Abstract
A facultative bacterium producing cellulolytic and hemicellulolytic enzymes was isolated from the rumen of a native Korean goat. The bacterium was identified as a Bacillus licheniformis on the basis of biochemical and morphological characteristics and 16S rDNA sequences, and has been designated Bacillus licheniformis JK7. Endoglucanase activities were higher than those of β-glucosidase and xylanase at all temperatures. Xylanase had the lowest activity among the three enzymes examined. The optimum temperature for the enzymes of Bacillus licheniformis JK7 was 70°C for endoglucanase (0.75 U/ml) and 50°C for β-glucosidase and xylanase (0.63 U/ml, 0.44 U/ml, respectively). All three enzymes were stable at a temperature range of 20 to 50°C. At 50°C, endoglucanse, β-glucosidase, and xylanase had 90.29, 94.80, and 88.69% residual activity, respectively. The optimal pH for the three enzymes was 5.0, at which their activity was 1.46, 1.10, and 1.08 U/ml, respectively. The activity of all three enzymes was stable in the pH range of 3.0 to 6.0. Endoglucanase activity was increased 113% by K+, while K+, Zn+, and tween 20 enhanced β-glucosidase activity. Xylanase showed considerable activity even in presence of selected chemical additives, with the exception of Mn2+ and Cu2+. The broad range of optimum temperatures (20 to 40°C) and the stability under acidic pH (4 to 6) suggest that the cellulolytic enzymes of Bacillus licheniformis JK7 may be good candidates for use in the biofuel industry.
Keywords: Bacillus licheniformis, Endoglucanase, β-Glucosidase, Xylanase, Goat
INTRODUCTION
Lignocellulosic materials are the most abundant resource for the production of renewable bioenergy and fermented products. Cellulosic materials need to be first hydrolyzed into fermentable sugars since they are not useful in their polysaccharide form (Li et al., 2009). The biohydrolysis of cellulose through the use of cellulolytic microorganisms is an attractive approach since the degradation of cellulose by chemical agents produces environmental pollution (Rizzatti et al., 2001). Cellulase, which is produced by fungi and bacteria, can be divided into three major types: endoglucanase (endo-1,4-β-D-glucanase, EC 3.2.1.4), cellobiohydrolase (exo-1,4-β-D-glucanase, EC 3.2.1.91), and β-glucosidase (1,4-β-D-glucosidase EC 3.2.1.21) (Hong et al., 2001). Endoglucanases randomly hydrolyze the internal β-1,4-glysidic bonds of cellulose chains so that new chain ends are produced. In contrast, cellobiohydrolases cleave cellulose chains at the ends to produce cellobiose or glucose. β-glucosidase only hydrolyzes cellobiose, and releases glucose units (Percival Zhang et al., 2006; Kumar et al., 2008).
Fungal species have been primarily used commercially for cellulase production because of their capacity to secrete cellulolytic enzymes into their medium, which allows for easy purification and extraction (Maki et al., 2009). Among the cellulolytic fungi, Trichoderma spp. and Aspergillus spp. have been extensively investigated since they can produce all three types of cellulose-degrading enzymes (Wang et al., 2008). However, bacterial cellulases have several advantages. First, bacteria have higher growth rates than fungi and can easily grow to high cell densities in inexpensive nutrient sources (Maki et al., 2009). Second, the enzyme expression system of bacteria is more convenient. Third, bacteria can not only survive harsh conditions but can also excrete enzymes that are stable under extreme conditions of high temperature and low or high pH.
Several bacterial genera show cellulolytic activity, including Bacillus, Clostridium, Cellulomonas, Rumminococcus, Alteromonas, Acetivibrio, and Bacteriodes (Roboson and Chambliss, 1989). Among these, Bacillus species produce a variety of extracellular cellulolytic enzymes. Bacillus licheniformis is a facultative and a Gram-positive endospore-forming bacterium (Sneath et al., 1986) which is used extensively in large-scale commercial enzyme production since it can excrete proteins in large quantities of up to 20 to 25 g/L (Schallmey et al., 2004).
Many cellulolytic or xylanolytic Bacillus species have been isolated from compost (Archana and Satyanarayana, 1997; Rastogi et al., 2010), milled paper (Geetha and Gunasekaran, 2010), swine waste (Liang et al., 2009), and hot springs (Mawadza et al., 2000). However, the isolation of cellulolytic and xylanolytic Bacillus sp. from the rumen of goats has not previously been reported as the rumen environment is a strictly anaerobic environment, which can make it difficult for aerobic bacteria to survive. In this study, we isolated the facultative anaerobic bacteria Bacillus licheniformis JK7, which can secrete endoglucanase, β-glucosidase, and xylanase, in the rumen of a native Korean goat which can survive on harsh condition such as provision of low quality roughage as a sole feed source (Son, 1999). The objectives of this study were i) to isolate and identify the microorganism responsible for degrading cellulose and xylan, and ii) to characterize the endoglucanase, β-glucosidase, and xylanase released by selected Bacillus sp.
MATERIALS AND METHODS
Materials
All chemicals, media components and reagents used in these experiments were purchased from Sigma (Sigma and Aldrich, St. Louis, USA) and Difco laboratories (Sparks, USA). Azo-CM-Cellulose (Megazyme co. Ltd., Ireland) was used as a substrate to screen cellulolytic bacteria.
Isolation and screening of cellulose-degrading bacteria
The ruminal fluid of goats was collected before their morning feeding from rumen fistulas. The rumen fluid was diluted with modified Dehority (MD) medium (Scott and Dehority, 1965) using 1% carboxymethylcellulose (CMC) as the sole carbon source and anaerobically cultured overnight at 37°C. The fluid was then spread onto MD agar plates containing 1% Azo-CMC and anaerobically cultured overnight at 39°C to screen for bacteria with endoglucanase activity. The colonies forming clear zones were then carefully picked and re-streaked onto Azo-CMC agar plate to check for enzyme activity and isolate single strains. The strains which showed consistent endoglucanase activity were transferred to aerobic conditions and cultured on Luria-Bertani (LB) medium overnight at 37°C. Surviving strains which were facultative anaerobic cellulolytic bacteria were selected. The isolated strain was analyzed by Gram staining as described by Moaledj (1986). Spore formation was examined using phase-contrast microscopy (Nikon Optiphot-2, Japan).
16s rDNA sequencing for strain identification
A total of 1.5 ml of LB culture was centrifuged (10,000 g×1 min) to obtain a cell pellet for DNA extraction, which was performed using a DNeasy Blood & Tissue Kit (Qiagen, Seoul, South Korea). PCR amplification of the 16s rDNA gene fragments was performed using the universal primers 27f (5’-AGAGTTTGATCMTGGCTCAG-3’) and 1492R (5’-ACGGCTACCTTGTTACGACTT-3’). The amplified PCR product was visualized by gel electrophoresis. The 16s rDNA band was cut and purified using a Gel DNA extraction kit (Qiagen, Seoul, South Korea). The purified PCR product was then cloned using pGEM-T Easy Vector and transformed into E. coli top10 competent cells (Promega, USA) as per the manufacturer’s protocol. Plasmids were isolated using a plasmid extraction kit (Bioneer, Korea). A sequence similarity search was carried out using BLAST with the NCBI database (http://www.ncbi.nlm.nih.gov) and alignment was carried out using V-NTI (Life Science Technology, Co. Ltd., USA).
Biochemical analysis of strain identification
Exponentially growing cells were biochemically analyzed using the API 50 CHB Kit (Biomeriux, USA) following the manufacturer’s instructions.
Growth curve
The culture medium used in this experiment was liquid LB medium containing 1% CMC. The seed culture was developed prior to measurement of growth phase using same media. The culture media (100 ml) in 500 ml shake flasks was inoculated with 1% of seed culture showing 0.5 of OD600 value. Aliquots of the bacterial cultures were taken from the growth medium at two hour intervals, and absorbance was measured at 600 nm. Growth curves were plotted as absorbance vs time. Enzyme activity was also calculated at the two hour intervals.
Enzyme assays
Cellulase and xylanase activity were measured by spectrometric determination of reducing sugars by the 3, 5-dinitrosalicylic acid (DNS) method (Ghose, 1987). Briefly, a mixture of the enzyme and a 1% CMC solution (1:1) was prepared in 50 mM phosphate buffer (pH 6). Endoglucanase activity was assayed using CMC as a substrate. β-glucosidase activity was determined using salicin (2-hydroxymethyl-phenyl-β-D-glucopyranoside) as a substrate and xylanase activity was determined by measuring the release of xylose from birch wood xylan. For crude enzyme preparation, Bacillus licheniformis JK7 was cultured in the basal medium (g/L, 2.5 KH2PO4, 2.5 K2HPO4, 0.1 NaCl, 0.2 MgSO4·7H2O, 0.01 FeSO4·7H2O, 0.007 MnSO4·7H2O, 0.05 CaCl2·2H2O, 1.0 (NH4)2SO4, 2.5 yeast extract, 5.0 CMC, 5.0 birchwood xylan) at 37°C for 24 h. The cultures were centrifuged at 13,000 g×10 min at 4°C and the supernatant was used for the enzyme assay. The reaction mixture was incubated at 37°C for 30 min. After incubation, 300 μl of DNS reagent was added and the mixture was heated to 99°C for 5 min in a boiling water bath. The release of reducing sugars was calculated from the OD measured at 546 nm. One unit of enzymatic activity was defined as the amount of enzyme that released 1 μmol of reducing sugar per minute. All assays were performed in triplicate and average values are reported.
Optimum pH and temperature of cellulase and xylanase and their stability
The optimum pH for crude enzyme preparations was measured in different buffers (50 mM acetate buffer for pH 3 to 5, 50 mM phosphate buffer for pH 6 to 8) at 37°C. The stability of the enzymes at different pH values was determined by pre-incubating crude enzyme in various pH buffer solutions for 4 h at 4°C (Dong et al., 2010). Relative activity was expressed as the percentage of enzyme activity that remained after incubation in comparison to the maximum observed activity at each pH. To determine the optimum temperature for cellulolytic and xylanolytic enzymes, crude enzyme preparations were incubated at a range of temperatures (20 to 80°C) in 50 mM phosphate buffer (pH 6). Thermal stability was determined by incubating crude enzyme at selected temperatures (20 to 80°C) for one hour. The relative activity was calculated in comparison to the maximum observed activity at respective temperature.
All assays were carried out in triplicate, and average values are reported.
Effects of ions and detergents on enzyme activity
The effect of various metal ions and detergents on the activity of the crude enzyme preparations was investigated. The additives used in this study were 5 mM of nine different metal ions (CaCl2, CoCl2, KCl, MnCl2, NiCl2, MgCl2, FeCl2, CuCl2, ZnCl2) and 0.25% detergent (TritonX-100, Tween20). The reaction mixtures were incubated with the additives for 60 min at 37°C and pH 6, and enzyme activities were assayed as described previously. Residual activity was calculated as relative (%) value to control. All assays were performed in triplicate.
Statistical analysis
Data from the characterization of the enzymes at different temperatures and pH values were analyzed statistically using the MIXED procedure in SAS (SAS, 1996). The effects of enzymes, treatments, and the interactions between enzymes and treatments were considered fixed. Significant differences (p<0.05) in treatment least square means were reported only if the Tukey-test (SAS, 1996) for treatments was also significant (p<0.05). The relative enzyme activities of different chemical additives were analyzed using the GLM procedure (SAS, 1996). Differences between treatments were considered significant if p<0.05.
RESULTS AND DISCUSSION
Isolation and identification of cellulolytic bacteria
The majority of rumen bacteria are anaerobic as the rumen maintains an obligate anaerobic environment. Representatives of many Bacillus strains are known to grow even under anaerobic conditions (Williams and Withers, 1983), but there have been few reports of the isolation of Bacillus spp. from the rumen ecosystem. In this study, ten spore-forming facultative microorganisms were screened on LB agar plates containing 1% Azo-CMC. Of these, bacteria JK7 showed maximum endoglucanase activity (data not shown). This strain was found to be a facultative, spore forming, Gram-positive bacteria. The physiological and biochemical characteristics of this organism are listed in Table 1. This bacteria was found to be able to hydrolyze various carbohydrates, including L-arabinose, galactose, fructose, mannose, α-methyl-D-glucoside, N-acethyl-glucosamine, D-turanose, salicin, cellobiose, β-gentiobiose, and D-xylose (Table 1), but did not utilize D-arabinose, erythritol, sorbose, dulcitol, inositol, α-methyl-D-mannoside, Lactose, D, L-arabitol, 2-keto-gluconate, or 5-keto-gluconate (Table 1). Based on these results, JK7 was preliminarily identified as Bacillus licheniformis. Strain JK7 was found by 16S rDNA sequence alignment to be closely related to the Bacillus genus, with the highest similarity with Bacillus licheniformis ATCC14580 (99%). Therefore, this strain was identified as a Bacillus licheniformis and designated to Bacillus licheniformis JK7 on the basis of biochemical and morphological characteristics and 16S rDNA sequences.
Table 1.
Physiologic and biochemical characteristics of Bacillus licheniformis JK7
| Characteristics | Result | Characteristics | Result |
|---|---|---|---|
| Gram stain | + | Esculine | + |
| Spore formation | + | Salicin | + |
| Glycerol | + | Cellobiose | + |
| Erythritol | − | Maltose | + |
| D-arabinose | − | Lactose | − |
| L-arabinose | + | Melibiose | − |
| Ribose | + | Sucrose | + |
| D-xylose | + | Trehalose | + |
| L-xylose | − | Inuline | − |
| Adonitol | − | Melezitose | − |
| β-methyl-D-xylose | − | D-raffinose | − |
| Galactose | + | Starch | + |
| Glucose | + | Glycogen | + |
| Fructose | + | Xylitol | − |
| Mannose | + | β-Gentiobiose | + |
| L-sorbose | − | D-turanose | + |
| Rhamnose | + | D-lyxose | − |
| Dulcitol | − | D-tagatose | + |
| Inositol | − | D-fucose | − |
| Mannitol | + | L-fucose | − |
| Sorbitol | + | D-arabitol | − |
| α-methyl-D-mannoside | − | L-arabitol | − |
| α-methyl-D-glucoside | + | Gluconate | + |
| N-acethyl-glucosamine | + | 2-keto-gluconate | − |
| Amygdaline | + | 5-keto-gluconate | − |
| Arbutine | + |
Growth curve
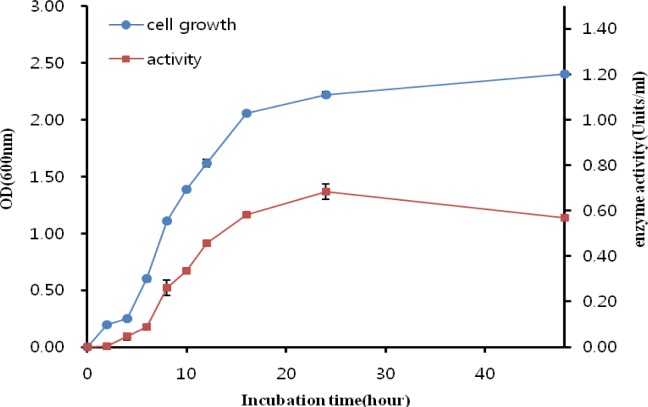
The endoglucanase production and cell growth of Bacillus licheniformis JK7 was measured by culturing in a 500 ml shake flask with 100 ml of LB media containing 1% CMC at pH 6 and 37°C (Figure 1). The growth phase of Bacillus licheniformis JK7 started at time zero (0 h) and has grown to sixteen hours later (16 h). It started faster than previously studied Bacillus sp. (Peixoto et al., 2011; Samiullah et al., 2009; Yang et al., 1995). It might be due to different culture condition and the amount of inoculums population.
Figure 1.
Bacterial growth curve (
 ) and endoglucanase activity (
) and endoglucanase activity (
 ) of Bacillus licheniformis JK7. The cell growth was determined by measuring the OD600 of the cell culture. Enzyme activity was determined using the culture supernatants. All experiments were performed in triplicate. The data points and error bars indicate the average values and standard errors.
) of Bacillus licheniformis JK7. The cell growth was determined by measuring the OD600 of the cell culture. Enzyme activity was determined using the culture supernatants. All experiments were performed in triplicate. The data points and error bars indicate the average values and standard errors.
The stationary began at hour sixteen, which was similar to another Bacillus sp. (Peixoto et al., 2011) and Geobacillus thermoleovorans (Sharma et al., 2007). Bacterial growth was maintained up to 48. The OD600 values were around 2.1 at the stationary phase, and maximum values reached around 2.3 at 48 h. The stationary phase started faster than seen in the growth curves of the previously described Bacillus licheniformis 77-2 (Damiano et al., 2003) and Bacillus licheniformis SVD1 (van Dyk et al., 2009) and lasted for 30 h, which was longer than other described Bacillus sp. (Samiullah et al., 2009) and Bacillus sp. V1-4 (Yang et al., 1995).
Endoglucanase production increased rapidly from h 6 up to h 16, with a maximum value of 0.68 U/ml at 24 h and a steady decrease thereafter. The increase in enzyme production was associated with an increase in cell growth, which indicated that cellulose was actively utilized by Bacillus licheniformis JK7 during the growth phase. There have been several studies of endoglucanase production which reported similar patterns (Ariffin et al., 2008; Ko et al., 2011; Rastogi et al., 2010; Saratale and Oh, 2011). For example, Rastogi et al. (2010) showed that Bacillus sp. DUSELR13 had maximum CMCase activity (0.12 U/ml) at d 9, when the culture had reached the dying phase. The Geobacillus strain WSUCF1 also produced maximum CMCase activity (0.13 U/ml) on d 7 at the end of stationary phase (Rastogi et al., 2010). Saratale and Oh (2011) reported that the decrease in cellulolytic enzyme production at the stationary phase was caused by metabolite repression by molecules released after the hydrolysis such as glucose or cellobiose.
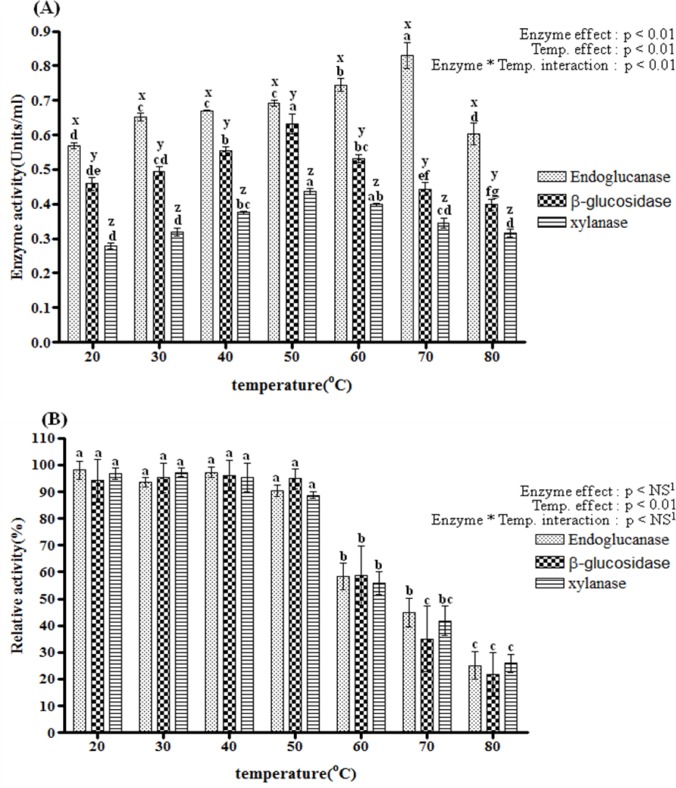
Effect of temperature on endoglucanase, β-glucosidase and xylanase activity and stability
The effect of temperature on endoglucanase, β-glucosidase, and xylanase activity of the crude enzyme was determined over a temperatures range of 20 to 80°C at pH 6.0 (Figure 2A). At all temperatures, endoglucanase activity was higher than that of either β-glucosidase or xylanase. In the present study, the xylanase activity of Bacillus licheniformis JK7 was lower than those reported by others at all temperatures. According to van Dyk et al. (2009), Bacillus licheniformis SVD1 predominantly produced xylanase, and showed minimal production of mannanase, CMCase and avicelase. This difference might be due to the culture conditions (Geetha and Gunasekaran, 2010; Saratale and Oh, 2010). Van Dyk et al. (2009) also used complex media containing 1% xylan, which could induce increased xylanase production.
Figure 2. i).
Temperature and pH effects on endoglucanase, β-glucosidase and xylanase of Bacillus licheniformis JK7 activity (A and C) their stability (B and D). Enzyme activity of the culture supernatants was determined at 24 h. All assays were performed in triplicate. The data points and error bars indicate the average values and standard errors. a,b,c,d,e,f,g Indicates a significantly (p<0.05) different activity influenced by temperature or pH in the same enzyme group. x,y,z Indicates a significantly (p<0.05) different activity between different enzymes within same temperature or pH. 1 NS means not significant.
The optimum temperature for Bacillus licheniformis JK7 endoglucanase activity was 70°C, at which activity was 0.75 U/ml. Activity increased linearly with increased temperature, up to 70°C, and declined thereafter. The previously described Bacillus sp. CH43 (Mawadza et al., 2000) showed a similar optimal temperature for endoglcucanase. In another study, Bacillus DUSELR13 (Rastogi et al., 2009) also showed maximum endoglucanase activity at 75°C. However, in that study, endoglucanase activity was very low at low temperatures (20 to 40°C). In comparison, Bacillus licheniformis JK7 showed endoglucanase activity at broad range of temperatures in our study. Thermophilic cellulose degrading enzymes have great potential for the biofuel, leather, textile, food and agriculture industry, since high temperatures are often required in these processes (Rastogi et al., 2009; Trivedi et al., 2011).
Bacillus licheniformis JK7 showed maximum β-glucosidase and xylanase activity (0.63 U/ml, 0.44 U/ml respectively) at 50°C (Figure 2A). This is consistent with the β-glucosidase of Bacillus licheniformis KCTC1918 (Choi et al., 2009), which showed a similar optimal temperature of 47°C. The optimum temperature of various Bacillus sp. xylanases have also been reported in the literature, with similar results (Archana and Satyanarayana, 1997; Ko et al., 2010; Ko et al., 2011, Yang et al., 1995; Yin et al., 2010).
The thermo-stability of endoglucanase, β-glucosidase, and xylanase was assessed at selected temperatures ranging from 20 to 80°C, as shown Figure 2(B). The relative activity was calculated as the relative enzyme activity compared to the maximum value observed across the range of temperatures. All three enzymes were stable at a range from 20 to 50°C. At 50°C, endoglucanse, β-glucosidase and xylanase had 90.29, 94.80, and 88.69% residual activity, respectively. However, the residual activity of endoglucanse, β-glucosidase and xylanase declined after 50°C. In the case of endoglucanase, maximum activity was observed at 70°C, but it maintained 44.68% residual activity after one hour of pre-incubation.
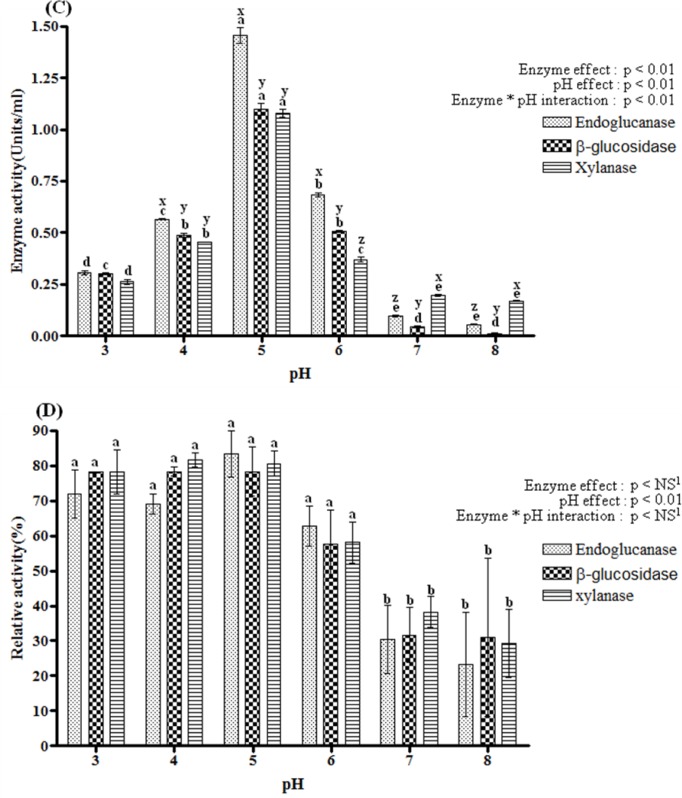
Effect of pH on endoglucanase, β-glucosidase and xylanase activity, and stability
The effect of pH on enzyme activity was investigated at various pH levels ranging from a pH of 3.0 to 8.0 as shown Figure 2(C). The optimal pH for all three enzymes was 5.0 and their activity at that pH was 1.46, 1.10 and 1.08 U/ml, respectively. Endoglcucanase retained 39% and 46% of its maximum activity across the pH range of 4.0 to 6.0, and β-glucosidase retained more than 45% residual activity in the same range. Xylanase maintained 41% and 34% of its maximum activity between pH 4.0 and 6.0. This is consistent with Bishoff et al. (2007), who reported on a cloned glycoside hydrolase family 5 endoglucanase gene from Bacillus licheniformis B-41361; their recombinant gene had maximum endoglucanase activity at pH 5.5. The optimum pH of xylanase in the multi enzyme complex of Bacillus licheniformis SVD1 was also 5.0 (van Dyk et al., 2010). Many industrial processes involving cellulase need to use extreme pH conditions to reduce contamination by other bacteria (Dong et al., 2010). Since these processes often require acidophilic enzymes to degrade fiber efficiently under low pH conditions, the relatively high acidophilic nature of the enzymes examined in this study might be considered beneficial for industrial application. All three enzymes were strongly inhibited at pH 7.0 to 8.0. Figure 2(D) shows the pattern of pH stability of selected enzymes. Relative activity was calculated as the percentage of the maximum observed activity for each enzyme. Endoglucanase, β-glucosidase and xylanase activities were found to be stable in the pH range of 3 to 6. They maintained more than 58% of their maximum activity at selected pH after four hour pre-incubation at 4°C. At pH 7 and 8, relative enzyme activity of all three enzymes dramatically declined, with remaining endoglucanase β-glucosidase and xylanase activity of only 23, 30, 29% of their maximum activity, respectively.
Figure 2. ii).
Temperature and pH effects on endoglucanase, β-glucosidase and xylanase of Bacillus licheniformis JK7 activity (A and C) their stability (B and D). Enzyme activity of the culture supernatants was determined at 24 h. All assays were performed in triplicate. The data points and error bars indicate the average values and standard errors. a,b,c,d,e,f,g Indicates a significantly (p<0.05) different activity influenced by temperature or pH in the same enzyme group. x,y,z Indicates a significantly (p<0.05) different activity between different enzymes within same temperature or pH. 1 NS means not significant.
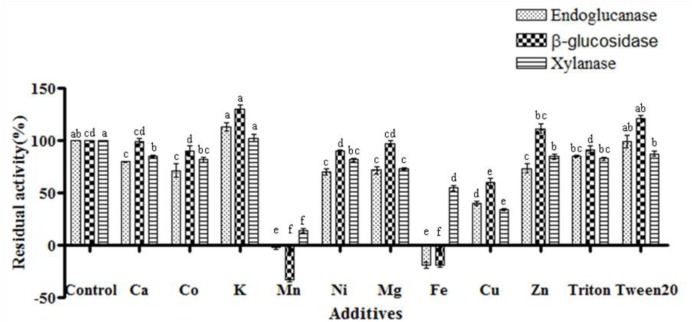
Effects of various chemical additives on endoglucanase, β-glucosidase and xylanase activity
The effects of various chemicals on endoglcucanase, β-glucosidase, and xylanase were investigated by the DNS assay method (Figure 3). Crude enzyme was incubated for one hour with 5 mM of each of the metal ions and 0.25% of TritonX-100 and Tween20 before determining the residual activity of the three enzymes. Residual activity was expressed as the relative amount (%) compared to control (100%). Understanding the effect of various metal ions and reagents on enzyme activity is important since many industrial applications need to increase enzyme activity, which is often accomplished by addition of chemical additives at various stages of the process (Dong et al., 2010). The majority of Bacillus spp. which produce cellulase showed responses ranging from stimulation to inhibition depending on the specific cation (Christakopoulos et al., 1999).
Figure 3.
Effect of chemical additives on endoglucanase, β-glucosidase, xylanase activity of Bacillus licheniformis JK7. Residual activity was calculated as relative (%) considering control as 100%. All assays were performed in triplicate. The data points and error bars indicate the average values and standard errors. a,b,c,d,e,f Indicates a significantly (p<0.05) different enzyme activity compared to control.
In this study, endoglucanase activity was found to be stimulated by K+ to 113% of the control (Figure 3). GH5 endoglucanase from Martelella mediterranea (Dong et al., 2010) was previously reported to show increased relative activity when K+ was added. K+ may stimulate enzyme activity due to its ability to alter the structure of the enzyme itself (Kui et al., 2009). However, in our study the enzyme activity was inhibited by Ca2+, Co2+, Mn2+, Ni2+, Mg2+, Fe2+, Cu2+, Zn2+, and TritonX-100. In particular, Mn2+ and Fe2+ both strongly inhibited endoglucanase activity (2.18, and 19.0%, respectively). The strong inhibitory effect of Mn2+ on endoglucanase activity is consistent with previous reports of Bacillus amyloliquefaciens DL-3 and Bacillus flexus (Lee et al., 2008; Trivedi et al., 2011). An inhibitory effect on enzyme activity by metal ions usually suggests the presence of a sulfhydryl group in the active site, where oxidation by the metal ions destabilizes the conformational folding of the enzymes (Karnchanatat et al., 2007).
In present study, the relative activities of β-glucosidase with 5 mM Ca2+, Co2+, K+, Mn2+, Ni2+, Mg2+, Fe2+, Cu2+, Zn2+, and 0.25% of TritonX-100 and tween20 were 99, 90, 130, −33, 90, 96, −19, 60, 111, 91 and 120%, respectively (Figure 3). Detergents such as tween20 have been implicated in altering the conformational or structural characteristics of selected enzymes (Bajaj et al., 2009). Xylanase was not influenced by selected chemical additives, with the exception of Mn2+ and Cu2+ (Figure 3). The strong inhibition of Mn2+ on the xylanase activity of Bacillus species was also reported in a previous study (Mamo et al., 2006). The slight stimulatory effect of K+ and the inhibition of the xylanase enzyme activity of Bacillus licheniformis by tritonX-100 is also similar to what was observed by Archana et al. (2003).
CONCLUSION
The broad range of optimum temperatures (20 to 40°C) and the stability under acidic pH (4 to 6) suggest that the cellulolytic enzymes of Bacillus licheniformis JK7 may be good candidates for use in the biofuel industry, which requires that celluloses be able to be hydrolyzed by acids at high temperature (Gao et al., 2008).
Acknowledgments
This research was supported by the Technology Development Program for Agriculture and Forestry (Project No. 109024032CG000), Ministry for Food, Agriculture, Forestry and Fisheries, Republic of Korea.
REFERENCES
- Archana A, Satyanarayana T. Xylanase production by thermophilic Bacillus licheniformis A99 in solid-state fermentation. Enzyme and Microb Technol. 1997;21:12–17. [Google Scholar]
- Ariffin H, Hassan MA, Shah UKM, Abdullah N, Ghazali FM, Shirai Y. Production of bacterial endoglucanase from pretreated oil palm empty fruit bunch by bacillus pumilus EB3. J Biosci Bioeng. 2008;106:231–236. doi: 10.1263/jbb.106.231. [DOI] [PubMed] [Google Scholar]
- Bajaj BK, Pangotra H, Wani MA, Sharma P, Sharma A. Partial purification and characterization of a highly thermostable and pH stable endoglucanase from a newly isolated Bacillus strain M-9. Indian J Chem Technol. 2009;16:382–387. [Google Scholar]
- Bischoff KM, Liu S, Hughes SR. Cloning and characterization of a recombinant family 5 endoglucanase from Bacillus licheniformis strain B-41361. Process Biochem. 2007;42:1150–1154. [Google Scholar]
- Choi I, Wi S, Jung S, Patel D, Bae H-J. Characterization and application of recombinant β-glucosidase (BglH) from Bacillus licheniformis KCTC 1918. J Wood Sci. 2009;55:329–334. [Google Scholar]
- Christakopoulos P, Hatzinikolaou DG, Fountoukidis G, Kekos D, Claeyssens M, Macris BJ. Purification and mode of action of an alkali-resistant endo-1,4-β-glucanase from Bacillus pumilus. Arch Biochem Biophys. 1999;364:61–66. doi: 10.1006/abbi.1999.1102. [DOI] [PubMed] [Google Scholar]
- Damiano VB, Bocchini DA, Gomes E, da Silva R. Application of crude xylanase from Bacillus licheniformis 77-2 to the bleaching of eucalyptus Kraft pulp. World J Microbiol Biotechnol. 2003;19:139–144. [Google Scholar]
- Dong J, Hong Y, Shao Z, Liu Z. Molecular cloning, purification, and characterization of a novel, acidic, pH-stable endoglucanase from Martelella mediterranea. J Microbiol. 2010;48:393–398. doi: 10.1007/s12275-010-9361-0. [DOI] [PubMed] [Google Scholar]
- Gao J, Weng H, Zhu D, Yuan M, Guan F, Xi Y. Production and characterization of cellulolytic enzymes from the thermoacidophilic fungal Aspergillus terreus M11 under solid-state cultivation of corn stover. Bioresour Technol. 2008;99:7623–7629. doi: 10.1016/j.biortech.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Geetha K, Gunasekaran P. Optimization of nutrient medium containing agricultural waste for xylanase production by Bacillus pumilus B20. Biotechnol Bioprocess Eng. 2010;15:882–889. [Google Scholar]
- Ghose TK. Measurement of cellulase activities. Pure Appl Chem. 1987;59:257–268. [Google Scholar]
- Hong J, Tamaki H, Akiba S, Yamamoto K, Kumagai H. Cloning of a gene encoding a highly stable endo-b-1,4-glucanase from Aspergillus niger and its expression in yeast. J Biosci Bioeng. 2001;92:434–441. doi: 10.1263/jbb.92.434. [DOI] [PubMed] [Google Scholar]
- Karnchanatat A, Petsom A, Sanvanich P, Piaphukiew J, Whalley AJ, Reynolds CD, Sihanonth P. Purification and biochemical characterization of an extracellular β-glucosidase from the wood-decaying fungus Daldinia eschscholzii (Ehrenb.:Fr.) Rehm. FEMS Microbiol Lett. 2007;270:162–170. doi: 10.1111/j.1574-6968.2007.00662.x. [DOI] [PubMed] [Google Scholar]
- Ko C-H, Lin Z-P, Tu J, Tsai C-H, Liu C-C, Chen H-T, Wang T-P. Xylanase production by Paenibacillus campinasensis BL11 and its pretreatment of hardwood kraft pulp bleaching. Int. Biodeterior. Biodegradation. 2010;64:13–19. [Google Scholar]
- Ko C-H, Tsai C-H, Tu J, Yang B-Y, Hsieh D-L, Jane W-N, Shih T-L. Identification of Paenibacillus sp. 2S-6 and application of its xylanase on biobleaching. Int. Biodeterior. Biodegradation. 2011;65:334–339. [Google Scholar]
- Kui H, Luo H, Shi P, Bai Y, Yuan T, Wang Y, Yang P, Dong S, Yao B. Gene cloning, expression, and characterization of a thermostable xylanase from Nesterenkonia xinjiangensis CCTCC AA001025. Appl Biochem Biotechnol. 2009;162:953–965. doi: 10.1007/s12010-009-8815-5. [DOI] [PubMed] [Google Scholar]
- Kumar R, Singh S, Singh OV. Bioconversion of lignocellulosic biomass: biochemical and molecular perspectives. J Ind Microbiol Biotechnol. 2008;35:377–391. doi: 10.1007/s10295-008-0327-8. [DOI] [PubMed] [Google Scholar]
- Lee Y-J, Kim B-K, Lee B-H, Jo K-I, Lee N-K, Chung C-H, Lee Y-C, Lee J-W. Purification and characterization of cellulase produced by Bacillus amyoliquefaciens DL-3 utilizing rice hull. Bioresour Technol. 2008;99:378–386. doi: 10.1016/j.biortech.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Li W, Huan X, Zhou Y, Ma Q, Chen Y. Simultaneous cloning and expression of two cellulase genes from Bacillus subtilis newly isolated from Golden Takin (Budorcas taxicolor Bedfordi) Biochem Biophys Res Commun. 2009;383:397–400. doi: 10.1016/j.bbrc.2009.04.027. [DOI] [PubMed] [Google Scholar]
- Liang Y, Yesuf J, Schmitt S, Bender K, Bozzola J. Study of cellulases from a newly isolated thermophilic and cellulolytic Brevibacillus sp. strain JXL. J Ind Microbiol Biotechnol. 2009;36:961–970. doi: 10.1007/s10295-009-0575-2. [DOI] [PubMed] [Google Scholar]
- Maki M, Leung KT, Qin W. The prospects of cellulase-producing bacteria for the bioconversion of lignocellulosic biomass. Int J Biol Sci. 2009;5:500–516. doi: 10.7150/ijbs.5.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamo G, Hatti-Kaul R, Mattiason B. Athermostable alkaline active endo-β-1,4-xylanase from Bacillus halodurans S7: purification and characterization. Enzyme Microb Biotechnol. 2006;39:1492–1498. [Google Scholar]
- Mawadza C, Hatti-Kaul R, Zvauya R, Mattiasson B. Purification and characterization of cellulases produced by two Bacillus strains. J Biotechnol. 2000;83:177–187. doi: 10.1016/s0168-1656(00)00305-9. [DOI] [PubMed] [Google Scholar]
- Moaledh K. Comparison of Gram-stainig and alternate methods, KOH test and aminopeptidase activity in aquatic bacteria: their application to numerical taxonomy. J. Microbiol. Methods. 1986;5:303–310. [Google Scholar]
- Peixoto SB, Cladera-Olivera F, Daroit DJ, Brandelli A. Cellulase-producing Bacillus strains isolated from the intestine of Amazon basin fish. Aquac Res. 2011;42:887–891. [Google Scholar]
- Percival Zhang YH, Himmel ME, Mielenz JR. Outlook for cellulase improvement: Screening and selection strategies. Biotechnol Adv. 2006;24:452–481. doi: 10.1016/j.biotechadv.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Rastogi G, Bhalla A, Adhikari A, Bischoff KM, Hughes SR, Christopher LP, Sani RK. Characterization of thermostable cellulases produced by Bacillus and Geobacillus strains. Bioresour Technol. 2010;101:8798–8806. doi: 10.1016/j.biortech.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Rastogi G, Muppidi GL, Gurram RN, Adhikari A, Bischoff KM, Hughes SR, Apel WA, Bang SS, Dixon DJ, Sani RK. Isolation and characterization of cellulose-degrading bacteria from the deep subsurface of the Homestake gold mine, Lead, South Dakota, USA. J Ind Microbiol Biotechnol. 2009;36:585–598. doi: 10.1007/s10295-009-0528-9. [DOI] [PubMed] [Google Scholar]
- Rizzatti AC, Jorge JA, Terenzi HF, Rechia CG, Polizeli ML. Purification and properties of a thermostable extracellular beta-D-xylosidase produced by a thermotolerant Aspergillus phoenicis. J Ind Microbiol Biotechnol. 2001;26:156–160. doi: 10.1038/sj.jim.7000107. [DOI] [PubMed] [Google Scholar]
- Roboson LM, Chambliss GH. Celluases of bacterial origin. Enzyme Microb Technol. 1989;11:626–644. [Google Scholar]
- Samiullah TR, Bakhsh A, Rao AQ, Naz M, Saleem M. Isolation, Purification and characterization of extracellular β-glucosidase from Bacillus sp. Adv Environ Biol. 2009;3:269–277. [Google Scholar]
- Saratale GD, Oh SE. Production of thermotolerant and alkalotolerant cellulolytic enzymes by isolated Nocardiopsis sp. KNU. Biodegradation. 2011 doi: 10.1007/s10532-010-9450-0. [DOI] [PubMed] [Google Scholar]
- SAS . SAS user’s guide: Statistics, Version 6. 12th edition. SAS Institute Inc; Cary, NC, USA: 1996. [Google Scholar]
- Scott HW, Dehority BA. Vitamin requirements of several cellulolytic rumen bacteria. J Bacteriol. 1965;89:1169–1175. doi: 10.1128/jb.89.5.1169-1175.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shallmey M, Singh A, Ward OP. Developments in the use of Bacillus species for industrial production. Can J Microbiol. 2004;50:1–17. doi: 10.1139/w03-076. [DOI] [PubMed] [Google Scholar]
- Sharma A, Adhikari S, Satyanarayana T. Alkali-thermostable and cellulase free xylanase production by an extreme thermophile Geobacillus thermoleovorans. World J Microbiol Biotechnol. 2007;23:483–490. [Google Scholar]
- Sneath PHA, Mair NS, Sharpe ME, Holt JG. Bergy’s Manual of Systematic Bacteriology. Vol. 2. Baltimore: Williams & Wilkins; 1986. [Google Scholar]
- Son YS. Production and uses of Korean Native Black Goat. Small Rumin Res. 1999;34:303–308. [Google Scholar]
- Trivedi N, Gupta V, Kumar M, Kumari P, Reddy CRK, Jha B. An alkali-halotolerant cellulase from Bacillus flexus isolated from green seaweed Ulva lactuca. Carbohydr Polym. 2011;83:891–897. [Google Scholar]
- van Dyk JS, Sakka M, Sakka K, Pletschke BI. The cellulolytic and hemi-cellulolytic system of Bacillus licheniformis SVD1 and the evidence for production of a large multi-enzyme complex. Enzyme Microb Technol. 2009;45:372–378. [Google Scholar]
- van Dyk JS, Sakka M, Sakka K, Pletschke BI. Identification of endoglucanases, xylanases, pectinases and mannanases in the multi-enzyme complex of Bacillus licheniformis SVD1. Enzyme Microb Technol In Press, Corrected Proof. 2010;47:112–118. [Google Scholar]
- Wang CM, Shyu CL, Ho SP, Chiou SH. Characterization of a novel thermophilic, cellulose-degrading bacterium Paenibacillus sp. strain B39. Lett Appl Microbiol. 2008;47:46–53. doi: 10.1111/j.1472-765X.2008.02385.x. [DOI] [PubMed] [Google Scholar]
- Williams AG, Withers SE. Bacillus spp. in the rumen ecosystem. Hemicellulose depolymerases and glycoside hydrolases of Bacillus spp. and rumen isolates grown under anaerobic conditions. J Appl Bacteriol. 1983;55:283–292. [Google Scholar]
- Yang VW, Zhuang Z, GffElegir G, Jeffries TW. Alkaline-active xylanase produced by an alkaliphilic Bacillus sp. isolated from kraft pulp. J Ind Microbiol. 1995;15:434–441. [Google Scholar]
- Yin L-J, Lin H-H, Chiang Y-I, Jiang S-T. Bioproperties and Purification of Xylanase from Bacillussp. YJ6. J Agric Food Chem. 2010;58:557–562. doi: 10.1021/jf902777r. [DOI] [PubMed] [Google Scholar]