Abstract
Four, lactating dairy crossbreds (50%×50% Holstein Friesian×Native Zebu cattle) were randomly assigned according to a 2×2 factorial arrangement (two protein levels and two levels of mangosteen peel pellets (Mago-pel)) in a 4×4 Latin square design to receive four dietary treatments. All cows received concentrate at a proportion of 1 kg concentrate per 2 kg of milk yield, and urea-treated 5% rice straw (UTRS) was given ad libitum. It was found that total dry matter intakes, nutrient digestibility, ruminal pH and NH3-N concentrations were not affected (p>0.05) by treatments. Concentrations of ruminal pH and NH3-N were not affected by dietary treatments although the concentration of BUN varied significantly (p<0.05) between protein levels (p<0.05). The populations of rumen bacteria and fungal zoospores did not differ among treatments (p>0.05); however, the population of protozoa was decreased (p<0.05) when cows received Mago-pel supplementation. The composition of the population of bacteria, identified by real-time PCR technique, including total bacteria, methanogens, Fibrobacter succinogenes and Ruminococcus albus was similar (p>0.05) among dietary treatments (p>0.05); however, copy numbers of Ruminococcus flavefaciens was increased when protein level increased (p<0.05). Microbial protein synthesis, in terms of both quantity and efficiency, was enriched by Mago-pel supplementation. Milk yield was greatest in cows fed UTRS based diets with concentrate containing protein at 16% CP with Mago-pel, but were lowest without Mago-pel (p<0.05). In addition, protein level and supplementation of Mago-pel did not affect (p>0.05) milk composition except solids-not-fat which was higher in cows fed the diet with 19% CP. Therefore, feeding a concentrate containing 16% CP together with 300 g/hd/d Mago-pel supplementation results in changes in rumen fermentation and microbial population and improvements in milk production in lactating dairy crossbreds fed on UTRS.
Keywords: Protein Level, Mangosteen Peel Pellets, Rumen Fermentation, Milk Production, Dairy Crossbreds
INTRODUCTION
Currently, there is increasing interest in exploiting natural products as feed additives to solve problems in animal nutrition and livestock production (Wanapat et al., 2000a). Plant secondary compounds such as condensed tannin (CT) or saponins (SP) are able to impede the ruminal digestion of protein by forming reversible complexes with proteins and ultimately contribute to by-pass proteins for ruminants (Perez-Maldonado and Norton, 1996; McNeill et al., 1998). Furthermore, decreasing ruminal degradation of protein will result in decreased ruminal ammonia concentrations which could negatively impact on microbial growth and efficiency. Tannin-protein complexes are stable in the rumen (pH 5.0 to 7.0); however, the low pH (2.5 to 3.5) in the abomasum as well as the high pH (8.0 to 9.0) in the small intestine can stimulate dissociation for further absorption and utilization (Perez-Maldonado et al., 1995; McNeill et al., 1998).
Tropical plants or wastes containing CT and/or SP are numerous, for example mangosteen peel, guava leaf, and sesban leaf which contain 100, 20 to 100, and 20 gCT/kg DM, respectively. Mangosteen peel is a fruit by-product containing high levels of CT; as well as SP which also affects rumen fermentation (Poungchompu et al., 2009). Wang et al. (2000) reported that CT and SP exert a specific effect against rumen protozoa while the rest of the rumen biomass remains unaltered. Similarly, Ngamsaeng et al. (2006) found that supplementation with mangosteen powder decreased protozoa populations and the calculated methane production. Supplementation with mangosteen peel and soap berry fruit pellet, which are high in CT and SP, have been shown to alter rumen fermentation by lowering the protozoa population and methane production (Sliwinski et al., 2002; Poungchompu et al., 2009). Moreover, Carrula et al. (2005) suggested that the inhibition of methanogenesis by tannins was primarily the result of suppressing fiber degradation. However, the effects of CT and SP in ruminants vary with the type and concentration in the plants (Wanapat et al., 2000b; 2000c). Studies on the effects of protein levels and mangosteen peel pellets have been quite limited. Therefore, this study was undertaken to determine their effects on nutrient digestibility, rumen ecology and milk production in lactating crossbred cows.
MATERIALS AND METHODS
Animals, treatments, and experimental design
Four crossbreds (50%×50% Holstein Friesian×Native Zebu cattle) lactating dairy cows with average live weight of 413±25 kg and 120±21 DIM (initial milk production of 12±2 kg/cow/d) were randomly assigned according to a 2×2 factorial arrangement in a 4×4 Latin square. Two protein levels, 16% and 19% CP, and two mangosteen peel pellet levels, 0 and 300 g/hd/d, were tested. The four dietary treatments were thus: T1) 16% CP of concentrate without Mago-pel; T2) 16% CP of concentrate with Mago-pel 300 g/hd/d; T3) 19% CP of concentrate without Mago-pel; and T4) 19% CP of concentrate with Mago-pel 300 g/hd/d. Mago-pel products were prepared by combining 99.5% mangosteen peel meal 99.5% with 0.5% cassava starch. After mixing well, then using 5 kg of the mixtures was combined with 800 ml of and pelleted by machine. Urea-treated rice straw (UTRS) was prepared by pouring 5% (wt/wt) urea mixed with 100 kg of water over 100 kg stacks of RS and then covering with a plastic sheet for a minimum of 10 days before feeding directly to the animals (Wanapat et al., 2009).
All animals were kept in individual pens (4×6 m), and mineral block and water were available at free choice. The experiment was conducted for four periods, and each period lasted for 21 days. During the first 14 days, all animals were fed on respective treatment diets while during the last 7 days, samples were collected (diets, feces, milk, blood, and rumen fluid). The chemical composition and components of the concentrate and UTRS are shown in Table 1.
Table 1.
Chemical composition of concentrate and urea-treated rice straw (UTRS) used in the experiment (% DM)
| Ingredients | Dietary treatments
|
UTRS | |||
|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | ||
| Feed ingredients | |||||
| Cassava chip | 50.0 | 50.0 | 48.0 | 48.0 | – |
| Rice bran | 15.0 | 15.0 | 15.0 | 15.0 | – |
| Coconut meal | 12.5 | 12.5 | 14.9 | 14.9 | – |
| Palm kernel meal | 10.0 | 10.0 | 8.7 | 8.7 | – |
| Sunflower oil | 6.0 | 6.0 | 6.0 | 6.0 | – |
| Urea | 2.5 | 2.5 | 3.4 | 3.4 | – |
| Molasses | 2.0 | 2.0 | 2.0 | 2.0 | – |
| Salt | 0.5 | 0.5 | 0.5 | 0.5 | – |
| Sulfur | 0.5 | 0.5 | 0.5 | 0.5 | – |
| Di-calcium | 0.5 | 0.5 | 0.5 | 0.5 | – |
| Premix | 0.5 | 0.5 | 0.5 | 0.5 | – |
| Total | 100 | 100 | 100 | 100 | – |
| Chemical composition | |||||
| DM (%) | 91.2 | 91.3 | 91.0 | 90.1 | 59.8 |
| ----------------------% dry matter----------------- | |||||
| OM | 95.1 | 95.3 | 94.7 | 94.3 | 87.4 |
| Ash | 4.9 | 4.7 | 5.3 | 5.7 | 12.6 |
| CP | 16.4 | 16.5 | 19.0 | 19.1 | 6.5 |
| EE | 10.5 | 10.4 | 11.3 | 11.6 | 0.7 |
| NDF | 18.3 | 18.8 | 16.8 | 17.2 | 72.3 |
| ADF | 16.2 | 15.8 | 15.3 | 16.9 | 52.1 |
| TDN* | 83.0 | 83.0 | 82.4 | 82.4 | 55.1 |
UTRS = Urea-treated rice straw, DM = Dry matter, OM = Organic matter, CP = Crude protein, EE = Ether extract, NDF = Neutral detergent fiber, ADF = Acid detergent fiber, TDN = Total digestible nutrients.
Calculated values.
Data collection and sampling procedures
Feeds samples were randomly collected, and fecal samples were taken by rectal sampling from each individual cow during the last 7 days of each period. Composited samples were dried at 60°C and ground (1-mm screen using Cyclotech Mill, Teactor, Sweden) and then analyzed for DM, ether extract, ash, CP content (AOAC, 1990), NDF, ADF (Van Soest et al., 1991), and acid-insoluble ash (AIA). The AIA was used to estimate digestibility of nutrients as described by Van Keulen and Young (1977). Condensed tannins content in Mago-pel was analyzed by using the vanillin-HCl method as modified by Wanapat and Poungchompu (2001).
Cows were milked twice daily using a bucket-type milking system, and milk was weighed at each milking period. Milk samples were composited daily, for both the morning and evening milking, and preserved with 2-bromo-2 nitropropane-1, 3-dial and stored at 4°C until analysed for milk composition (fat, protein, lactose, total solids, and solid-not-fat) by infrared method using Milko-Scan 33 (Foss Electric, Hillerod, Denmark). Milk urea nitrogen (MUN) was determined using Sigma kits #640 (Sigma Diagnostics, St. Louis, MO, USA).
Rumen fluid was collected by stomach tube connected with vacuum pump at 0 and 4 h post feeding on the last day of each period. The pH and temperature of the rumen fluid was immediately measured using a portable pH and temperature meter (HANNA instrument HI 8424 microcomputer, Singapore). Rumen fluid samples were then filtered through four layers of cheesecloth. Samples were divided into three portions; the first portion was used for volatile fatty acids (VFA) and NH3-N analysis where 5 ml of H2SO4 solution (1 M) was added to 45 ml of rumen fluid. The mixture was centrifuged at 1,600×g for 15 min, and the supernatant was stored at −20°C prior to VFA analyses using high-performance liquid chromatography (HPLC; Instruments by controller water model 600E, water model 484 UV detector, column novapak C18, column size 4×150 mm, mobile phase 10 mM H2SO4 (pH 2.5); ETL Testing Laboratory, Inc., Cortland, NY, USA) according to Samuel et al. (1997). The concentration of NH3-N was determined using the micro-Kjeldahl method (AOAC, 1990). The second portion was fixed with 10% formalin solution in sterilized 0.9% saline solution and then analyzed for total direct count of bacteria, protozoa, and fungal zoospores using the methods of (Galyean, 1989) based on the use of a hemacytometer (Hausser Scientific, Horsham, PA). The last portion was taken to study cultured groups of viable bacteria using the roll-tube technique (Hungate, 1966) for identifying rumen bacteria groups (cellulolytic, proteolytic, amylolytic, and total viable bacteria). A blood sample (about 10 ml) was drawn from the jugular vein at the same time as rumen fluid sampling, separated by centrifugation at 1,600×g for 10 min (Table Top Centrifuge PLC-02, USA) and stored at −20°C for subsequent analysis of blood urea nitrogen (BUN) according to the method of Crocker (1967). Urine samples were analyzed for allantoin and creatinine by HPLC as described by Chen and Gomes (1995). The amount of microbial purines absorbed was calculated from purine derivative excretion based on the relationship derived by Chen and Gomes (1995).
Community DNA was extracted from 0.25 ml aliquots of each sample by the RBB+ C method (Yu and Morrison, 2004), which was shown to substantially increase DNA yields. In total, 16 samples were obtained, belonging to four treatments, four periods and two times of rumen fluid sampling (0 and 4 h post-feeding). The primers used for the real-time quantitative PCR (qPCR) are as follows: Primers for Fibrobacter succinogenes, Fs219f (5′-GGTATGGGATGAGCTTGC-3′) and Fs654r (5′-GCCTGCCCCTGAACTATC-3′), were selected to allow amplification (446-bp product) of all 10 F. succinogenes strains deposited in GenBank. For Ruminococcus albus primers, Ra1281f (5′-CCCTAAAAGCAGTCTTAGTTCG-3′) and Ra1439r (5′-CCTCCTTG CGGTTAGAACA-3′) (175-bp product). Ruminococcus flavefaciens primers, Rf154f (5′-TCT GGAAACGGATGGTA-3′) and Rf425r (5′-CCTTTAAGACAGGAGTTTACAA-3′), were also selected to allow species–species amplification (295 bp) of all seven R. flavefaciens strains deposited in GenBank. All these primer sets were previously published by Koike and Kobayashi (2001). Regular PCR conditions for Fsuccinogenes were as follows: 30 s at 94°C for denaturing, 30 s at 60°C for annealing and 30 s at 72°C for extension (48 cycles), except for the 9 min denaturation in the first cycle and the 10 min extension in the last cycle. Amplification of 16S rDNA for the other two species was carried out similarly except an annealing temperature of 55°C was used. Quantification of total bacteria population, primer and condition, was previously published by Kongmun et al. (2010). Four sample derived standards were prepared from a treatment pool set of community DNA. The regular PCR was used to generate sample derived DNA standards for each qPCR assay. Then the PCR product was purified using a QIA quick PCR purification kit (QIAGEN, Inc., Valencia, CA, USA) and quantified using a spectrophotometer. For each sample derived standard, copy number concentration was calculated based on the length of the PCR product and the mass concentration. Tenfold serial dilution was made in Tris-EDTA prior to real-time PCR (Yu et al., 2005). In total, four qPCR standards were prepared. The conditions of the qPCR assays of target genes were the same as those of the regular PCR described earlier. Biotools QuantiMix EASY SYGKIT (B&M Labs, S.A., Spain) was used for real-time PCR amplification. All PCRs were performed in duplicate.
Statistical analysis
The data were analyzed in a 2×2 factorial arrangement in a 4×4 Latin square design by analysis of variance using GLM procedure the (SAS, 1996). The results are presented as mean values and standard error of the means. Differences between treatment means were determined by orthogonal contrast. Differences among means with p<0.05 were accepted as representing statistically different.
RESULTS AND DISSCUSSION
The chemical composition of UTRS and concentrate diets used in the experiment is shown in Table 1. Mago-pel contained 17.7% CT; therefore, cows received 53.2 g CT/hd/d. The effect of protein levels and Mago-pel on total dry matter intake and nutrient digestibility are shown in Table 2. Feed intakes and nutrient digestibility were not significantly different (p>0.05) among protein levels and Mago-pel supplementation (p>0.05). However, digestibility of dry matter, ether extract, and crude protein tended (p = 0.056) to be higher in Mago-pel supplemented group while fiber digestion (NDF, ADF) tended (p = 0.051) to be lower with Mago-pel supplementation. These results agreed with Pakmaluek et al. (2005) who found that reducing crude protein from 18 to 14% did not affect voluntary feed intake or digestibility in dairy cow fed with TMR. However, insufficient protein may influence rumen fermentation and decrease of nutrient digestibility. Puchala et al. (2005) found that forage containing CT at 0.196 kg/d resulted in relatively high intake.
Table 2.
Effect of protein level and mangosteen peel pellets (Mago-pel) in concentrate on voluntary feed intake and nutrient digestibility
| Items | 16% CP
|
19% CP
|
SEM | Contrasts1
|
||||
|---|---|---|---|---|---|---|---|---|
| −MSP | +MSP | −MSP | +MSP | Pro | MSP | Pro×MSP | ||
| UTRS DMI intake | ||||||||
| kg/d | 5.5 | 6.6 | 5.2 | 6.6 | 0.18 | NS | NS | * |
| % BW | 1.4 | 1.5 | 1.3 | 1.6 | 0.03 | NS | NS | * |
| g/kg BW0.75 | 60.6 | 69.9 | 56.5 | 70.5 | 1.50 | NS | NS | * |
| Total DMI intake | ||||||||
| kg/d | 11.1 | 12.0 | 11.0 | 12.4 | 0.45 | NS | NS | NS |
| % BW | 2.7 | 2.8 | 2.6 | 2.9 | 0.10 | NS | NS | NS |
| g/kg BW0.75 | 121.7 | 126.9 | 119.1 | 132.7 | 4.44 | NS | NS | NS |
| Digestibility (%) | ||||||||
| DM | 57.0 | 57.4 | 56.5 | 58.1 | 3.27 | NS | NS | NS |
| OM | 61.3 | 61.2 | 60.1 | 62.0 | 3.60 | NS | NS | NS |
| NDF | 50.1 | 50.1 | 40.3 | 38.8 | 5.42 | NS | NS | NS |
| ADF | 46.8 | 45.2 | 43.6 | 47.0 | 4.64 | NS | NS | NS |
| EE | 84.6 | 89.9 | 89.9 | 91.7 | 8.30 | NS | NS | NS |
| CP | 47.4 | 53.7 | 57.7 | 60.1 | 5.63 | NS | NS | NS |
Pro = Protein, MSP = Mago-pel, Pro×MSP = Protein×Mago-pel, DM = Dry matter, OM = Organic matter, NDF = Neutral detergent fiber, ADF = Acid detergent fiber, EE = Ether extract, CP = Crude protein. SEM = Standard error of the means, NS = Not significant.
p<0.05.
Table 3 shows effects of protein levels and Mago-pel in concentrates on rumen fermentation, and BUN. Ruminal pH, temperature and NH3-N concentration were not affected by either the protein levels or Mago-pel supplementation (p>0.05). This could be due to effect of Mago-pel on protecting the protein, thus ruminal protein degradation did not occur. Rumen pH at 0 and 4 h post-feeding were unchanged by dietary treatments and the values were quite stable at 6.7 to 6.8. According to Wanapat (1999), ruminal pH of cattle fed on urea-treated rice straw ranges from 6.5 to 7.2, with the optimum level of pH in the rumen 6.5 to 7.0. However, supplementation of protein levels and Mago-pel in concentrates with high fat did not affect on NH3-N in the rumen (p>0.05). However, Nguyen and Preston (1999) also found rumen NH3-N was increased 8 to 18 mg/dl by adding urea-treated rice straw. Moreover, concentrate containing 19% CP tended to higher the in concentration of NH3-N (p = 0.06). Perez-Maldonado and Norton (1996) reported also that rumen NH3-N concentrations were not affected by dietary CT intake which could relate to the lower level of CT used in the experiment. Protein levels and supplementation of Mago-pel had no effect on volatile fatty acids concentrations in the rumen (p>0.05). Total volatile fatty acids were produced in normal range of 70 to 130 mM in animals fed with roughage based diet (France and Siddons, 1993). The average of acetic acid, propionic acid and butyric acid concentrations in this study were 67.8, 21.2 and 11.1% of total VFA, respectively which is similar to those reported by Hungate (1966) (62, 22 and 16% for acetic acid, propionic and butyric acid proportion in the rumen respectively). Ngamsaeng et al. (2006) found no significant effect (p>0.05) of the feeding level of magosteen peel (MSP) on total VFAs and individual VFA concentrations.
Table 3.
Effect of protein level and mangosteen peel pellets (Mago-pel) in concentrate diet on ruminal pH, temperature, NH3-N concentration, and volatile fatty acids (VFA)
| Items | 16% CP
|
19% CP
|
SEM | Contrasts1
|
||||
|---|---|---|---|---|---|---|---|---|
| −MSP | +MSP | −MSP | +MSP | Pro | MSP | Pro×MSP | ||
| Ruminal pH | 6.7 | 6.7 | 6.7 | 6.8 | 0.06 | NS | NS | NS |
| Temperature | 39.0 | 38.8 | 39.0 | 38.9 | 0.22 | NS | NS | NS |
| NH3-N (mg/dl) | 11.1 | 11.8 | 13.4 | 12.9 | 0.95 | NS | NS | NS |
| BUN (mg/dl) | 9.9 | 9.5 | 13.8 | 14.1 | 0.86 | ** | NS | NS |
| Total VFA (mmol/L) | 106.3 | 107.9 | 102.3 | 105.9 | 4.07 | NS | NS | NS |
| Acetic acid (%) | 68.9 | 66.8 | 68.1 | 67.3 | 0.82 | NS | NS | NS |
| Propionic acid (%) | 20.6 | 21.9 | 21.8 | 20.6 | 1.18 | NS | NS | NS |
| Butyric acid (%) | 10.5 | 11.3 | 10.2 | 12.2 | 1.11 | NS | NS | NS |
| C2 to C3 ratio | 3.4 | 3.1 | 3.2 | 3.3 | 0.20 | NS | NS | NS |
| CH4 (mol/100 mol)2 | 29.6 | 28.6 | 28.7 | 29.5 | 0.8 | NS | NS | NS |
Pro = Protein, MSP = Mago-pel, Pro×MSP = Protein×Mago-pel, SEM = Standard error of the means, NS = Not significant,
p<0.01.
Calculated according to Moss et al. (2000) CH4 production = 0.45(acetate)−0.275(propionate)+0.4(butyrate).
The BUN concentration was highest in the cows receiving the 19% CP concentrate with 300 g/hd/d.
Fungal zoospores population, cellulolytic, amylolytic and proteolytic bacterial groups did not differ (p>0.05) among treatments (p>0.05). However, including Mago-pel in the concentrate decreased the protozoal population. This is similar to the findings of Samuel et al. (1997) who reported that supplementation of 150 g/hd/d of MSP reduced the population of protozoa in the rumen. Ngamsaeng et al. (2006) also suggested that supplementation of mangosteen peel (100 g/DM/d) in cattle can decrease the protozoal population, increase rumen bacteria and maintain the fungal zoospore population.
The accuracy of each qPCR was validated by quantifying known numbers of target species templates (total bacteria, F. succinogenes, R. flavefaciens and R. albus) and results are presented in Table 5. Then, those templates were used for generated standard curve. In this study, those standard curves showed a highly linear relationship between ct value and known number template dilution with highly correlations value r2 of standard curve in each species as shown in Figure 1. The target DNAs for qPCR were prepared as described earlier for rumen fluid. For each standard a linear regression derived from the C(T) cycle of each DNA dilution and the log quantity was presented. As obtained, the r2 values of the regressions from methanogen total and cellulolytic bacteria were relatively high. Application of qPCR to quantify methanogen, total bacteria, predominant cellulolytic bacteria (16S rDNA) targets revealed that treatments did not have an effect on total bacteria, methanogen, F. succinogenes and R. albus. In contrast, R. flavefaciens was significantly increased (p<0.05) when cows received 19% CP (6.25 log copies/ml rumen fluid). When compared among treatments, R. flavefaciens was highest in 19% CP while lowest in 16% CP diets. Similarly, Wanapat and Cherdthong (2009), who studied rumen cellulolytic bacteria population using realtime PCR, also found that the three cellulolytic bacterial numbers were 3.0×109, 3.1×107 and 2.9×106 copies/ml of the rumen content for F. succinogenes, R. flavefaciens and R. albus, respectively. Fibolytic species, such as R. albus and R. flavefaciens, F. succinogenes can digest fiber faster and to a greater extent, in fact, these species even digest crystalline cellulose more actively than ruminococcal species as reported by Koike and Kobayashi (2001).
Table 5.
Effect of protein level and mangosteen peel pellets (Mago-pel) in concentrate diet on rumen total bacterial methanogen and predominant cellulolytic bacteria using real-time PCR technique
| Items | 16% CP
|
19% CP
|
SEM | Contrasts1
|
||||
|---|---|---|---|---|---|---|---|---|
| −MSP | +MSP | −MSP | +MSP | Pro | MSP | Pro×MSP | ||
| ------------------- copies/ml of rumen fluid -------------------- | ||||||||
| Total bacteria (×109) | 2.4 | 1.8 | 2.1 | 3.1 | 0.42 | NS | NS | NS |
| Methanogens (×106) | 1.1 | 1.1 | 1.2 | 1.3 | 0.13 | NS | NS | NS |
| F. succinogenes (×107) | 11.6 | 7.6 | 10.2 | 13.0 | 0.84 | NS | NS | NS |
| R. flavefaciens (×106) | 1.6 | 1.6 | 1.8 | 1.8 | 0.07 | * | NS | NS |
| R. albus (×106) | 2.6 | 2.5 | 2.6 | 2.6 | 0.08 | NS | NS | NS |
SEM = Standard error of the means, NS = Not significant,
p<0.05.
Pro = Protein, MSP = Mago-pel, Pro×MSP = Protein×Mago-pel, SEM = Standard error of the means, CFU = Colony forming unit, NS = No significant, *p<0.05.
Figure 1.
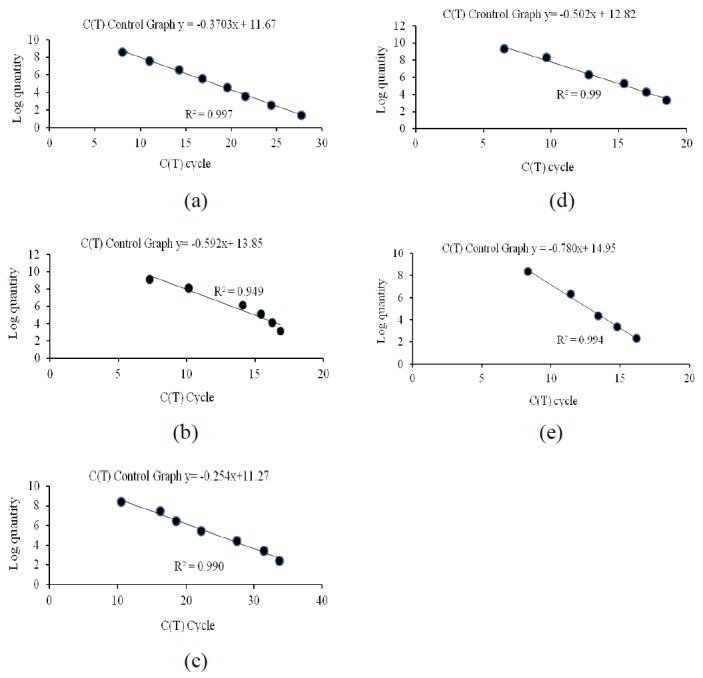
The standard curves obtained by plotting the logarithm of DNA concentration for total bacteria (a), methanogen (b), F. succinogenes (c), R. flavefaciens (d) and R. albus (e) versus threshold cycle (Ct) for population quantification by using real time PCR.
The effects of protein level and Mago-pel supplementation in lactating dairy cows on the excretion of urinary purine derivatives (PD) and microbial crude protein supply in lactating dairy cows are presented in Table 6. N balance was significantly different among treatment (p<0.05). The excretions of creatinine and allantoin concentrations in urine were not affected in all treatments. In this regard, the positive N retention observed in this study indicated the positive influence of protein level with Mago-pel based diet supplements and urea-treated rice straw based feeding systems of lactating dairy cows. With regards to N utilization, Chen and Gomes (1995) stated that N excretion and N retention should reflect differences in N metabolism, because N retention was the most important index of the protein nutrition status of ruminants (Ørskov, 1999; Firkins et al., 2007). Microbial efficiencies of the dietary treatments are shown in Table 6. One indicator of the efficiency of rumen nitrogen use is the amount of microbial crude protein (MCP) flow from the rumen, which is a consequence of microbial growth and its washout from the rumen (Weisbjerg et al., 1996). Microbial protein synthesis in the rumen provides the majority of protein supplied to the small intestine of ruminants, accounting for 50 to 80% of total absorbable protein (Firkins et al., 2007). In the present study, MCP protein flow from the rumen as calculated from purine derivative excretion using the equation (Chen and Gomes, 1995; Galo et al., 2003) ranged from 350.1 to 542.8 g/d, respectively. Moreover, supplementation of 16% protein with Mago-pel resulted in the greatest efficiency of microbial N synthesis (EMNS) (19.3 g N/d). However, ruminal protozoa count was reduced through the addition of MSP in agreement with the our previous work (Pilajan and Wanapat, 2011). The sensitivity of protozoa towards plant secondary compounds may be explained by the presence of sterols in cell membranes (Newbold et al., 1997). This result accorded with the bacterial population from roll-tube technique in which the 16% protein with Mago-pel and 19% protein with Mago-pel ratio had the highest and lowest viable bacteria population respectively.
Table 6.
Effect of protein level and mangosteen peel pellets (Mago-pel) in concentrate diet on excretion of urinary derivatives and microbial nitrogen supply in lactating dairy crossbreds
| Items | 16% CP
|
19% CP
|
SEM | Contrasts1
|
||||
|---|---|---|---|---|---|---|---|---|
| −MSP | +MSP | −MSP | +MSP | Pro | MSP | Pro×MSP | ||
| Allantoin excretion (mmol/d) | 199.7 | 291.1 | 215.9 | 283.1 | 8.86 | NS | ** | NS |
| Allantoin absorption (mmol/d) | 150.9 | 242.2 | 166.5 | 234.0 | 8.62 | NS | ** | NS |
| Urine creatinine (mg/dl) | 69.4 | 63.8 | 65.6 | 65.7 | 2.31 | NS | NS | NS |
| MCP1 (g/d) | 350.1 | 561.8 | 368.3 | 542.8 | 19.9 | NS | ** | NS |
| EMNS2 (g/kg OMDR) | 14.1 | 19.3 | 15.0 | 17.0 | 0.69 | NS | ** | NS |
SEM = Standard error of the means, NS = Not significant,
p<0.01.
Microbial crude protein (MCP) (g/d) = 3.99×0.856×mmol of purine derivatives excreted (Galo et al., 2003).
Efficiency of microbial nitrogen supply (EMNS), g/kg of OM digested in the rumen (OMDR) = ((MCP (g/d)×1,000)/DOMR(g)), assuming that rumen digestion = 65% of digestion in total tract.
All cows were able to maintain levels of milk yield during the 84 days of the experiment (Table 7). Yield of milk and 3.5% FCM were greatest in cows fed UTRS based diets with concentrate containing protein at 16% CP with Mago-pel, but were lowest when receiving concentrate containing protein at 16% CP without Mago-pel. However, Milk yield was also affected by a MSP interaction with CP so that milk yield was higher in the 16% CP plus MSP than in the 19% concentrate treatment. In addition, the supplementation of protein levels in concentrate and Mago-pel did not significantly affect on milk compositions including milk fat, milk protein, lactose and total solid. However, solids-not-fat was higher in cow fed with 19% CP than those on 16% CP (Table 5). Supplementation of Mago-pel tended to increase milk yield and 3.5% FCM as compared to non-supplementation group.
Table 7.
Effect of protein level and mangosteen peel pellets (Mago-pel) in concentrate diet on milk yield, milk composition and milk urea nitrogen
| Items | 16% CP
|
19% CP
|
SEM | Contrasts1
|
||||
|---|---|---|---|---|---|---|---|---|
| −MSP | +MSP | −MSP | +MSP | Pro | MSP | Pro×MSP | ||
| Milk yield (kg/d) | 11.1 | 12.7 | 11.2 | 11.5 | 0.18 | * | NS | * |
| 3.5% FCM (kg/d) | 11.3 | 12.4 | 11.7 | 12.3 | 0.08 | * | NS | * |
| Milk composition | ||||||||
| Protein | 2.9 | 3.1 | 3.2 | 3.3 | 0.16 | NS | NS | NS |
| Fat | 3.5 | 3.8 | 3.2 | 3.6 | 0.53 | NS | NS | NS |
| Lactose | 4.9 | 4.8 | 5.2 | 5.0 | 0.19 | NS | NS | NS |
| Solids-not-fat | 8.5 | 8.5 | 9.4 | 9.2 | 0.29 | * | NS | NS |
| Total solids | 11.7 | 11.3 | 11.9 | 12.5 | 0.32 | NS | NS | NS |
| MUN (mg/dl) | 10.3 | 11.5 | 13.0 | 11.5 | 1.27 | NS | NS | NS |
Pro = Protein, MSP = Mago-pel, Pro×MSP = Protein×Mago-pel, SEM = Standard error of the means, NS = Not significant,
p<0.05.
CONCLUSIONS AND RECOMMENDATIONS
Protein level and supplementation of Mago-pel had no effect on feed intake, digestibility of nutrient, and volatile fatty acids; however, blood urea nitrogen was higher in cow receiving concentrate containing 19% CP. Ruminococcus flavefaciens abundance was impacted by protein levels, while the population of protozoa was decreased by Mago-pel supplementation. Microbial protein synthesis was improved with Mago-pel supplementation. Milk yields were greatest in cows fed with concentrate containing 16% CP. Therefore, a combined use of concentrates containing 16% CP with supplementation of Mago-pel at 300 g/hd/d could have potential for lactating dairy crossbreds especially when fed on rice straw as a roughage source.
Table 4.
Effect of protein level and mangosteen peel pellets (Mago-pel) in concentrate diet on microbial population in the rumen of lactating dairy crossbreds
| Items | 16% CP
|
19% CP
|
SEM | Contrasts1
|
||||
|---|---|---|---|---|---|---|---|---|
| −MSP | +MSP | −MSP | +MSP | Pro | MSP | Pro×MSP | ||
| Total direct counts, cell/ml | ||||||||
| Protozoa (×105) | 4.5 | 3.2 | 4.7 | 3.4 | 0.33 | NS | * | NS |
| Fungi zoospores (×105) | 3.0 | 2.6 | 2.4 | 2.6 | 0.41 | NS | NS | NS |
| Grouping of bacteria (×106 CFU/ml) | ||||||||
| Total viable bacteria | 21.0 | 30.8 | 31.5 | 26.0 | 9.75 | NS | NS | NS |
| Cellulolytic bacteria | 8.2 | 9.8 | 9.6 | 10.2 | 2.17 | NS | NS | NS |
| Amylolytic bacteria | 4.4 | 4.6 | 4.2 | 2.9 | 0.66 | NS | NS | NS |
| Proteolytic bacteria | 3.3 | 5.4 | 3.5 | 2.5 | 0.64 | NS | NS | NS |
Pro = Protein, MSP = Mago-pel, Pro×MSP = Protein×Mago-pel, SEM = Standard error of the means, CFU = Colony forming unit, NS = Not significant,
p<0.05.
ACKNOWLEDGEMENTS
The authors would like to express their most sincere thanks to Tropical Feed Resources Research and Development Center (TROFREC), Department of Animal Science, Faculty of Agriculture, Khon Kaen University, Thailand and The Thailand Research Fund in collaboration with Khon Kaen University (TRF Master Research Grants: TRF-MAG Window II) for their kind financial support and the use of research facilities.
REFERENCES
- AOAC. Official methods of analysis. Association of Official Analysis Chemists; DC, USA: 1990. [Google Scholar]
- Carulla JE, Kreuzer M, Machmller A, Hess HD. Supplementation of Acacia mearnsii tannins decreases methanogenesis and urinary nitrogen in forage-fed sheep. Aust J Agric Res. 2005;56:961–970. [Google Scholar]
- Crocker CL. Rapid determination of urea nitrogen in serum or plasma without deproteinzation. Am J Med Technol. 1967;33:361–365. [PubMed] [Google Scholar]
- Chen XB, Gomes MJ. Estimation of microbial protein supply to sheep and cattle based on urinary excretion of purine derivative-an overview of the technique details. International Feed Resources Unit, Rowett Research Institute; Aberdeen, UK: 1995. (Occasional Publication 1992). [Google Scholar]
- Fievez V, Piattoni F, Mbanzamihigo L, Demeyer D. Reductive acetogenesis in the hindgut and attempts to its induction in the rumen- a review. J Appl Anim Res. 1999;16:1–22. [Google Scholar]
- Firkins JL, Yu Z, Morrison M. Ruminal nitrogen metabolism: perspectives for integration of microbiology and nutrition for dairy. J Dairy Sci. 2007;90(E Suppl):E1–E16. doi: 10.3168/jds.2006-518. [DOI] [PubMed] [Google Scholar]
- France J, Siddons RC. Volatile fatty acid production. In: Forbes JM, France J, editors. Quantitative Aspects Ruminant Digestion and Metabolism. C. A. B. International; Willingford, UK: 1993. pp. 107–122. [Google Scholar]
- Galyean M. Laboratory procedure in animal nutrition research. Department of Animal and Life Science; New Mexico State University, USA: 1989. [Google Scholar]
- Galo E, Emanuele SM, Sniffen CJ, White JH, Knapp JR. Effects of a polymer-coated urea product on nitrogen metabolism in lactating Holstein dairy cattle. J Dairy Sci. 2003;86:2154–2162. doi: 10.3168/jds.S0022-0302(03)73805-3. [DOI] [PubMed] [Google Scholar]
- Hungate RE. The rumen and its microbes. Academic Press; New York and London: 1966. [Google Scholar]
- Kafi M, Mirzaei A. Effect of first postpartum progesterone rise, metabolites, milk yield, and body condition score on the subsequent ovarian activity and fertility in lactating Holstein dairy cows. Trop Anim Health Prod. 2010;42:761–767. doi: 10.1007/s11250-009-9484-7. [DOI] [PubMed] [Google Scholar]
- Koike S, Kobayashi Y. Development and use of competitive PCR assays for the rumen cellulolytic bacteria: Fibrobacter succinogenes, Ruminococcus albus and Ruminococcus flavefaciens. FEMS Microbiol Lett. 2001;204:361–366. doi: 10.1111/j.1574-6968.2001.tb10911.x. [DOI] [PubMed] [Google Scholar]
- Kongmun P, Wanapat M, Pakdee P, Navanukraw C. Effect of coconut oil and garlic powder on in vitro fermentation using gas production technique. Livest Sci. 2010;127:38–44. [Google Scholar]
- McNeill DM, Osborne N, Komolong M, Nankervis D. Condensed tannins in the Leucaena genus and their nutritional significance for ruminants. In: Shelton HM, Gutteridge RC, Mullin BF, Bray RA, editors. Leucaena-Adaptation, Quality and Farming Systems; ACIAR Proceedings; 1998. pp. 205–214. [Google Scholar]
- Newbold CJ, Hassan SM, Wang J, Ortega ME, Wallace RJ. Influence of foliage from African multipurpose trees on activity of rumen protozoa and bacteria. Br J Nutr. 1997;78:237–249. doi: 10.1079/bjn19970143. [DOI] [PubMed] [Google Scholar]
- Van Thu Nguyen, Preston TR. Rumen environment and feed degradability in swamp buffaloes fed different supplements. Livest Res Rural Dev. 1999;11(3) http://www.Cipav.Org.co/ieed/Irrd11/3/thu113.htm. [Google Scholar]
- Orskov ER. Supplement strategies for ruminants and management of feeding to maximize utilization of roughages. J Anim Vet Adv. 1999;38:179–185. doi: 10.1016/s0167-5877(98)00123-8. [DOI] [PubMed] [Google Scholar]
- Pakmaluek P, Wachirapakorn C, Wanapat M, Pakdee P. Effect of level of crude protein in Total Mixed Ration (TMR) with corn cobs and rice straw as roughage sources on rumen fermentation, milk yield and composition in lactating dairy cows. KKU Res J (GS) 2005;5:2. [Google Scholar]
- Perez-Maldonado RA, Norton BW. The effects of condensed tannins from Desmodium intorturn and Calliandra calothyrsus on protein and carbohydrate digestion in sheep and goats. Br J Nutr. 1996;76:515–533. doi: 10.1079/bjn19960060. [DOI] [PubMed] [Google Scholar]
- Pilajan R, Wanapat M. Effect of coconut oil and mangosteen peel supplementation on ruminal fermentation, microbial protein synthesis in swamp buffaloes. Livest Sci. 2011 doi: 10.1016/j.livsci.2011.05.013. [DOI] [Google Scholar]
- Poungchompu O, Wanapat M, Wachirapakorn C, Wanapat S, Cherdthong A. Manipulation of ruminal fermentation and methane production by dietary saponins from mangosteen peel and soapberry fruit. J Anim Nutr. 2009;63:389–400. doi: 10.1080/17450390903020406. [DOI] [PubMed] [Google Scholar]
- Puchala R, Min BR, Goetsch AL, Sahlu T. The effect of a condensed tannin-containing forage on methane emission by goats. J Anim Sci. 2005;83:182–186. doi: 10.2527/2005.831182x. [DOI] [PubMed] [Google Scholar]
- Samuel M, Sagathewan S, Thomas J, Mathen G. An HPLC method for estimation of volatile fatty acids of ruminal fluid. Indian J Anim Sci. 1997;67:805–807. [Google Scholar]
- SAS. User’s guide: Statistic, Version 5 Edition. SAS; Inst Cary, NC, USA: 1996. 1996. [Google Scholar]
- Satter LD, Styler LL. Effect of ammonia concentration on ruminal microbial protein production in vitro. Br J Nutr. 1974;32:199–208. doi: 10.1079/bjn19740073. [DOI] [PubMed] [Google Scholar]
- Sliwinski BJ, Kreuzer M, Wettatein HR, Machmuller A. Rumen fermentation and nitrogen balance of lambs fed diets containing plant extracts rich in tannins and saponins, and associated emissions of nitrogen and methane. Arch Anim Nutr. 2002;56:379–392. doi: 10.1080/00039420215633. [DOI] [PubMed] [Google Scholar]
- Van Keulen J, Young BA. Evaluation of acid-insoluble ash as a marker in ruminant digestibility. J Anim Sci. 1977;44:282–287. [Google Scholar]
- Van Soest PJ, Robertson JB, Lewis BA. Methods for dietary fiber neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci. 1991;74:3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2. [DOI] [PubMed] [Google Scholar]
- Wanapat M. Feeding of ruminants in the tropicals base on local feed resources. Khon Kaen Publishing Company Ltd; Khon Kaen, Thailand: 1999. p. 236. [Google Scholar]
- Wanapat M. Potential uses of local feed resources for ruminants. Trop Anim Health Prod. 2009;41:1035–1049. doi: 10.1007/s11250-008-9270-y. [DOI] [PubMed] [Google Scholar]
- Wanapat M, Poungchompu O. Method for estimation of tannin by vanillin-HCl method (A modified method of Burns, 1971) Department of Animal Science, Khon Kaen University; Khon Kaen 4002, Thailand: 2001. [Google Scholar]
- Wanapat M, Pimpa O, Sripuek W, Puramongkol T, Petlum A, Boontao U, Wachirapakorn C, Sommart K. Cassava hay: An important on-farm feed for ruminants. In: Brooker JD, editor. Tannins in livestock and human nutrition; Proceedings of an international workshop; Adelaide. May 31–June 2, 1999; 2000a. pp. 71–74. ACIR. No. 92. [Google Scholar]
- Wanapat M, Puramongkol T, Siphuak W. Feeding of cassava hay for in lactating dairy cows. Asian-Aust J Anim Sci. 2000b;13:478–482. [Google Scholar]
- Wanapat M, Petlum A, Pimpa O. Supplementation of cassava hay to replace concentrate use in lactating Holstein Friesian crossbreds. Asian-Aust J Anim Sci. 2000c;13:600–604. [Google Scholar]
- Wang Y, McAllister TA, Yanke LJ, Xu ZJ, Cheeke PR, Cheng KJ. In vitro effects of steroidal saponins from Yucca schidigera extract on rumen microbial protein synthesis and ruminal fermentation. J Sci Food Agric. 2000;80:2114–2122. [Google Scholar]
- Weisbjerg MR, Hvelplund T, Hellberg S, Olsson S, Same S. Effective rumen degradability and intestinal digestibility of individual amino acids in different concentrates determined in situ. Anim Feed Sci Technol. 1996;62:179–188. [Google Scholar]
- Yu Z, Michel FC, Jr, Hansen G, Wittum T, Morrison M. Development and application of real-time PCR assays for quantification of genes encoding tetracycline resistance. Appl Environ Microbiol. 2005;71:6926–6933. doi: 10.1128/AEM.71.11.6926-6933.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z, Morrison M. Improved extraction of PCR-quality community DNA from digesta and fecal samples. BioTechniques. 2004;36:808–812. doi: 10.2144/04365ST04. [DOI] [PubMed] [Google Scholar]


