Abstract
The objective of this study was to determine the effect of slaughter season on the fatty acid profile in four types of fat deposits in crossbred (Polish Holstein Friesian Black-and-White×Limousine) beef bulls. The percentage share of fatty acids was determined by gas chromatography and were divided into the following categories of fatty acids: saturated (SFAs), unsaturated (UFAs), monounsaturated (MUFAs), polyunsaturated (PUFAs), desirable hypocholesterolemic (DFAs) and undesirable hypercholesterolemic (OFAs), n-3 and n-6. Perinephric fat was characterized by the highest SFA concentrations (59.89%), and subcutaneous fat had the highest MUFA content (50.63%). Intramuscular fat was marked by a high percentage share of PUFAs and the highest PUFA/SFA ratio. The slaughter season had a significant effect on the levels of C18:3, C20:4 (p≤0.01) and conjugated linoleic acid (p≤0.05). There was an interaction between the slaughter season and fat type for the content of C20:4 (p≤0.01) and C20:5 (p≤0.05). The results of this study show that beef from cattle slaughtered in the summer season has a higher nutritional value and more health-promoting properties.
Keywords: Beef, Fatty Acids, Gas Chromatography, Slaughter Season, Fat Deposit
INTRODUCTION
Animal fats are believed to contain relatively high concentrations of nutritionally undesirable saturated fatty acids and high cholesterol levels (Schaefer, 2002). However, many research studies show that only selected saturated fatty acids have adverse effects on human health. Animal fats contain also biologically active substances that deliver health benefits. In Poland, beef production is based mainly on dairy cattle herds. Dairy cows are also increasingly crossbred with beef bulls to improve fattening performance, slaughter value and meat quality. Commercial crossbreeding supports the production of prime quality beef that can adequately meet market demand (Ducatti et al., 2009; Prado et al., 2009).
Meat consumers give importance to the content, distribution and composition of fat in beef carcasses. Consumers tend to prefer meat of low intramuscular fat content to other ones with higher fat of even better palatability (Holló et al., 2001). Fat quality is determined by the levels and proportions of fatty acids. The fatty acid profile of beef is influenced, among others, by the body weight, gender and breed of animals, age at slaughter, and geographical factors (Holló et al., 2001; Moreno et al., 2006; Xie et al., 2012). The fatty acid composition of adipose tissue is also affected by the nutritional regime, the length of the fattening period and the type of adipose tissue (Duckett et al., 1993; Aharoni et al., 1995; Scollan et al., 2006). According to Callow (1962), subcutaneous fat and muscles with a similar anatomical location may have different fatty acid profiles. Dietary manipulation in ruminants can lead to an increase in the concentrations of conjugated linoleic acid (CLA), n-3 polyunsaturated fatty acids (PUFAs) and monounsaturated fatty acids (MUFAs) in meat (French et al., 2000; Williams, 2000). The CLA content of fat may be increased through grass feeding and pasture grazing (Enser et al., 1998; Nuernberg et al., 2005). According to Alfaia et al. (2007a; 2007b), very few papers deal with seasonal changes in CLA levels in different beef muscles. The possibility to alter the PUFA content of beef through dietary manipulation remains relatively poorly investigated, in comparison with milk. Dietary PUFAs from different families can interact to modify the neoplastic process. The recommended dietary n-6/n-3 PUFA ratio is 4–5:1, and a higher ratio is associated with an increased risk of cancer. There is also a scarcity of published data on seasonal changes in the fatty acid profile of meat. As demonstrated by Rhee et al. (2006), temperature changes may contribute to improving meat quality by enhancing cardiopulmonary blood flow, oxygen utilization and the metabolic rate in animals in response to lower oxygen saturation and colder temperatures.
In view of the above, the objective of this study was to determine the effect of slaughter season on the fatty acid profile in four types of fat deposits in crossbred bulls.
MATERIALS AND METHODS
Experimental material
The experimental materials comprised fat samples collected from the carcasses of 50 crossbred beef bulls produced by mating Polish Holstein Friesian Black-and-White (PHF) cows to Limousine (LM) bulls. The animals were slaughtered in two seasons, winter (January to March, n = 27) and summer (June to August, n = 23). Feeding was typical for North-East Poland: semi intensive fattening based on pasture (spring and summer) and on forage (autumn and winter). The average age of bulls was 21.10±2.94 months, and their average body weight at slaughter was 645±9.8 kg. Slaughter and post-slaughter processing were carried out in accordance with the relevant meat industry regulations. After 96 h of carcass chilling, samples of four types of fat deposits were collected. Intramuscular fat samples were collected from the loin (m. longissimus dorsi, between the 11th and 13th thoracic vertebra). Samples of intermuscular and subcutaneous fat were collected from the leg, and perinephric fat samples - from the kidney region. Vacuum-packaged samples were transported (+4°C) to the laboratory of the Department of Cattle Breeding and Milk Quality Evaluation, University of Warmia and Mazury in Olsztyn.
Fat extraction
Fat was extracted from ground meat samples by the Soxhlet method using the Büchi B-811 extraction system, with hexane as a solvent. Crude fat content and the percentage share of fatty acids were determined based on the following standards: PN-EN ISO 5509: 2001. Animal and vegetable fats and oils. Preparation of methyl esters of fatty acids, and PN-EN ISO 5508:1996. Animal and vegetable fats and oils.
Fatty acid profile
Fatty acid methyl esters were obtained by dissolving the extracted fat in a methanol-chloroform-H2SO4 mixture, followed by methylation according to the modified Peisker method (Żegarska et al., 1991). The percentage share of 31 fatty acids was determined by gas chromatography, using the Varian CP 3800 system with a split/splitless injector and a flame-ionization detector (FID). Samples (1 μl) of fatty acid methyl esters were placed on a CP-Sil 88 capillary column (length: 100 m, inner diameter: 0.25 mm). Data were processed using the GALAXIE Chromatography Data System. Fatty acids were identified by comparing their retention times with those of commercially available reference standards purchased from Supelco, Inc. Analyses of samples and reference standards were performed under identical conditions, i.e. carrier gas - helium, injector temperature −260°C, detector temperature −260°C, initial oven temperature −110°C, raised to 249°C. The fatty acids were divided into the following categories: saturated fatty acids (SFAs), unsaturated fatty acids (UFAs), including monounsaturated fatty acids (MUFAs) and polyunsaturated fatty acids (PUFAs), desirable hypocholesterolemic fatty acids (DFAs) and undesirable hypercholesterolemic fatty acids (OFAs). The following ratios were calculated: UFA/SFA, MUFA/SFA, PUFA/SFA and n-6/n-3 PUFA.
Statistical analysis
The results were processed statistically using the STATISTICA data analysis software system Ver. 9.0 (StatSoft, Inc., 2009). One-way and two-way analysis of variance with interactions was performed. The significance of differences between mean values in groups was estimated by LSD (least significant differences) test.
RESULTS AND DISCUSSION
Functional fatty acid
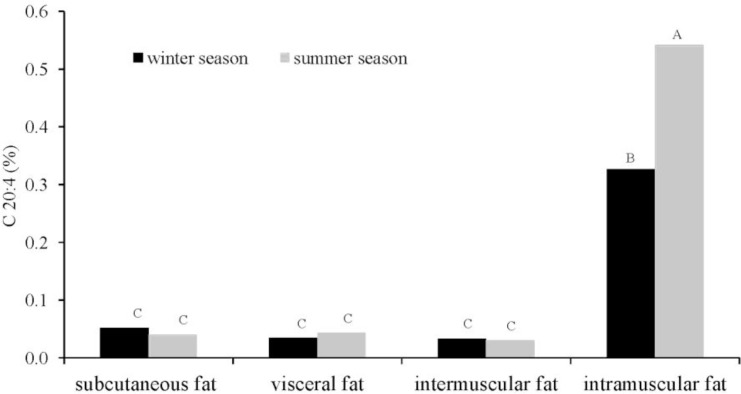
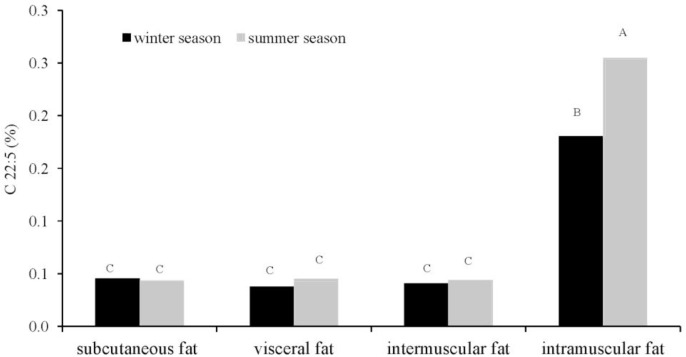
The slaughter season had a significant effect on the levels of linoleic acid C18:2, arachidonic acid C20:4 (p≤0.01) and CLA (p≤0.05) (p≤0.01) (Table 1). Higher concentrations of those fatty acids were noted in the carcasses of bulls slaughtered in the summer. A similar trend was observed for vaccenic acid and docosapentaenoic acid C22:5 (DPA), but the noted differences were statistically non-significant. The above acids are known to have disease preventing and health-promoting properties. Therefore, beef from cattle slaughtered in the summer is characterized by a more desirable fatty acid profile, compared with the meat of animals slaughtered in the winter. The concentrations of functional fatty acids were determined by the type of fat deposits (p≤0.01). The lowest CLA levels were found in intramuscular fat, whose content is an important determinant of beef quality. The highest CLA concentrations were reported for subcutaneous fat, and the difference in CLA content between subcutaneous fat and the other three types of fat deposits was statistically significant (p≤0.01). Our results are consistent with the findings of Aldai et al. (2007) who compared three types of adipose tissue (subcutaneous, intramuscular and intermuscular) and reported that CLA content was highest in subcutaneous fat (0.42%), followed by intramuscular fat (0.22%) and intermuscular fat (0.37%). According to Kazala et al. (1999) and Raes et al. (2003), the above could be due to the fact that CLA isomers are found mostly in the fraction of neutral lipids. Despite the lowest CLA content, intramuscular fat had higher concentrations of PUFAs (in particular C20:4, C20:5 and C22:5) than the other analyzed fats. The average levels of those fatty acids in intramuscular fat were several-fold higher than in the remaining types of fat deposits, which corroborates the findings of Aldai et al. (2007). Bulls slaughtered in the summer were characterized by a higher content of arachidonic acid and DPA in intramuscular fat. The concentrations of C18:1 T10+11, C18:2, CLA, C20:4 and C22:5 fatty acids were higher in the summer, thus suggesting that beef from cattle slaughtered in the summer season has a higher nutritional value and more health-promoting properties, compared with beef produced in the winter season. The above is due to seasonal variations in the quality of green forage and its availability to cattle. As demonstrated by many authors, the content of n-3 C18:3, eicosapentaenoic acid C20:5 (EPA), C22:5 (DPA) and docosahexaenoic acid (DHA) in muscle lipids is higher in grass-fed and pasture-fed cattle (Warren et al., 2002; Dannenberger et al., 2004). The predominant functional MUFA was oleic acid (C18:1 cis9), which had a significantly (p≤0.01) higher share of the total fatty acid pool in intramuscular fat and subcutaneous fat (37.74%) than in intermuscular fat (30.64%) and perinephric fat (28.86%). There was an interaction between the slaughter season and fat type for the content of C20:4 (p≤0.01; Figure 1) and C20:5 (p≤0.05; Figure 2) fatty acids. Significantly higher levels of those fatty acids were noted in the intramuscular fat deposits of animals slaughtered in the summer (C20:4 0.54%, C22:5 0.33%). Alfaia et al. (2007b) also observed an interaction between the slaughter season and muscle type for the concentrations of some fatty acids, including C20:4 and C20:5, which could result from modifications of muscle metabolic type caused by adaptations to the different grazing periods, followed by changes in fatty acid composition.
Table 1.
The functional of fatty acids in different adipose tissues and slaughter season
| Specification | Subcutaneous fat | Perinephric fat | Intermuscular fat | Intramuscular fat | SEM | Winter season | Summer season | SEM | Influence
|
|||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Season | Adipose tissue | Interaction season × adipose tissue | ||||||||||
| C18:1 T10+11 | x̄ | 1.14B | 2.18A | 2.24A | 1.23B | 0.08 | 1.59 | 1.75 | 0.07 | ns | ** | ns |
| C18:1 C9 | x̄ | 37.74A | 28.86Bb | 30.64Ba | 37.74A | 0.41 | 34.29 | 33.49 | 0.41 | ns | ** | ns |
| C18:2 | x̄ | 1.62C | 2.04B | 2.04B | 2.56A | 0.04 | 1.99B | 2.22A | 0.04 | ** | ** | ns |
| C18:3 | x̄ | 0.48Bb | 0.65A | 0.59a | 0.62A | 0.02 | 0.61 | 0.55 | 0.02 | ns | ** | ns |
| CLA | x̄ | 0.43Aa | 0.30B | 0.34b | 0.27Ba | 0.01 | 0.31b | 0.37a | 0.01 | * | ** | ns |
| C20:4 | x̄ | 0.05B | 0.04B | 0.03B | 0.41A | 0.14 | 0.13 | 0.16 | 0.02 | ** | ** | ** |
| C20:5 EPA | x̄ | 0.02B | 0.02B | 0.01B | 0.08A | 0.01 | 0.04 | 0.03 | 0.01 | ns | ** | ns |
| C22:5 DPA | x̄ | 0.04B | 0.04B | 0.04B | 0.21A | 0.01 | 0.08 | 0.10 | 0.01 | ns | ** | * |
Man values denoted by different letters in rows within trait are significantly different at:
p≤0.05;
p≤0.01. Man values are significantly different at:
* p≤0.05;
** p≤0.01, ns p>0.05.
Figure 1.
Simultaneous effect of slaughter season and the fat deposit on the C 20:4 acid content mean values denoted by different letters are significantly different at: A,B p≤0.01.
Figure 2.
Simultaneous effect of slaughter season and the fat deposit on the C22:5 acid content mean values denoted by different letters are significantly different at: A,B p≤0.01.
Fatty acids groups and ratios
Subcutaneous fat had the lowest SFA content (46.64%) and the highest concentrations of UFAs, MUFAs and hypercholesterolemic OFAs (Table 2). Intramuscular fat had the highest content of essential PUFAs (4.48%) and the most desirable PUFA/SFA ratio (0.09), compared with the other types of adipose tissue. The high PUFA content of intramuscular fat can be attributed to the presence of small adipocytes (Harper and Pethick, 2004; Pethick et al., 2004) and a higher ratio of phospholipids to neutral lipids (Nurnberg et al., 1998, Arana et al., 2006). In our study, the PUFA/SFA ratio was similar to that reported by Enser et al. (1998) of 0.11 and lower than that noted by Aldai et al. (2007) and Muchenje et al. (2009), respectively 0.45 and 0.49. The PUFA/SFA and n-6/n-3 PUFA ratios are good indicators of the nutritional value of dietary fat (Alfaia et al., 2007a; 2007b). Xie et al. (2012) reported that an increase in PUFA and lower n-6:n-3 ratios are desirable for human health. According to the current nutritional recommendations, the PUFA/SFA ratio in human diet should be above 0.45, and the n-6/n-3 PUFA ratio should not exceed 4.0 (British Department of Health, 1994). In our study, the values of the n-6/n-3 PUFA ratio were within this range in all types of adipose tissue and in both slaughter seasons, while the PUFA/SFA ratio was below the recommended levels in all samples. Similar results were reported by Alfaia et al. (2007a; 2007b) who, similarly as French et al. (2000), attribute those values to the ruminal biohydrogenation of dietary unsaturated fatty acids. Scollan et al. (2006) found that beef from pasture-fed cattle contains higher levels of beneficial n-3 PUFAs, compared with beef from young bulls raised in conventional or intensive production system. In a study by Varela et al. (2004), the intramuscular fat of steers fed maize silage and concentrate had a less favorable n-6/n-3 PUFA ratio, in comparison with pasture-finished steers. In our experiment, the n-6/n-3 PUFA ratio ranged from 2.28 to 2.68, and it reached the highest value in intramuscular fat. According to Bartnikowska and Kulasek (1994), the optimal n-6/n-3 PUFA ratio in human diet is 2–5:1, whereas according to Wijendran and Hayes (2004) it should oscillate around 6:1. The n-6/n-3 PUFA ratios determined for four types of fat deposits in the present experiment were lower than the values recommended by nutrition experts, but similar to those reported by Alfaia et al. (2007a) for Barrosă-PDO calves (2.9 to 3.1), and by Enser et al. (1998) for UK steers fed grass (2.0 to 2.3). No significant differences in the n-6/n-3 PUFA ratio were found between slaughter seasons, but the noted values were lower in the winter (2.31) than in the summer (2.60). Our findings are consistent with those of Costa et al. (2006) and Varela et al. (2004) who noted higher n-6/n-3 PUFA ratios in the spring than in the autumn. Changes in the n-6/n-3 PUFA ratio may be due to differences in diet composition between feeding periods (Marmer et al., 1984, Elmore et al., 2004; Costa et al., 2006), since fats supplied by green forage have a higher content of C18:3 fatty acid, the precursor of essential n-3 PUFAs. In the current study, the slaughter season had a significant (p≤0.01) effect on the percentage share of all analyzed PUFAs and the PUFA/SFA ratio. The adipose tissue of animals slaughtered in the summer contained higher amounts of essential PUFAs (3.74%) and hypocholesterolemic DFAs (66.78%). Since the above fatty acids have a beneficial influence on human health, it can be stated that beef from cattle slaughtered in the summer delivers more health benefits.
Table 2.
The fatty acids groups and ratios in different adipose tissues and slaughter season
| Specification | Subcutaneous fat | Perinephric fat | Intermuscular fat | Intramuscular fat | SEM | Winter season | Summer season | SEM | Influence
|
|||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Season | Adipose tissue | Interaction season × adipose tissue | ||||||||||
| SFA | x̄ | 46.64C | 59.89A | 57.84A | 49.45B | 0.54 | 53.06 | 53.45 | 0.54 | ns | ** | ns |
| UFA | x̄ | 53.36A | 40.25C | 42.26C | 50.58B | 0.53 | 47.00 | 46.63 | 0.53 | ns | ** | ns |
| MUFA | x̄ | 50.63A | 36.34Cb | 38.45Ca | 46.34B | 0.54 | 43.53 | 42.66 | 0.54 | ns | ** | ns |
| PUFA | x̄ | 2.99Bb | 3.38Ba | 3.37Ba | 4.48A | 0.07 | 3.47b | 3.74a | 0.07 | ** | ** | ns |
| DFA | x̄ | 65.61B | 65.99B | 66.64 | 67.89A | 0.34 | 66.40 | 66.78 | 0.34 | ns | ns | ns |
| OFA | x̄ | 34.38a | 34.15a | 33.46 | 32.13b | 0.34 | 33.65 | 33.29 | 0.34 | ns | ns | ns |
| ΣUFA/SFA | x̄ | 1.18A | 0.68C | 0.74C | 1.04B | 0.02 | 0.93 | 0.91 | 0.02 | ns | ** | ns |
| ΣPUFA/SFA | x̄ | 0.06B | 0.05B | 0.06B | 0.09A | 0.00 | 0.06 | 0.07 | 0.00 | ** | ** | ns |
| ΣMUFA/SFA | x̄ | 1.12A | 0.62C | 0.68C | 0.95B | 0.02 | 0.86 | 0.83 | 0.02 | ns | ** | ns |
| n-3 | x̄ | 0.49Bb | 0.66a | 0.61A | 0.70A | 0.02 | 0.65 | 0.58 | 0.02 | ns | ** | ns |
| n-6 | x̄ | 1.04B | 1.31b | 1.18B | 1.67Aa | 0.90 | 1.38 | 1.24 | 0.06 | ns | ** | ** |
| n-6/n-3 | x̄ | 2.37 | 2.28 | 2.40 | 2.68 | 0.15 | 2.31 | 2.60 | 0.15 | ns | ns | ns |
| PUFA ratio | ||||||||||||
Mean values denoted by different letters in rows within trait are significantly different at:
p≤0,05;
p≤0.01. Mean values are significantly different at:
p≤0.05;
p≤0.01, ns p>0.05.
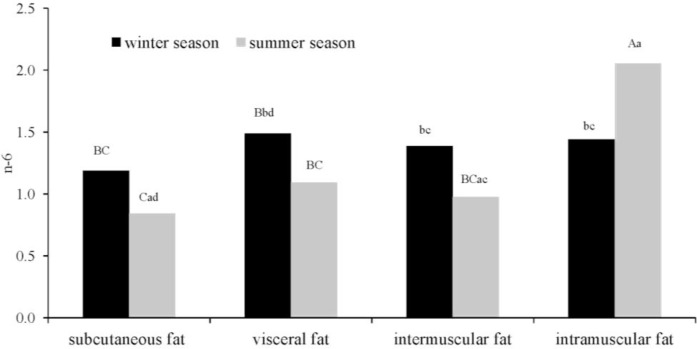
The concentrations of n-6 PUFAs were determined by both the slaughter season and fat type (p≤0.01, Figure 3). The subcutaneous fat of bulls slaughtered in the summer had the lowest n-6 PUFA content (0.84%). In the winter, n-6 PUFA levels were similar in all types of adipose tissue, and they were higher than those noted in subcutaneous, perinephric and intermuscular fat in the summer. The intramuscular fat of animals slaughtered in the summer had the highest concentrations of essential n-6 PUFAs (2.05%). Aldai et al. (2007) also noted significant differences in n-6 PUFA content between three types of adipose tissue (subcutaneous, intramuscular and intermuscular) in young bulls. Costa et al. (2006) reported higher n-6 PUFA levels in calves slaughtered in the spring than in those sacrificed in the autumn, which corroborates our results. The cited authors also observed an interaction between muscle type and slaughter season. The fatty acid composition of meat can be influenced by a variety of factors. According to De Smet et al. (2004), the levels of n-6 and n-3 PUFAs in beef are determined by dietary factors to a greater extent than by genetic factors and the type of adipose tissue.
Figure 3.
Simultaneous effect of slaughter season and the fat deposit on the PUFA n-6acid content mean values denoted by different letters are significantly different at: A,B p≤0.01.
The results of our study show that fatty acid composition was affected by both the slaughter season and the type of fat deposits. Perinephric fat was characterized by the highest SFA concentrations, and subcutaneous fat had the highest MUFA content. Intramuscular fat, whose content was relatively low, was marked by a high percentage share of PUFA, the highest PUFA/SFA ratio, and a desirable n-6/n-3 PUFA ratio. Beef from cattle slaughtered in the summer season had a higher nutritional value and more health-promoting properties, compared with beef produced in the winter season.
Acknowledgments
Research was realized within the project “Optymalizacja produkcji wołowiny w Polsce zgodnie ze strategią “od widelca do zagrody” (“Optimising of beef production in Poland according to fork-to-farm strategy”) no. PO IG 01.03.01-00-204/ co-financed by the European Union from the European Regional Development Fund within the Innovative Economy Operational Programme 2007–2013”.
REFERENCES
- Aharoni Y, Nachtomi E, Holstein P, Brosh A, Holzer Z, Nitsan Z. Dietary effects on fat deposition and fatty acid profiles in muscle and fat depots of Friesian bull calves. J Anim Sci. 1995;73:2712–2720. doi: 10.2527/1995.7392712x. [DOI] [PubMed] [Google Scholar]
- Aldai N, Najera AI, Dugan MER, Celaya R, Osoro K. Characterisation of intramuscular, intermuscular and subcutaneous adipose tissues in yearling bulls of different genetic groups. Meat Sci. 2007;76:682–691. doi: 10.1016/j.meatsci.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Alfaia CPM, Castro MLF, Martins SIV, Portugal APV, Alves SPA, Fontes CMGA, Bessa RJB, Prates JAM. Effect of slaughter season on fatty acid composition, conjugated linoleic acid isomers and nutritional value of intramuscular fat in Barrosa-PDO veal. Meat Sci. 2007a;75:44–52. doi: 10.1016/j.meatsci.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Alfaia CPM, Castro MLF, Martins SIV, Portugal APV, Alves SPA, Fontes CMGA, Bessa RJB, Prates JAM. Influence of slaughter season and muscle type on fatty acid composition, conjugated linoleic acid isomeric distribution and nutritional quality of intramuscular fat in Aroquesa-PDO veal. Meat Sci. 2007b;76:787–795. doi: 10.1016/j.meatsci.2007.02.023. [DOI] [PubMed] [Google Scholar]
- Arana A, Mendizabal JA, Alzo′n M, Eguinoa P, Beriain MJ, Purroy A. Effect of feeding lambs oleic acid calcium soaps on growth, adipose tissue development and composition. Small Rumin Res. 2006;63:75–83. [Google Scholar]
- Bartnikowska E, Kulasek G. Znaczenie nienasyconych kwasów tłuszczowych w żywieniu człowieka i zwierząt (cz. II) Niedobory i dietetyczne leczenie niedoborów. 1994;5:34–38. Magazyn Weterynaryjny (in Polish) [Google Scholar]
- British Department of Health . Nutritional aspects of cardiovascular disease Report of Health and Social Subjects No 46. London, HMSO: 1994. [PubMed] [Google Scholar]
- Callow EH. Comparative studies of meat. VIII. The percentage of fat in the fatty and muscular tissues of steers and the iodine number of the extracted fat, as affected by breed and level of nutrition. J Agric Sci. 1962;58:295–307. [Google Scholar]
- Costa P, Roseiro LC, Partidario A, Alves V, Bessa RJB, Calkins CR, Santos C. Influence of slaughter season and sex on fatty acid composition, cholesterol and α-tocopherol contents on different muscles of Barrosa-PDO veal. Meat Sci. 2006;72:130–139. doi: 10.1016/j.meatsci.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Dannenberger D, Nuernberg G, Scollan N, Schabbel W, Steinhart H, Ender K, et al. Effect of diet on the deposition of n-3 fatty acids, conjugated linoleic- and C18:1trans fatty acid isomers in muscle lipids of German Holstein bulls. J Agric Food Chem. 2004;52:6607–6615. doi: 10.1021/jf049511l. [DOI] [PubMed] [Google Scholar]
- De Smet S, Raes K, Demeyer D. Meat fatty acid composition as affected by fatness and genetic factors: a review. Anim Res. 2004;53:81–98. [Google Scholar]
- Ducatti T, Prado IN, Rotta PP, Prado RM, Perotto D, Maggioni D, Visentainer JV. Chemical composition and fatty acid profile in crossbred (Bos taurus vs. Bos indicus) young bulls finished in feedlot. Asian-Aust J Anim Sci. 2009;22:433–439. [Google Scholar]
- Duckett SK, Wagner DG, Yates LD, Dolezal HG, May SG. Effects of time on feed on beef nutrient composition. J Anim Sci. 1993;71:2079–2088. doi: 10.2527/1993.7182079x. [DOI] [PubMed] [Google Scholar]
- Elmore JS, Warren HE, Mottram DS, Scolan ND, Enser M, Richardson RI, Wood JD. A comparison of the aroma volatiles and fatty acid compositions of grilled beef muscle from Aberdeen Angus and Holstein-Friesian steers fed diets based on silage or concentrates. Meat Sci. 2004;68:27–33. doi: 10.1016/j.meatsci.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Enser M, Hallet B, Hewitt G, Fursey J, Wood D, Harrington G. Fatty acid content and composition of UK beef and lamb muscle in relation to production system and implications for human nutrition. Meat Sci. 1998;49:329–341. doi: 10.1016/s0309-1740(97)00144-7. [DOI] [PubMed] [Google Scholar]
- French P, Stanton C, Lawless F, O’Riordan EG, Monahan FJ, Caffrey PJ. Fatty acid composition, including conjugated linoleic acid, of intramuscular fat from steers offered grazed grass, grass silage, or concentrate based diets. J Anim Sci. 2000;78:2849–2855. doi: 10.2527/2000.78112849x. [DOI] [PubMed] [Google Scholar]
- Harper GS, Pethick DW. How might marbling Begin? Aust J Exp Agric. 2004;44:653–662. [Google Scholar]
- Holló G, Csapó J, Szűcs E, Tözsér J, Repa I, Holló I. Influence of breed, slaughter weight and gender on chemical composition of beef. Part 2. Fatty acid composition of fat in rib samples. Asian-Aust J Anim Sci. 2001;14:1719–1723. [Google Scholar]
- Kazala EC, Lozeman FJ, Mir PS, Laroche A, Bailey DRC, Weselake RJ. Relationship of fatty acid composition to intramuscular fat content in beef from crossbred Wagyu cattle. J Anim Sci. 1999;77:1717–1725. doi: 10.2527/1999.7771717x. [DOI] [PubMed] [Google Scholar]
- Marmer WN, Maxwell RJ, Williams JE. Effects of dietary regimen and tissue site on bovine fatty acid profiles. J Anim Sci. 1984;59:109–121. [Google Scholar]
- Moreno T, Varela A, Oliete B, Carballo J, Sa′nchez L, Montserrat L. Nutritional characteristics of veal from weaned and unweaned calves: Discriminatory ability of the fat profile. Meat Sci. 2006;73:209–217. doi: 10.1016/j.meatsci.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Muchenje V, Hugo A, Dzama K, Chimonyo M, Strydom PE, Raats JG. Cholesterol levels and fatty acid profiles of beef from three cattle breeds raised on natural pasture. J Food Compost Anal. 2009;22:354–358. [Google Scholar]
- Nuernberg KD, Dannenberger D, Nuernberg G, Ender K, Voigt J, Scollan ND, Wood JD, Nute GR, Richardson RI. Effect of a grass-based and a concentrate feeding system on meat quality characteristics and fatty acid composition of longissimus muscle in different cattle breeds. Livest Prod Sci. 2005;94:137–147. [Google Scholar]
- Nurnberg K, Wegner J, Ender K. Factors influencing fat composition in muscle and adipose tissue of farm animals. Livest Prod Sci. 1998;56:145–156. [Google Scholar]
- Pethick DW, Harper GS, Oddy VH. Growth, development and nutritional manipulation of marbling in cattle: a review. Aust J Exp Agric. 2004;44:705–715. [Google Scholar]
- PN-EN ISO 5508 Oleje i tłuszcze roślinne oraz zwierzęce. Analiza estrów metylowych kwasów tłuszczowych metodą chromatografii gazowej (Animal and vegetable fats and oils -Analysis by gas chromatography of metyl esters of fatty acids) 1996.
- PN-EN ISO 5509 Oleje i tłuszcze zwierzęce. Przygotowanie estrów metylowych kwasów tłuszczowych (Animal and vegetable fats and oils - Preparation of methyl esters of fatty acids) 2001.
- Prado IN, Oliveira AN, Rotta PP, Perotto D, Prado RM, Silva RR, Souza NE, Molettas JL. Chemical and Fatty Acid Composition of Longissimus Muscle of Crossbred Bulls Finished in Feedlot. Asian-Aust J Anim Sci. 2009;22:1054–1059. [Google Scholar]
- Raes K, De Smet S, Balcaen A, Clayes E, Demeyer D. Effect of diets rich in n-3 polyunsaturated fatty acids on muscle lipids and fatty acids in Belgian Blue double-muscled young bulls. Reprod Nutr Dev. 2003;43:331–345. doi: 10.1051/rnd:2003029. [DOI] [PubMed] [Google Scholar]
- Rhee YJ, Hyun CB, Kim JT, Lee SK, Song YH. Altitude influenced hematological and biochemical differences in Hanwoo. Proceedings of the XIIth AAAP Science Congress; 2006; Busan, Korea. 2006. p. 630. [Google Scholar]
- Schaefer EJ. Lipoproteins, nutrition, and disease. Am J Clin Nutr. 2002;75:191–212. doi: 10.1093/ajcn/75.2.191. [DOI] [PubMed] [Google Scholar]
- Scollan N, Hocquette J-F, Nuernberg K, Dannenberger D, Richardson I, Moloney A. Innovations in beef production systems that enhance the nutritional and health value of beef lipids and their relationship with meat quality. Meat Sci. 2006;74:17–33. doi: 10.1016/j.meatsci.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Statsoft Inc . STATISTICA (data analysis software system), Version 9.0. 2009. www.statsoft.com [Google Scholar]
- Varela A, Oliete B, Moreno T, Portela C, Monserrat L, Carballo J, Sanchez L. Effect of pasture finishing on the meat characteristics and intramuscular fatty acid profile of steers of the Rubia Gallega breed. Meat Sci. 2004;67:512–522. doi: 10.1016/j.meatsci.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Warren HE, Scollan ND, Hallett K, Enser M, Richardson RI, Nute GR, Wood JD. The effects of breed and diet on the lipid composition and quality of bovine muscle. Proceedings of the 48th congress of meat science and technology; 2002. pp. 370–371. [Google Scholar]
- Wijendran V, Hayes KC. Dietary n-6 and n-3 fatty acid balance and cardiovascular health. Annu Rev Nutr. 2004;24:597–615. doi: 10.1146/annurev.nutr.24.012003.132106. [DOI] [PubMed] [Google Scholar]
- Williams C. Dietary fatty acids and human health. Ann Zootech. 2000;49:165–180. [Google Scholar]
- Xie X, Meng Q, Cui Z, Ren L. Effect of cattle breed on meat quality, muscle fiber characteristics, lipid oxidation and fatty acids in China. Asian-Aust J Anim Sci. 2012;25:824–831. doi: 10.5713/ajas.2011.11462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Żegarska Z, Jaworski J, Borejszo Z. Ocena zmodyfikowanej metody Peiskera otrzymywania estrów metylowych kwasów tłuszczowych. Acta Acad. Agricult. Techn. Olst (in Polish) 1991;24:25–33. [Google Scholar]