Abstract
The effects of dietary additives and holding time on resistance and resilience of broiler chickens to Clostridium perfringens challenge were investigated by offering four dietary treatments. These were a negative control (basal), a positive control (Zn-bacitracin) and two dietary additives, mannanoligosaccharides (MOS), and acidifier. Two holding times included (a) immediate access to feed and water post hatch (FED) and (b) access to both feed and water 48 h post hatch (HELD). Chicks fed Zn-bacitracin had no intestinal lesions attributed to necrotic enteritis (NE), whereas chicks fed both MOS or acidifier showed signs of NE related lesions. All dietary treatments were effective in reducing the numbers of C. perfringens in the ileum post challenge. The FED chicks had heavier body weight and numerically lower mortality. The FED chicks also showed stronger immune responses to NE challenge, showing enhanced (p<0.05) proliferation of T-cells. Early feeding of the MOS supplemented diet increased (p<0.05) IL-6 production. The relative bursa weight of the FED chicks was heavier at d 21 (p<0.05). All the additives increased the relative spleen weight of the HELD chicks at d 14 (p<0.05). The FED chicks had increased villus height and reduced crypt depth, and hence an increased villus/crypt ratio, especially in the jejunum at d 14 (p<0.05). The same was true for the HELD chicks given dietary additives (p<0.05). It may be concluded that the chicks with early access to dietary additives showed enhanced immune response and gut development, under C. perfringens challenge. The findings of this study shed light on managerial and nutritional strategies that could be used to prevent NE in the broiler industry without the use of in-feed antibiotics.
Keywords: Necrotic Enteritis, Early Feeding, Gut Morphology, Alternative to Antibiotics, Mannanoligosaccharides (MOS)
INTRODUCTION
Necrotic enteritis (NE) is a costly poultry disease caused by the bacterium Clostridium perfringens (Parish, 1961; Hofacre, 2001). The C. perfringens is found in the intestine of healthy chickens and the environment (Ficken and Wages, 1997), and it only causes NE when it grows unchecked and produces toxins (Al-Sheikhly and Truscott, 1977). Dietary and husbandry factors contribute to the outbreak of this disease, including a high level of viscous cereal grains (for example wheat, barley and oat) and animal protein (i.e., fish meal and meat meal) in the diets, and factors that cause damage to the intestinal mucosa (i.e., coccidiosis) (Branton et al., 1987; Kaldhusdal and Skjerve, 1996; Kaldhusdal, 2000). Stress factors, such as stocking density may also contribute the pathogenesis of the disease (McDevitt et al., 2006). However, the single most important factor believed to be responsible for the disease is a toxin, netB, produced by some isolates of C. perfringens (Keyburn et al., 2006).
Traditionally, antibiotics such as bacitracin, virginiamycin, avoparcin, lincomycin, tylosin, and penicillin, have been used as a successful means to control NE in broilers (Ficken and Wages, 1997; Williams, 2005). However, there is a worldwide trend that more and more chickens will be produced under an antibiotic-free environment in the future. Such a demand will require not only nutriceutical alternatives (organic acids, enzymes, probiotics, prebiotics, nucleotides, functional carbohydrates, betaine, spice and plant extracts, and so on) but also novel managerial and environmental measures. A hygienic environment will prevent the exposure of animals to potential pathogens, thus alleviating the pressure on their immune system. During the last two decades, early nutrition has been studied intensively. Early access to nutrients can improve the immunity and enhance the health status of chicks (Dibner et al., 1998). However, in practice, newly hatched chicks are held for a long time, sometimes more than 48 h without access to feed and water, due to processing at hatchery and transportation to farms (Noy and Sklan, 1999). Under some circumstances, chicks are held without access to nutrients deliberately in an attempt to minimise possible bacterial contamination of incompletely healed navels of young chicks and initiate a vaccine response (Dibner et al., 1998). Early feeding, either given access to feed and water to newly hatched birds, or injected in ovo has been demonstrated to be beneficial (Hornasio et al., 2011). When antibiotics are not included in the diet, a sound immune competence is of paramount importance in maintaining the health of animals. Chicken cytokines have been considered as another candidate for replacing in-feed antibiotics (Lowenthal et al., 1994) However, it is not known whether the use of feed additives either under normal feeding practices or in an early feeding regime will impact on IL-6 production.
This study investigated the effects of early nutrition and dietary additives, used solely or in combination, on the performance, immune competence and gut morphology in broilers under NE challenge.
MATERIALS AND METHODS
Experimental diets
A 4×2 factorial arrangement of treatments was used in this study. Four dietary treatments consisted of a negative control (basal diet, designated as “control”), positive control (Zn-bacitracin, designated as “Antibiotic”), mannan-oligosaccharides (MOS) (Bio-Mos®, Alltech Inc., USA), and MOS+acidifier (Acid-Pak 4-way, Alltech Inc., USA, designated as “acidifier”). The chicks were fed under two holding times, immediate access to both feed and water after hatch (FED) and 48 h delayed access to both feed and water post hatch (HELD). Bio-MOS and Acid-Pak 4-way were supplemented at a rate of 2.0 and 3.0 kg per tonne of feed, respectively.
All commercial products used in this study were kindly supplied by the manufacturers. Zn-bacitracin was purchased from a local feedstuff supplier (Ridley AgriProducts, Tamworth, NSW, Australia). The chicks were fed starter diets for 0 to 21 d and then finisher diets until d 42. All diets were wheat-SBM based and were cold-pelleted (60–75°C). The chicks were fed a high-protein diet containing 40% fish meal from d 8 to d 14 prior to the inoculation with C. perfringens, to trigger the outbreak of NE (Table 1).
Table 1.
Ingredient and composition (as-fed basis) of the basal starter, grower/finisher, and the high-protein diets
| Ingredient (g/kg) | Starter | Hi-protein starter | Grower/finisher |
|---|---|---|---|
| Wheat | 474.5 | 441.0 | 499.5 |
| Oats | 100.0 | 100.0 | 100.0 |
| Wheat offal | - | 40.0 | - |
| Rice pollard | 26.5 | - | 26.0 |
| Tallow | 35.0 | - | 62.0 |
| Soybean meal 48% | 190.0 | - | 150.0 |
| Meat-bone meal 50% | 80.0 | - | 75.0 |
| Peas | 75.0 | - | 72.0 |
| Fish meal | - | 400.0 | - |
| Limestone 38% | 5.0 | 5.0 | 4.0 |
| Sodium bicarbonate | 3.4 | 3.4 | 2.0 |
| Salt | 1.0 | 1.0 | 1.5 |
| Lysine-HCl | 2.2 | 2.2 | 2.3 |
| DL-methionine | 3.3 | 3.3 | 2.5 |
| L-threonine | 1.0 | 1.0 | 0.3 |
| Choline chloride | 0.6 | 0.6 | 0.4 |
| Vitamin/mineral premix1 | 2.0 | 2.0 | 2.0 |
| Xylanase2 | 0.5 | 0.5 | 0.5 |
| Nutrients | ———Calculated value——— | ||
| Protein | 225.0 | 330.0 | 205.0 |
| ME (MJ/kg) | 12.6 | 12.1 | 13.4 |
| Lysine | 12.0 | 24.1 | 10.9 |
| Met+cys | 9.3 | 14.7 | 8.6 |
| Calcium | 9.8 | 18.5 | 9.0 |
| Available P | 4.8 | 13.4 | 4.6 |
Provided per kg of diet: vitamin A (as all-trans retinol) 3.03 mg, cholecalciferol 0.09 mg; vitamin E (as d-α-tocopherol) 20 mg; vitamin K3 2 mg; thiamine 2 mg; riboflavin 6 mg; pyridoxine hydrochloride 5 mg; vitamin B12 0.2 mg; biotin 0.1 mg; niacin 50 mg; D-calcium pantothenate 12 mg; folic acid 2 mg; Mn 80 mg; Fe 60 mg; Zn 80 mg; Cu 8 mg; I 1 mg; Co 0.3 mg; Se 0.1 mg; Mo 1 mg (obtained from Ridley AgriProducts, Tamworth, New South Wales, Australia).
Allzyme PT, Alltech Inc., USA.
Bird management
Four hundred (400) male Cobb 500 broiler chicks were obtained from a local hatchery, with a special arrangement to take chicks out of hatchery within 2 h of hatch. The chicks were reared in floor pens in groups of 50 chicks per pen in the same environmentally controlled facility. All the chicks were wing-tagged and weighed. Half the chicks were given access to feed and water immediately after they were weighed, while the other half was held in chicken boxes at 33°C for 48 h to mimic the condition in incubator prior to giving access to feed and water.
On d 9 (five days before C. perfringens inoculation), all chicks were given a one-off dose of 1 ml Eimeria inoculation containing 4,500 E. maxima, 6,000 E. tenella and 10,000 E. acervulina (obtained from the Animal Research Institute, Queensland Department of Primary Industries and Fisheries, Brisbane, Australia). On days 14, 15 and 16 all the chicks were orally infected with 2 ml C. perfringens culture (approx. 108 CFU/ml) in a thioglycolate broth (Oxoid, UK). The C. perfringens inoculant was prepared using a subculture of a C. perfringens field isolate of C. perfringens type A (obtained from the CSIRO, Geelong, Victoria, Australia). Necropsies of all dead chicks from d 14 onwards were conducted to determine the cause of death. Mortality associated with lesions of confluent necrosis or sloughing of the epithelial lining of the intestinal tract was considered to be caused by NE (Helmboldt and Bryant, 1971). For chicks died as a result of NE, intestinal swab samples from the infected area were taken and plated onto C. perfringens agar (Oxoid, UK). Total mortality and mortality due to NE were recorded daily.
The experiment was approved by the Animal Ethics Committee of the University of New England. Health and animal husbandry practices complied with the “Australian Code of Practice for the Care and Use of Animals for Scientific Purposes” issued by the Australian Government (National Health and Medical Research Council, 2004).
Live weight, feed intake and mortality
Body weight and feed intake on a cage basis were recorded weekly for calculation of weight gain during the entire experimental period. Mortality was recorded daily.
C. perfringens counts
On days 17 and 20, four chicks from each treatment group were randomly selected, weighed, and killed by cervical dislocation. The numbers of C. perfringens (CFU/g digesta) in the ileum were measured by plating onto C. perfringens agar plate.
NE lesion scores
On days 17 and 20, the small intestine from each killed bird was incised longitudinally and examined for evidence of gross NE lesion (0 = none, 1 = mild, 2 = moderate, 3 = marked/severe).
Lymphoid organ development
On sample collection days, the bursa of Fabricius and spleen were removed and weighed. The weights were expressed as a percentage of body weight.
Chicken interleukin-6 bioassay
Production of Chicken interleukin-6 (ChIL-6) was investigated using a murine IL-6 7TD1 bioassay as described by van Snick et al. (1986) with some minor modifications. The bioassay of ChIL-6 activity was conducted at the Australian Animal Health Laboratory (AAHL), CSIRO Livestock Industries, Geelong, Victoria, Australia.
Approximately 5 ml of blood were taken from each bird by wing bleeding on d 26, 10 days post infection (p.i.), using 5 ml vacutainers containing Lithium Heparin (Becton Dickinson, NJ 07417, USA). The blood samples were stored in room temperature and analysed within 24 h. The IL-6 secretion was expressed by titres (U/ml). A titre is defined as the reciprocal of the lowest dilution of a sample that gives half the maximum response. Maximum response equals the maximum count minus the background count.
Chicken T-cell proliferation assay
The same blood samples used for ChIL-6 bioassay were used for T-cell proliferation assay as described by Lowenthal et al. (1994). This assay was also conducted at the AAHL, CSIRO Livestock Industries, Geelong, Victoria, Australia. A mitogen called Concanavalin A (ConA, Sigma, Israel) was used to stimulate the proliferation of the T-cells. Two concentrations of ConA, 50 μg/ml and 10 μg/ml, were used in the assay and made by adding ConA into DMEM medium. DMEM medium was designated as 0μg/mL and used as a negative control. For each sample the assay was conducted in triplicate. An aliquot of 200 μl of each medium, 0 μg/ml, 10 μg/ml and 50 μg/ml, were pipetted into pre-labelled 96-well plates (Nunc, Kamstrup, Denmark) and 5 μl of whole blood was added to each well. The plates were gently mixed using a Dynatech Micro Shaker (IKA® Works, Selangor, Malaysia) and then placed in a humidified cell incubator at 37°C with 5% CO2 (Forma Scientific, USA) for three days. On day three, the wells were pulsed with 25 μl of 1:50 dilution of 3H thymidine and mixed gently. The plates were incubated for 18 h. On day four, the plates were removed and shaken again on a micro shaker for approximately three min to ensure that the cells were suspended in solution. The cells were harvested onto glass fibre filter mats (Wallac, Finland) using a plate harvester (Wallac Harvester 96 Mach III M, Tomtec, USA), saturated with scintillant fluid (Wallac Betaplate Scint, Wallac, Finland), and the radioactivity was measured (MicroBeta 1450 Trilux Liquid Scintillantion and Luminescence Counter, Wallac, Finland). The ability of T-cells to proliferate is expressed by Stimulation Index (SI) relative to cells cultured in the absence of mitogen.
Gut morphology
On sampling days, four chicks from each treatment group were killed by cervical dislocation. The body cavity was opened, and 20 mm segments from the jejunum (20 mm distal to the point of entry of the bile ducts) and the ileum (20 mm proximal to the ileo-caecal junction) were removed for morphometric analyses. The intestinal samples were flushed clean with phosphate buffered saline (PBS, pH 7.4) and fixed in 10% neutral buffered formalin solution for at least 24 h. The fixed tissue samples were processed in an automatic tissue processor (TOSCO, Thomas Optical and Scientific Co., Melbourne and Sydney, Australia) and embedded in paraffin using a Histo Embedding Centre (Leica EG 1160, Leica Microsystems, Bensheim, D-64625, Germany). Embedded samples were subsequently sectioned sagittally with a Rotary Microtome (Leitz 1516, Leica Microsystems, Bensheim, D-64625, Germany) at 5 μm. The tissue sections on the slides were stained using Harris’s haematoxylin (Gurr’s, UK) and eosin Y (Chroma, Germany) stains and mounted with DPX Mountant for histology (Aldrich Chemical Company, Inc., Milwaukee, WI 53255, USA). Morphometric indices were determined using computer-aided light microscope image analyses (SPOT 3.1, Diagnostic Instruments, Inc., Sterling Heights, MI 48314, USA) as described by Bird et al. (1994). The morphometric variables analysed included: villus length (from the tip of the villus to muscularis mucosa), villus height using the lamina propria as the base (from the tip of the villus to the villus crypt junction), villus width at half height, crypt depth as the depth of the invagination between adjacent villi, and thickness of the muscularis (the external muscle layer) and muscularis mucosa. Villus area was calculated from the villus height and width at half height. Values are means from 12 different villi and only vertically oriented villi and crypts were measured (Uni et al., 1999).
Statistical analysis
All data were analysed using Statgraphics software (Manugistics, Inc., 2000). ANOVA (by the general linear model procedure) was used to determine the significance of the main effects and interactions. Duncan’s multiple-range test was used to separate means when significant effects (p<0.05) were detected by multifactorial analyses of variance (Duncan, 1955).
RESULTS
Growth performance
Growth performance of chicks before and after C. perfringens challenge is presented in Table 2. FED chicks had higher (p<0.001) body weight throughout the experiment. Chicks given the MOS and acidifier both had heavier (p<0.001) body weight at d 14 than the antibiotic-supplemented chicks and the control chicks (before C. perfringens challenge). Furthermore, the HELD chicks given antibiotic had lighter (p<0.001) body weight than the control chicks at d 14.
Table 2.
Effects of post hatch holding time and dietary supplements on body weight at d 7, 14 and 211
| Body weight (g) | |||
|---|---|---|---|
|
|
|||
| d 7 | d 14 | d 21 | |
| FED | |||
| Control | 114.2ab | 302.4b | 662.4b |
| Antibiotic | 111.2b | 305.9b | 804.9a |
| MOS | 117.0a | 321.6a | 685.2b |
| Acidifier | 116.3ab | 317.3a | 707.5b |
| HELD | |||
| Control | 87.5d | 254.0d | 583.3c |
| Antibiotic | 77.4e | 233.7e | 654.6b |
| MOS | 87.6d | 259.0cd | 537.5c |
| Acidifier | 93.7c | 276.5c | 586.6c |
| SEM | 1.8 | 6.7 | 18.9 |
| ———Significance of treatment effect2——— | |||
| Diet | *** | *** | *** |
| Holding time | *** | *** | *** |
| Diet×holding time | * | NS | *** |
Values are means of 20 replicates.
NS = Not significant;
p<0.05;
p<0.01;
p<0.001.
Means within a column without a common superscript differ significantly.
The HELD chicks lost 12.3% of their initial body weight during the first 48 h post hatch, while the FED chicks gained 25.6% of the initial body weight during the same period. A higher relative growth occurred in HELD chicks immediately after the chicks were given access to nutrients from d 3 to d 28 (p<0.001) (Table 3). However, the growth rate of FED chicks became greater during week 5 (p<0.001). Chicks given dietary supplements had higher body growth during the first two weeks before C. perfringens infection (p<0.001). The chicks given the antibiotic exhibited higher body growth from second week post hatch (p<0.001). During wk 3, when clinical signs of NE occurred, only the chicks given the antibiotic-supplemented diet had a greater growth rate, compared to the other treatment groups (p<0.001).
Table 3.
Effects of post hatch holding time and dietary supplements on relative growth rate of chicks at d 3, 7, 14, 21, 28 and 351
| Treatment | Relative growth (%)A | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| 0–2 d | 3–7 d | 8–14 d | 15–21 d | 22–28 d | 29–35 d | |
| FED | ||||||
| Control | 26.5ab | 92c | 166d | 127b | 76b | 55a |
| Antibiotic | 19.5b | 96c | 176c | 166a | 70c | 52ab |
| MOS | 30.1a | 90c | 173c | 122b | 76b | 53ab |
| Acidifier | 28.4a | 93c | 175c | 125b | 74bc | 53ab |
| HELD | ||||||
| Control | −12.22 | 115b | 184b | 128b | 84a | 47b |
| Antibiotic | 95c | 204a | 190a | 84a | 43c | |
| MOS | 115b | 197ab | 119b | 83a | 46b | |
| Acidifier | 132a | 194ab | 114b | 77b | 33d | |
| SEM | 1.3 | 3 | 5 | 7 | 3 | 2 |
| ———Significance of treatment effect3——— | ||||||
| Diet | *** | *** | ** | *** | ** | *** |
| Holding time | *** | *** | *** | NS | *** | *** |
| Diet×holding time | *** | NS | NS | NS | ** | |
Values are means of 20 replicates.
Value is mean of HELD chicks 48 h post hatch.
NS = Nnot significant;
p<0.05;
p<0.01;
p<0.001.
Means within a column without a common superscript differ significantly.
Weight gain relative to initial body weight at d 0.
The NE-related and non-NE related mortality figures for the HELD chicks were 14.6 and 6.9%, respectively, and both were numerically higher than that of the FED chicks, which were 12.2 and 2.8%, respectively.
C. perfringens counts and necrotic enteritis lesion scores
Only the antibiotic offered a complete protection against NE (Table 4). There seemed to have an interaction between early nutrition and dietary treatments on the C. perfringens counts for chicks at d 17 (3 d after challenge), with the FED chicks given MOS and acidifier having numerically lower C. perfringens counts in the ileum.
Table 4.
Effects of post hatch holding time and dietary supplements on NE lesion scores and C. perfringens counts 3 and 6 days after challenge1
| d-17 (3 d after challenge) | d-20 (6 d after challenge) | |||
|---|---|---|---|---|
|
|
|
|||
| NE lesion scores | C. perfringens counts (105 CFU/g) | NE lesion scores | C. perfringens counts (105 CFU/g) | |
| FED | ||||
| Control | 2.17a | 6.38a | 1.38a | 0.98a |
| Antibiotic | 0.00c | 0.00b | 0.13bc | 0.00b |
| MOS | 1.88a | 1.20ab | 0.75ab | 0.68ab |
| Acidifier | 1.88a | 1.44ab | 0.75ab | 0.33ab |
| HELD | ||||
| Control | 2.00a | 1.24ab | 0.75ab | 0.03b |
| Antibiotic | 0.25bc | 0.00b | 0.00c | 0.00b |
| MOS | 1.63ab | 2.58ab | 1.75a | 1.10a |
| Acidifier | 2.38a | 2.56ab | 1.00ab | 0.30ab |
| SEM | 0.53 | 1.84 | 0.30 | 0.31 |
| ———Significance of treatment effect2——— | ||||
| Diet | *** | NS | ** | NS |
| Holding time | NS | NS | NS | NS |
| Diet×holding time | NS | NS | NS | NS |
Values are means of 4 replicates for each treatment group.
NS = Not significant;
p<0.05;
p<0.01;
p<0.001.
Means within a column without a common superscript differ significantly.
Lymphoid organs and immune responses
As shown in Table 5, the FED chicks had a significantly heavier relative bursa weight at d 21 (p<0.05). MOS supplementation tended to increase the spleen weight at d 14 (p = 0.056). There was an interaction between early nutrition and dietary treatments on IL-6 production (p<0.05), with the FED chicks given MOS producing more IL-6 than other treatments (Figure 1). Early nutrition had no effect on IL-6 production in birds given the control, antibiotic and acidifier diets. T-cells proliferation was enhanced by early access to nutrients (p<0.001) (Figure 2). In the FED chicks, both MOS and acidifier reduced T-cell proliferation, compared to antibiotic (p<0.05).
Table 5.
Effects of post hatch holding time and dietary supplements on lymphoid organ weights as a percentage of body weight at d 14 and 21 1
| d 14 | d 21 | |||
|---|---|---|---|---|
|
|
|
|||
| Bursa/BW (g/kg) | Spleen/BW (g/kg) | Bursa/BW (g/kg) | Spleen/BW (g/kg) | |
| FED | ||||
| Control | 2.307 | 0.863abc | 2.182ab | 1.167ab |
| Antibiotic | 1.830 | 0.957abc | 2.340ab | 1.060ab |
| MOS | 1.713 | 1.200a | 2.953a | 0.890ab |
| Acidifier | 1.890 | 0.710bc | 2.237ab | 1.260a |
| HELD | ||||
| Control | 1.897 | 0.590c | 1.947b | 0.975ab |
| Antibiotic | 1.723 | 1.083ab | 2.367ab | 1.025ab |
| MOS | 1.790 | 0.960abc | 1.932b | 0.967ab |
| Acidifier | 1.713 | 0.963abc | 2.262ab | 0.790b |
| SEM | 0.254 | 0.120 | 0.281 | 0.123 |
| ———Significance of treatment effect2——— | ||||
| Diet | NS | NS | NS | NS |
| Holding time | NS | NS | * | NS |
| Diet×holding time | NS | NS | NS | NS |
Values are means of 4 replicates.
NS = Not significant;
p<0.05;
p<0.01;
p<0.001.
Means within a column without a common superscript differ significantly.
Figure 1.
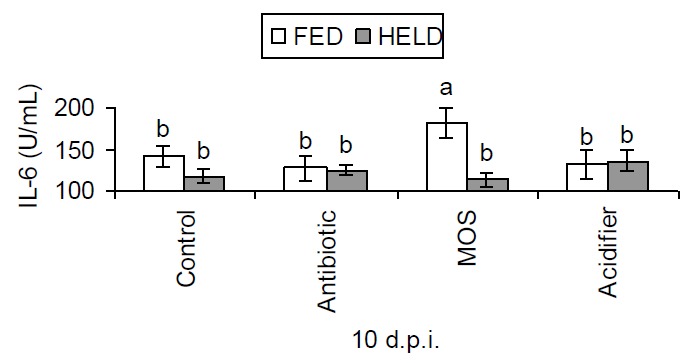
Effects of post hatch holding time and dietary supplements on Chicken IL-6 production 10 days post infection (d.p.i.) (Mean values, n = 6; error bars indicate SEM; bars without a common superscript differ significantly).
Figure 2.
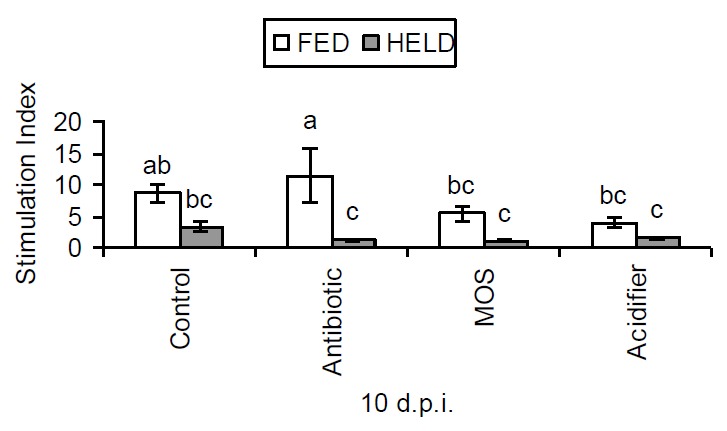
Effects of post hatch holding time and dietary supplements on T-cell proliferation of broilers 10 days post infection (d.p.i.) when stimulated with different levels of Con A, expressed by Stimulation Index (Mean values, n = 6; error bars indicate SEM; bars without a common superscript differ significantly).
Gut morphology
The morphology of intestinal segments was studied before and after the C. perfringens challenge, and the data are presented in Table 6 and 7. At d 14, before C. perfringens challenge, the FED chicks regardless of dietary treatment and the HELD chicks given dietary supplements had shallower crypts and greater villus/crypt ratio in the jejunum (p<0.01). Dietary supplements also increased villus height in the jejunum (p<0.001), but not for FED chicks given the MOS or the HELD bird given the acidifier. Early feeding and dietary supplements had a similar effect on the ileal segment, albeit less apparent. The HELD chicks had thicker submucosa in both the jejunum and ileum (p<0.001). Early feeding increased villus height in the jejunum (p<0.05), but not in the ileum.
Table 6.
Effects of post hatch holding time and dietary supplements on the morphology of small intestine at d 141
| Jejunum | Ileum | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Submucosa (μm) | Villus height (μm) | Crypt depth (μm) | Villus/crypt ratio | Submucosa (μm) | Villus height (μm) | Crypt depth (μm) | Villus/crypt ratio | |
| FED | ||||||||
| Control | 16.9bc | 1,240.2b | 195.6ab | 6.7a | 20.0bcd | 555.6b | 164.9ab | 3.5b |
| Antibiotic | 15.2c | 1,221.4b | 182.6bc | 7.1a | 22.3abc | 685.6a | 186.8a | 4.0ab |
| MOS | 16.5bc | 1,069.6c | 158.0c | 7.3a | 18.5d | 577.8b | 147.3b | 4.1ab |
| Acidifier | 17.7ab | 1,366.7a | 187.8bc | 7.4a | 23.0abc | 684.2a | 162.6ab | 4.4a |
| HELD | ||||||||
| Control | 20.2a | 1,059.5c | 225.0a | 4.9b | 25.2a | 605.0b | 190.4a | 3.5ab |
| Antibiotic | 19.5a | 1,241.9b | 203.4ab | 6.4a | 23.7ab | 684.9a | 189.7a | 3.7ab |
| MOS | 17.9ab | 1,219.2b | 180.5bc | 7.2a | 23.6ab | 686.2a | 178.9a | 3.9ab |
| Acidifier | 18.9ab | 1,166.8bc | 178.9bc | 7.1a | 19.3cd | 592.7b | 166.8ab | 3.9ab |
| SEM | 0.8 | 36.8 | 10.3 | 0.4 | 1.2 | 18.5 | 10.1 | 0.3 |
| ———Probability of greater F value in analysis of variance2——— | ||||||||
| Diet | NS | *** | *** | ** | NS | *** | * | NS |
| Holding time | *** | * | * | * | * | NS | * | NS |
| Diet×holding time | NS | *** | NS | NS | *** | *** | NS | NS |
Values are means of 12 replicates.
NS = Not significant;
p<0.05;
p<0.01;
p<0.001.
Means within a column without a common superscript differ significantly.
Table 7.
Effects of post hatch holding time and dietary supplements on the morphology of small intestine at d 211
| Jejunum | Ileum | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Submucosa (μm) | Villus height (μm) | Crypt depth (μm) | Villus/crypt ratio | Submucosa (μm) | Villus height (μm) | Crypt depth (μm) | Villus/crypt ratio | |
| FED | ||||||||
| Control | 30.0a | 1,417.9ab | 280.5a | 5.14e | 28.8a | 893.2a | 198.3a | 4.81c |
| Antibiotic | 23.2bc | 1,542.8a | 200.4bcd | 8.93b | 25.8abc | 772.2bc | 102.0b | 8.04ab |
| MOS | 21.2cde | 1,239.6c | 174.9cd | 7.39c | 24.4abc | 782.4b | 103.6b | 8.23ab |
| Acidifier | 26.6ab | 1,418.7ab | 227.1abc | 6.61cd | 28.9a | 890.4a | 116.7b | 7.65b |
| HELD | ||||||||
| Control | 19.0de | 1,321.3bc | 189.8bcd | 7.14c | 26.4ab | 803.5ab | 108.3b | 7.73b |
| Antibiotic | 18.6e | 1,435.7ab | 135.0d | 10.88a | 21.7c | 694.5c | 79.1c | 9.33a |
| MOS | 19.7cde | 1,062.9d | 264.1ab | 5.32de | 21.8c | 835.1ab | 115.9b | 7.34b |
| Acidifier | 22.8bcd | 1,566.2a | 262.1ab | 6.12cde | 23.9bc | 893.3a | 114.6b | 8.05ab |
| SEM | 1.3 | 59.6 | 24.6 | 0.45 | 1.4 | 29.2 | 6.4 | 0.49 |
| ———Probability of greater F value in analysis of variance2——— | ||||||||
| Diet | *** | *** | * | *** | ** | *** | *** | *** |
| Holding time | *** | NS | NS | NS | *** | NS | *** | ** |
| Diet×holding time | ** | * | *** | *** | NS | * | *** | *** |
Values are means of 12 replicates.
NS = Not significant;
p<0.05;
p<0.01;
p<0.001.
Means within a column without a common superscript differ significantly.
At d 21, only the FED chicks given dietary supplements had thinner crypts and greater villus/crypt ratio in the jejunum and ileum (p<0.001). In the HELD chicks, the antibiotic and acidifier gave greater villus height, but only the antibiotic gave shallower crypts and higher villus/crypt ratio in the jejunum (p<0.001). Again in the HELD chicks, the acidifier increased villus height, while the antibiotic reduced it in the ileum (p<0.001). The FED chicks given dietary supplements had shallower crypts in the ileum (p<0.001), while in the HELD chicks, only the antibiotic had such an effect (p<0.001).
DISCUSSION
The current NE model was designed to mimic real-life situation where mortality from NE should be moderate and performance of unaffected chicks relevant to commercial standard (Wu et al., 2010). In the current study, only the antibiotic, Zn-bacitracin, offered a complete protection against the outbreak of the disease. The birds used in the study performed to commercial standard, suggesting that there were no lingering health problems with the chicks. Some of the feed additives as well as early nutrition were helpful in preventing performance loss through effects on gut development and immunity. These effects are detailed in the following sections.
Growth performance
As growth rate increases and market age decreases, the percentage of time a broiler bird spends as a neonate increases (De Beer et al., 2011). The impact of nutrition at an early age on the chicks has been studied intensively. It is now well accepted that early access to nutrients post hatch has a major impact on chicks’ both immediate and long-term development. Early growth is directly correlated with the final body weight and feed efficiency. Heavier body weight on placement usually results in heavier body weight at marketing (Wilson, 1991). Research in early life of chickens has led to the conclusion that the immediate post hatch period is critical for the development of all major organs in chicks (Uni, 1998; Hornasio et al., 2011). Early feeding has been reported to enhance the body growth, uniformity and health status of the chicks (Moran, 1990; Casteel et al., 1994; Sklan et al., 2000).
In this study, body weight of the FED chicks increased by 25.6% compared with their initial body weight during the first 48 h post hatch, while the HELD chicks lost 12.3% of the initial body weight during the same period. The HELD chicks demonstrated superior growth relative to body weight from d 3 to d 14, before the C. perfringens infection. However, after the chicks were given C. perfringens challenge, the FED chicks seemed to be better able to grow than the HELD chicks. This was not the case in a previous study (Ao and Choct, 2003) where the chicks were reared under a higher standard of hygiene without NE challenge, suggesting an interaction between early feeding and disease challenge. Thus delayed access to nutrients up to 48h post hatch may have caused a long-term compromise on the competence of chicks to resist disease challenge and to gain compensatory growth. Both the MOS and acidifier used in the current study failed to prevent the outbreak of NE. This is of practical importance because under commercial production systems, broiler chickens face many stressors like delayed access to feed, dirty litter and a frequent change of diets. Therefore it might be worthwhile to consider early feeding strategies, in combination with good environmental management, where antibiotics are not allowed in feed.
NE lesion scores and C. perfringens counts
Feed additives such as acidifiers, enzymes, probiotics and prebiotics have all been reported to inhibit the pathogen colonisation of the intestine and subsequently reduce the incidence of some diseases, such as NE, with varying levels of success (Corrier et al., 1995; Thompson and Hinton, 1997; Finuance et al., 1999; Kaldhusdal, 2000). Whilst some of these additives are claimed to have a direct effect on the organisms themselves, others are deemed to have a more indirect impact, such as enhancing immunity. It is reported frequently that early access to nutrients post hatch benefit the health status of chicks through aiding the immune system. But it was not known whether birds with a stronger immune system would be better able to resist disease, for example NE. In the current study, the HELD chicks had numerically higher NE scores and C. perfringens counts in the intestine, especially three days after C. perfringens challenge. These results appeared to indicate that under the condition of this study, early feeding offered a degree of protection even though the reduction of NE lesion scores and C. perfringens counts was not statistically significant.
Lymphoid organs
The protective immunity of chicks plays an important role in preventing infectious diseases, especially when in-feed antibiotics are not used. The first week post hatch is a critical period and the nutritional status of chicks can impact the immune system (Klasing, 1998). The bursa of Fabricius, a primary immune organ, plays a major role in creating antibody diversity (Uni, 1998), and inhibition of bursal development was found to result in the failure of normal development of the spleen, the secondary immune organ (Glick, 1967). The bursa and spleen were both found to be responsive to environmental stress of chicks at an early age (Wyatt et al., 1986). Dibner et al. (1998) found that chicks had early access to feed and water show a higher relative bursa weight, earlier appearance of germinal centres, and better disease resistance than their HELD hatch mates. This is also supported by the data of Wyatt et al. (1986) who found that broiler chicks held in incubators for 30 h without access to feed and water before placement showed significantly lower bursa and spleen weights. This suggests that early stress to young broiler chicks, such as holding without feed and water post hatch, alters the immune capabilities and growth rate later in their life. In the current study, under a challenged environment, the FED chicks given the MOS diet had heavier bursas. This appears to suggest that when chicks are exposed to certain environmental stressors, early feeding and certain dietary additives might have a greater ability to modulate the development of these lymphoid organs and enhance the immune competence of the bird.
Immune responses
The immune response to infection is controlled by a complex interplay between various cytokines (Kelso and Metcalf, 1990). IL-6 is a multifunctional cytokine that plays a major role in regulating immune responses, acute phase reactions and haematopoiesis (Hirano et al., 1986). IL-6 is produced by many different cell types and acts on B-cells, T-cells, hepatocytes, haematopoietic progenitor cells and cells of the central nervous system, immediately following infection or vaccination (Hirano et al., 1986; Gauldie et al., 1987; Ikebuchi et al., 1987).
Since T-cells are the major source of cytokines, the ability of these cells to proliferate in response to their mitogens has been used to determine the development of the immune responses of chickens (Lowenthal et al., 1994). Studies conducted by these authors suggested that susceptibility of newly hatched chicks to infection is due to a period of transient T-cell unresponsiveness to immune stimulation, a deficiency that may be amenable to cytokine therapy. In this study, only the FED chicks given MOS diet had elevated IL-6 production. This indicates that there is an interaction between early nutrition and dietary supplements. Early administration of MOS might augment the production of IL-6 under challenged conditions. In the current study, early feeding significantly increased T-cell proliferation. Interestingly, the T-cells of the chicks given the control or the antibiotic diet had a significantly enhanced ability to proliferate, while all the other additives had the opposite effects. Such an effect has not been demonstrated elsewhere. The mechanism by which this occurs is not understood. This result, together with the data on IL-6 production, suggests that early access to nutrients post hatch might enhance the immune responses to infectious challenge.
Gut morphology
Hydrolysis of macromolecules in the small intestine is achieved, to a large extent, by pancreatic enzyme activities, which are correlated with body weight and intestinal weight (Sklan and Noy, 2000). Undoubtedly early access to nutrients stimulates the gastro-intestinal tract (GIT) activity (Uni et al., 1998; Noy and Sklan, 1999). It was reported that the GIT increases in size and weight more rapidly in relation to body weight, during early post hatching period, than other organs and tissues of chickens (Lilja, 1983; Katanbaf et al., 1988). The preferential early growth of the small intestine occurs both in the presence and absence of the feed, although in the absence of exogenous feed both the absolute and the relative growth rates are lower (Noy and Sklan, 1999). The intestine is also the largest immunological organ and contains a large number of lymphocytes, most of them are T-cells located predominantly in the Lamina propria (Perdigon et al., 1991).
The interaction between the microflora and the morphology of the intestinal wall is clearly shown by alteration in the structure and morphology of the GIT of germ-free animals compared with that in conventional animals (Heneghan, 1965). The small intestine villi of germ-free species are usually slender and uniform in shape, whereas crypts are shorter (Gordon and Bruckner-Kardoss, 1961). Cook and Bird (1973) associated the presence of pathogenic micro-organisms with a change in the intestinal wall and in the surface area for nutrient absorption. They demonstrated a shortening of the villi and a decrease in their epithelial layers when counts of pathogenic bacteria increased, in company with a deeper crypt. Schneeman (1982) suggests that shorter villi relative to crypt depths result in less absorptive and more secretory cells. This change in the intestinal morphology due to pathogenic organisms is more pronounced in the upper than in the lower parts of the intestinal tract. It was reported that Coliform bacteria (Truscott and Al-Sheikhly, 1977) and Clostridia (Kaldhusdal and Hofshagen, 1992) in the intestinal tract cause damage to the mucosal layer of the intestine of broiler chicks. Based on these results it is possible that morphological changes in the intestinal wall are indicative of a disturbance in the balance between non-pathogenic and pathogenic micro-organisms. In addition, research showed that chicks with immediate access to feed and water post hatch have increased villus height and surface area of the small intestine, and enhanced crypt development (Uni et al., 1998). The length of villi within the jejunum and ileum of chicks receiving an amylase-supplemented corn-soy based diet was significantly increased, with a concomitant improvement in growth rate (Ritz et al., 1995). This might suggest an increased absorptive area capable of greater absorption of available nutrients (Caspary, 1992).
In the current study, when the chicks were 14 days old, just before the C. perfringens infection, all the dietary supplements tended to increase villus height and reduce crypt depth in the jejunum, thus increasing the villus height/crypt depth ratio. This was particularly obvious in HELD chicks. On d 21, seven days after the initial C. perfringens challenge, the FED chicks given the supplements still showed reduced jejunal crypt depth and increased villus/crypt ratio; while in the HELD chicks, the supplements increased the crypt depth and reduced the villus/crypt ratio. The morphology of the ileum followed a similar trend, although less pronounced. The results on the intestinal morphology suggest that under unchallenged conditions, both dietary supplements and early access to nutrients may enhance the absorptive capacity and reduce the secretion in the small intestine. But under pathogenic challenge, early feeding and dietary supplements gave the FED chicks reduced crypt depth and thus increased villus/crypt ratio, but not the HELD chicks, indicated the gut microflora community might be disturbed for HELD chicks given the supplements.
The present study indicated that the supplementation of MOS or acidifier to broiler diets enhanced the absorptive surface area of the small intestine, and improved the growth performance of chicks before C. perfringens challenge. However, neither of these additives gave the chicks the same degree of protection against C. perfringens infection as an antibiotic did.
Early access to certain supplements post hatch, such as MOS and acidifier, improved the development of intestinal morphology and immune competency of the chicks, thus enhanced the absorptive potential and the immune response of chicks under pathogenic infection.
In conclusion, under challenged condition, chicks with early access to feed and water exhibit superior disease resistance and growth performance through enhanced immunity and intestinal development.
ACKNOWLEDGEMENTS
The authors would like to thank Mark Porter, Shuyu Song, Annette McLeod from the University of New England, and Mary Broadway from CSIRO Livestock Industries for their technical support. The valuable comments from Dr. John Lowenthal (CSIRO Livestock Industries), Professor Steven Walkden-Brown (UNE), the late Professor David Sklan and Dr. Yael Noy (Hebrew University of Jerusalem, Israel) are also gratefully acknowledged. The University of New England and Alltech Biotechnology Australia Pty Ltd provided a scholarship to Mr. Z. Ao during his PhD study.
REFERENCES
- Al-Sheikhly, Truscott RB. The interaction of Clostridium perfringens and its toxins in the production of necrotic enteritis of chickens. Avian Dis. 1977;21:256–263. [PubMed] [Google Scholar]
- Ao Z, Choct M. Early nutrition for broilers-A two edged sword? Aust Poult Sci Symp. 2003;15:149–153. [Google Scholar]
- Bird AR, Croom WJ, Jr, Fan YK, Daniel LR, Black BL, McBride BW, Eisen EJ, Bull LS, Taylor LL. Jejunal glucose absorption is enhanced by epidermal growth factor in mice. J Nutr. 1994;124:231–240. doi: 10.1093/jn/124.2.231. [DOI] [PubMed] [Google Scholar]
- Branton SL, Reece FN, Hagler WM., Jr Influence of a wheat diet on mortality of broiler chickens associated with necrotic enteritis. Poult Sci. 1987;66:1326–1330. doi: 10.3382/ps.0661326. [DOI] [PubMed] [Google Scholar]
- Caspary WF. Physiology and pathophysiology of intestinal absorption. Am J Clin Nutr. 1992;55:299–308. doi: 10.1093/ajcn/55.1.299s. [DOI] [PubMed] [Google Scholar]
- Casteel ET, Wilson JL, Buhr RJ, Sander JE. The influence of extended posthatch holding time and placement density on broiler performance. Poult Sci. 1994;73:1679–1684. doi: 10.3382/ps.0731679. [DOI] [PubMed] [Google Scholar]
- Cook RH, Bird FH. Duodenal villus area and epithelial cellular migration in conventional and germ-free chicks. Poult Sci. 1973;52:2276–2280. doi: 10.3382/ps.0522276. [DOI] [PubMed] [Google Scholar]
- Corrier DE, Nisbet DJ, canlan CM, Hollister AG, Caldwell DJ, Thomas LA, Hargis BM, Tomkins T, Deloach JR. Treatment of commercial broiler chickens with a characterized culture of cecal bacteria to reduce Salmonella colonisation. Poult Sci. 1995;74:1093–1101. doi: 10.3382/ps.0741093. [DOI] [PubMed] [Google Scholar]
- De Beer M, Elfick D, Emmerson DA. Is a feed conversion ratio of 1:1 a realistic and appropriate goal for broiler chickens in the next 10 years? In: Cronje P, editor. Recent Advances in Animal Nutrition-Australia. University of New England; Armidale, Australia: 2011. pp. 9–14. [Google Scholar]
- Dibner JJ, Knight CD, Kitchell ML, Atwell CA, Downs AC, Ivey FJ. Early feeding and development of the immune system in neonatal poultry. J App Poult Res. 1998;7:425–436. [Google Scholar]
- Duncan DB. Multiple range test and multiple F-tests. Biometrics. 1955;11:1–42. [Google Scholar]
- Ficken MD, Wages DP. Necrotic enteritis. In: Calnek BW, editor. Diseases of Poultry. Iowa State University Press; 1997. pp. 261–264. [Google Scholar]
- Finucane M, Spring P, Newman KE. Incidence of mannose sensitive adhesins in enteric bacteria. Poult Sci. 1999;78:139. [Google Scholar]
- Gauldie J, Richards C, Harnish D, Lansdorp P, Baumann H. Interferon beta 2/B-cell stimulatory factor type 2 shares identity with monocyte-derived hepatocyte-stimulating factor and regulates the major acute phase protein response in liver cells. Proc Nat Acad Sci USA. 1987;84:7251–7255. doi: 10.1073/pnas.84.20.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick B. Antibody and gland studies in cortisone and ACTH-injected birds. J Immunol. 1967;98:1076–1084. [PubMed] [Google Scholar]
- Gordon HA, Bruckner-Kardoss E. Effect of normal microbial flora on intestinal surface area. Am J Physiol. 1961;201:175–178. doi: 10.1152/ajplegacy.1961.201.1.175. [DOI] [PubMed] [Google Scholar]
- Helmboldt CF, Bryant ES. The pathology of necrotic enteritis in domestic fowl. Avian Dis. 1971;15:775–780. [PubMed] [Google Scholar]
- Heneghan JB. Imbalance of the normal microbial flora: The germfree alimentary tract. Am J Digest Dis. 1965;10:864–869. doi: 10.1007/BF02236095. [DOI] [PubMed] [Google Scholar]
- Hirano T, Yasukawa K, Harada H, Taga T, Watanabe Y, Matsuda T, Kashiwamura S, Nakajima K, Koyama K, Iwamatsu A, Tsunasawa S, Sakiyama F, Matsui H, Takahara Y, Taniguchi T, Kishimoto T. Complementary DNA for novel human interleukin (BSF-2) that induces lymphocytes-B to produce immunoglobulin. Nature. 1986;324:73–76. doi: 10.1038/324073a0. [DOI] [PubMed] [Google Scholar]
- Hofacre CL. Necrotic enteritis, currently a billion dollar disease: is there anything new on the horizon? In: Lyons TP, Jacques KA, editors. Science and Technology in the Feed Industry: Proceedings of Alltech’s 17th Annual Symposium. Nottingham University Press; Nottingham, UK: 2001. pp. 79–86. [Google Scholar]
- Kornasio R, Halevy O, Kedar O, Uni Z. Effect of in ovo feeding and its interaction with timing of first feed on glycogen reserves, muscle growth, and body weight. Poult Sci. 2011;90:1467–1477. doi: 10.3382/ps.2010-01080. [DOI] [PubMed] [Google Scholar]
- Ikebuchi K, Wong GG, Clark SC, Ihle JN, Hirai Y, Ogawa M. Interleukin 6 enhancement of interleukin 3-dependent proliferation of multipotential hemopoietic progenitors. Proc Nat Acad Sci USA. 1987;84:791–798. doi: 10.1073/pnas.84.24.9035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaldhusdal MI. Necrotic enteritis as affected by dietary ingredients. World Poult. 2000;16:42–43. [Google Scholar]
- Kaldhusdal M, Hofshagen M. Barley inclusion and avoparcin supplementation in broiler diets. 2. Clinical, pathological, and bacteriological findings in a mild form of necrotic enteritis. Poult Sci. 1992;71:1145–1153. doi: 10.3382/ps.0711145. [DOI] [PubMed] [Google Scholar]
- Kaldhusdal M, Skjerve E. Association between cereal contents in the diet and the incidence of necrotic enteritis in broiler chickens in Norway. Prev Vet Med. 1996;28:1–16. [Google Scholar]
- Katanbaf MN, Dunnington EA, Siegel PB. Allomorphic relationships from hatching to 56 days in parental lines and F1 crosses of chickens selected over 27 generations for high or low BW. Growth Dev Aging. 1988;52:11–22. [PubMed] [Google Scholar]
- Kelso A, Metcalf D. T lymphocyte-derived colony-stimulating factors. Adv Immunol. 1990;48:69–105. doi: 10.1016/s0065-2776(08)60752-x. [DOI] [PubMed] [Google Scholar]
- Keyburn AL, Sheedy SA, Ford ME, Williamson MM, Awad MM, Rood JI, Moore RJ. Alpha-toxin of Clostridium perfringens is not an essential virulence factor in necrotic enteritis in chickens. Infect Immun. 2006;74:6496–6500. doi: 10.1128/IAI.00806-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klasing KC. Nutritional modulation of resistance to infectious diseases. Poult Sci. 1998;77:1119–1125. doi: 10.1093/ps/77.8.1119. [DOI] [PubMed] [Google Scholar]
- Lilja C. A comparative study of postnatal growth and organ development in some species of birds. Growth. 1983;47:317–339. [PubMed] [Google Scholar]
- Lowenthal JW, Connick TE, McWaters PG, York JJ. Development of T-cell immune responsiveness in the chicken. Immunol Cell Biol. 1994;72:115–122. doi: 10.1038/icb.1994.18. [DOI] [PubMed] [Google Scholar]
- Manugistics Inc. Statgraphics Plus for Windows. Rockville, MD, USA: 2000. [Google Scholar]
- McDevitt RM, Brooker JD, Acamovic T, Sparks NHC. Necrotic enteritis: a continuing challenge for the poultry industry. World’s Poult Sci J. 2006;62:221–247. [Google Scholar]
- Moran ET., Jr Effects of egg weight, glucose administration at hatch, and delayed access to feed and water on the poult at 2 weeks of age. Poult Sci. 1990;69:1718–1723. doi: 10.3382/ps.0691718. [DOI] [PubMed] [Google Scholar]
- National Health and Medical Research Council. Australia code of practice for the care and use of animals for scientific purposes. National Health and Medical Research Council, Commonwealth Scientific and Industrial Research Organisation, Australia Agricultural Council, Australian Government Publication Service; Canberra, Australia: 2004. [Google Scholar]
- Noy Y, Sklan D. Different types of early feeding and performance in chicks and poults. J Appl Poult Res. 1999;8:16–24. [Google Scholar]
- Parish WE. Necrotic enteritis in the fowl. I. Histopathology of the disease and isolation of a strain of Clostridium welchii. J Comp Pathol. 1961;71:377–393. [PubMed] [Google Scholar]
- Perdigon G, Alvarez S, Pesce De Ruiz Holgado A. Immunoadjuvant activity of oral Lactobacillus casei: influence of dose on the secretory immune response and protective capacity in intestinal infections. J Dairy Res. 1991;58:485–496. doi: 10.1017/s0022029900030090. [DOI] [PubMed] [Google Scholar]
- Ritz CW, Hulet RM, Self BB, Denbow DM. Growth and intestinal morphology of male turkeys as influenced by dietary supplementation of amylase and xylanase. Poult Sci. 1995;74:1329–1334. doi: 10.3382/ps.0741329. [DOI] [PubMed] [Google Scholar]
- Schneeman BD. Pancreatic and digestive function. In: Vahoung GV, Kritchevsky D, editors. Dietary Fibre in Health and Disease. Plenum Press; New York: 1982. pp. 73–83. [Google Scholar]
- Sklan D, Noy Y. Hydrolysis and absorption in the small intestines of posthatch chicks. Poult Sci. 2000;79:1306–1310. doi: 10.1093/ps/79.9.1306. [DOI] [PubMed] [Google Scholar]
- Sklan D, Noy Y, Hoyzman A, Rozenboim I. Decreasing weight loss in the hatchery by feeding chicks and poults in hatching trays. J Appl Poult Res. 2000;9:142–148. [Google Scholar]
- Thompson JL, Hinton M. Antibacterial activity of formic and propionic acids in the diet of hens on Salmonella s in the crop. Br Poult Sci. 1997;38:59–65. doi: 10.1080/00071669708417941. [DOI] [PubMed] [Google Scholar]
- Truscott RB, Al-Sheikhly F. Reproduction and treatment of necrotic enteritis in broilers. Am J Vet Res. 1977;38:857–861. [PubMed] [Google Scholar]
- Uni Z. Impact of early nutrition on poultry: Review of presentations. J Appl Poult Res. 1998;7:452–455. [Google Scholar]
- Uni Z, Ganot S, Sklan D. Posthatch development of mucosal function in the broiler small intestine. Poult Sci. 1998;77:75–82. doi: 10.1093/ps/77.1.75. [DOI] [PubMed] [Google Scholar]
- Uni Z, Noy Y, Sklan D. Posthatch development of small intestinal function in the poult. Poult Sci. 1999;78:215–222. doi: 10.1093/ps/78.2.215. [DOI] [PubMed] [Google Scholar]
- van Snick J, Cayphas S, Vink A, Uyttenhove C, Coulie PG, Rubira MR, Simpson RJ. Purification and NH2-terminal amino acid sequence of a T-cell-derived lymphokine with growth factor activity for B-cell hybridomas. Proc Nat Acad Sci USA. 1986;83:9679–9683. doi: 10.1073/pnas.83.24.9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RB. Intercurrent coccidiosis and necrotic enteritis of chickens:rational, integrated disease management by maintenance of gut integrity. Avian Pathol. 2005;34:159–180. doi: 10.1080/03079450500112195. [DOI] [PubMed] [Google Scholar]
- Wilson HR. Interrelationships of egg size, chick size, posthatching growth, and hatchability. World’s Poult Sci J. 1991;47:5–16. [Google Scholar]
- Wu SB, Rodgers N, Choct M. Optimized necrotic enteritis model producing clinical and subclinical infection of Clostridium perfringens in broiler chickens. Avian Dis. 2010;54:1058–1065. doi: 10.1637/9338-032910-Reg.1. [DOI] [PubMed] [Google Scholar]
- Wyatt CL, Weaver WD, Jr, Beane WL, Denbow DM, Gross WB. Influence of hatcher holding times on several physiological parameters associated with the immune system of chickens. Poult Sci. 1986;65:2156–2164. doi: 10.3382/ps.0652156. [DOI] [PubMed] [Google Scholar]


