Abstract
Intestinal phosphate (Pi) absorption across the apical membrane of small intestinal epithelial cells is mainly mediated by the type IIb Na-coupled phosphate co-transporter (NaPi-IIb), but its expression and regulation in the chicken remain unclear. In the present study, we investigated the mRNA and protein levels of NaPi-IIb in three regions of chicken small intestine, and related their expression levels to the rate of net phosphate absorption. Our results showed that maximal phosphate absorption occurs in the jejunum, however the highest expression levels of NaPi-IIb mRNA and protein occurs in the duodenum. In response to a low-Pi diet (TP 0.2%), there is an adaptive response restricted to the duodenum, with increased brush border membrane (BBM) Na-Pi transport activity and NaPi-IIb protein and mRNA abundance. However, when switched from a low- (TP 0.2%) to a normal diet (TP 0.6%) for 4 h, there is an increase in BBM NaPi-IIb protein abundance in the jejunum, but no changes in BBM NaPi-IIb mRNA. Therefore, our study indicates that Na-Pi transport activity and NaPi-IIb protein expression are differentially regulated in the duodenum vs the jejunum in the chicken.
Keywords: Chicken, NaPi-IIb, Small Intestine, Duodenum, Jejunum
INTRODUCTION
Inorganic phosphate (Pi) plays a major role in growth, development, bone formation and cellular metabolism. The kidney and the small intestine are important regulatory sites which maintain extracellular Pi concentrations. Sodium-coupled phosphate transport is the major form of Pi absorption in both kidney and intestine. Phosphate uptake by renal and intestinal brush-border membrane vesicles (BBMVs) has been studied previously in human (Borowitz et al., 1989), rat (Ghishan et al., 1990), rabbit (Borowitz et al., 1992) and mouse (Nakagawa et al., 1994). The molecular basis of Pi uptake in kidney has been identified (sodium-phosphate [Na+-Pi] transporters types I and II) and well characterized. Type II Na+-Pi transporter is the major transport pathway of Pi reabsorption in kidney (Magagnin et al., 1993; Collins et al., 1994). However, intestinal absorption of Pi has drawn considerably less attention.
To date, two Na-dependent Pi cotransporters, type IIb (NaPi-IIb; GenBank accession number AAC80007) and type III Na/Pi cotransporter (also called PiT-2; GenBank accession number AF 196774), have been identified in mouse small intestine (Hilfiker et al., 1998; Bai et al., 2000). Na/Pi IIb is expressed in enterocytes and is located in BBMVs (Hilfiker et al., 1998). Apical location, kinetic characteristics, and pH dependency suggest that type IIb protein is likely a major Na/Pi cotransporter in the mammalian intestine, this transporter is thought to be the rate-limiting step for trans-epithelial phosphate absorption (Murer et al., 2004).
Although the identification and characterization of a chicken homolog for the NaPi-IIb cotransporter has been extensively studied (Yan et al., 2007), the regulating mechanism of intestinal absorption of Pi in chickens has remain unknown. Earlier studies has shown that the dietary phosphate deprivation is an important physiological regulator of intestinal phosphate uptake as observed in BBM vesicles (BBMVs) in rats, mice, and pigs (Quamme, 1985; Hattenhaur et al., 1999; Kari et al., 2010). Kinetic parameters indicate increases in Na-dependent phosphate uptake are due to a 50% increase in maximal capacity for absorption (Vmax) with no change in affinity, indicating the maximal capacity for phosphate transport is increasing and not the affinity of NaPi-IIb transporter for phosphate (Segawa et al., 2004). The increase in Vmax in mice fed a low-P diet has been accompanied by an increase in abundance of membrane-bound NaPi-IIb cotransporter protein in the small intestine (Hattenhaur et al., 1999; Segawa et al., 2004). However, mixed results have been reported on the effect of low-P diets on NaPi-IIb mRNA expression and it appears to be dependent upon both the severity and length of dietary Pi deprivation.
The severity of dietary Pi restriction in rodent studies raised questions as to whether the NaPi-IIb is an important factor under physiologically relevant situations (Knochel et al., 1996; Heaney et al., 2002). However, severe Pi deprivation is often conducted without consideration for the intestinal segments of Pi absorption. Huber et al. (2002) indicated that at least two different mechanisms were involved in goat intestinal Pi absorption between the duodenum and the jejunum. Additionally, the distribution of transporters is different along the intestinal axis from proximal to distal segments and from the crypt to villus in chickens (Yan et al., 2007). In an attempt to determine the mechanism through which dietary Pi regulates intestinal P absorption, diets almost devoid of Pi (<10% of the total P (tPi) requirement), excess of Pi (>10% of the total Pi (tPi) requirement) and adapting from devoid Pi to excess Pi were utilized in the present chicken experiments. Our objectives were to determine whether modest reductions or adaption in dietary Pi results in stimulation of Na-dependent phosphate transport, mRNA expression and protein levels of NaPi-IIb in the distinct segments of chicken small intestine.
MATERIALS AND METHODS
Animals and diets
All animal work was approved by the University of Hunan Agricultural Animal Care Committee (Changsha, Hunan Province, China). To determine the effects of dietary Pi on the expression of the chicken intestinal Na-Pi co-transporter, experimental diets were formulated to be deficient in total Pi. A total of ninety 40-d-old Ross broilers with an average initial weight of about 1.5 kg were allotted to three treatments randomly, each treatment had five replicates of six broilers. Levels of dietary phosphorus were: low (TP 0.2%) Pi, normal (TP 0.6%) Pi, high (TP 1.0%) Pi. These diets were formulated based on a corn and soybean meal mash basal diet formulated to meet NRC (1994) broiler requirements and China broiler standard (Table 1). In the chronic adaptation studies, the chickens were fed the different Pi diets ad libitum for 7 d. In the acute adaptation studies, the chickens were fed the 0.2% Pi diet for 4 h in the morning for 7 consecutive days. On the day of the experiment, the chickens were fed the 0.2% or 1.0% Pi diet for 4 h before the experiments. Normal drinking water was supplied ad libitum.
Table 1.
Composition and nutrient levels of diets
| Ingrendients (%) | Low Pi | Normal Pi | Higher Pi |
|---|---|---|---|
| Maize | 60 | 56.2 | 54.1 |
| Soybean meal | 28.5 | 28.5 | 28.2 |
| Fish meal | 3.0 | 3.0 | 3.0 |
| Colza oil | 3.5 | 4.5 | 5.0 |
| Met | 0.1 | 0.1 | 0.1 |
| Limestone | 0.5 | 1.3 | 1.6 |
| Dicalcium phosphate | 0 | 2.0 | 3.5 |
| Salt | 0.4 | 0.4 | 0.4 |
| Premixa | 4.0 | 4.0 | 4.0 |
| Nutrient levels | |||
| ME (MJ/kg) | 12.66 | 12.55 | 12.59 |
| CP (%) | 19.38 | 19.08 | 19.01 |
| Lys (%) | 1.08 | 1.07 | 1.07 |
| Met (%) | 0.44 | 0.43 | 0.43 |
| Met+cys (%) | 0.69 | 0.67 | 0.67 |
| Ca (%) | 0.41 | 1.22 | 1.62 |
| Total P (%) | 0.43 | 0.82 | 1.48 |
| Available P (%) | 0.21 | 0.61 | 1.02 |
Premix provided the following per kilogram of diet: vitamin A 10,000 IU; vitamin D3 2,750 IU; vitamin E 20 IU; vitamin K3 2 mg; vitamin B1 1.5 mg; riboflavin 6 mg; pantothenic acid 12 mg; niacin 20 mg; vitamin B6 2.5 mg; vitamin B12 12 μg; choline 500 mg; n 75 mg; Zn 75 mg; Fe 95 mg; Cu 10 mg; I 0.6 mg; Se 0.3 mg.
Isolation of brush border membrane vesicles (BBMVs)
The methods used to prepare intestinal BBMVs have been described previously (Hector Giral et al., 2009). The purity of the membranes was assessed by measuring the levels of leucine amino peptidase, Na+, K+-ATPase and cytochrome c oxidase (Palmada et al., 2004). Ross broilers (1.5 kg) were euthanized with an intraperitoneal injection of 60 mg/kg pentobarbitone sodium ((Pentoject, Animalcare Ltd, York, UK) before removal of the distinct regions of the small intestine. Intestinal segments were opened longitudinally, and the mucosa scraped off using glass slides. The resulting mucosa was suspended in buffer containing (in mmol/L): 50 mannitol, 2Hepes (pH 7.1) and 0.25 phenylmethylsulphonyl fluoride (PMSF), and homogenized three times at half-speed for 20 s using a Ultra Turrax homogenizer (VWR, lutterworth, Leicestershire, UK), followed by the addition of MgCl2 to a concentration of 10 mmol/L, and then stirred on ice for 20 min. The suspension was then centrifuged at 3,000 g for 20 min, and the supernatant then re-centrifuged at 27,000 g for 30 min. The pellet was suspended in buffer containing (in mmol/L): 300 mannitol, 20Hepes, 0.1 MgSO4 (pH 7.2) and 0.25 PMSF, by passing six times through a 21 gauge needle. The suspension was then centrifuged for 15 min at 6,000 g, and the resulting supernatant centrifuged at 27,000 g for a further 30 min. The purified BBMVs pellet was finally resuspended in the same buffer to a protein concentration of 3 to 6 mg/ml using five or six passes through a 21 gauge needle. All steps were carried out at 4°C.
32P uptake into isolated BBMVs
Pi transport was measured by radioactive 32Pi uptake in freshly isolated BBMVs. 32Pi uptake of intestinal BBMVs was measured as previously described (McHaffie GS et al., 2007). Briefly, 10 μl of isolated BBMVs prewarmed to 37°C were incubated with 40 μl of uptake buffer (150 mM NaCl, 16 mM HEPES (pH 7.5), and 0.1 mM K2HPO4) containing K2H32PO4 tracer. After 30 s of incubation at 37°C, uptake buffer was quickly removed by rapid Millipore filtration and washed with ice-cold stop solution (100 mM NaCl, 100 mM mannitol, and 5 mM HEPES, pH 7.5). The incorporation of 32Pi was measured in a beta counter. The Na-dependent uptake of Pi was calculated as the difference between the incorporation of 32Pi in the presence and absence of NaCl.
Western blot analysis
Polyclonal antibodies for NaPi-IIb were raised in female New Zealand white rabbits against a synthetic peptide (CKNLEEEEKEQDVPVKAS) corresponding to the amino acids 644–661 residues of the C-terminus of the chicken NaPi-IIb protein (GenBank accession number AF AF29887). The methods used to develop antibodies have been described previously (Ramziya Kiyamova et al., 2008). For western blotting, samples (50 μg of intestinal BBMV) were separated on 10% SDS/PAGE gels, transferred to PVDF membranes and incubated overnight with antibodies against NaPi-IIb (1:3,000) and β-actin (1:10,000). After several washes, membranes were incubated for 2 h at room temperature with HRP-linked secondary antibodies (cat no.; NA931V, GE Healthcare) followed by incubation with a chemiluminescence reagent (ECL, Amersham Pharmacia Biotech). Immunoreactive signals were detected using the DIANA III-chemiluminescence detection system (Raytest, Straubenhardt, Germany) and quantification was performed with AIDA software (Advanced Image Data Analyser AIDA, Raytest). Data are shown as ratios between the protein of interest to β-actin.
Real-time PCR
Total RNA was extracted from mucosal scrapes of the distinct regions of the chick small intestine using a QIAamp RNA blood mini kit according to the manufacturer’s instructions (Qiagen, Crawley, UK). RNA was reverse transcribed with 0.5 μg of oligo-dT primer and a First Strand cDNA synthesis kit (Superscript II RNase H-reverse transcriptase; Life Technologies, Paisley, UK). Using QuantiTech SYBR® Green PCR kit (Qiagen) on a LightCycler Real-Time PCR instrument (version 3.5, Roche Diagnostics, Lewes, UK), we determined mRNA levels using the following primers: NaPi-IIb [5’-GAAAGTGGTGAAGATGCC-3’ (forward) and (reverse) 5’- AAGTATGAGACCGATGGC -3’], β-actin [5’-CAGCCATCTTTCTTGGGTAT-3’ (forward) and (reverse) 5’-GTTTAGAAGCATTTGCGGTG-3’]. For all primers, cycling conditions were as follows: 95°C for 10 min followed by 45 cycles of 95°C for 15 s, 60°C for 20 s and 72°C for 40 s with transition rates of 20°C/s and a single fluorescence acquisition at 81°C. A standard curve for each gene was established by performing the above procedure with serially diluted DNA samples of known concentrations. The relative amounts of the target and reference genes in each sample were then calculated based on the crossing-point analysis (Relquant, version 1.01, Roche Diagnostics); the second derivative maximum method was used to automatically determine the crossing point for individual samples. The sequences of the PCR products were confirmed by DNA sequencing.
Statistics
All data (means±SD) were tested for significance by applying the unpaired Student’s t-test and ANOVA. Differences at the level of p<0.01 and p<0.05 were considered as significant.
RESULTS
Expression of NaPi-IIb in distinct regions of the chicken small intestine
The profile of phosphate absorption and the expression of NaPi-IIb mRNA and protein along three distinct intestinal regions of the chicken small intestine were examined using the following landmarks: duodenum (from distal of the gizzard to 1 cm distal of the bile duct), jejunum (1 cm distal from the bile duct to the Meckel’s diverticulum), and ileum (Meckel’s diverticulum to 5 cm proximal to the ileocecal junction). In normal dietary Pi levels (TP 0.6%), Pi uptake measurements of intestinal BBMV showed that Na-dependent Pi activity was significantly higher in the jejunum than in the ileum and duodenum (Figure 1A), with the latter two regions having the ability to absorb equivalent amounts of phosphate. Quantification of NaPi-IIb mRNA expression in the chicken small intestine using real-time PCR demonstrated that the transcript for this protein is expressed at highest levels in the duodenum followed by the jejunum and the ileum (Figure 1B). The protein levels of NaPi-IIb were examined using Western blotting of intestinal BBM vesicles isolated from the distinct regions of the chicken small intestine. The ratio of this protein to β-actin enabled us to access the regional distribution of the sodium-phosphate co-transporter. In accordance with the in vivo uptake data and the mRNA expression profile, quantification of NaPi-IIb protein in relation to β-actin protein levels revealed that the transporter was most abundant in the duodenum and that lower levels were evident in the jejunum and in the ileum (Figure 1C and 1D).
Figure 1.
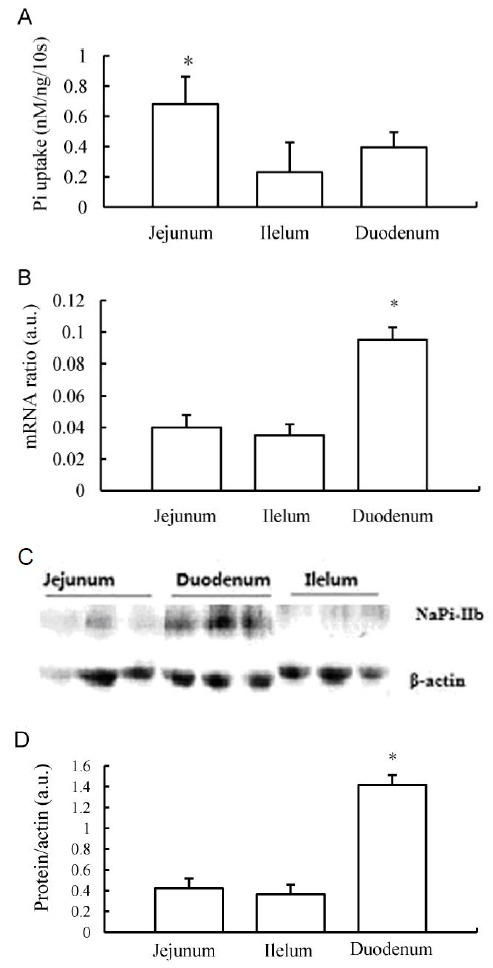
Expression profile of NaPi transport activity, protein, and mRNA along the small intestine of chickens fed the 0.6% Pi diet ad libitum. (A) brush border membrane (BBM) vesicle (BBMV) Na-dependent Pi uptake activity in small intestinal segments. (B) NaPi-IIb mRNA abundance determined by RT-PCR in chicken intestinal segments. (C) Western blot analysis of NaPi-IIb protein in distinct regions of the chicken small intestine. (D) Quantification of NaPi-IIb protein relative to β-actin. a.u., arbitrary units. * p<0.05.
Effects of dietary Pi restriction on regulation of intestinal Na-Pi transporter activity, protein and Mrna
Intestinal BBMV were isolated from chickens chronically fed the 0.2, 0.6, or 1.0% Pi diets. Pi uptake activity was determined in BBMV isolated from the duodenum, the jejunum and the ileum. Duodenal BBMV isolated from birds chronically fed the low-Pi (0.2%) diet showed a 68% increase in the Na-dependent Pi uptake compared with BBMV isolated from the birds fed the normal-Pi (0.6%) or high-Pi (1.0%) diet (Figure 2). However, there were no significant differences in the jejunal and ileac BBMV Pi transport activity among the three Pi diets (Figure 2).
Figure 2.

Effects of dietary Pi on chicken intestinal BBMV Pi transport activity. NaPi transport uptake was measured in BBMV of duodenum, ileum and jejunum of chickens chronically fed high-Pi (1.0%), normal Pi (0.6%), or low-Pi (0.2%) diet. * p<0.05.
NaPi-IIb levels were determined in BBMV isolated from three distinct regions of the chicken small intestine by Western blotting. Jejunal and ileac BBM did not show significant changes in NaPi-IIb expression (Figure 3A). However, BBM isolated from the duodenum fed the 0.2% Pi diet showed an up-regulation of NaPi-IIb total protein expression (Figure 3B).
Figure 3.
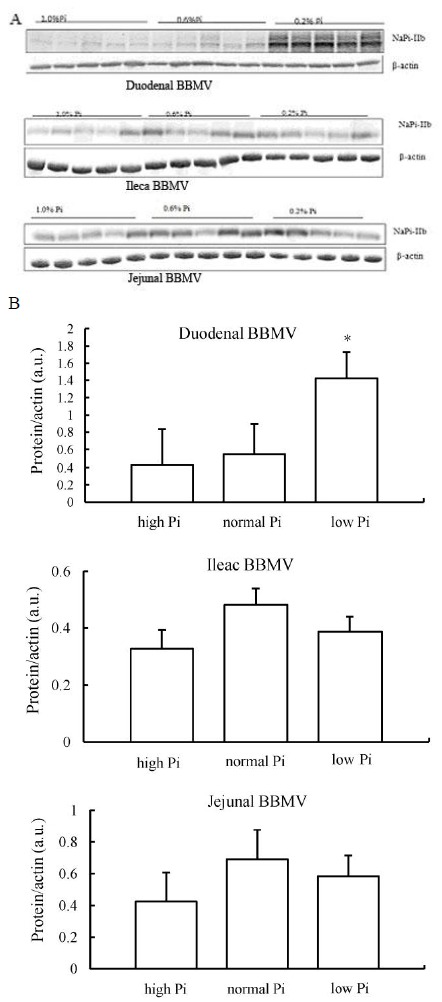
BBMV NaPi-IIb protein regulation in chickens chronically fed high-, normal-, or low-Pi diet. (A) detection of NaPi-IIb protein by Western blots analysis in BBM vesicles prepared from intestinal mucosa of chicken duodenum, jejunum and ileum. (B) quantification of NaPi-IIb protein relative to β-actin, results is expressed as the means±SEM of Western blots. The abundance of NaPi-IIb is given as a ratio of NaPi-IIb protein to β-actin protein, in a.u.. * p<0.05.
Quantification of mRNA levels of the Na-Pi co-transporters showed a significant increase of NaPi-IIb mRNA levels in the ileum of chickens fed the 0.2% Pi diet. This implies that chronic adaptation to the low-Pi diet is at least in part mediated by transcriptional mechanisms (Figure 4).
Figure 4.

Chicken intestinal NaPi-IIb mRNA regulation chronically fed high-, normal-, or low Pi diet. In duodenum, NaPi-IIb mRNA abundance is increased in parallel with increases in BBMV NaPi-IIb protein abundance and Pi uptake in response to chronic low-Pi diet. There are no changes in jejunal or ileac NaPi-IIb mRNA abundance in response to low-Pi diet. * p<0.05.
Changes of NaPi-IIb protein and mRNA levels when adapted low-Pi diet to normal Pi diet for 4 h
For these studies, chickens were trained to eat their diets for 7 d. On the 8th day, chickens that were chronically adapted on a low-Pi diet were fed acutely a normal-Pi diet for 4 h. In BBMV isolated from the jejunum, there was a fivefold increase in the Pi uptake rate when the chickens were switched from a chronic low-Pi to an acute high-Pi diet. In contrast, in BBMV isolated from the duodenum, there was no change in Pi uptake under the same conditions, indicating a regulatory adaptation different from that in chronic conditions (Figure 5).
Figure 5.
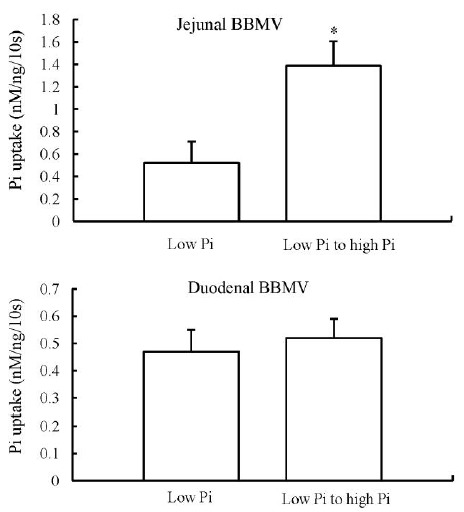
Na-dependent Pi uptake in BBMV of chickens chronically adapted to a low-Pi diet and then fed low- to high-Pi diet for 4 h. Jejunal BBMV shows an increased NaPi transport activity. Whereas duodenal BBMV demonstrates no significant changes in NaPi transport activity. * p<0.05.
The increase in BBMV Pi uptake in the jejunum was associated with an increase in NaPi-IIb protein abundance (Figure 6A and 6B). There were no changes in NaPi-IIb protein abundance in BBM isolated from the duodenum, and also there were no changes in NaPi-IIb mRNA abundance in the duodenum or jejunum (Figure 7).
Figure 6.

NaPi-IIb protein regulation in chickens chronically adapted to low-Pi diet and then fed low- or high-Pi diet for 4 h. (A) detection of NaPi-IIb protein by Western blots analysis in chicken duodenum and jejunum BBMV. (B) quantification of NaPi-IIb protein relative to β-actin. There is a significant increase in jejunal BBMV NaPi-IIb protein abundance. No significant changes in NaPi-IIb protein in duodenal BBMV. * p<0.05.
Figure 7.
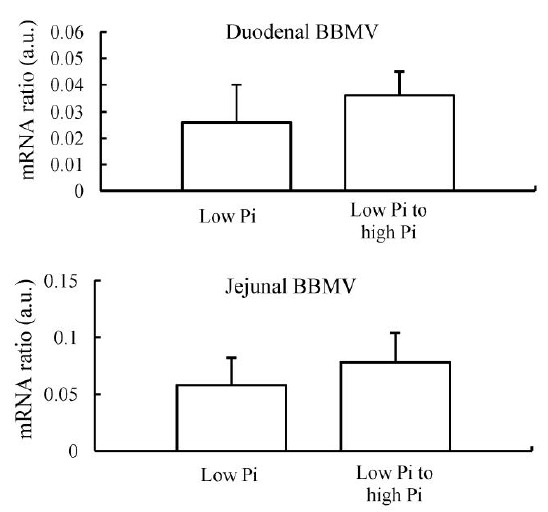
NaPi-IIb mRNA regulation in chicken small intestine when chronically adapted to low-Pi diet and then fed low- or high-Pi diet for 4 h. There are no significant changes in NaPi-IIb mRNA levels both duodenal BBMV and jejunal BBMV in response to acute changes in dietary Pi.
DISCUSSION
Dietary phosphate deprivation is an important physiological regulator of intestinal phosphate absorption. Previously, in a series of studies with rodent species, dietary Pi restriction acutely and chronically increased intestinal NaPi-IIb transporter expression (Cross et al., 1990; Hattenhauer et al., 1999, Segawa et al., 2004). Similar responses have been reported in goats, humans and fishes (Huber et al., 2002; Sugiura et al., 2007; Villa-Bellosta et al., 2010). In our study, the reduction of Pi in the diet had a significant effect on the Pi uptake and the expression of the Na-Pi cotransporter by stimulating increase in the mRNA levels only in the duodenum. However, in mice it was shown that significant increases of NaPi-IIb mRNA abundance in duodenum, jejunum, and ileum when fed low-P diet for 5 d. Furthermore, Hattenhauer et al. (1999) saw NaPi-IIb protein and Pi transport were both increased across all segments of the intestine by dietary P restriction in mice after 5 d of feeding with low-P diet, although there was not any increase of NaPi-IIb mRNA. In rat, the NaPi-IIb transporter was found to be increased from duodenum to ileum when fed a low-P diet compared to fed an adequate-P diet. It is unclear why the broiler NaPi-IIb cotransporter expression was found not to be increased with dietary P restriction in as many segments as it is in rodent species. It is possibly due to the specific length and the movement of nutrients through different segments of small intestine. However, it appears that the severity of dietary restriction, and length of time over which restriction is imposed may affect the mechanism though which intestinal Na-dependent phosphate uptake is stimulated.
In this study, we found that the chicken small intestine has the ability to absorb phosphate in all segments examined, with the highest NaPi-IIb protein expression in the BBMVs of chicken duodenum under normal dietary Pi. This finding is in agreement with a previous study that showed the highest NaPi-IIb mRNA expression in chicken duodenum as well (Miyamoto et al., 1995). However, it was surprising that BBMVs Na-Pi co-transport activity was highest in the jejunum, despite lower NaPi-IIb protein and mRNA expression than in the duodenum. The equilibrium values of Pi uptake determined in duodenal and jejunal BBMVs under three different dietary Pi levels were very similar, suggesting that this discrepancy was not induced by differences in intravascular space/total protein rates. Posttranslational modification of NaPi-IIb protein and/or the presence of additional Na-Pi transporters playing different roles in the duodenum and the jejunum could explain the discrepancy between Na-Pi co-transport activity and NaPi-IIb protein expression. Posttranslational modifications of NaPi-IIb described in previous studies include glycosylation (Arima et al., 2002), ubiquitination (Xu et al., 2002), and palmitoylation (Hashimoto et al., 2000). However, the role of these modifications in modulation of the activity or trafficking of the intestinal Na-Pi transporters needs to be determined.
The jejunum has been considered to be the major site for Pi absorption in chicks (Hurwitz et al., 1970; Blahos et al., 1981). The duodenum, however, may have a greater ability to absorb Pi than the jejunum, but its short length and short transit time of digesta through the duodenum possibly limits its role in chicken intestinal Pi absorption (Yan et al., 2007). Our studies involved in the regulation of NaPi-IIb with dietary Pi deprivation showed that jejunal BBMV NaPi intake activity is increased, when birds adapted to a chronic low-Pi (0.2%) for 7 d compared with animals adapted to high-Pi (1.0%) diets. The increase in NaPi transport activity is associated with parallel increases in NaPi-IIb protein and mRNA in the jejunum. In contrast, no significant response is observed in ileac and duodenal BBMV. The increase in Na-dependent Pi transport activity in the jejunum after dietary Pi restriction treatment is paralleled to the increase in NaPi-IIb protein and mRNA levels, suggesting that the increase induced by low-Pi regulation likely involves the synthesis of new NaPi-IIb mRNA in the jejunum. This finding indicates that the effect of chronically dietary Pi regulation on chicken jejunal NaPi-IIb gene expression can be mediated by increased transcriptional activation.
In contrast, birds fed a low-Pi diet for 7 d and then acutely adapted to a high-Pi diet for 4 h shows an unexpected significantly increase in jejunal BBMV NaPi transport activity associated with a parallel increase in jejunal BBMV NaPi-IIb protein abundance. However, there are no significant changes in duodenal BBMV NaPi transport activity and NaPi-IIb protein expression. More interestingly, there were no significant changes in the jejunal and duodenal BBMV NaPi-IIb mRNA abundance in response to acutely dietary Pi adaptation. This inconsistency might be a consequence of regional differences in the stability of the mRNA, or the rate of translation and/or degradation of the protein, or might relate to differences in post-translational modification or the intrinsic activity of the protein in the different segments of the small intestine.
In summary, we determined the protein expression of the NaPi-IIb in the apical membrane of chicken enterocytes. NaPi-IIb plays a major role in the adaptation to chronic and acute alterations in dietary phosphate. The dietary adaptation of NaPi-IIb in the duodenum is different from that in the jejunum, suggesting different regulatory mechanisms. Although the duodenum seems to play a more important role in the chronic adaptation to a low-Pi diet, the jejunum plays a major role in the acute response to a high-Pi diet.
ACKNOWLEDGEMENTS
This research was supported by grants from the National Natural Science Foundation of China (No.31172218), National Scientific and Technological Supporting Project (No. 2011BAD26B03), the Program for Science and Technology Innovative Research Team in Higher Educational Institutions of Hunan Province. Thanks also be given to Dr. Zhai Hengxiao from Purdue University of America for his kindness revised of this manuscript.
REFERENCES
- Arima K, Hines ER, Kiela PR, Drees JB, Collins JF, Chishan FK. Glucocorticoid regulation and glycosylation of mouse intestinal type IIb Na-Pi cotransporter during ontogeny. Am J Physiol Gastrointest Liver Physiol. 2002;283:G426–G434. doi: 10.1152/ajpgi.00319.2001. [DOI] [PubMed] [Google Scholar]
- Bai L, Collins JF, Ghishan FK. Cloning and characterization of a type III Na-dependent phosphate cotransporter from mouse intestine. Am J Physiol Cell Physiol. 2000;279:C1135–C1143. doi: 10.1152/ajpcell.2000.279.4.C1135. [DOI] [PubMed] [Google Scholar]
- Blahos J, Care AD. The jejunumis the site of maximal rate of intestinal absorption of phosphate in chicks. Physiol Bohemoslov. 1981;30:157–159. [PubMed] [Google Scholar]
- Borowitz SM, Ghishan FK. Phosphate transport in human jejunal bnrsh border membrane vesicles. Gastroenterology. 1989;96:4–10. doi: 10.1016/0016-5085(89)90757-9. [DOI] [PubMed] [Google Scholar]
- Borowitz SM, Granrud GS. Ontogency of intestinal phosphate absorption in rabbits. Am J Physiol. 1992;262:G847–G853. doi: 10.1152/ajpgi.1992.262.5.G847. [DOI] [PubMed] [Google Scholar]
- Collins JF, Ghishan FK. Molecular cloning, functional expression, tissue distribution, and in situ hybridization of the renal sodium phosphate (Na+/Pi) transporter in the control and hypophosphatemic mouse. FASEB J. 1994;8:862–868. doi: 10.1096/fasebj.8.11.8070635. [DOI] [PubMed] [Google Scholar]
- Cross HS, Debiec H, Peterlik M. Mechanism and regulation of intestinal phosphate absorption. Miner Electrolyte Metab. 1990;16:115–124. [PubMed] [Google Scholar]
- Ghishan FK, Arab N, Shibata H. Intestinal phosphate transport in spontaneously hypertensive rats and genetically matched controls. Gastroenterology. 1990;99:106–112. doi: 10.1016/0016-5085(90)91236-y. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Wang D, Kamo T, Zhu Y, Tsujiuchi T, Konishi Y, Tanaka M, Sugimura H. Isolation and localization of type IIb Na/Pi cotransporter in the developing rat lung. Am J Pathol. 2000;157:21–27. doi: 10.1016/S0002-9440(10)64512-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattenhaur O, Traebert M, Murer H, Biber J. Regulation of small intestinal Na-Pi type IIb cotransporter by dietary phosphate intake. Am J Physiol Gasreoinrest Liver Physiol. 1999;277:G756–762. doi: 10.1152/ajpgi.1999.277.4.G756. [DOI] [PubMed] [Google Scholar]
- Heaney RP, Nordin BEC. Calcium effects nn phnsphoius absorption:implications for the prevention and co-therapy of osteoporosis. J Am Coll Nutr. 2002;21:239–204. doi: 10.1080/07315724.2002.10719216. [DOI] [PubMed] [Google Scholar]
- Giral Hector, Caldas Yupanqui, Sutherland Eileen, Wilson Paul, Breusegem Sophia, Barry Nicholas, Blaine Judith, Jiang Tao, Wang Xiaoxin X, Levi Moshe. Regulation of rat intestinal Na-dependent phosphate transporters by dietary phosphate. Am J Physiol Renal Physiol. 2009;297:1466–1475. doi: 10.1152/ajprenal.00279.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilfiker H, Hattenhauer O, Traebert M, Forster I, Murer H, Biber J. Characterization of a murine type II sodiumphosphate cotransporter expressed in mammalian intestine. Proc Natl Acad Sci USA. 1998;95:14564–14569. doi: 10.1073/pnas.95.24.14564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber K, Walter C, Schroder B, Breves G. Phosphate transport in the duodenum and jejunum of goats and its adaptation by dietary phosphate and calcium. Am J Physiol Regul Integr Comp Physiol. 2002;283:R296–R302. doi: 10.1152/ajpregu.00760.2001. [DOI] [PubMed] [Google Scholar]
- Hurwitz S, Bar A. The sites of calcium and phosphate absorption in the chick. Poult Sci. 1970;49:324–325. doi: 10.3382/ps.0490324. [DOI] [PubMed] [Google Scholar]
- Kiyamova R, Gryshkova V, Ovcharenko G, Lituyev D, et al. Development of monoclonal antibodis specific for the human sodium-dependent phosphate cotransporter NaPi2b. Hybridoma. 2008;27:277–284. doi: 10.1089/hyb.2008.0015. [DOI] [PubMed] [Google Scholar]
- Magagnin S, Werner A, Markovich D, Sorribas V, Stange G, Biber J, Murer H. Expression cloning of human and rat renal cortex Na/Pi cotransport. Proc Natl Acad Sci USA. 1993;90:5979–5983. doi: 10.1073/pnas.90.13.5979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHaffie GS, Graham C, Kohl B, Strunck-Warnecke U, Werner A. The role of an intracellular cysteine stretch in the sorting of the type II Na/phosphate cotransporter. Biochim Biophys Acta. 2007;1768:2099–2106. doi: 10.1016/j.bbamem.2007.05.017. [DOI] [PubMed] [Google Scholar]
- Miyamoto K, Tatsumi S, Sondoda T, Yamamoto H, Minami H, Taketani Y, Takeda E. Cloning and functional expression of a Na-dependent phosphate cotransporter from human kidney: cDNA cloning and functional expression. Biochem J. 1995;301:81–85. doi: 10.1042/bj3050081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murer H, Forster I, Biber J. The sodium phosphate cotransporter family SLC34. Pflugers Arch. 2004;447:763–767. doi: 10.1007/s00424-003-1072-5. [DOI] [PubMed] [Google Scholar]
- Nakagawa N, Ghishan FK. Low phosphate diet upregulates the renal and intestinal sodium-dependent phosphate transporter in vitamin D-resistant hypophosphatemic mice. Proc Soc Exp Biol Med. 1994;205:162–167. doi: 10.3181/00379727-205-43692. [DOI] [PubMed] [Google Scholar]
- Palmada M, Dieter M, Speil A, Bohmer C, Mack AF, Wagner HJ, Klingel K, Kandolf R, Murer H, Biber J, Closs EI, Lang F. Regulation of intestinal phosphate cotransporter NaPi IIb by ubiquitin ligase Nedd4-2 and by serum- and glucocorticoid-dependent kinase 1. Am J Physiol Gastrointest Liver Physiol. 2004;287:G143–G150. doi: 10.1152/ajpgi.00121.2003. [DOI] [PubMed] [Google Scholar]
- Quamme GA. Phosphate transport in intestinal brush-border membrane vesicles: effect of pH and dietary phosphate. Am J physiol. 1985;249:G168–G176. doi: 10.1152/ajpgi.1985.249.2.G168. [DOI] [PubMed] [Google Scholar]
- Radanovic T, Wagner CA, Murer H, Biber J. Regulation of intestinal phosphate transport. I. Segmental expression and adaptation to low-P (i) diet of the type IIb Na+-Pi cotransporter in mouse small intestine. Am J Physiol. 2005;288:G496–G500. doi: 10.1152/ajpgi.00167.2004. [DOI] [PubMed] [Google Scholar]
- Saddoris KL, Fleet JC, Radcliffe JS. Sodium-dependent phosphate uptake in the jejunum is post-transcriptionally regulated in pigs fed a low-phosphorus diet and is independent of dietary calcium concentration. J Nutr. 2010;4:731–736. doi: 10.3945/jn.109.110080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segawa H, Kaneko I, Yamanaka S, Ito M, Kuwahata M, Inoue Y, Kato S, Miyamoto K. Intestinal Na-Pi cotranspotter adaptation to dietuy Pi content in vitamin D receptor null mice. Am J Physiol Renal Physiol. 2004;287:F39–47. doi: 10.1152/ajprenal.00375.2003. [DOI] [PubMed] [Google Scholar]
- Sugiura SH, Kelsey K, Ferraris RP. Molecular and conventional responses of large rainbow trout to dietary phosphorus restriction. J Comp Physiol B. 2007;177:461–472. doi: 10.1007/s00360-007-0144-9. [DOI] [PubMed] [Google Scholar]
- Villa-Bellosta R, Sorribas V. Compensatory regulation of the sodium/phosphate cotransporters NaPi-IIc (SCL34A3) and Pit-2 (SLC20A2) during Pi deprivation and acidosis. Pflugers Arch. 2010;459:499–508. doi: 10.1007/s00424-009-0746-z. [DOI] [PubMed] [Google Scholar]
- Xu H, Bai L, Collins JF, Ghishan FK. Age-dependent regulation of rat intestinal type IIb sodium-phosphate cotransporter by 1, 25-(OH) 2 vitamin D3. Am J Physiol Cell Physiol. 2002;282:C487–C493. doi: 10.1152/ajpcell.00412.2001. [DOI] [PubMed] [Google Scholar]
- Yan F, Angel R, Ashwell CM. Characterization of the chicken small intestine type IIb sodium phosphate cotransporter. Poult Sci. 2007;86:67–76. doi: 10.1093/ps/86.1.67. [DOI] [PubMed] [Google Scholar]


