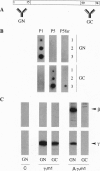
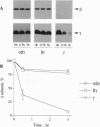
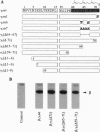
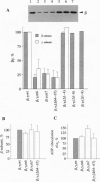
Abstract
Receptor-G protein interaction is characterized by cycles of association and dissociation. We present evidence which indicates that during receptor-G protein interaction, the C-terminal tail of the G protein gamma subunit, which is masked in the beta gamma complex, is exposed and establishes high-affinity contact with the receptor. This potential conformational switch provides a mechanism to regulate receptor-G protein coupling. This switch may also be significant for the role of the beta gamma complex in regulation of effector function.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett N., Dupont Y. The G-protein of retinal rod outer segments (transducin). Mechanism of interaction with rhodopsin and nucleotides. J Biol Chem. 1985 Apr 10;260(7):4156–4168. [PubMed] [Google Scholar]
- Bennett N., Michel-Villaz M., Kühn H. Light-induced interaction between rhodopsin and the GTP-binding protein. Metarhodopsin II is the major photoproduct involved. Eur J Biochem. 1982 Sep;127(1):97–103. doi: 10.1111/j.1432-1033.1982.tb06842.x. [DOI] [PubMed] [Google Scholar]
- Bornancin F., Pfister C., Chabre M. The transitory complex between photoexcited rhodopsin and transducin. Reciprocal interaction between the retinal site in rhodopsin and the nucleotide site in transducin. Eur J Biochem. 1989 Oct 1;184(3):687–698. doi: 10.1111/j.1432-1033.1989.tb15068.x. [DOI] [PubMed] [Google Scholar]
- Bourne H. R., Sanders D. A., McCormick F. The GTPase superfamily: conserved structure and molecular mechanism. Nature. 1991 Jan 10;349(6305):117–127. doi: 10.1038/349117a0. [DOI] [PubMed] [Google Scholar]
- Carty D. J. Pertussis toxin-catalyzed ADP-ribosylation of G proteins. Methods Enzymol. 1994;237:63–70. doi: 10.1016/s0076-6879(94)37053-2. [DOI] [PubMed] [Google Scholar]
- Clapham D. E., Neer E. J. New roles for G-protein beta gamma-dimers in transmembrane signalling. Nature. 1993 Sep 30;365(6445):403–406. doi: 10.1038/365403a0. [DOI] [PubMed] [Google Scholar]
- Conklin B. R., Bourne H. R. Structural elements of G alpha subunits that interact with G beta gamma, receptors, and effectors. Cell. 1993 May 21;73(4):631–641. doi: 10.1016/0092-8674(93)90245-l. [DOI] [PubMed] [Google Scholar]
- Conklin B. R., Farfel Z., Lustig K. D., Julius D., Bourne H. R. Substitution of three amino acids switches receptor specificity of Gq alpha to that of Gi alpha. Nature. 1993 May 20;363(6426):274–276. doi: 10.1038/363274a0. [DOI] [PubMed] [Google Scholar]
- Dratz E. A., Furstenau J. E., Lambert C. G., Thireault D. L., Rarick H., Schepers T., Pakhlevaniants S., Hamm H. E. NMR structure of a receptor-bound G-protein peptide. Nature. 1993 May 20;363(6426):276–281. doi: 10.1038/363276a0. [DOI] [PubMed] [Google Scholar]
- Emeis D., Hofmann K. P. Shift in the relation between flash-induced metarhodopsin I and metarhodpsin II within the first 10% rhodopsin bleaching in bovine disc membranes. FEBS Lett. 1981 Dec 28;136(2):201–207. doi: 10.1016/0014-5793(81)80618-7. [DOI] [PubMed] [Google Scholar]
- Emeis D., Kühn H., Reichert J., Hofmann K. P. Complex formation between metarhodopsin II and GTP-binding protein in bovine photoreceptor membranes leads to a shift of the photoproduct equilibrium. FEBS Lett. 1982 Jun 21;143(1):29–34. doi: 10.1016/0014-5793(82)80266-4. [DOI] [PubMed] [Google Scholar]
- Florio V. A., Sternweis P. C. Mechanisms of muscarinic receptor action on Go in reconstituted phospholipid vesicles. J Biol Chem. 1989 Mar 5;264(7):3909–3915. [PubMed] [Google Scholar]
- Fukada Y., Ohguro H., Saito T., Yoshizawa T., Akino T. Beta gamma-subunit of bovine transducin composed of two components with distinctive gamma-subunits. J Biol Chem. 1989 Apr 5;264(10):5937–5943. [PubMed] [Google Scholar]
- Fung B. K. Characterization of transducin from bovine retinal rod outer segments. I. Separation and reconstitution of the subunits. J Biol Chem. 1983 Sep 10;258(17):10495–10502. [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Hamm H. E., Deretic D., Arendt A., Hargrave P. A., Koenig B., Hofmann K. P. Site of G protein binding to rhodopsin mapped with synthetic peptides from the alpha subunit. Science. 1988 Aug 12;241(4867):832–835. doi: 10.1126/science.3136547. [DOI] [PubMed] [Google Scholar]
- Hamm H. E. Molecular interactions between the photoreceptor G protein and rhodopsin. Cell Mol Neurobiol. 1991 Dec;11(6):563–578. doi: 10.1007/BF00741446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargrave P. A., Hamm H. E., Hofmann K. P. Interaction of rhodopsin with the G-protein, transducin. Bioessays. 1993 Jan;15(1):43–50. doi: 10.1002/bies.950150107. [DOI] [PubMed] [Google Scholar]
- Hekman M., Holzhöfer A., Gierschik P., Im M. J., Jakobs K. H., Pfeuffer T., Helmreich E. J. Regulation of signal transfer from beta 1-adrenoceptor to adenylate cyclase by beta gamma subunits in a reconstituted system. Eur J Biochem. 1987 Dec 1;169(2):431–439. doi: 10.1111/j.1432-1033.1987.tb13630.x. [DOI] [PubMed] [Google Scholar]
- Higashijima T., Ferguson K. M., Sternweis P. C., Smigel M. D., Gilman A. G. Effects of Mg2+ and the beta gamma-subunit complex on the interactions of guanine nucleotides with G proteins. J Biol Chem. 1987 Jan 15;262(2):762–766. [PubMed] [Google Scholar]
- Higgins J. B., Casey P. J. In vitro processing of recombinant G protein gamma subunits. Requirements for assembly of an active beta gamma complex. J Biol Chem. 1994 Mar 25;269(12):9067–9073. [PubMed] [Google Scholar]
- Huff R. M., Neer E. J. Subunit interactions of native and ADP-ribosylated alpha 39 and alpha 41, two guanine nucleotide-binding proteins from bovine cerebral cortex. J Biol Chem. 1986 Jan 25;261(3):1105–1110. [PubMed] [Google Scholar]
- Hurley J. B., Fong H. K., Teplow D. B., Dreyer W. J., Simon M. I. Isolation and characterization of a cDNA clone for the gamma subunit of bovine retinal transducin. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6948–6952. doi: 10.1073/pnas.81.22.6948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iñiguez-Lluhi J. A., Simon M. I., Robishaw J. D., Gilman A. G. G protein beta gamma subunits synthesized in Sf9 cells. Functional characterization and the significance of prenylation of gamma. J Biol Chem. 1992 Nov 15;267(32):23409–23417. [PubMed] [Google Scholar]
- Katada T., Kusakabe K., Oinuma M., Ui M. A novel mechanism for the inhibition of adenylate cyclase via inhibitory GTP-binding proteins. Calmodulin-dependent inhibition of the cyclase catalyst by the beta gamma-subunits of GTP-binding proteins. J Biol Chem. 1987 Sep 5;262(25):11897–11900. [PubMed] [Google Scholar]
- Kisselev O. G., Ermolaeva M. V., Gautam N. A farnesylated domain in the G protein gamma subunit is a specific determinant of receptor coupling. J Biol Chem. 1994 Aug 26;269(34):21399–21402. [PubMed] [Google Scholar]
- Kisselev O., Gautam N. Specific interaction with rhodopsin is dependent on the gamma subunit type in a G protein. J Biol Chem. 1993 Nov 25;268(33):24519–24522. [PubMed] [Google Scholar]
- Kühn H. Light- and GTP-regulated interaction of GTPase and other proteins with bovine photoreceptor membranes. Nature. 1980 Feb 7;283(5747):587–589. doi: 10.1038/283587a0. [DOI] [PubMed] [Google Scholar]
- Lambright D. G., Noel J. P., Hamm H. E., Sigler P. B. Structural determinants for activation of the alpha-subunit of a heterotrimeric G protein. Nature. 1994 Jun 23;369(6482):621–628. doi: 10.1038/369621a0. [DOI] [PubMed] [Google Scholar]
- Neer E. J., Schmidt C. J., Nambudripad R., Smith T. F. The ancient regulatory-protein family of WD-repeat proteins. Nature. 1994 Sep 22;371(6495):297–300. doi: 10.1038/371297a0. [DOI] [PubMed] [Google Scholar]
- Noel J. P., Hamm H. E., Sigler P. B. The 2.2 A crystal structure of transducin-alpha complexed with GTP gamma S. Nature. 1993 Dec 16;366(6456):654–663. doi: 10.1038/366654a0. [DOI] [PubMed] [Google Scholar]
- Phillips W. J., Cerione R. A. Rhodopsin/transducin interactions. I. Characterization of the binding of the transducin-beta gamma subunit complex to rhodopsin using fluorescence spectroscopy. J Biol Chem. 1992 Aug 25;267(24):17032–17039. [PubMed] [Google Scholar]
- Pronin A. N., Gautam N. Characterization of antibodies for various G-protein beta and gamma subunits. Methods Enzymol. 1994;237:482–498. doi: 10.1016/s0076-6879(94)37085-0. [DOI] [PubMed] [Google Scholar]
- Pronin A. N., Gautam N. Interaction between G-protein beta and gamma subunit types is selective. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6220–6224. doi: 10.1073/pnas.89.13.6220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pronin A. N., Gautam N. Proper processing of a G protein gamma subunit depends on complex formation with a beta subunit. FEBS Lett. 1993 Aug 9;328(1-2):89–93. doi: 10.1016/0014-5793(93)80971-v. [DOI] [PubMed] [Google Scholar]
- Shinozawa T., Uchida S., Martin E., Cafiso D., Hubbell W., Bitensky M. Additional component required for activity and reconstitution of light-activated vertebrate photoreceptor GTPase. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1408–1411. doi: 10.1073/pnas.77.3.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M. I., Strathmann M. P., Gautam N. Diversity of G proteins in signal transduction. Science. 1991 May 10;252(5007):802–808. doi: 10.1126/science.1902986. [DOI] [PubMed] [Google Scholar]
- Stryer L. Cyclic GMP cascade of vision. Annu Rev Neurosci. 1986;9:87–119. doi: 10.1146/annurev.ne.09.030186.000511. [DOI] [PubMed] [Google Scholar]
- Willardson B. M., Pou B., Yoshida T., Bitensky M. W. Cooperative binding of the retinal rod G-protein, transducin, to light-activated rhodopsin. J Biol Chem. 1993 Mar 25;268(9):6371–6382. [PubMed] [Google Scholar]