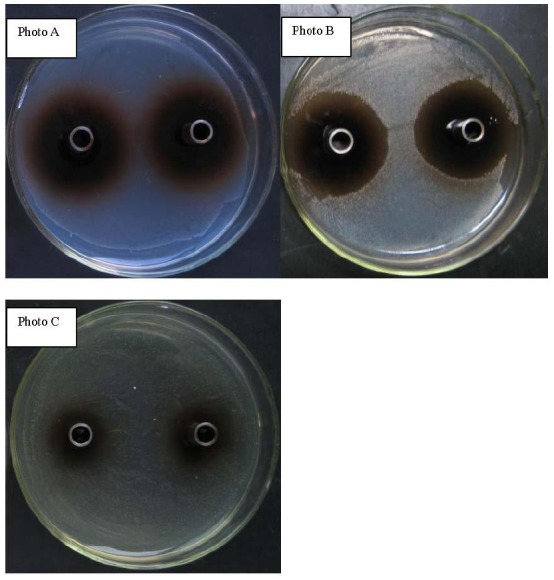
Photo of bacteriostatic activities of Forsythia suspensa extract on the Escherichia coli K88, Staphylococcus aureus, and Salmonella. Concentration of FSE was 200 mg/ml, photo A was for Escherichia coli K88, photo B was for Staphylococcus aureus, photo C was for Salmonella.
Table 2.
Bacteriostatic circle diameter of Forsythia suspensa extract on the Escherichia coli K88, Staphylococcus aureus, and Salmonella1
| Items | Two-fold dilution | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| 1:1 | 1:2 | 1:4 | 1:8 | 1:16 | 1:32 | 1:64 | 1:128 | 1:256 | 1:512 | |
| Bacteriostatic circle diameter of Staphylococcus aureus (mm) | 38.00 | 32.63 | 32.50 | 29.67 | 27.50 | 23.67 | 13.17 | 10.33 | ||
| Bacteriostatic circle diameter of Escherichia coli K88 (mm) | 16.50 | 13.25 | 10.83 | 8.17 | ||||||
| Bacteriostatic circle diameter of Salmonella (mm) | 22.25 | 19.88 | 14.17 | 11.33 | 9.12 | |||||
The initial concentration of FSE solution was 200 mg/ml.
Growth performance
In the starter phase, no treatment effect of FSE or chlortetracycline on ADG, ADFI, and FCR of broiler chickens was observed (Table 3). In the finisher phase, ADG of FC and PC was greater (16.2% and 12.2% respectively, p<0.01) than NC, a similar effect of FSE and chlortetracycline supplementation on ADFI was also observed (9.6% and 11.5% respectively, p<0.01). However, FCR was not affected by dietary treatment. No difference between PC and FSE was observed on ADG, ADFI, and FCR of broiler chickens. For the entire period, dietary supplementation with FSE and chlortetracycline improved ADG (12.4% and 8.6% respectively, p<0.01) and ADFI (7.9% and 8.4% respectively, p<0.01) compared with NC, FCR was still not affected by dietary treatment.
Table 3.
Effects of dietary FSE and chlortetracycline supplement on the growth performance in broilers
| Items | Treatments | SEM | p value | ||
|---|---|---|---|---|---|
|
| |||||
| NC | FC | PC | |||
| ADG (g) | |||||
| 1–21 d | 32 | 34 | 33 | 0.01 | 0.44 |
| 22–42 d | 67b | 78a | 75a | 1.57 | <0.01 |
| 1–42 d | 50b | 56a | 54a | 1.15 | <0.01 |
| ADFI (g) | |||||
| 1–21 d | 45 | 46 | 45 | 0.34 | 0.59 |
| 22–42 d | 124b | 136a | 138a | 2.15 | <0.01 |
| 1–42 d | 85b | 91a | 92a | 0.85 | <0.01 |
| FCR | |||||
| 1–21 d | 1.40 | 1.36 | 1.37 | 0.01 | 0.66 |
| 22–42 d | 1.86 | 1.75 | 1.84 | 0.03 | 0.19 |
| 1–42 d | 1.71 | 1.64 | 1.71 | 0.02 | 0.17 |
Means within a row with different letter differ (p<0.05); SEM is standard error of the mean.
Apparent digestibility
At the end of starter phase, the apparent digestibility of calcium was improved (14.4%, p<0.05) by FSE supplementation compared with NC (Table 4), no dietary treatment effect was observed on the apparent digestibility of DM, energy, CP, and phosphorus. At the end of finisher phase, the apparent digestibility of energy (p<0.05) and calcium (p<0.05) were improved by FSE and chlortetracycline supplementation compared with NC. No difference between FC and PC was observed.
Table 4.
Effects of dietary Forsythia suspensa extract (FSE) supplement on the apparent digestibility (%) of nutrients and bacterial concentrations (log CFU/g wet digesta) in broilers
| Items | Treatments | SEM | p value | ||
|---|---|---|---|---|---|
|
| |||||
| NC | FC | PC | |||
| d 21 | |||||
| Dry matter | 71.32 | 72.53 | 72.41 | 0.51 | 0.80 |
| Energy | 78.32 | 76.18 | 77.48 | 0.45 | 0.18 |
| Crude protein | 69.60 | 68.01 | 69.12 | 0.82 | 0.74 |
| Calcium | 51.31b | 58.72a | 55.24ab | 1.76 | <0.05 |
| Phosphorus | 53.95 | 55.85 | 56.24 | 1.31 | 0.77 |
| Lactobacillus | 5.47 | 5.48 | 5.57 | 0.11 | 0.45 |
| Escherichia coli | 6.74a | 5.85b | 5.58b | 0.15 | <0.05 |
| d 42 | |||||
| Dry matter | 72.16 | 73.31 | 73.25 | 0.57 | 0.85 |
| Energy | 77.53b | 80.52a | 79.40ab | 0.49 | <0.05 |
| Crude protein | 60.06 | 63.25 | 60.07 | 0.93 | 0.28 |
| Calcium | 46.47b | 62.12a | 52.97b | 2.15 | <0.05 |
| Phosphorus | 46.23 | 53.53 | 53.17 | 1.73 | 0.15 |
| Lactobacillus | 7.12 | 7.44 | 7.42 | 0.09 | 0.56 |
| Escherichia coli | 6.19a | 5.41b | 5.31b | 0.12 | <0.05 |
Histological measurements
As determined at d 42, dietary FSE supplementation improved (p<0.05) villus height and villus height to crypt depth ratios in both duodenum and ileum, decreased (p<0.05) crypt depth in duodenum compared with NC (Table 5). Duodenum villus height and villus height to crypt depth ratios in both duodenum and ileum from FC were also greater (p<0.05) than PC. In jejunum, similar effects were also observed but not significantly.
Table 5.
Effects of dietary Forsythia suspensa extract (FSE) supplement on small intestinal morphology in broilers
| Items | Treatments | SEM | p value | ||
|---|---|---|---|---|---|
|
| |||||
| NC | FC | PC | |||
| Villus height (μM) | |||||
| Duodenum | 1,198.7b | 1,379.7a | 1,199.0b | 34.11 | 0.03 |
| Jejunum | 579.6 | 631.8 | 601.6 | 19.62 | 0.58 |
| Ileum | 473.8b | 609.0a | 547.6ab | 20.73 | <0.05 |
| Crept depth (μM) | |||||
| Duodenum | 203.4 | 163.9b | 192.4ab | 6.81 | <0.05 |
| Jejunum | 178.4 | 167.9 | 160.8 | 5.72 | 0.47 |
| Ileum | 161.0 | 139.7 | 164.6 | 5.53 | 0.13 |
| Villus height/crept depth ratio | |||||
| Duodenum | 6.00b | 8.67a | 6.27b | 0.42 | <0.01 |
| Jejunum | 3.26 | 3.76 | 3.74 | 0.22 | 0.43 |
| Ileum | 2.99b | 4.44a | 3.40b | 0.23 | <0.05 |
Cecum microflora and serum GH and IGF-1
No effect of dietary treatment on cecum lactobacilli counts was observed on d 21 or d 42 for broiler chicken. Both FSE and chlortetracycline supplementation decreased (p<0.05) cecum E. coli counts on d 21 and d 42 compared with NC group (Table 4). No effect of dietary treatment on serum GH concentration or IGF-I concentration was observed during the experimental period (p>0.05) (Table 6).
Table 6.
Effects of dietary Forsythia suspensa extract (FSE) supplement on serum growth hormone and IGF- I concentrations (ng/ml) in broilers
| Items | Treatments | SEM | p value | ||
|---|---|---|---|---|---|
|
| |||||
| NC | FC | PC | |||
| Growth hormone (ng/ml) | |||||
| d 21 | 6.20 | 6.27 | 5.85 | 0.39 | 0.91 |
| d 42 | 4.24 | 5.01 | 4.19 | 3.63 | 0.63 |
| Insulin-like growth factor-I (ng/ml) | |||||
| d 21 | 209.63 | 209.04 | 217.22 | 0.37 | 0.61 |
| d 42 | 206.31 | 228.48 | 180.66 | 9.48 | 0.12 |
DISCUSSION
The bacteria selected in this study were three of the most dangerous bacteria to the poultry industry. Escherichia coli has several virulence attributes which result in variety of diseases in poultry, including salpingities, sinovities, omphalitis, and chronic respiratory disease (Gutierrez et al., 2010). These virulence attributes could promote colonization or adhesion to the mucosa, cause metabolic dysfunction or death of enterocytes, affect the local or systemic vasculature; they promote invasion and septicemia (Jubb et al., 1993). Salmonella infection is another major problem in poultry production. The infections can sometimes lead from moderate illness all the way to death at high frequencies especially for young poultry (Gast and Holt, 1998; Oh et al., 2010). Staphylococcus aureus is also an important human and veterinary pathogen that causes great economic loss in the poultry industry. The diseases associated with this bacterium vary in severity from superficial skin and ophthalmic infections to life-threatening endocardities, septic arthritis, and septicemia. The inhibition of FSE in vitro provided evidence for the positive effect of dietary supplemental FSE. These results of the bacteriostatic activities for FSE were consistent with previous literature (Niu et al., 2002; Li et al., 2007; Liu et al., 2007). The functioning component, forsythiaside, which was isolated from FSE, has been reported to inhibit growth of Escherichia coli-10B, Pseudomonas aeruginosa and Staphylococcus aureus-Rn4220 (Qu et al., 2008), but research on the other bacteriostatic activities is rare because of the great difficulty in purifying single components from FSE. The mechanism for such an effect is still unreported. However, the effect of dietary supplementation with FSE on maintaining health and promoting growth can be ascribed to such bacteriostatic activities.
Unlike antibiotics, FSE has also been reported to be an antioxidant. Phenylethanoid glycosides like forsythoside A, forsythoside B, and lignans such as phillyrin, forsythialan A, forsythialan B, phillygenin, and 8-hydroxypinoresinol were reported as antioxidant components of FSE (Piao et al., 2008a ; Qu et al., 2008). Protective effects of FSE against oxidative stress either peroxynitrite-induced in LLC-PK1 cells or diquat-induced in rats, and high ambient temperature induced in broiler chicken were also reported (Piao et al., 2008a; Wang et al., 2008; Lu et al., 2010). Oxidative stress is related to a number of aging-dependent pathogenic processed including cancers, arteriosclerosis, arthritis, neurodegenerative disorders and other diseases (Valko et al., 2006). At the same time, FSE was also reported to alleviate the hypersensitivity reaction induced by soybean β-conglycinin in a swine model (Hao et al., 2010). Such an effect of FSE played an important role in achieving the better intestine histological morphology compared with PC and NC in this experiment, by the way of both benefiting the repair of epidermis damage caused by pathogen and antigen protein in diet and by reducing the presence of these harmful factors. The improvement in gut health could theoretically increase the digestibility of nutrients, which was also detected in our experiment.
Unexpectedly, the effect of FSE and chlortetracycline supplementation on the growth performance and nutrient digestibility was not observed in the starter phase in this experiment. A possible reason for this result may due to the way that broiler chicks grow. About 70% of body weight is gained in the finisher phase, which is to say growth performance is easier to be influenced in broiler chickens during the finisher phase (Wang et al., 2008). Also, developments of broiler chicken in the starter phase were mainly focused on the growth from hatching of organs such as pancreas, small intestine and liver (Nitsan et al., 1991). The positive effect was expressed until the end of the finisher phase.
IGF-1 could enhance rates of net Na+ and Na+-dependent nutrient absorption and stimulate electrolyte transport in animals (Alexander and Carey, 1999). It is also well established that IGF-I can influence gastrointestinal growth in adults, Intravenous or subcutaneous administration of IGF-I in adults increases intestinal mucosal weight, protein and DNA content, villus height, and epithelial proliferation (Lemmey et al., 1991; Peterson et al., 1997). GH was an important hormone which can affect growth of chickens directly (Burke et al., 1982). Effect of FSE and chlortetracycline supplementation on these hormones seems to be variable, which may exclude the possible mechanism of FSE to function in such a way.
Considering the bacteriostatic activities of FSE both in vitro and vivo, as well as its positive effect on the growth performance and histological morphology in broiler chicken, FSE has the potential to be a substitute for antibiotic. Further research on the functional mechanism, optimum dose in diets, methods of lowering production cost needs to be done; followed by evaluating its effect in large populations in commercial production before FSE can be used by industry.
ACKNOWLEDGEMENTS
This study was completed at the State Key Laboratory of Animal Nutrition (2004DA125184-0810) of China. The authors thank their effort to make the experiment get finished successfully.
Acknowledgments
Acknowledgement: financial supported by the National Natural Science Foundation of China (No. 31072040), State Key Laboratory of Animal Nutrition of China (No. 2004DA125184-0810), Ministry of Science and Technology of China (No. nyhyzx07-034), and Guangdong cooperation project between Industry-Academia-Research of China (No. 2009B090300110).
REFERENCES
- Alexander AN, Carey HV. Oral IGF-I enhances nutrient and electrolyte absorption in neonatal piglet intestine. Am J Physiol Gastrointest Liver Physiol. 1999;277:619–625. doi: 10.1152/ajpgi.1999.277.3.G619. [DOI] [PubMed] [Google Scholar]
- AOAC. Official methods of analysis. 17th edn. Association of Official Analytical Chemists; Arlington. Virginia, USA: 2000. [Google Scholar]
- Barton MD. Antibiotic use in animal feed and its impact on human health. Nutr Res Rev. 2000;13:279–299. doi: 10.1079/095442200108729106. [DOI] [PubMed] [Google Scholar]
- Bach Knudsen KE. Development of antibiotic resistance and options to replace antimicrobials in animal diets. Proc Nutr Soc. 2001;60:291–299. doi: 10.1079/pns2001109. [DOI] [PubMed] [Google Scholar]
- Burke WH, Marks HL. Growth hormone and prolactin levels in non selected and selected broiler lines of chickens from hatch to eight weeks of age. Growth. 1982;46:283–295. [PubMed] [Google Scholar]
- Cassel GH. ASM task force urges broad program on antimicrobial resistance. ASM News. 1995;61:116–120. [Google Scholar]
- Chinivasagam HN, Tran T, Maddock L, Gale A, Blackall PJ. Mechanically ventilated broiler sheds: a possible source of aerosolized salmonella, Campylobacter, and Escherichia coli. Appl Environ Microbiol. 2009;75:7417–7425. doi: 10.1128/AEM.01380-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimov SG. A novel bacteriocin-like substance produced by Enterococcus faecium 3587. Curr Microbiol. 2007;55:323–327. doi: 10.1007/s00284-007-0018-0. [DOI] [PubMed] [Google Scholar]
- Gast RK, Holt PS. Persistence of salmonella enteritidis from one day of age until maturity in experimentally infected layer chickens. Poult Sci. 1998;77:1759–1762. doi: 10.1093/ps/77.12.1759. [DOI] [PubMed] [Google Scholar]
- Gutierrez L, Ocampo L, Rosario C, Sumano H. Pharmacokinetics of disodium fosfomycin in broilers and dose strategies to comply with its pharmacodynamics versus Escherichia coli. Poult Sci. 2007;89:2106–2115. doi: 10.3382/ps.2010-00892. [DOI] [PubMed] [Google Scholar]
- Hao Y, Piao XS, Li DF. Forsythia suspensa extract alleviates hypersensitivity induced by soybean β-conglycinin in weaned piglets. J Ethnopharmacol. 2010;128:412–418. doi: 10.1016/j.jep.2010.01.035. [DOI] [PubMed] [Google Scholar]
- Hu JY, Lei L, Yu Y, Deng WL. The research of Forsythia suspensa on anti-inflammatory and relieving fever activities. Pharmacol Clin Chin Mater Med. 2007;23:51–52. [Google Scholar]
- Jubb K, Kennedy P, Palmer N. Nutritional myopathy. In: Jubb K, Kennedy P, Palmer N, editors. Pathology of domestic animals. 4th Ed. Academic Press; San Diego, California: 1993. pp. 228–242. [Google Scholar]
- Kong X, Hu Y, Rui R, Wang D, Li X. Effects of chinese herbal medicinal ingredients on peripheral lymphocyte proliferation and serum antibody titer after vaccination in chicken. Int Immunopharmacol. 2004;4:975–982. doi: 10.1016/j.intimp.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Lemmey AB, Martin AA, Read LC, Tomas FM, Owens PC, Ballard FJ. IGF-I and the truncated analogue des-1–3 IGF-I enhance growth in rats after gut resection. Am J Physiol. 1991;260:213–219. doi: 10.1152/ajpendo.1991.260.2.E213. [DOI] [PubMed] [Google Scholar]
- Li ZX, Wang XH, Zhao JH, Yang JF, Wang X. In vitro study of antibacterial activity of Forsythia suspensa against staphylococcus aureus and sepidermidis. Tianjin J Traditional Chin Med. 2007;24:328–331. [Google Scholar]
- Lien TF, Horng YM, Wu CP. Feasibility of replacing antibiotic feed promoters with the Chinese traditional herbal medicine Bazhen in weaned piglets. Livest Sci. 2007;107:97–102. [Google Scholar]
- Lima FL, de Carvalho MAR, Apolônio ACM, Bemquerer MP, Santoro MM, Oliveira JS, Alviano CS, Farias LM. Actinomycetemcomitin: a new bacteriocin produced by Aggregatibacter(Actinobacillus) actinomycetemcomitans. J Ind Microbiol Biotechnol. 2008;35:103–110. doi: 10.1007/s10295-007-0271-z. [DOI] [PubMed] [Google Scholar]
- Liu YJ, Yang ZQ, Xiao H, Wen L. Inhibitory effect of four kinds of extract of Chinese herbs - Forsythia on herpes simplex virus type. J Hubei College Trad Chin Med. 2004;6:36–38. [Google Scholar]
- Liu WS, Xu YX, Zheng YL. Effect of the ethanol extract of Forsythia suspensa(thunb.) Vahl. on the growth curve of bacterium. J Anhui Agric Sci. 2007;35:7383–7387. [Google Scholar]
- Lu T, Piao XL, Zhang Q, Wang D, Piao XS, Kim SW. Protective effects of Forsythia suspensa extract against oxidative stress induced by diquat in rats. Food Chem Toxicol. 2010;48:764–770. doi: 10.1016/j.fct.2009.12.018. [DOI] [PubMed] [Google Scholar]
- Nitsan Z, Ben-Avraham G, Zoref Z, Nir I. Growth and development of the digestive organs and some enzymes in broiler chicks after hatching. Br Poult Sci. 1991;32:515–523. doi: 10.1080/00071669108417376. [DOI] [PubMed] [Google Scholar]
- Niu XH, Qiu SC, Di DL, Bai XL, Gao F. The in vitro growth inhibition effect of Forsythia suspensa(thunb.) Vahi (FSV) on bacteria. Li Shizhen Med Mater Med Res. 2002;13:342–343. [Google Scholar]
- Oh JY, Kang MS, An BK, Song EA, Kwon JH, Kwon YK. Occurrence of purulent arthritis broiler vertically infected with Salmonellla enterica serovar Enteritidis in Korea. Poult Sci. 2010;89:2116–2122. doi: 10.3382/ps.2010-00918. [DOI] [PubMed] [Google Scholar]
- Peterson CA, Carey HV, Hinton PS, Lo HC, Ney DM. GH elevates serum IGF-I levels but does not alter mucosal atrophy in parenterally-fed rats. Am J Physiol. 1997;272:1100–1108. doi: 10.1152/ajpgi.1997.272.5.G1100. [DOI] [PubMed] [Google Scholar]
- Piao XL, Jang MH, Cui J, Piao XS. Lignans from the fruits of Forsythia suspensa. Bioorg Med Chem Lett. 2008a;18:1980–1984. doi: 10.1016/j.bmcl.2008.01.115. [DOI] [PubMed] [Google Scholar]
- Piao XL, Cho EJ, Jang MH, Cui J. Cytoprotective effect of lignans from Forsythia suspensa against peroxynitrite-induced LLC-PK1cell damage. Phytother Res. 2009;23:938–942. doi: 10.1002/ptr.2834. [DOI] [PubMed] [Google Scholar]
- Qu H, Zhang Y, Wang Y, Li B, Sun W. Antioxidant and antibacterial activity of two compounds (forsythiaside and forsythin) isolated from Forsythia suspensa. J Pharm Pharmacol. 2008;60:261–266. doi: 10.1211/jpp.60.2.0016. [DOI] [PubMed] [Google Scholar]
- Revolledo L, Ferreira AJP. Salmonella antibiotic-mutant strains reduce fecal shedding and organ invasion in broiler chicks. Poult Sci. 2010;89:2130–2140. doi: 10.3382/ps.2010-00920. [DOI] [PubMed] [Google Scholar]
- Institute SAS. SAS user’s guide: statisitcs Version 7.0. SAS Institute; Cary, North Carolina: 1996. [Google Scholar]
- Schinella GR, Tournier HA, Prieto JM, de Mordujovich BP, Rios JL. Antioxidant activity of anti-inflammatory plant extracts. Life Sci. 2002;70:1023–1033. doi: 10.1016/s0024-3205(01)01482-5. [DOI] [PubMed] [Google Scholar]
- Shen YB, Piao XS, Kim SW, Wang L, Liu P, Yoon I, Zhen YG. Effects of yeast culture supplementation on growth performance, intestinal health, and immune response of nursery pigs. J Anim Sci. 2009;87:2614–2624. doi: 10.2527/jas.2008-1512. [DOI] [PubMed] [Google Scholar]
- Shen YB, Piao XS, Kim SW, Wang L, Liu P. The effects berberine on the magnitude of the acute inflammatory response induced by Escherichia coli lipopolysaccharide in broiler chickens. Poult Sci. 2010;89:13–19. doi: 10.3382/ps.2009-00243. [DOI] [PubMed] [Google Scholar]
- Smith DL, Harris AD, Johnson JA, Silbergeld EK, Jr, Morris JG. Animal antibiotic use has an early but important impact on the emergence of antibiotic resistance in human commensal bacteria. Proc Natl Acad Sci. 2002;99:6434–6439. doi: 10.1073/pnas.082188899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Wang L, Piao XL, Kim SW, Piao XS, Shen YB, Lee HS. Effects of forsythia suspensa extract on growth performance, nutrient digestibility, and antioxidant activities in broiler chickens under high ambient temperature. Poult Sci. 2008;87:1287–1294. doi: 10.3382/ps.2008-00023. [DOI] [PubMed] [Google Scholar]
- Yang J, Li ZH, Huang L, Li JL, Wen R, Cui PW. Antimicrobial activity of the volatile oil from Valeriana officinalis L. Li Shizhen Med Mater Med Res. 2009;20:1651–1652. [Google Scholar]
- Zhang HY. The advanced research of Forsythia Suspensa on its chemical components and pharmacological activities. J Chin Med Mater. 2000;23:657–660. [Google Scholar]
- Zhou HY, Mineshita S. The effect of berberine chloride on experimental colitis in rats in vivo and in vitro. J Pharmacol Exp Ther. 2000;294:822–829. [PubMed] [Google Scholar]


