Abstract
Unfertilized oocytes age inevitably after ovulation, which limits their fertilizable life span and embryonic development. Rapamycin affects mammalian target of rapamycin (mTOR) expression and cytoskeleton reorganization during oocyte meiotic maturation. The goal of this study was to examine the effects of rapamycin treatment on aged porcine oocytes and their in vitro development. Rapamycin treatment of aged oocytes for 24 h (68 h in vitro maturation [IVM]; 44 h+10 μM rapamycin/24 h, 47.52±5.68) or control oocytes (44 h IVM; 42.14±4.40) significantly increased the development rate and total cell number compared with untreated aged oocytes (68 h IVM, 22.04±5.68) (p<0.05). Rapamycin treatment of aged IVM oocytes for 24 h also rescued aberrant spindle organization and chromosomal misalignment, blocked the decrease in the level of phosphorylated-p44/42 mitogen-activated protein kinase (MAPK), and increased the mRNA expression of cytoplasmic maturation factor genes (MOS, BMP15, GDF9, and CCNB1) compared with untreated, 24 h-aged IVM oocytes (p<0.05). Furthermore, rapamycin treatment of aged oocytes decreased reactive oxygen species (ROS) activity and DNA fragmentation (p<0.05), and downregulated the mRNA expression of mTOR compared with control or untreated aged oocytes. By contrast, rapamycin treatment of aged oocytes increased mitochondrial localization (p<0.05) and upregulated the mRNA expression of autophagy (BECN1, ATG7, MAP1LC3B, ATG12, GABARAP, and GABARAPL1), anti-apoptosis (BCL2L1 and BIRC5; p<0.05), and development (NANOG and SOX2; p<0.05) genes, but it did not affect the mRNA expression of pro-apoptosis genes (FAS and CASP3) compared with the control. This study demonstrates that rapamycin treatment can rescue the poor developmental capacity of aged porcine oocytes.
Keywords: Porcine Oocyte, Age, Rapamycin, Reactive Oxygen Species (ROS), Mammalian Target of Rapamycin (mTOR)
INTRODUCTION
Oocyte meiotic resumption leads to the completion of the first meiotic asymmetric division, after which oocytes enter meiosis II before arresting at metaphase II (MII) until fertilization. A disruption in any one of these cellular events usually results in aged oocytes of poor quality, which may affect fertilization outcomes and result in mammalian infertility. Despite substantial progress in assisted reproduction technologies (ART), many failures are the result of oocyte aging (Miao et al., 2009). Chromosomal abnormalities, spindle defects, mitochondrial dysfunction, and a decrease in the levels of maturation-promoting factor (MPF) and mitogen-activated protein kinase (MAPK) are directly implicated in age-related declines in fertility and embryonic development (Dodson et al., 1989; Xu et al., 1997; Kikuchi et al., 2000; Pellestor et al., 2003; Baird et al., 2005). Lee et al. (2007) reported that the mitogen-activated protein kinase kinase (MEK)/MAPK pathway regulates microtubule and centrosome assembly. In the absence of centrosome and microtubule stability, oocytes cannot be fertilized or, alternatively, fertilized oocytes display aneuploidy and developmental abnormalities.
While there is little knowledge on the mechanism of oocyte aging, there is a general consensus that it associates with an increase in intracellular oxidative damage (Shigenaga et al., 1994). An increase in reactive oxygen species (ROS) production over time may lead to a decrease in the intracellular adenosine triphosphate (ATP) concentration and the glutathione/glutathione disulfide ratio as well as to a concomitant increase in cytosolic calcium ions, which are all detrimental to oocyte health and embryo development (Tarin, 1996). Harman (1956) proposed a theory of aging which states that damage caused by ROS leads to cell senescence. Since ROS are produced mainly as by-products of mitochondrial respiration, mitochondria play a fundamental role in aging and represent putative targets of anti-aging strategies (Mammucari and Rizzuto, 2010).
Recently, it has been proposed that the main driver of aging is target of rapamycin (TOR) signaling rather than ROS (Blagosklonny, 2008). Inhibition of TOR, either pharmacologically with rapamycin or genetically, extends the life span of yeast, C. elegans (Kenyon, 2010), D. melanogaster (Bjedov et al., 2010), and mice (Harrison et al., 2009). Furthermore, TOR is a negative regulator of autophagy in organisms from yeast to man because knockout or knockdown of ATG genes can abolish the lifespan-extending effects of rapamycin in all species investigated (Bjedov et al., 2010). However, the mechanism behind the positive effects of rapamycin on lifespan remains largely unexplored.
Rapamycin, a bacterial macrolide with antifungal and immunosuppressant activities (Dumont et al., 1990), can form a complex with FK506 binding protein 12 and then bind mTOR, selectively inhibiting its kinase activity and function (Guertin and Sabatini, 2007). Intensive studies have focused on the crucial roles of mTOR in controlling cell proliferation, growth, and survival. Rapamycin also inhibits F-actin reorganization and cell motility, at least in part, by downregulating the level and activity of RhoA through mTORC1-mediated S6K1- and 4E-BP1-signaling pathways. In particular, the inhibition of mTOR by rapamycin affected the formation of the actin cap and the cortical granules-free domain (CGFD), and disrupted peripheral spindle migration and asymmetric division during oocyte meiotic maturation (Lee et al., 2012). However, it is still not known whether rapamycin treatment affects cytoskeleton dynamics in developing porcine early embryos.
The objective of this in vitro study was to investigate the effects of appropriate concentrations of rapamycin on nuclear and cytoplasmic maturation and the developmental capacity of aged porcine oocytes, and to better understand the link between the production of mitochondrial ROS and mTOR signaling. We demonstrate that rapamycin can stabilize microtubules and centrosomes, possibly by maintaining a balance between molecules involved in the maturation of metaphase II (MII) oocytes. These findings may be applicable to IVF procedures, and they may help to overcome oocyte aging.
MATERIALS AND METHODS
Chemicals and reagents
All chemicals and reagents were purchased from Sigma (St. Louis, MO, USA) unless otherwise stated.
Antibodies
Rabbit polyclonal anti-mTOR and mouse monoclonal anti-α-tubulin–FITC antibodies were purchased from Abcam (Cambridge, UK) and Sigma, respectively. Rabbit polyclonal anti-p44/42 MAP Kandanti-phospho-p44/42 MAPK antibodies and anti-rabbit IgG–HRP were purchased from Cell Signaling Technology (Danvers, MA, USA). An Alexa Fluor488 goat anti-rabbit secondary antibody was purchased from Invitrogen (Carlsbad, CA, USA).
In vitro maturation and aging of porcine oocytes
Prepubertal porcine ovaries were collected from a local slaughterhouse and transported to the laboratory at 25°C in Dulbecco’s phosphate-buffered saline (DPBS) supplemented with 75 μg/L penicillin G and 50 μg/L streptomycin sulfate. Cumulus-oocyte complexes (COCs) were aspirated from follicles 2 to 8 mm in diameter with an 18-gauge needle and a disposable 10 mL syringe. The COCs were washed three times in tissue culture medium (TCM)-199-HEPES containing 0.1% (w/v) bovine serum albumin (BSA) (TCM-HEPES-BSA). Groups of 50 COCs were matured in 500 μL TCM-199 (Gibco, Grand Island, NY, USA) containing Earle’s salts, 0.57 mM cysteine, 10 ng/mL epidermal growth factor (EGF), 0.5 μ/mL follicle stimulating hormone (FSH), 0.5 μg/mL luteinizing hormone (LH), and 10% (v/v) porcine follicular fluid under mineral oil for 44 h at 38.8°C. Oocyte aging was initiated by culturing oocytes with cumulus cells for an additional 24 or 48 h in TCM-199.
Rapamycin treatment
Mature oocytes were covered with mineral oil and cultured in wells of a four-well multidish containing 500 μL TCM-199 at 38.8°C in a humidified atmosphere of 5% CO2 and 95% air. After maturation, MII stage oocytes were transferred into TCM-199 containing 0, 0.1, 1, 10, and 50 μM rapamycin (Sigma) and cultured for 24 or 48 h (68 or 92 h for in vitro maturation [IVM]) as described above. After treatment, oocytes were collected and aging was assessed.
Parthenogenetic activation and embryo culture
Following maturation, cumulus cells were removed by pipetting in the presence of 1 mg/mL hyaluronidase for 2 to 3 min. Oocytes were parthenogenetically activated with 5 μM Ca2+ ionomycin (Sigma) for 5 min. After 3 h of culture in porcine zygote medium-5 (PZM-5) containing 7.5 μg/mL cytochalasin B (Sigma), embryos were washed three times in PZM-5 containing 0.4% (w/v) BSA and cultured in the same medium for 7 days at 38.8°C in a humidified atmosphere of 5% CO2 and 95% air. The oocytes and embryos were washed in DPBS, and depending on the experiment, either fixed in 3.7% (w/v) paraformaldehyde for 20 min and stored at 4°C, or snap-frozen in liquid nitrogen and stored at −70°C.
Measurement of intracellular reactive oxygen species
Intracellular ROS activity in oocytes and embryos was measured by a 2,7-dichlorofluorescene assay, as previously described (Gupta et al., 2010). In brief, oocytes and embryos were incubated with 100 μM 2,7-dichlorodihydrofluorescein diacetate (DCHFDA) for 20 min at 38.8°C, washed three times in PZM-5 to remove excess dye, and immediately analyzed by epifluorescence microscopy (Olympus, Tokyo, Japan) using excitation and emission wavelengths of 450 to 490 nm and 515 to 565 nm, respectively. Gray scale images were acquired with a digital camera (Nikon, Tokyo, Japan) attached to the microscope, and mean gray values were measured with Image J software (NIH, Bethesda, MD, USA). Background fluorescent values were subtracted from the final values before statistical analysis. The experiment was repeated four independent times with each experiment consisting of 25 to 30 oocytes.
Confocal microscopy
Confocal microscopy was performed as previously described (Lee et al., 2012). In brief, oocytes were fixed with 3.7% (w/v) paraformaldehyde in phosphate buffered saline (PBS) overnight at 4°C and then transferred to membrane permeabilization solution (0.5% (v/v) Triton X100 in PBS) for 30 min. After 1 h in blocking buffer (1% (w/v) BSA in PBS), oocytes were incubated with an anti-mTOR (1:100) or anti-α-tubulin-FITC (1:200) antibody in blocking buffer overnight at 4°C. After three washes in PBS containing 0.5% (v/v) Tween 20 and 0.5% (v/v) Triton X-100, the oocytes were labeled with Alexa Fluor 488 goat anti-rabbit IgG (1:100) for 1 h at room temperature to visualize mTOR. To visualize α-tubulin–FITC, oocytes were washed three times in PBS containing 0.5% (v/v) Tween 20 and 0.5% (v/v) Triton X-100 for 2 min each. A 30 min incubation of porcine blastocysts with MitoTracker Green FM (Molecular Probes, Eugene, OR, USA) stained mitochondria. To detect fragmented DNA, embryos were incubated with fluorescein-conjugated dUTP and terminal deoxynucleotidyl transferase (in situ Cell Death Detection Kit, Roche, Mannheim, Germany) in the dark for 1 h at 37°C. The total number of mitotic and apoptotic cells was scored. The percentage of apoptotic cells per embryo was expressed as follows: apoptotic index = (no. of apoptotic nuclei/total no. of nuclei)×100. Nuclei were stained with Hoechst 33342 (1 μg/mL) for 30 min, and embryos were washed in PBS/polyvinyl alcohol (PVA). Oocytes were mounted onto glass slides and examined under a model FV500 confocal laser-scanning microscope (Olympus). At least 20 oocytes were examined from each group.
Real-time quantitative PCR
mRNA was isolated from groups of 20 in vitro-cultured embryos using the Dynabeads mRNA Direct Kit (DynalAsa, Oslo, Norway). First-strand cDNA synthesis was achieved by the reverse transcription of mRNA using an oligo(dT)12–18 primer and SuperScript III reverse transcriptase (Invitrogen, Grand Island, NY, USA). Real-time PCR was performed in a DNA Engine OPTICON 2 system (MJ Research, Waltham, MA, USA) in a final reaction volume of 20 μL that contained SYBR Green and a double-stranded DNA-binding fluorophore (qPCR Kit; FINNZYMES, Espoo, Finland). The primers used for PCR are listed in Table 1. The PCR conditions were as follows: 10 min at 94°C, followed by 39 cycles of 30 s at 94°C, 30 s at 60°C and 55 s at 72°C, and a final extension of 5 min at 72°C. Relative gene expression was analyzed by the 2-ΔΔCt method (Livak and Schmittgen, 2001) after normalization against the GAPDH mRNA level.
Table 1.
Primers used for real-time PCR
| Gene | GenBank accession no. | Primer sequence | Annealing temp. (EC) | Product size (bp) |
|---|---|---|---|---|
| GAPDH | AF017079 | F:GGGCATGAACCATGAGAAGT R:AAGCAGGGATGATGTTCTGG |
60 | 230 |
| MOS | NM_001113219 | F:TGGGAAGAAACTGGAGGACA R:TTCGGGTCAGCCCAGGTTCA |
60 | 121 |
| BMP15 | NM_001005155 | F:CCCTCGGGTACTACACTATG R:GGCTGGGCAATCATATCC |
60 | 192 |
| GDF9 | AY_626786 | F:GAGCTCAGGACACTCTAAGCT R:CTTCTCGTGGATGATGTTCTG |
60 | 272 |
| CCNB1 | NM_001170768.1 | F:CCAACTGGTTGGTGTCACTG R:GCTCTCCGAAGAAAATGCAG |
60 | 195 |
| MTOR | XM_003127584.3 | F:AGGAGACCTCCTTTAACCAG R:ATGTACTTCCTGCACCACTC |
55 | 70 |
| RICTOR | XM_003483813.1 | F:GGTGCTAAAATTGAAAGTGG R:TGCTTGTGTCCTCTCTACCT |
55 | 82 |
| LOC100512677 | XM_003131151.3 | F:GTCACTGCCATGGAGTATCT R:CAAGTCAGCAAAGTTCTTCC |
55 | 99 |
| ATG5 | NM_001037152.1 | F:GAATATGAAGGCACACCACT R:AAATTGAGGCAAGAAGATCA |
55 | 70 |
| BECN1 | NM_001044530.1 | F:TTTTCTGGGACAACAAGTTT R:CAACCTCTTCTTTGAACTGC |
55 | 75 |
| ATG7 | NM_001190285.1 | F:GAACGGGAAGGATTTAATTT R:CAGTCAAGTCCTCCAAGAAG |
55 | 70 |
| MAP1LC3B | NM_001190290 | F:CCGAACCTTCGAACAGAGAG R:AGGCTTGGTTAGCATTGAGC |
60 | 206 |
| ATG12 | NM_001190282.1 | F:CATCCTACTAAAGGCTGTGG R:AGTCAATGAGTCCTTGGATG |
55 | 92 |
| GABARAP | NM_001190288.1 | F:CCTCCAGTACTCCTTTCCTT R:TGACAGAGATGGACAATCAA |
55 | 78 |
| GABARAPL1 | NM_001190287.1 | F:TAGGAAACGTTGAGAGGGTA R:TAACCCCGAAATGAAAATAA |
55 | 76 |
| BCL2L1 | AF216205 | F:ACTGAATCAGAAGCGGAAAC R:AAAGCTCTGATACGCTGTCC |
60 | 249 |
| BIRC5 | NM_214141 | F:CCTGGCAGCTCTACCTCAAG R:GAAAGCACAACCGGATGAAT |
60 | 233 |
| FAS | AJ001202 | F:AAGTTCCCAAGCAAGGGATT R:AATTTCCCATTGTGGAGCAG |
60 | 207 |
| CASP3 | NM_214131.1 | F:GAGGCAGACTTCTTGTATGC R:ACAAAGTGACTGGATGAACC |
55 | 93 |
| POU5F1 | NM_001113060 | F:AGTGAGAGGCAACCTGGAGA R:TCGTTGCGAATAGTCACTGC |
60 | 166 |
| NANOG | DQ447201.1 | F:GAACTTTCCAACATCCTGAA R:TTTCTGCCACCTCTTACATT |
55 | 87 |
| SOX2 | EU503117 | F:GCCCTGCAGTACAACTCCAT R:GCTGATCATGTCCCGTAGGT |
60 | 216 |
F, forward; R, reverse.
Western blot analysis
The protocol was basically the same as the one described previously (Lee et al., 2012). In brief, oocytes (40 oocytes per sample) were solubilized in 20 mL 1× sodium dodecyl sulfate (SDS) sample buffer (62.5 mM Tris-HCl pH 6.8, containing 2% (w/v) SDS, 10% (v/v) glycerol, 50 mM DTT, and 0.01% (w/v) bromophenol blue or phenol red) and heated for 5 min at 95°C. For western blotting, proteins were resolved on a 5% to 12% Tris-SDS-PAGE gel for 1.5 h at 80 to 100 V. Samples were then transferred onto a nitrocellulose membrane (Amersham, Hybond-ECL, Buckinghamshire, UK) at 300 mA for 2 h in transfer buffer (25 mM Tris, pH 8.5, containing 200 mM glycine and 20% (v/v) methanol). After blocking with 5% (w/v) nonfat milk in PBS for 1 h, the membrane was incubated for at least 2 h with an anti-p44/42 MAPK or anti-phospho-p44/42 MAPK antibody diluted 1:500 in blocking solution (1× TBS, pH 7.5, containing 0.1% (v/v) Tween-20 and 5% (w/v) nonfat milk). Thereafter, the membrane was washed three times in Tris-Buffered Saline and Tween 20 (TBST, 20 mM Tris-HCl, pH 7.5, containing 250 mM NaCl and 0.1% (v/v) Tween-20) and incubated for 1 h with anti-rabbit IgG-HRP diluted 1:2,000 in blocking solution. After three washes with TBST, antibody binding was visualized with chemiluminescence luminal reagent (Invitrogen).
Statistical analysis
The general linear model (GLM) procedure embedded in the Statistical Analysis System (SAS User’s Guide, 1985, Statistical Analysis System Inc., Cary, NC. USA) was used to analyze data from all experiments. Significant differences were determined by Tukey’s multiple range test. P-values of <0.05 were considered significant.
RESULTS
Rapamycin enhances the in vitro development of aged porcine oocytes
To determine the optimal concentration of rapamycin to use, 44 h IVM porcine oocytes were further cultured in IVM medium supplemented with different concentrations (0, 0.1, 1, 10, and 50 μM) of rapamycin for 24 h (total IVM time: 68 h) or 48 h (total IVM time: 96 h). As shown in Table 2, there were no differences in the percentage of MII oocytes among control IVM (44 h, 76.00±4.53%), untreated 24 h-aged IVM (68 h, 77.67±2.33%), and rapamycin-treated (75.00±8.74 to 77.56±8.25%) groups. However, in the 48 h-aged IVM group (44 h+wo/w rapamycin/48 h), the percentage of MII oocytes was significantly lower than those of control IVM and 24 h-aged IVM groups, except for 1 μM and 10 μM rapamycin-treated groups (p<0.05). Porcine IVM oocytes in control, 24 h- and 48 h-aged, and rapamycin-treated groups were parthenoted. There was no difference in the frequency of cleavage into 2 to 4 cells in 10 μM rapamycin-treated 24 h-aged IVM and 48 h-aged IVM groups (44 h+10 μM rapamycin/24 h; 66.10±0.29% and 44 h+10 μM rapamycin/48 h; 68.01±6.04%) compared with the control IVM group (63.97±3.11%). However, there were significant decreases (p<0.05) in 50 μM rapamycin-treated 48 h-aged IVM (43.97±14.15 to 52.01±4.88%) and untreated 48 h-aged IVM (92 h, 45.44±7.51%) groups compared with the control IVM group.
Table 2.
Effect of rapamycin treatment on the development of aged porcine IVM oocytes
| Treatment group | Rapamycin conc. (μM) | No. of oocytes | Rate* (%) of | ||
|---|---|---|---|---|---|
|
| |||||
| Metaphase II1 | Cleavage | Blastocyst2 | |||
| Control IVM (44 h) | 0 | 296 | 76.00±4.53c | 63.97±3.11b | 42.14±4.40d |
| 24 h-aged IVM (68 h; 44 h+ wo/w R 24 h) | 0 | 200 | 77.67±2.33c | 68.24±9.22a,b | 22.04±5.68b,c |
| 0.1 | 200 | 75.33±7.69c | 68.36±9.76a,b | 28.54±2.49c | |
| 1 | 200 | 75.00±8.74c | 68.32±10.67a,b | 28.39±2.76c | |
| 10 | 200 | 76.67±5.70c | 66.10±0.29b | 47.52±5.68d | |
| 50 | 207 | 77.56±8.25c | 52.01±4.88a | 14.14±3.17a,b | |
| 48 h-aged IVM (92 h; 44 h+ wo/w R 48 h) | 0 | 244 | 60.19±5.66b | 45.44±7.51a | 13.50±1.26a,b |
| 0.1 | 242 | 62.55±5.52b,c | 59.01±6.73a,b | 9.23±1.92a | |
| 1 | 242 | 63.45±9.66b,c | 59.78±5.17a,b | 5.13±5.13a | |
| 10 | 242 | 58.49±8.31a,b | 68.01±6.04b | 10.28±1.33a,b | |
| 50 | 242 | 44.70±8.42a | 43.97±14.15a | 4.95±2.48a | |
R, rapamycin; IVM, in vitro maturation.
Assessed by the presence of a polar body.
Percentages were calculated from cleavage numbers.
p<0.05.
The frequency of development into blastocysts was calculated as the number of blastocysts on day 2 that developed from cleaved embryos. Compared with the control IVM group, only the 10 μM rapamycin-treated 24 h-aged IVM group had no difference in the frequency of development into blastocysts (42.14±4.40% and 47.52±5.68%, respectively). Furthermore, this frequency of development into blastocysts was significantly higher than those of the other groups (24 h-aged groups: 44 h+rapamycin/24 h [14.14±3.17 to 28.54±2.49%]; and 48 h-aged groups: 44 h+rapamycin/48 h [4.95±2.48% to 13.50±1.26%]) (p<0.05).
Rapamycin treatment improves the quality of blastocysts developed from rapamycin-treated porcine oocytes
The quality of blastocysts developed from 10 μM rapamycin-treated 24 h-aged IVM oocytes (44 h+10R/24 h, Figure 1A, c) was better than that of blastocysts developed from the untreated 24 h-aged IVM group (68 h, Figure 1A, b). Blastocyst quality was also better than the control IVM group (44 h, Figure 1A, a). The total number of cells in control IVM (n = 67.25±4.18) and 10 μM rapamycin-treated 24 h-aged IVM (n = 57.75±1.52, p<0.05) groups was significantly higher than that of the untreated 24 h-aged IVM group (n = 47.00±3.90, p<0.01, Figure 1B). When genomic DNA fragmentation (i.e., an indicator of apoptosis) was measured in individual embryos with the TUNEL assay, the index was significantly lower in the 10 μM rapamycin-treated 24 h-aged IVM group (2.53±0.66%) than those of untreated 24 h-aged IVM (4.88±0.87%, p<0.05) and control IVM (2.10±0.60%) groups (Figure 1C).
Figure 1.
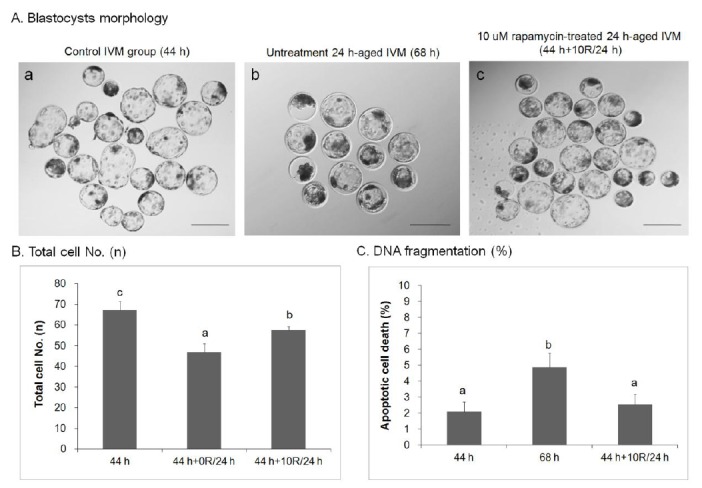
Effect of rapamycin (R) treatment on the developmental capacity of aged porcine oocytes. (A) Blastocyst morphology in control IVM (44 h; a), untreated 24 h-aged IVM (68 h; b), and 10 μM rapamycin-treated 24 h-aged IVM (44 h+10R/24 h; c) oocytes. (B) Total cell number and (C) DNA fragmentation in control IVM, untreated 24 h-aged IVM, and 10 μM rapamycin-treated 24 h-aged IVM oocytes. Significant differences from control oocytes are indicated (a–c p<0.05). Values are presented as means±SEM of independent experiments. Bar = 200 μm. IVM, in vitro maturation; SEM, standard error of the mean.
Rapamycin rescues aberrant spindle organization and chromosomal misalignment of aged porcine oocytes in vitro
To further investigate the effect of rapamycin on spindle organization, we treated oocytes with 10 μM rapamycin. As shown in Figure 2A, there were no spindle abnormalities in 10 μM rapamycin-treated 24 h-aged IVM oocytes (44 h+10R/24 h, Figure 2A, d), similar to control IVM oocytes (Figure 2A, a), usually the failure of chromosomes to align at the metaphase plate, compared with aged oocytes (Figure 2A, b–c). There was a significant difference in the frequency of oocytes showing normal meiotic spindles among untreated 24 h-aged IVM (68 h, 28.57±6.21%), 10 μM rapamycin-treated 24 h-aged IVM (44 h+10R/24 h, 71.43±7.68%), and control IVM (44 h, 66.67±5.46%) groups (p<0.05) (Figure 2B).
Figure 2.
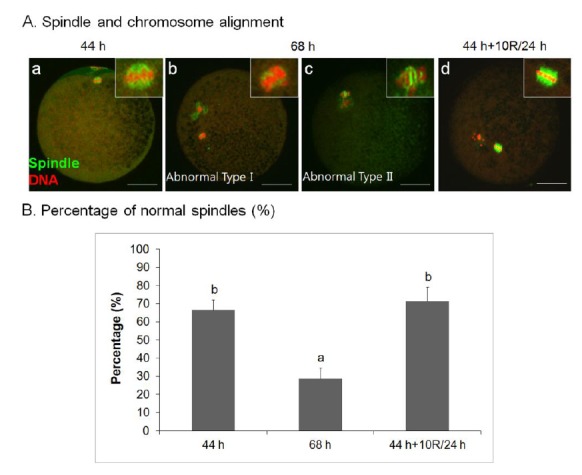
Effect of rapamycin (R) treatment on the nuclear maturation of aged porcine oocytes. (A) Spindle position in control IVM (44 h; a), untreated 24 h-aged IVM (68 h; b–c), and 10 μM rapamycin-treated 24 h-aged IVM (44 h+10R/24 h; d) oocytes. (B) Percentage of aligned chromosomes and normalspindles in control IVM, untreated 24 h-aged IVM, and 10 μM rapamycin-treated 24 h-aged IVM oocytes. Significant differences from control oocytes are indicated (a–b p<0.05). Values are presented as means±SEM of independent experiments. α-Tubulin, green; chromatin, red. Bar = 40 μm. IVM, in vitro maturation; SEM, standard error of the mean.
Rapamycin increases molecular maturation factors in porcine oocytes in vitro
To examine the effect of rapamycin treatment on the maturation of aged oocytes at the molecular level, we investigated MAPK activity and maternal gene expression (Figure 3). Active phosphorylated p44/42 MAPK (phospho-p44/42 MAPK) appeared as a doublet in maturing porcine oocyte lysates by western blotting (Figure 3A). The level of phospho-p44/42 MAPK increased in 10 μM rapamycin-treated 24 h-aged IVM oocytes compared with untreated 24 h-aged IVM oocytes. The mRNA expression of cytoplasm maturation marker genes (MOS, BMP15, GDF9, and CCNB1) was analyzed by real-time RT-PCR (Figure 3B). Although the levels of most genes in untreated 24 h-aged IVM and 10 μM rapamycin-treated 24 h-aged IVM oocytes were lower than those in control IVM oocytes, rapamycin-treated aged IVM oocytes expressed higher levels of MOS, BMP15, GDF9, and CCNB1 (p<0.05).
Figure 3.
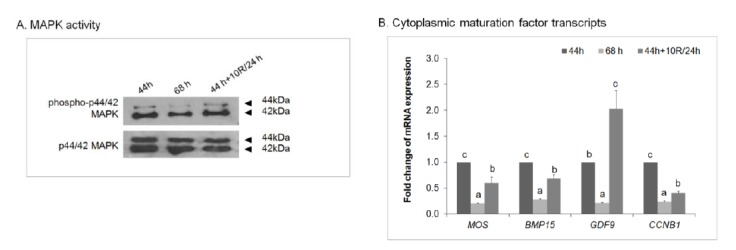
Effect of rapamycin (R) treatment on the cytoplasmic maturation of aged porcine oocytes. (A) Phosphorylated MAPK activity and (B) maternal gene expression in control IVM (44 h), untreated 24 h-aged IVM (68 h), and 10 μM rapamycin-treated 24 h-aged IVM (44 h+10R/24 h) oocytes. GAPDH was used as an internal standard. Significant differences from control oocytes are indicated (a–b p<0.05). Values are presented as means±SEM of independent experiments. IVM, in vitro maturation; SEM, standard error of the mean.
Rapamycin reduces reactive oxygen species activity in aged porcine oocytes in vitro
The effect of rapamycin treatment on ROS activity in oocytes was analyzed. Control IVM (44 h), untreated 24 h-aged IVM (68 h), and 10 μM rapamycin-treated 24 h-aged IVM (44 h+10R/24 h) oocytes were analyzed for ROS activity by the DCHFDA assay in three separate experiments. ROS activity in control IVM (50.00±2.34, Figure 4A, a; 4B) and 10 μM rapamycin-treated 24 h-aged IVM (40.89±1.37, Figure 4A, c; 4B) oocytes was significantly lower than that of untreated aged oocytes (125.89±1.03, p<0.05, Figure 4A, b; 4B).
Figure 4.
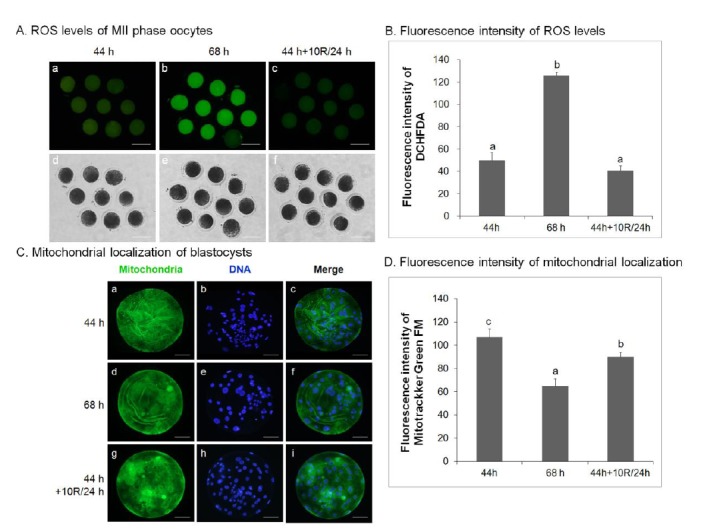
Effect of rapamycin (R) treatment on ROS activity and the localization of mitochondria in aged porcine oocytes. (A) ROS activity incontrol IVM (44 h; a, d), untreated 24 h-aged IVM (68 h; b, e), and 10 μM rapamycin-treated 24 h-aged IVM (44 h+10R/24 h; c, f) oocytes. ROS activity was detected with DCHFDA, green. Bar = 200 μm. (B) Quantification of DCHFDA fluorescence intensity in control IVM, untreated 24 h-aged IVM, and 10 μM rapamycin-treated 24 h-aged IVM oocytes. Significant differences from control oocytes are indicated (a–b p<0.05). (C) The localization of mitochondria in blastocysts derived from control IVM, untreated 24 h-aged IVM, and 10 μM rapamycin-treated 24 h-aged IVM oocytes. Bar = 60 μm. Mitochondria were stained with MitoTracker, green; chromatin, blue. (D) Quantification of MitoTracker fluorescence intensity in control IVM, untreated 24 h-aged IVM, and 10 μM rapamycin-treated 24 h-aged IVM oocytes. Significant differences from control oocytes are indicated (a–c p<0.05). Values are presented as means±SEM of independent experiments. ROS, reactive oxygen species; IVM, in vitro maturation; DCHFDA, dichlorodihydrofluorescein diacetate; SEM, standard error of the mean.
To investigate the effect of rapamycin treatment on mitochondrial distribution, blastocysts were stained with Hoechst 33342 to label nuclei and with MitoTracker Green FM to label mitochondria, and analyzed by epifluorescent and confocal microscopy. Blastocysts developed from 10 μM rapamycin-treated aged IVM oocytes (91.33±2.40, p<0.05, Figure 4C, g–I; 4D) showed a significant increase in green fluorescence compared with those from untreated aged oocytes (64.33±3.48, Figure 4C, d–f; 4D). These values were significantly lower than those from control IVM oocytes (107.00±4.04, p<0.05, Figure 4C, a–c; 4D).
Rapamycin inhibits mTOR expression in aged porcine oocytes in vitro
The mRNA expression of mTOR in MII oocytes treated with rapamycin was measured by real-time quantitative PCR (Figure 5). It revealed a decrease in mTOR synthesis in 10 μM rapamycin-treated 24 h-aged IVM oocytes (Figure 5A, g–i) compared with control IVM oocytes (Figure 5A, a–c) and untreated 24 h-aged IVM oocytes (Figure 5A, d–f). Specifically, the mRNA expression of MTOR complex genes (MTOR, RICTOR, and LOC100512677) was downregulated in 10 μM rapamycin-treated 24 h-aged IVM oocytes compared with untreated 24 h-aged IVM oocytes (p<0.05, Figure 5B).
Figure 5.
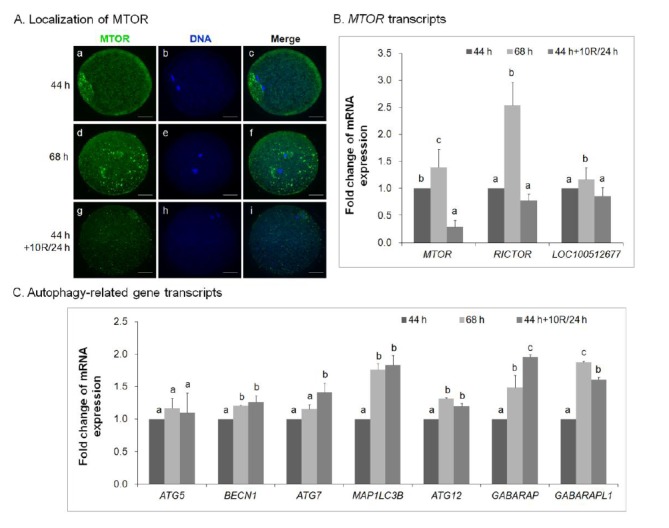
Effect of rapamycin (R) treatment on mTOR signaling in aged porcine oocytes. (A) Subcellular localization of mTOR and (B) MTOR complex mRNA levels in control IVM (44 h; a–c), untreated 24 h-aged IVM (68 h; d–f), and 10 μM rapamycin-treated 24 h-aged IVM (44 h+10R/24 h; g–i) oocytes. (C) Relative mRNA expression of autophagy-related genes in blastocysts derived from control IVM, untreated 24 h-aged IVM, and 10 μM rapamycin-treated 24 h-aged IVM oocytes. GAPDH was used as an internal standard. Significant differences from control oocytes are indicated (a–c p<0.05). Values are presented as means±SEM of independent experiments. mTOR = green; chromatin = blue. Bar = 40 μm. mTOR, mammalian target of rapamycin; IVM, in vitro maturation; SEM, standard error of the mean.
To investigate whether the treatment of 10 μM rapamycin in 24 h-aged IVM oocytes could affect the mRNA expression of autophagy-related genes, the expression of ATG5, BECN1, ATG7, MAP1LC3B, ATG12, GABARAP, and GABARAPL1 was quantified by real-time PCR (Figure 5C). In 10 μM rapamycin-treated 24 h-aged IVM oocytes, there was no difference in ATG5 expression compared with both control IVM and untreated aged IVM oocytes; however, there was a significant increase in the mRNA expression of other autophagy-related genes such as BECN1, ATG7, MAP1LC3B, ATG12, GABARAP, and GABARAPL1 compared with control IVM oocytes (p<0.05, Figure 5C).
Treatment with rapamycin affects the relative expression of developmentally important genes in blastocysts developed from aged porcine oocytes
mRNA expression was quantified by real-time PCR (Figure 6). After treatment of 24 h-aged IVM oocytes with 10 μM rapamycin, the mRNA expression of pro-apoptosis genes (FAS and CASP3) decreased in blastocysts compared with untreated 24 h-aged IVM oocytes; however, the expression of anti-apoptosis genes (BCL2L1 and BIRC5) increased compared with blastocysts developed from control IVM or untreated 24 h-aged IVM oocytes (p<0.05, Figure 6). Rapamycin treatment in 24 h-aged IVM oocytes did not affect the mRNA expression of the development-related gene, POU5F1; however, it did increase the expression of NANOG and SOX2 in blastocysts compared with control IVM and untreated 24 h-aged IVM oocytes (p<0.05, Figure 6).
Figure 6.
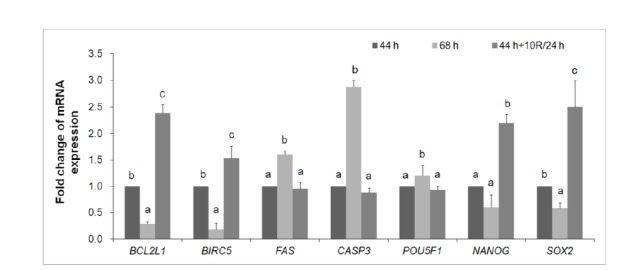
Relative mRNA expression of apoptosis- (BCL2L1, BIRC5, FAS, and CASP3) and development-(POU5F1, NANOG, and SOX2) related genes in blastocysts derived from control IVM (44 h), untreated 24 h-aged IVM (68 h), and 10 μM rapamycin (R)-treated 24 h-aged IVM (44 h+10R/24 h) oocytes. GAPDH was used as an internal standard. Significant differences from control oocytes are indicated (a–c p<0.05). Values are presented as means±SEM of independent experiments. IVM, in vitro maturation; SEM, standard error of the mean.
DISCUSSION
Until now, our knowledge of anti-aging effectors in oocytes has been limited, and the mechanism of oocyte rescue during aging is unknown. To better understand how rapamycin rescues aged oocytes, the present study investigated the role of this agent in the nuclear and cytoplasmic maturation of oocytes, the production of mitochondrial ROS, and mTOR signaling. Aged IVM oocytes were treated with increasing concentrations of rapamycin (0, 0.1, 1, 10, or 50 μM) for 24 h (68 h; 44 h+R/24 h) or 48 h (92 h; 44 h+R/48 h), respectively. Our results demonstrate that developmental capacity was highest in the 10 μM rapamycin-treated 24 h-aged IVM group, similar to the control IVM group, indicating that rapamycin treatment can rescue the poor developmental capacity of aged porcine oocytes. Furthermore, rapamycin treatment (10 μM) increased total cell number and development-related gene expression, but decreased DNA fragmentation in the 24 h-aged IVM group compared with the untreated 24 h-aged IVM group. Rapamycin-treated aged oocytes showed microtubule rearrangement and an increased phospho-p44/42 MAPK level compared with untreated aged oocytes. There was a significant decrease of ROS activity in rapamycin-treated aged oocytes, and blastocysts showed a normal distribution of mitochondria. During mTOR signaling, MTOR mRNA and protein levels were reduced, and autophagy was induced in rapamycin-treated aged oocytes. Moreover, anti-apoptosis processes increased in blastocysts developed from rapamycin-treated aged oocytes, but pro-apoptosis events decreased. The results illustrate that ROS- and mTOR-mediated autophagy affected apoptosis. Therefore, this study shows that rapamycin improves the developmental capacity of blastocysts, and it also reveals that mitochondrial ROS activity and mTOR signaling are involved in oocyte aging.
Instability of centrosomes and microtubules plays a major role in oocyte aging as these ultrastructures are responsible for proper separation of chromosomes at meiotic poles. The adverse effects of suboptimal IVM culture conditions on the structure of the meiotic spindle have been reported in several mammalian species (George et al., 1996), including the cow, pig (Somfai et al., 2011), and mouse (Ono et al., 2011). These adverse effects appear to be caused by the reduced expression of specific spindle-related genes during aging (Ma et al., 2005). In this study, rapamycin treatment increased the frequency of oocytes with normal meiotic spindles compared with untreated aged oocytes. Meiotic spindles in oocytes lack true centrosomes, indicating that specialized mechanisms may be responsible for the off-center positioning of spindles. Furthermore, rapamycin enhanced the rearrangement of abnormal spindles in aged oocytes.
Although we do not yet fully understand the molecular mechanisms in freshly isolated oocytes that are needed to maintain MII spindle dynamics, we know that MAPK and MPF are important. MAPK is a signaling molecule that we have previously shown to associate with centrosome components (Sun et al., 2002), and it is thought to be involved in centrosome and microtubule stabilization. Moreover, MAPK activity is gradually reduced during pig oocyte aging (Ma et al., 2005; Steuerwald et al., 2005). The present study shows that rapamycin-treated aged oocytes exhibit an increase in phosphorylated p44/42 MAPK. Also, there was an increase in the maternal gene expression of MOS, BMP15, and GDF9 in rapamycin-treated aged oocytes compared with untreated aged oocytes. Based on these findings, we hypothesize that rapamycin treatment blocked the reduction in maturation by maintaining the ooplasmic status of MII oocytes, and that the enhanced expression of maternal genes may be involved. Collectively, these results indicate that treatment with rapamycin significantly influences MAPK activity and maternal gene expression. Therefore, rapamycin affects the cytoplasmic maturation process in porcine oocytes and improves embryonic developmental competence.
Consequently, in vitro blastocyst development and quality was enhanced in rapamycin-treated aged oocytes. The expression of NANOG and SOX2 was also increased in blastocysts developed from rapamycin-treated aged oocytes compared with blastocysts developed from untreated aged oocytes. SOX2 and NANOG interact with POU5F1 to regulate the transcriptional hierarchy that specifies embryonic stem (ES) cell identity (Nichols et al., 1998; Chambers et al., 2003; Mitsui et al., 2003). A previous study reported that embryos treated with rapamycin exhibited morphological defects and elevated levels of POU5F1 protein in mouse early embryos (Lee et al., 2011). Taken together, rapamycin regulates nuclear and cytoplasmic maturation by rearranging microtubules and rescuing MAPK activity in porcine oocytes in vitro. Furthermore, these results indicate that rapamycin is a potent compound that can improve in vitro oocyte culture conditions and maintain healthy oocytes.
ROS is a key signaling molecule in several physiological processes such as meiotic resumption. It also plays a role in pathological processes such as cell apoptosis and senescence (Agarwal et al., 2005; Tripathi et al., 2009). In the present study, we demonstrate that rapamycin treatment effectively reduced intracellular ROS levels and increased the surface area of mitochondrial. During embryonic genome activation, mitochondria progressively undergo functional and structural changes, and also generate higher levels of ATP to supply the increasing energy demands of the embryo that result from RNA and protein synthesis and blastocoel formation (Khurana and Niemann, 2000). Since ROS are generated mainly as by-products of mitochondrial respiration, mitochondria play a fundamental role in aging and represent putative targets of anti-aging strategies (Mammucari and Rizzuto, 2010). These results illustrate that rapamycin affects mitochondrial function in porcine early embryos developing in vitro.
mTOR is an evolutionarily conserved Ser/Thr protein kinase that controls many cellular processes such as cell cycle progression, cell size, transcription, cytoskeleton dynamics, and autophagy in response to a variety of environmental cues (Harris and Lawrence, 2003; Jacinto and Hall, 2003; Wullschleger et al., 2006). Inhibition of the TOR pathway extends lifespan in yeast, worms, and flies (Vellai et al., 2003; Kapahi et al., 2004; Kaeberlein et al., 2005), and a recent study showed that rapamycin, an inhibitor of mTOR, administered late in life extends lifespan in genetically heterogeneous mice (Toth et al., 2008; Harrison et al., 2009). A recent study using loss of function mutational analysis in C. elegans showed a clear acceleration in tissue aging and a reduced lifespan in worms with loss of function mutations in the autophagy genes BEC1, UNC-51, and ATG-18 (Toth et al., 2008). This shows the importance of autophagy in regulating normal lifespan. In the present study, we showed that rapamycin-treated aged oocytes significantly decreased mTOR protein synthesis and mRNA expression, and that it also affected the expression of the MTOR complex gene, RICTOR, compared with untreated aged oocytes. Moreover, we observed that autophagy-related gene expression (MAP1LC3B, ATG12, GABARAP, and GABARAPL1) increased significantly. These results indicate that rapamycin is involved in the inhibition of mTOR and induced or maintained autophagy via the same signaling pathways to affect oocyte aging. Collectively, these findings suggest that mitochondrial ROS- and mTOR-mediated autophagy may be essential for the regulation of oocyte aging.
Our data show that rapamycin treatment significantly decreased apoptosis in blastocysts developed from rapamycin-treated aged oocytes. Rapamycin also decreased the mRNA levels of both FAS and CASP3; however, the expression of BCL2L1 and BIRC5 increased in blastocysts compared with untreated aged oocytes. BCL2, another well-characterized anti-apoptotic gene, can bind BECN1/ATG6 to inhibit BECN1-mediated autophagy and cell death (Luo and Rubinsztein, 2007). A previous study reported that autophagy modulators 3-methyladenine and rapamycin affect the interplay between autophagy and apoptosis in mouse early embryos (Lee et al., 2011). These results show that treatment with autophagy modulators increased apoptosis, disrupted mitochondrial morphology, and reduced mitochondrial numbers, as indicated by mtDNA copy number (Lee et al., 2011). These results also illustrate that rapamycin plays a role in anti-apoptosis and supports crosstalk between autophagy and apoptosis during porcine early embryo development.
In conclusion, our results show that rapamycin treatment in aged oocytes improved their poor developmental capacity, and that ROS activity and mTOR signaling are involved in porcine oocyte aging. Our results also illustrate that treatment of aged oocytes with rapamycin improved in vitro development by rearranging the cytoskeleton and increasing the level of phospho-p44/42 MAPK compared with untreated aged oocytes. Specifically, this study demonstrated that rapamycin increased the quality of blastocysts by affecting the developmental rate and the total cell number, and by decreasing ROS activity, which modulated mitochondrial distribution, apoptosis, autophagy, and mTOR signaling. Thus, rapamycin is a good agent that can be used to prevent the poor developmental competence of porcine oocytes in ART.
ACKNOWLEDGMENTS
This research was supported by grants from the Next-Generation BioGreen 21 Program (PJ009075) and the Cooperative Research Program for Agriculture Science & Technology Development (PJ009103), Rural Development Administration, Korea.
REFERENCES
- Agarwal A, Gupta S, Sharma R. Oxidative stress and its implications in female infertility - A clinician’s perspective. Reprod. Biomed Online. 2005;11(5):641–650. doi: 10.1016/s1472-6483(10)61174-1. [DOI] [PubMed] [Google Scholar]
- Baird DT, Collins J, Egozcue J, Evers LH, Gianaroli L, Leridon H, Sunde A, Templeton A, Van Steirteghem A, Cohen J, Crosignani PG, Devroey P, Diedrich K, Fauser BC, Fraser L, Glasier A, Liebaers I, Mautone G, Penney G, Tarlatzis B. Fertility and ageing. Hum. Reprod Update. 2005;11(3):261–276. doi: 10.1093/humupd/dmi006. [DOI] [PubMed] [Google Scholar]
- Bjedov I, Toivonen JM, Kerr F, Slack C, Jacobson J, Foley A, Partridge L. Mechanisms of life span extension by rapamycin in the fruit fly Drosophila melanogaster. Cell Metab. 2010;11:35–46. doi: 10.1016/j.cmet.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagosklonny MV. Aging: ROS or TOR. Cell Cycle. 2008;7(21):3344–3354. doi: 10.4161/cc.7.21.6965. [DOI] [PubMed] [Google Scholar]
- Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, Smith A. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113:643–655. doi: 10.1016/s0092-8674(03)00392-1. [DOI] [PubMed] [Google Scholar]
- Dodson MG, Minhas BS, Curtis SK, Palmer TV, Robertson JL. Spontaneous zona reaction in the mouse as a limiting factor for the time in which an oocyte may be fertilized. J. In Vitro Fert Embryo Transf. 1989;6:101–106. doi: 10.1007/BF01130735. [DOI] [PubMed] [Google Scholar]
- Dumont FJ, Staruch MJ, Koprak SL, Melino MR, Sigal NH. Distinct mechanisms of suppression of murine T cell activation by the related macrolides FK-506 and rapamycin. J Immunol. 1990;144:251–258. [PubMed] [Google Scholar]
- George MA, Pickering SJ, Braude PR, Johnson MH. The distribution of alpha- and gamma-tubulin in fresh and aged human and mouse oocytes exposed to cryoprotectant. Mol Hum Reprod. 1996;2:445–456. doi: 10.1093/molehr/2.6.445. [DOI] [PubMed] [Google Scholar]
- Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Gupta MK, Uhm SJ, Lee HT. Effect of vitrification and beta-mercaptoethanol on reactive oxygen species activity and in vitro development of oocytes vitrified before or after in vitro fertilization. Fertil Steril. 2010;93:2602–2607. doi: 10.1016/j.fertnstert.2010.01.043. [DOI] [PubMed] [Google Scholar]
- Harman D. Aging: A theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Harris TE, JrLawrence JC. TOR signaling. Science’s STKE: signal transduction knowledge environment. 2003;2003(212):re15. doi: 10.1126/stke.2122003re15. [DOI] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacinto E, Hall MN. Tor signalling in bugs, brain and brawn. Nat Rev Mol Cell Biol. 2003;4:117–126. doi: 10.1038/nrm1018. [DOI] [PubMed] [Google Scholar]
- Kaeberlein M, 3rd, Powers RW, Steffen KK, Westman EA, Hu D, Dang N, Kerr EO, Kirkland KT, Fields S, Kennedy BK. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science. 2005;310(5751):1193–1196. doi: 10.1126/science.1115535. [DOI] [PubMed] [Google Scholar]
- Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol. 2004;14:885–890. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon CJ. The genetics of ageing. Nature. 2010;464:504–512. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- Khurana NK, Niemann H. Energy metabolism in preimplantation bovine embryos derived in vitro or in vivo. Biology of reproduction. 2000;62(4):847–856. doi: 10.1095/biolreprod62.4.847. [DOI] [PubMed] [Google Scholar]
- Kikuchi K, Naito K, Noguchi J, Shimada A, Kaneko H, Yamashita M, Aoki F, Tojo H, Toyoda Y. Maturation/M-phase promoting factor: a regulator of aging in porcine oocytes. Biol Reprod. 2000;63:715–722. doi: 10.1095/biolreprod63.3.715. [DOI] [PubMed] [Google Scholar]
- Lee SE, Hwang KC, Sun SC, Xu YN, Kim NH. Modulation of autophagy influences development and apoptosis in mouse embryos developing in vitro. Mol Reprod Dev. 2011;78:498–509. doi: 10.1002/mrd.21331. [DOI] [PubMed] [Google Scholar]
- Lee SE, Kim JH, Kim NH. Inactivation of MAPK affects centrosome assembly, but not actin filament assembly, in mouse oocytes maturing in vitro. Mol Reprod Dev. 2007;74:904–911. doi: 10.1002/mrd.20695. [DOI] [PubMed] [Google Scholar]
- Lee SE, Sun SC, Choi HY, Uhm SJ, Kim NH. mTOR is required for asymmetric division through small GTPases in mouse oocytes. Mol Reprod Dev. 2012;79:356–366. doi: 10.1002/mrd.22035. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Luo S, Rubinsztein DC. Atg5 and Bcl-2 provide novel insights into the interplay between apoptosis and autophagy. Cell Death Differ. 2007;14:1247–1250. doi: 10.1038/sj.cdd.4402149. [DOI] [PubMed] [Google Scholar]
- Ma W, Zhang D, Hou Y, Li YH, Sun QY, Sun XF, Wang WH. Reduced expression of MAD2, BCL2, and MAP kinase activity in pig oocytes after in vitro aging are associated with defects in sister chromatid segregation during meiosis II and embryo fragmentation after activation. Biol Reprod. 2005;72:373–383. doi: 10.1095/biolreprod.104.030999. [DOI] [PubMed] [Google Scholar]
- Mammucari C, Rizzuto R. Signaling pathways in mitochondrial dysfunction and aging. Mech Ageing Dev. 2010;131:536–543. doi: 10.1016/j.mad.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao YL, Kikuchi K, Sun QY, Schatten H. Oocyte aging: cellular and molecular changes, developmental potential and reversal possibility. Human Reprod Update. 2009;15:573–585. doi: 10.1093/humupd/dmp014. [DOI] [PubMed] [Google Scholar]
- Mitsui K, Tokuzawa Y, Itoh H, Segawa K, Murakami M, Takahashi K, Maruyama M, Maeda M, Yamanaka S. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 2003;113:631–642. doi: 10.1016/s0092-8674(03)00393-3. [DOI] [PubMed] [Google Scholar]
- Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, Chambers I, Scholer H, Smith A. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95:379–391. doi: 10.1016/s0092-8674(00)81769-9. [DOI] [PubMed] [Google Scholar]
- Ono T, Mizutani E, Li C, Yamagata K, Wakayama T. Offspring from intracytoplasmic sperm injection of aged mouse oocytes treated with caffeine or MG132. Genesis. 2011;49:460–471. doi: 10.1002/dvg.20756. [DOI] [PubMed] [Google Scholar]
- Pellestor F, Andreo B, Arnal F, Humeau C, Demaille J. Maternal aging and chromosomal abnormalities: new data drawn from in vitro unfertilized human oocytes. Hum Genet. 2003;112:195–203. doi: 10.1007/s00439-002-0852-x. [DOI] [PubMed] [Google Scholar]
- Shigenaga MK, Hagen TM, Ames BN. Oxidative damage and mitochondrial decay in aging. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:10771–10778. doi: 10.1073/pnas.91.23.10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somfai T, Kikuchi K, Kaneda M, Akagi S, Watanabe S, Mizutani E, Haraguchi S, Dang-Nguyen TQ, Inaba Y, Geshi M, Nagai T. Cytoskeletal abnormalities in relation with meiotic competence and ageing in porcine and bovine oocytes during in vitro maturation. Anat Histol Ebryol. 2011;40:335–344. doi: 10.1111/j.1439-0264.2011.01079.x. [DOI] [PubMed] [Google Scholar]
- Steuerwald NM, Steuerwald MD, Mailhes JB. Post-ovulatory aging of mouse oocytes leads to decreased MAD2 transcripts and increased frequencies of premature centromere separation and anaphase. Mol Hum Reprod. 2005;11:623–630. doi: 10.1093/molehr/gah231. [DOI] [PubMed] [Google Scholar]
- Sun QY, Wu GM, Lai L, Bonk A, Cabot R, Park KW, Day BN, Prather RS, Schatten H. Regulation of mitogen-activated protein kinase phosphorylation, microtubule organization, chromatin behavior, and cell cycle progression by protein phosphatases during pig oocyte maturation and fertilization in vitro. Biol Reprod. 2002;66:580–588. doi: 10.1095/biolreprod66.3.580. [DOI] [PubMed] [Google Scholar]
- Tarin JJ. Potential effects of age-associated oxidative stress on mammalian oocytes/embryos. Mol Hum Reprod. 1996;2:717–724. doi: 10.1093/molehr/2.10.717. [DOI] [PubMed] [Google Scholar]
- Toth ML, Sigmond T, Borsos E, Barna J, Erdelyi P, Takacs-Vellai K, Orosz L, Kovacs AL, Csikos G, Sass M, Vellai T. Longevity pathways converge on autophagy genes to regulate life span in Caenorhabditis elegans. Autophagy. 2008;4:330–338. doi: 10.4161/auto.5618. [DOI] [PubMed] [Google Scholar]
- Tripathi A, Khatun S, Pandey AN, Mishra SK, Chaube R, Shrivastav TG, Chaube SK. Intracellular levels of hydrogen peroxide and nitric oxide in oocytes at various stages of meiotic cell cycle and apoptosis. Free Radic Res. 2009;43(3):287–294. doi: 10.1080/10715760802695985. [DOI] [PubMed] [Google Scholar]
- Vellai T, Takacs-Vellai K, Zhang Y, Kovacs AL, Orosz L, Muller F. Genetics: influence of TOR kinase on lifespan in C. elegans. Nature. 2003;426(6967):620. doi: 10.1038/426620a. [DOI] [PubMed] [Google Scholar]
- Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Xu Z, Abbott A, Kopf GS, Schultz RM, Ducibella T. Spontaneous activation of ovulated mouse eggs: time-dependent effects on M-phase exit, cortical granule exocytosis, maternal messenger ribonucleic acid recruitment, and inositol 1,4,5-trisphosphate sensitivity. Biol Reprod. 1997;57:743–750. doi: 10.1095/biolreprod57.4.743. [DOI] [PubMed] [Google Scholar]


