Abstract
Steroidogenesis requires coordination of the anabolic and catabolic pathways of lipid metabolism, but the profile of proteins associated with progesterone synthesis in cyclic and pregnant corpus luteum (CL) is not well-known in cattle. In Experiment 1, plasma progesterone level was monitored in cyclic cows (n = 5) and pregnant cows (n = 6; until d-90). A significant decline in the plasma progesterone level occurred at d-19 of cyclic cows. Progesterone level in abbatoir-derived luteal tissues was also determined at d 1 to 5, 6 to 13 and 14 to 20 of cyclic cows, and d-60 and -90 of pregnant cows (n = 5 each). Progesterone level in d-60 CL was not different from those in d 6 to 13 CL and d-90 CL, although the difference between d 6 to 13 and d-90 was significant. In Experiment 2, protein expression pattern in CL at d-90 (n = 4) was compared with that in CL of cyclic cows at d 6 to 13 (n = 5). Significant changes in the level of protein expression were detected in 32 protein spots by two-dimensional polyacrylamide gel electrophoresis (2-DE), and 23 of them were identified by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS). Six proteins were found only in pregnant CL, while the other 17 proteins were found only in cyclic CL. Among the above 6 proteins, vimentin which is involved in the regulation of post-implantation development was included. Thus, the protein expression pattern in CL was disorientated from cyclic luteal phase to mid pregnancy, and alterations in specific CL protein expression may contribute to the maintenance of pregnancy in Korean native cows.
Keywords: Corpus Luteum, Korean Native Cows, Pregnancy, Progesterone, Proteome Analysis
INTRODUCTION
The function of corpus luteum (CL) formed in the ovary following ovulation is a temporary endocrine gland, and contributes to the regulation of estrus/menstrual cycle and the successful maintenance of pregnancy (Axelson et al., 1975). The CL develops, functions, and begins to regress within 21 d after ovulation in non-pregnant women, but retains a functional lifespan for more than 200 d during pregnancy (Bersinger et al., 2009). Actually in most placental mammals, the continuation of luteal function associated with pregnancy extends well beyond the period of a single estrus or menstrual cycle, and is required for the establishment of pregnancy. Low concentrations of progesterone have been implicated as a causative factor in the low pregnancy rates in dairy cows (Diskin et al., 2008). More recent evidence has shown that lower circulating concentrations of progesterone in postpartum dairy cows are associated with an impaired ability of the oviduct/uterus to support embryo development compared with that of dairy heifers (Rizos et al., 2010). The kinetics of steroidogenic enzymes, such as cytochrome P450 side-chain cleavage (Rodgers et al., 1986; Rodgers et al., 1987), 3β-hydroxysteroid dehydrogenase (Couet et al., 1990), and P45017α hydroxylase (Rodgers et al., 1987), has been reported for the CL during the luteal phase of cyclic cows. In general, level of mRNA coding for the steroidogenic enzymes increases during the luteal development, especially at the mid-luteal phase of estrous cycle (McCord et al., 1969; Li et al., 1993).
As attempts to understand how the biochemistry of the ovary and uterus adapts to the presence of embryos and supports the maintenance of pregnancy, proteins in the CL have been investigated in some mammalian species including sheep (Willcox, 1986), cattle (Knickerbocker et al., 1986) and human (Groten et al., 2006). However, the factors responsible for the progressive changes in CL between cyclic luteal cows and pregnant cows are still unknown. Therefore in the present study, protein expression pattern in CL during mid pregnancy was compared with that in CL at d 6 to 13 of cyclic Korean native cows, following progesterone measurement in blood plasma and CL tissues of cyclic or pregnant cows. Two-dimensional polyacrylamide gel electrophoresis (2-DE) and matrix-associated laser desorption/ionization time-to-flight mass spectrometry (MALDI-TOF-MS) were used to separate complex polypeptide mixtures and to identify proteins in CL that are associated with establishment of the pregnancy, respectively.
MATERIALS AND METHODS
Experimental design
Experiment 1
To monitor the plasma progesterone level, pubertal Korean native cows were used. Cows were either artificially inseminated with frozen semen to become pregnant (n = 6) or non-treated to serve as cyclic cows (n = 5). Their plasma samples were subjected to ELISA for progesterone measurement. In an additional experiment, progesterone levels were determined from CL tissues of the abbatoir-derived non-pregnant ovaries (d 1 to 5, 6 to 13 and 14 to 20; n = 5 each) or pregnant ovaries (d-60 and -90; n = 5 each).
Experiment 2
Proteomic analysis of the luteal tissues at d 6 to 13 of cyclic cows (n = 5) and Day-90 of pregnant cows (n = 4) was performed using the 2-DE and the MALDI-TOF-MS. Protein spots exhibiting two-folds or more expression in the 2-DE patterns when compared with their counterparts were selected for the MALDI-TOF-MS, and the search program “ProFound” was used for protein identification by peptide mass fingerprinting.
Plasma progesterone level during estrus cycle and pregnancy
The National Institute of Animal Science Agricultural Animal Care and Use Committee approved the procedures used in the experiments. A total of 11 pubertal Korean native cows at 2 to 3 yrs old were used in this study. The Korean native cows under semi-intensive range condition were fed (07:00 and 16:00) with a mixture of locally available glass, corn silage, and concentrates (TDN 54.4% and CP 12.8% on DM basis). Cows were examined every 2 other days by palpation per rectum of genitalia and ovaries with transrectal ultrasonography (Aloka 500V, Aloka, Wallingford, CT, USA) equipped with a 5.0-MHz linear-array transducer probe. To determine the exact day of ovulation, all the cows received GnRH (0.01 mg Buserelin, 2.5 ml of Receptal™, Intervet, Unterschleißheim, Germany) and PGF2α (0.5 mg Cloprostenol, 2.0 ml Estrumate™, Essex Tierznei, Munich, Germany) at 7 d later, and then GnRH 48 h after the PGF2α injection. Cows were either artificially inseminated at 12 and 24 h after GnRH application (n = 6) or non-treated for cyclic CL (n = 5). Blood samples were collected into 10 ml tubes containing 0.3 M EDTA (BD Vacutainer™, Belliver Industrial Estste, Plymouth, UK) at 08:00 to 10:00 daily by puncture of the jugular veins, and immediately centrifuged for 20 min at 2,500×g. After storage of the plasma at −20°C, progesterone concentration was measured by a specific Immunoassay kit (DELFIA®, Boston, MA, USA) according to the protocol by manufacturer.
Progesterone level in luteal tissues at various stages
Ovaries were obtained from pubertal Korean native cows at the abattoir of National Institute of Animal Science in Suwon. The CLs from non-pregnant animals were classified into three stages by a careful examination of the reproductive tract as described and validated by Ireland et al. (1980) with slight modifications. Briefly, CL-1 phase (d 1 to 5) includes the interval between ovulation and time when the epithelium grows over the rupture point. During CL-2 phase (d 6 to 13), the CL is enclosed in epithelium, blood vessels are visible around the periphery of the CL and the color of apex is red. In CL-3 phase (d 14 to 20), the ovaries contain at least one large follicle and the CL has decreased in size and has no visible blood vessels on the CL surface. CLs were also collected from pregnant cows whose stages were estimated from the crown-rump length of the fetus (either d-60 or -90). The whole ovaries were frozen on solid CO2 immediately after collection (within 20 min from the slaughter) and stored at −20°C. After partial thawing, the CLs were dissected from the rest of the ovarian stroma and weighed. The individual CL (10 to 20 mg wet weight) was placed in cold extraction medium consisting of 15% (v/v) trifluoroacetic acid, 5% (v/v) formic acid, 1% (w/v) sodium chloride in 1 M HCl and homogenized as described by Osnes et al. (1993). Aliquots of each CL tissue extract were used to measure progesterone concentration in a specific ELISA. The progesterone content of the CL tissues was estimated by a scaled-down version of the technique described by Axelson et al. (1975).
Proteomic analysis of d 6 to13 and d-90 luteal tissues
2-DE
The corpus luteum tissues were homogenized directly by motor-driven homogenizer (PowerGen125, Fisher Scientific, Pittsburgh, PA, USA) in sample buffer containing 7 M urea, 2 M thiourea, 4% (w/v) 3-((3-cholamidopropy) dimethylammonio)-1-propanesulfonate (CHAPS), 1% (w/v) dithiothreitol (DTT), 2% (v/v) pharmalyte and 1 mM benzamidine. Proteins were extracted for 1 h at room temperature with vortexing. After centrifugation at 15,000×g for 1 h at 15°C, the soluble fraction was used for 2-DE normalized by Bradford assay (Bradford, 1976). The IPG dry strips were equilibrated for 12 to 16 h with a buffer containing 7 M urea, 2 M thiourea, 2% CHAPS, 1% DTT and 1% pharmalyte, and loaded with 200 μg of sample. Isoelectric focusing (IEF) was performed at 20°C using a Multiphor II electrophoresis unit and EPS 3500 XL power supply (Amersham Biosciences, Oslo, Oslo, Norway) according to the manufacturer’s instruction. For IEF, the voltage was linearly increased from 150 to 3,500 V during 3 h for sample entry followed by constant 3,500 V with focusing complete after 96 kVh. Prior to the second dimension, strips were incubated for 10 min in equilibration buffer (50 mM Tris-HCl, pH 6.8 containing 6 M urea, 2% SDS and 30% glycerol), first with 1% DTT and second with 2.5% iodoacetamide. Equilibrated strips were inserted onto SDS-PAGE gels (20 to 24 cm, 10 to 16%). The SDS-PAGE was performed using Hoefer DALT 2-DE system (Amersham Biosciences) according to the manufacturer’s instruction. The 2DE gels were run at 20°C for 1,700 Vh. And then, 2-DE gels were silver-stained as described by Oakley et al. (1980) with minor modifications as omitting fixation and sensitization steps with glutaraldehyde. Digitized images were quantitatively analyzed using the PDQuest software (version 7.1, Bio-Rad, Hercules, CA, USA) according to the protocols provided by the manufacturer. Quantities of each spot were selected for the significant expression variation over two folds in its expression level compared with non-pregnant CL tissues.
MALDI-TOF-MS
Protein analysis was performed by using Ettan MALDI-TOF (Amersham Biosciences). Peptides were evaporated with a N2 laser at 337 nm, using a delayed extraction approach. They were accelerated with 20 kV injection pulse for time of flight analysis. Each spectrum was the cumulative average of 300 laser shots. The search program “ProFound”, developed by the Rockefeller University (http://129.85.19.192/profound_bin/WebProfound.exe) was used for protein identification by peptide mass fingerprinting. Spectra were calibrated with trypsin auto-digestion Ion peak m/z (842.510, 2211.1046) as internal standards.
Statistical analysis
All data were presented as means±SEM. Plasma and CL progesterone concentrations were compared using Student’s t-test. A value of p<0.05 was considered statistically significant difference.
RESULTS
Experiment 1
Progesterone level
Plasma progesterone level was monitored in cyclic cows (n = 5) and pregnant cows (n = 6; until d-90), as shown in Figure 1A. The plasma progesterone concentrations increased equally in both the cyclic and pregnant cows up to d-18. However, in comparison with the pregnant cows, a significant decline in the plasma progesterone level occurred at d-19 of cyclic cows. Progesterone level in luteal tissues was also determined at d 1 to 5, 6 to 13 and 14 to 20 of cyclic cows, and d-60 and -90 of pregnant cows (n = 5 each), as shown in Figure 1B. In cyclic cows, the progesterone level of the CL increased from CL-1 to CL-2 stage (p<0.05), and then dropped precipitously by CL-3 stage (p<0.001). On the other hand, progesterone level in d-60 CL was not different from those in d 6 to 13 CL and d-90 CL, although the difference between d 6 to 13 and d-90 was significant.
Figure 1.
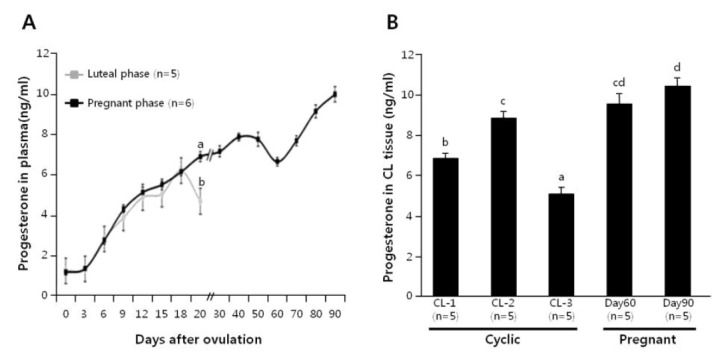
Time-dependent changes of plasma progesterone concentrations in cyclic and pregnant cows (A). Luteal progesterone concentrations in non-pregnant (CL-1, -2 and -3) and pregnant (d-60 and -90) cows (B). Different letters on the SEM bars indicate significant difference between the groups (p<0.05).
Experiment 2
Proteomic analysis
The proteins obtained from CL tissues at luteal phase (CL-2) and at d-90 of pregnancy were applied to 2-DE and visualized by silver staining. The number of spots estimated using PDQuest software was approximately 600 to 900, and quantity of each spot was normalized by total valid spot intensity. Protein spots (n = 32) were selected for the significant expression variation deviated over two-folds in pregnant CL proteins compared with luteal phase CL proteins. After the statistical analysis, MALDI-TOF-MS analysis was performed for all of the selected protein spots. This analysis was performed at least three times for each protein spot, and 23 proteins were clearly identified.
The identified 23 proteins are listed in Table 1, and their position on the 2-DE gel map was marked by SWISS-PROT accession number (Figure 2). Proteins listed in Table 1 are provided with their molecular mass, and experimental corresponding percentile in an estimated random match population (Est’dz score). A comparison of the density of the spots identified on the reference map between the luteal phase and pregnant CL tissues showed that 6 protein spots were significantly increased (Figure 2A and Table 1), while 17 protein spots were significantly decreased (Figure 2B and Table 1) in pregnant CL tissues.
Table 1.
Profiling of the 23 proteins for which the expression level differed in luteal tissues between luteal phase (d 6 to 13) and corpus luteum during pregnancy (d-90)
| Spot No. | AC* | Protein names | Est’dz** | |||
|---|---|---|---|---|---|---|
| --------------------------------------- Expression of luteal phase ---------------------------------- | ||||||
| 2324 | BAD32240 | mKIAA0518 protein (Mus musculus) | 1.84 | |||
| 2331 | 1IRUS | Chain S, crystal structure of the mammalian 20s proteasome at 2.75 A resolution | 1.67 | |||
| 2524 | A53661 | Protein-tyrosine-phosphates (EC 3.1.3.48), receptor type O precursor -rabbit | 0.87 | |||
| 3000 | AAG01993 | Similar to Homo sapiens mRNA for KIAA0120 gene with Genebank accession number D21261.1 | 1.30 | |||
| 3109 | XP 223443 | Similar to bM573K1.5 (novel UIp1 protease family member) (Rattus norvegicus) | 1.23 | |||
| 3407 | AAB37381 | IgG1 heavy chain constant region (Bos taurus) | 1.87 | |||
| 5113 | 3SODO | Chain O, Cu, Zn superoxide dismutase (E.C.1.15.1.1) Mutant with Cys 6 replaced by Ala (C6a) | 1.71 | |||
| 5215 | 1BWYA | Chain A, Nmr study of bovine heart fatty acid binding protein | 2.11 | |||
| 6004 | P02070 | Hemoglobin beta chain | 1.19 | |||
| 6523 | P33097 | Asparate aminotransferase, cytoplasmic (Transaminase A) (glutamate oxalocatate transferase-1) | 2.23 | |||
| 6606 | Q9XSJ4 | Alpha enolase (2-phospho-D-glycerate hydro-lyase) (NNE) (enolase 1) (phophopyruvate hydratase) | 2.39 | |||
| 6814 | Q29443 | Serotransferrin precursor (Transferrin) (Siderophilin) (Beta-1-metal binding globulin) | 2.37 | |||
| 7135_R | D41344 | Lutropin-choriogonadotropin receptor precursor (splice form D) | 1.02 | |||
| 7513 | AAC83646 | Dystrophin (Canis familiaris) | 1.17 | |||
| 7814 | CAA61864 | Put. 26s protease subunit (Sus scrofa) | 2.34 | |||
| 8633 | P10173 | Fumarate hydratase, mitochondrial (Fumarase) | 2.32 | |||
| 6226 | P00939 | Triosephosphate isomerase (TIM) | 1.45 | |||
| ------------------------------------ Expression of pregnancy phase ----------------------------- | ||||||
| 2509 | P48616 | Vimentin | 2.37 | |||
| 4223 | XP_218574 | Similar to BC013491 protein (Rattus norvegicus) | 1.03 | |||
| 5212 | P13620 | ATP synthase D chain, mitochondrial | 2.00 | |||
| 6311 | 1VSEB | Chain B, Crystal structure analysis of bovine carbonic anhydrase II | 2.30 | |||
| 6224 | P41976 | Superoxide dismutase (Mn), Mitochondrial precursor | 2.38 | |||
| 7813 | AAC60522 | Manganous superoxide dismutase; MnSOD (Bos taurus) | 2.37 | |||
AC = Accession Code (NCBI search).
Est’dz = Z score (corresponding percentile in an estimated random match population 2.326 = 99%, 1.65 = 95%, 1.282 = 90%).
Figure 2.
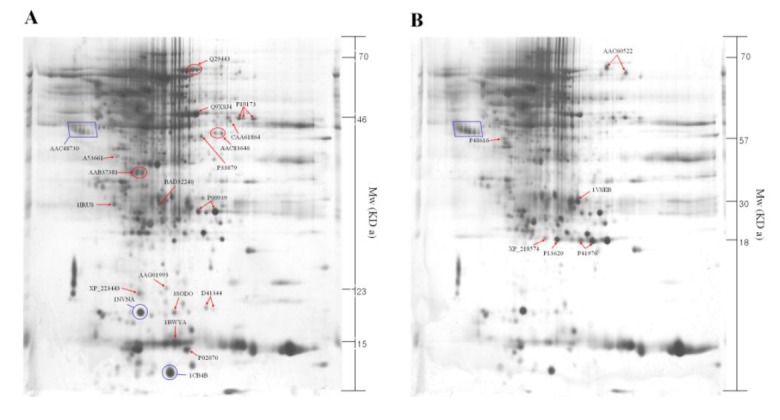
Two-dimensional polyacrylamide gel electrophoresis map of luteal proteins detected in cyclic cows at d 6 to 13 (A) and pregnant cows at d-90 (B). The square box shows the control marker.
DISCUSSION
Proteomics is becoming a powerful approach to study cellular mechanisms, and the research regarding proteins and post-translational modifications adds what can be learned from genomic studies, to our existing knowledge (Oakley et al., 1980). In the present study, protein expression profiles in bovine CL tissues were compared between luteal phase and pregnant phase using 2-DE and MALDI-TOF-MS as the proteomic tools (Figure 2). We identified a total of 23 proteins that expression levels of proteins were significantly different between luteal and pregnant phases. Our identification result has comprised of post-translational protein, Ulp 1 protease family (small ubiquitin-like modifier, SUMO), which has been identified in eukaryotic organisms (Dohmen, 2000; Melchior, 2000; Schwartz et al., 2003). The SUMOylation influences stability, interaction, cellular localization, and activity of proteins and thereby regulates processes, such as DNA repair (Hoege et al., 2002; Pfander et al., 2005), nuclear transport (Stade et al., 2002), transcription regulation (Ross et al., 2002; Sapetschnig et al., 2002), chromosome segregation (Nacerddine et al., 2005) and ion channel activity (Rajan et al., 2005). Interestingly, vimentin-related intermediated filament proteins were not detected in the luteal phase CL tissues on the 2-DE analysis (Figure 3A). It was reported that higher vimentin expression is required for the maintenance of functional CL in the pregnant cows (Perez-Martinez et al., 2001). Therefore, if vimentin expression was detected in the CL tissues during the earlier pregnancy phase (around the period for maternal recognition), vimentin may be used as a marker for the CL development during pregnancy in Korean native cows. Further proteomic analyses in the CL tissues during early pregnancy are required for understanding the cellular mechanisms by which pregnancy is established in domestic cattle.
The validity of the present study is dependent on the accuracy with which the CLs were dated. The kinetics of progesterone concentration in blood circulation and CL tissues in the present study matched well with the values reported previously (O’Malley et al., 1968; Axelson et al., 1975; Rekawiecki et al., 2008). During CL-1 phase the CL still has a soft consistency with a pool of blood, therefore harvesting all the CL tissues from the ovaries at the phase may be difficult, especially if the CL was removed at laparotomy rather than at slaughter. As shown in Figure 1, the plasma progesterone concentrations on d-19 of the pregnant cows significantly decreased from those of luteal phase cows on d-19, suggesting the significant deviation occurred at the time of expected onset of luteolysis. This result was consistent with the findings of Chagas and Lopes (2005) who indicated that the plasma progesterone concentrations of pregnant heifers (by artificial insemination or embryo transfer) were significantly deviated from those of non-pregnant and non-bred heifers on d-17. On the other hand, no change in the luteal progesterone concentrations was observed between the cyclic (CL-2 phase) and pregnant cows (d-60), which was in agreement with a previous report (Knickerbocker et al., 1986). Although luteal progesterone appears to be produced to maintain early pregnancy during the mid-luteal phase in cyclic cows, the secretion of progesterone into circulation may be independently regulated with the production of progesterone within the CL by the unknown mechanism.
In conclusion, it was noted that pregnant cows have functionally developed CL in which the profile of expressing proteins was partially different from that in cyclic cows. Based on an extensive proteomic analysis of luteal tissues from luteal phase and pregnant phase, some proteins showing significant differences in the expression level (e.g., vimentin or MnSOD) were identified, suggesting the possible use of these proteins as marker for the CL development during pregnancy in Korean native cows.
ACKNOWLEDGEMENTS
This study was supported by the Rural Development Administration (RDA) project number PJ006702.
REFERENCES
- Axelson M, Schumacher G, Sjovall J, Gustafsson B, Lindell JO. Identification and quantitative determination of steroids in bovine corpus luteum during oestrous cycle and pregnancy. Acta Endocrinol (Copenh) 1975;80:149–164. doi: 10.1530/acta.0.0800149. [DOI] [PubMed] [Google Scholar]
- Bersinger NA, Birkhauser MH, Yared M, Wunder DM. Serum glycodelin pattern during the menstrual cycle in healthy young women. Acta Obstet Gynecol Scand. 2009;88:1215–1221. doi: 10.3109/00016340903294264. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chagas ESJ, Lopes da CL. Luteotrophic influence of early bovine embryos and the relationship between plasma progesterone concentrations and embryo survival. Theriogenology. 2005;64:49–60. doi: 10.1016/j.theriogenology.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Couet J, Martel C, Dupont E, Luu-The V, Sirard MA, Zhao HF, Pelletier G, Labrie F. Changes in 3 beta-hydroxysteroid dehydrogenase/delta 5-delta 4 isomerase messenger ribonucleic acid, activity and protein levels during the estrous cycle in the bovine ovary. Endocrinology. 1990;127:2141–2148. doi: 10.1210/endo-127-5-2141. [DOI] [PubMed] [Google Scholar]
- Diskin MG, Morris DG. Embryonic and early foetal losses in cattle and other ruminants. Reprod Domest Anim. 2008;43(Suppl 2):260–267. doi: 10.1111/j.1439-0531.2008.01171.x. [DOI] [PubMed] [Google Scholar]
- Dohmen RJ. SUMO protein modification. Biochim Biophys Acta. 2000;1695:113–131. doi: 10.1016/j.bbamcr.2004.09.021. [DOI] [PubMed] [Google Scholar]
- Groten T, Fraser HM, Duncan WC, Konrad R, Kreienberg R, Wulff C. Cell junctional proteins in the human corpus luteum: changes during the normal cycle and after HCG treatment. Hum Reprod. 2006;21:3096–3102. doi: 10.1093/humrep/del286. [DOI] [PubMed] [Google Scholar]
- Hoege C, Pfander B, Moldovan GL, Pyrowolakis G, Jentsch S. RAD6-dependent DNA repair is linked to modification of PCNA by ubiquitin and SUMO. Nature. 2002;419:135–141. doi: 10.1038/nature00991. [DOI] [PubMed] [Google Scholar]
- Ireland JJ, Murphee RL, Coulson PB. Accuracy of predicting stages of bovine estrous cycle by gross appearance of the corpus luteum. J Dairy Sci. 1980;63:155–160. doi: 10.3168/jds.S0022-0302(80)82901-8. [DOI] [PubMed] [Google Scholar]
- Knickerbocker JJ, Thatcher WW, Bazer FW, Drost M, Barron DH, Fincher KB, Roberts RM. Proteins secreted by day-16 to -18 bovine conceptuses extend corpus luteum function in cows. J Reprod Fertil. 1986;77:381–391. doi: 10.1530/jrf.0.0770381. [DOI] [PubMed] [Google Scholar]
- Li XM, Juorio AV, Murphy BD. Prostaglandins alter the abundance of messenger ribonucleic acid for steroidogenic enzymes in cultured porcine granulosa cells. Biol Reprod. 1993;48:1360–1366. doi: 10.1095/biolreprod48.6.1360. [DOI] [PubMed] [Google Scholar]
- McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- Melchior F. SUMO-nonclassical ubiquitin. Annu. Rev. Cell Dev Biol. 2000;16:591–626. doi: 10.1146/annurev.cellbio.16.1.591. [DOI] [PubMed] [Google Scholar]
- Nacerddine K, Lehembre F, Bhaumik M, Artus J, Cohen-Tannoudji M, Babinet C, Pandolfi PP, Dejean A. The SUMO pathway is essential for nuclear integrity and chromosome segregation in mice. Dev Cell. 2005;9:769–779. doi: 10.1016/j.devcel.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Oakley BR, Kirsch DR, Morris NR. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980;105:361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- O’Malley BW, McGuire WL. Studies on the mechanism of action of progesterone in regulation of the synthesis of specific protein. J Clin Invest. 1968;47:654–664. doi: 10.1172/JCI105761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osnes T, Sandstad O, Skar V, Osnes M, Kierulf P. Total protein in common duct bile measured by acetonitrile precipitation and a micro bicinchoninic acid (BCA) method. Scand. J Clin Lab Invest. 1993;53:757–763. doi: 10.3109/00365519309092582. [DOI] [PubMed] [Google Scholar]
- Perez-Martinez C, Garcia-Fernandez RA, Escudero A, Ferreras MC, Garcia-Iglesias MJ. Expression of cytokeratins and vimentin in normal and neoplastic tissue from the bovine female reproductive tract. J Comp Pathol. 2001;124:70–78. doi: 10.1053/jcpa.2000.0430. [DOI] [PubMed] [Google Scholar]
- Pfander B, Moldovan GL, Sacher M, Hoege C, Jentsch S. SUMO-modified PCNA recruits Srs2 to prevent recombination during S phase. Nature. 2005;436:428–433. doi: 10.1038/nature03665. [DOI] [PubMed] [Google Scholar]
- Rajan S, Plant LD, Rabin ML, Butler MH, Goldstein SA. Sumoylation silences the plasma membrane leak K+ channel K2P1. Cell. 2005;121:37–47. doi: 10.1016/j.cell.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Rekawiecki R, Kowalik MK, Slonina D, Kotwica J. Regulation of progesterone synthesis and action in bovine corpus luteum. J Physiol Pharmacol. 2008;59(Suppl 9):75–89. [PubMed] [Google Scholar]
- Rizos D, Carter F, Besenfelder U, Havlicek V, Lonergan P. Contribution of the female reproductive tract to low fertility in postpartum lactating dairy cows. J Dairy Sci. 2010;93:1022–1029. doi: 10.3168/jds.2009-2605. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Waterman MR, Simpson ER. Cytochromes P-450scc, P-450(17)alpha, adrenodoxin, and reduced nicotinamide adenine dinucleotide phosphate-cytochrome P-450 reductase in bovine follicles and corpora lutea. Changes in specific contents during the ovarian cycle. Endocrinology. 1986;118:1366–1374. doi: 10.1210/endo-118-4-1366. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Waterman MR, Simpson ER. Levels of messenger ribonucleic acid encoding cholesterol side-chain cleavage cytochrome P-450, 17 alpha-hydroxylase cytochrome P-450, adrenodoxin, and low density lipoprotein receptor in bovine follicles and corpora lutea throughout the ovarian cycle. Mol Endocrinol. 1987;1:274–279. doi: 10.1210/mend-1-3-274. [DOI] [PubMed] [Google Scholar]
- Ross S, Best JL, Zon LI, Gill G. SUMO-1 modification represses Sp3 transcriptional activation and modulates its subnuclear localization. Mol Cell. 2002;10:831–842. doi: 10.1016/s1097-2765(02)00682-2. [DOI] [PubMed] [Google Scholar]
- Sapetschnig A, Rischitor G, Braun H, Doll A, Schergaut M, Melchior F, Suske G. Transcription factor Sp3 is silenced through SUMO modification by PIAS1. EMBO J. 2002;21:5206–5215. doi: 10.1093/emboj/cdf510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz DC, Hochstrasser M. A superfamily of protein tags: ubiquitin, SUMO and related modifiers. Trends Biochem Sci. 2003;28:321–328. doi: 10.1016/S0968-0004(03)00113-0. [DOI] [PubMed] [Google Scholar]
- Stade K, Vogel F, Schwienhorst I, Meusser B, Volkwein C, Nentwig B, Dohmen RJ, Sommer T. A lack of SUMO conjugation affects cNLS-dependent nuclear protein import in yeast. J Biol Chem. 2002;277:49554–49561. doi: 10.1074/jbc.M207991200. [DOI] [PubMed] [Google Scholar]
- Willcox DL. Progesterone-binding protein in the corpus luteum, blood and lymph of sheep. Biochim Biophys Acta. 1986;881:470–479. doi: 10.1016/0304-4165(86)90041-3. [DOI] [PubMed] [Google Scholar]


