Abstract
This review focuses on modulation of growth hormone (GH) and its downstream actions on periparturient dairy cows undergoing physiological and metabolic adaptations. During the periparturient period, cows experience a negative energy balance implicating that the feed intake does not meet the total energy demand for the onset of lactation. To regulate this metabolic condition, key hormones of somatotropic axis such as GH, IGF-I and insulin must coordinate adaptations required for the preservation of metabolic homeostasis. The hepatic GHR1A transcript and GHR protein are reduced at parturition, but recovers on postpartum. However, plasma IGF-I concentration remains low even though hepatic abundance of the GHR and IGF-I mRNA return to pre-calving value. This might be caused by alternation in IGFBPs and ALS genes, which consequently affect the plasma IGF-I stability. Plasma insulin level declines in a parallel manner with the decrease in plasma IGF-I after parturition. Increased GH stimulates the lipolytic effects and hepatic glucose synthesis to meet the energy requirement for mammary lactose synthesis, suggesting that GH antagonizes insulin-dependent glucose uptake and attenuates insulin action to decrease gluconeogenesis.
Keywords: Somatotropic Axis, Growth Hormone Receptor, IGF-I, Acid Labile Subunit
INTRODUCTION
Growth hormone (GH) is a peptide hormone secreted from the pituitary gland that controls normal postnatal growth and regulates physiological processes including carbohydrate and lipid metabolism (Le Roith et al., 2001; Lupu et al., 2001). GH also stimulates liver to release Insulin-like growth factor-I (IGF-I), which in turn controls growth and metabolism as well. In addition, GH antagonizes insulin action in several tissues and regulates a nutrient partitioning effect. GH activity can be regulated at the level of growth hormone receptor (GHR) or intracellular signal transduction and with catabolic situations such as parturition, malnutrition, infection and disease occurrence may lead to a state of GH resistance (Kopchick and Andry, 2000; Lucy et al., 2001; Zhu et al., 2001; Kim and Boisclair et al., 2008). GH resistance is characterized by reduced circulating IGF-I despite unchanged or increased plasma GH level. In circulation, most of the IGFs exist in ternary complexes composed of IGF-I or -II, IGF-binding protein (IGFBP)-3 or -5 and acid labile subunit (ALS) (Zapf et al., 1986; Baxter, 1988; Twigg and Baxter, 1998). ALS is synthesized primarily in liver in a GH-dependent manner (Ooi et al., 1998; Woelfle and Rotwein, 2004). Incorporation of free IGF-I in these ternary complexes extends its half-life from minutes to over 15 h (Ooi et al., 1998; Woelfle and Rotein, 2004). A complete understanding of growth and metabolism involves an appreciation of GH, IGF-I, IGFBPs, ALS and insulin due to their collective action on animals. In this review, I will address modulation of somatotropic axis during physiological and metabolic adaptations. In addition, implications for GHR will be explored for understanding modulation of GH and IGF action.
GROWTH HORMONE (GH)
GH is synthesized and stored by somatotroph cells within the anterior pituitary gland. GH contains approximately 200 amino acids with two disulfide bonds. The amino acid identity between bovine and ovine GH, or bovine and porcine GH is 99% and 90%, respectively. Two hypothalamic peptides, GH-releasing hormone (GHRH) and somatostatin (SS), regulate GH synthesis and secretion. Binding of GHRH to its G-protein coupled receptors increases cellular cAMP level (Mayo, 1992) and GH secretion. SS inhibits GH release, primarily by affecting the timing and amplitude of GH secretion rather than its synthesis (Brazeau et al., 1973). Another class of synthetic molecules called GH-releasing peptides (GHRPs) is able to stimulate GH release through activation of the GH secretagogue receptor (GHS-R) (Bowers, 1998). The endogenous ligand for GHS-R appears to be ghrelin, a 28-residue peptide expressed at high levels in stomach as well as lower levels in the hypothalamus (Kojima et al., 1999). Therefore, GH secretion can be regulated by both hypothalamic and peripheral signals Release of GH from pituitary gland is not constant over time, but rather is intermittent, or pulsatile. Species differ both in the temporal profiles of circulating GH concentration. Sex differences in the pulsatile profile of circulating GH and in mean GH concentrations are likely to contribute to sex differences in growth and other response to GH (Gatford et al., 1998).
GROWTH HORMONE RECEPTOR (GHR) AND ITS INTRACELLULAR SIGNALING
In all species, the GHR is a member of the class 1 hematopoietin receptor superfamily (Waters et al., 1999; Kopchick and Andry, 2000). The GHR consists of a ligand binding extracellular domain (N-terminus), a hydrophobic transmembrane domain, and a large intracellular domain (C-terminus). In cattle, there are three promoters, termed P1, P2, and P3 that transcribe three major classes of GHR transcripts, referred to as GHR1A, GHR1B, and GHR1C, respectively (Heap et al., 1995; Schwartzbauer and Menon, 1998; Jiang et al., 2000). The mRNA variants are produced by the alternative splicing of exon 1A, 1B or 1C onto a core transcript containing exon 2 to 10. All GHRs are identical proteins because the GHR protein is encoded in exon 2 to 10 of the mRNA (Edens and Talamantes, 1998). The GHR1A is expressed exclusively in liver where it accounts for ~50% of total hepatic GHR transcripts (Kobayashi et al., 1999; Jiang and Lucy 2001b; Lucy et al., 2001). The P1 promoter is up-regulated by the hepatic nuclear factor 4, HNF4 (Jiang and Lucy, 2001a) and the signal transducer and activator of transcription 5, STAT5 (Jiang et al., 2007). The P2 promoter transcribes GHR1B transcripts in a variety of tissues. GHR1B transcripts accounts for ~35% of total GHR transcripts in liver and ~70% in other tissues (Heap et al., 1996; Jiang aand Lucy, 2001b). The ubiquitous transcription factor Sp1 is required for efficient activity of the P2 promoter (Jiang et al., 2000). The GHR1C transcript is also produced in a variety of tissues by the P3 promoter. GHR1C transcript accounts for ~15% of total GHR in liver and ~30% in other tissues (Jiang et al., 1999; Jiang et al., 2000).
The working model of GH action involves numerous multi-step signal transduction cascades and has been described in the reviews (Herrington and Carter-Su, 2001; Kopchick and Andry, 2001; Zhu et al., 2001). GHR dimerization is a key requirement for receptor activation and leads to the activation of Janus kinase 2 (JAK2). JAK2 is a tyrosine kinase and phosphorylates tyrosine residue on itself and on the GHR. Thus, phosphorylated JAK2 and GHR provide multiple sites for signal molecules to bind. There are four major signaling pathway mediating GH actions have been studied: i) STAT signaling pathway, ii) mitogen-activated protein kinase (MAPK) pathway, iii) insulin receptor substrate (IRS) pathway, iv) phospholipase Cγ (PLCγ) pathway. These pathways are characterized by multiple points of intersection and convergence. In most physiological situations, there is an evidence of cross-talk between these pathways. Among these pathways, STAT is known to be the major pathway for regulation of IGF-I and ALS by GH actions. GH can activate four STATs, i.e. STAT1, 3, 5a, and 5b. JAK2 phosphorylates STATs on a single tyrosine residue, and then STATs are dimerized via their SH2 domain. Dimerized STATs translocate into the nucleus where they bind to cis-elements in the promoter regions of target genes. STAT5 has been implicated in the GH-regulation of IGF-I gene transcription. IGF-I expression is STAT5b-dependent (Davey et al., 2001) and a functional STAT5 response element on IGF-I gene has been identified (Woelfle et al., 2003; Woelfle and Rotwein, 2004).
PROPERTIES OF IGF-I TERNARY COMPLEXES
The IGF system contains two ligands, named IGF-I and -II (Daughaday and Rotwein, 1989). Only IGF-I will be discussed here because it is generally recognized that only IGF-I is correlated with postnatal growth and metabolism, and is regulated by GH (Le Roith et al., 2001). Although structurally similar to insulin, IGF-I differs with respect to its sites of origin, mode of action and circulating forms. Unlike the exclusive secretion of insulin by pancreatic β-cells, IGF-I is secreted by peripheral tissues in addition to its primary release form liver. After birth, IGF-I circulates in blood at high concentrations reflecting mostly hepatic production (Le Roith et al., 2001). Proteins with high affinity for IGFs called the insulin-like growth factor binding proteins (IGFBP) have been identified. Six IGFBPs have been isolated in the human and rat and shown to belong to a single family (Rechler, 1993; Jones and Clemmons, 1995). The major roles include shuttling IGFs from the circulation to tissues and modulation of IGFs action by controlling their access to specific receptors (Jones and Clemmons, 1995; Firth and Baxter, 2002). In rodents, humans and domestic species, IGFBP-1 and -2 constitute the largest proportion of plasma IGFBPs present during fetal life whereas IGFBP-3 is the predominant circulating IGFBP in the adult (Rajaram et al., 1997; Ooi and Boisclair, 1999). Most of the IGFs in fetal plasma appear at the 40 to 50 kDa (Butler and Gluckman, 1986). This agrees with circulation of IGF bound to IGFBPs of ~24 to 43 kDa (Cohick et al., 1992; McGuire et al., 1992). In plasma of adult animals, however, most of the IGFs are found at 150 kDa. The complex is composed of three units; one molecule of IGF-I or -II, one molecule of IGFBP-3 or IGFBP-5 and one molecule termed the acid-labile subunit (ALS) (Baxter, 1988; Baxter and Martin, 1989; Baxter et al., 1989; Twigg and Baxter, 1998). Genomic characteristics of the ALS has been identified in sheep, cattle, human, pig and mouse (Dai and Baxter, 1992; Leong et al., 1992; Boisclair et al., 1996; Lee et al., 2001; Kim et al., 2006). Three-dimensional structure predicted by computational modeling and rotary shadowing electron microscopy shows that ALS is a donut-shaped structure with negative-charged internal face which is thought to be important for binding with IGFBP-3 and -5 (Firth et al., 1998; Twigg et al., 1998; Janosi et al., 1999). In cattle, the abundance of ALS mRNA is five-fold in liver than that in lung, small intestine, adipose tissue, kidney and heart, but is almost absent in muscle and brain (Kim et al., 2006).
Incorporation of ALS in ternary complexes extends their half-lives from 10 min (free IGFs) or 30 min (IGF-IGFBPs in binary complexes) to over 15 h (Zapf et al., 1986; Twigg and Baxter, 1998). This evidence explains that IGFs cross the endothelial barrier when in free form or binary complexes, but are unable to do when in ternary complexes (Binoux and Hossenlopp, 1988). To understand the functionality and the action of ALS on development and growth, ALS knock-out mice model has been studied (Ueki et al., 2000). ALS deficient mice show a reduction of circulating IGF-I (62%) and IGFBP-3 (88%), and suffer at 15% growth retardation, but have normal plasma levels of glucose, insulin and GH. GH replacement therapy cannot restore in plasma IGF-I levels and growth (Ueki et al., 2000). This reflects that the ternary complexes are unable to be formed in ALS deficient mice and without ALS, overall IGF-I effects on development and growth is reduced.
CHANGES IN THE SOMATOTROPIC AXIS
In domestic animals, it is evident that GH stimulates IGF-I synthesis, and that a positive relationship exists between body weight gain and plasma IGF-I (Etherton and Bauman, 1998; Bauman, 1999; Renaville et al., 2002). It is also well established that GH inhibits fat deposition and increases fat mobilization Houseknecht et al., 1995). In early 1990’s, Bauman and Vernon proposed a model of GH action in lactating dairy cows representing undernutrition condition. In this model, GH actions are reduced in liver, limiting IGF-I production and its related anabolic effects (Bauman and Vernon, 1993). During the periparturient period, Cows in periparturient period are in negative energy balance the feed intake does not meet the total energy demand that increased by ~4-fold during the onset of lactation (Bell, 1995; Bauman, 2000). Under this condition, key hormones of somatotropic axis, i.e. GH, IGF-I and insulin must coordinate adaptations required for the preservation of metabolic homeostasis (Bell, 1995). Dairy cows during the parturition suffer a reduction of plasma IGF-I despite elevated plasma GH (Figures 1 and 2), a condition known as GH resistance. The somatotropic axis becomes uncoupled. Plasma insulin concentrations are low as well (Rhoads et al., 2004). Increased GH stimulates the lipolytic effects (elevated plasma NEFA and glycerol) and associates with adipose tissue mobilization. GH also stimulates hepatic gluconeogenesis to meet the energy requirement for mammary lactose synthesis (Knapp et al., 1992). It is suggested that GH antagonizes insulin-dependent glucose uptake and attenuates the ability of insulin to decrease gluconeogenesis. This insulin resistance conserves glucose for lactose synthesis in mammary gland (Hayirli, 2006). Therefore, elevated GH concentration drives nutrient partitioning to facilitate milk production.
Figure 1.
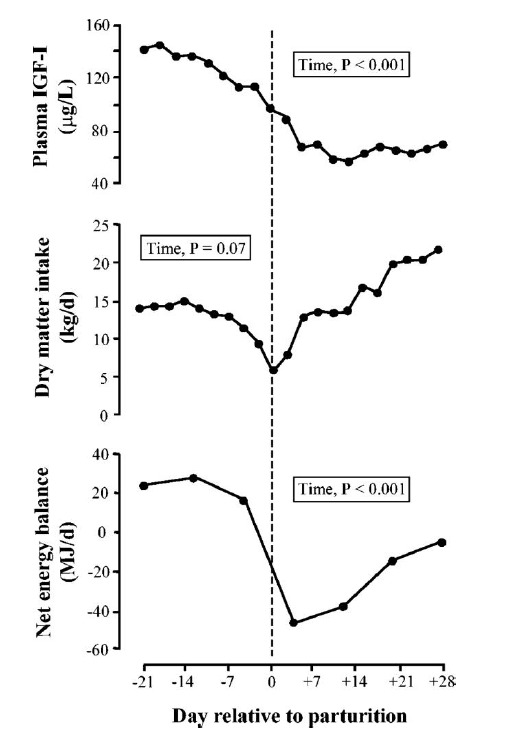
Profiles of plasma IGF-I, dry matter intake, and net energy balance during the transition from pregnancy to lactation in multiparous dairy cows. Cows (n = 9) were studied in the period from d 21 prepartum to d 8 postpartum. The day of parturition is denoted by the dashed vertical line (adapted from Rhoads et al., 2004).
Figure 2.
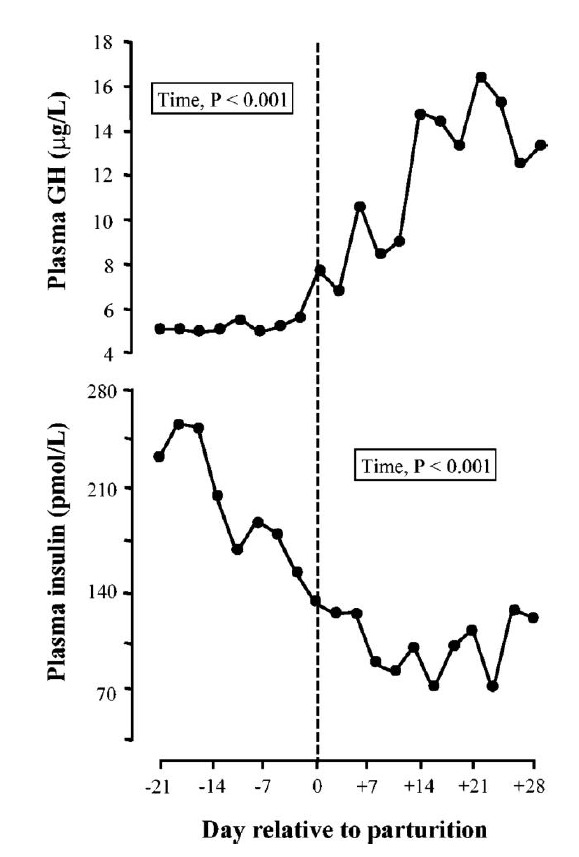
Profiles of plasma GH and insulin during the transition from pregnancy to lactation in multiparous dairy cows. Cows (n = 9) were studied in the period from d 21 prepartum to d 8 postpartum. The day of parturition is denoted by the dashed vertical line (adapted from Rhoads et al., 2004).
The actions of GH are regulated by the GHR. Regarding GHR regulation, hepatic GHR1A transcript of dairy cows changes dramatically before and after partutition (Lucy et al., 2001; Kim et al., 2004). The hepatic abundance of GHR1A transcript is lower on day +3 than on day −35 and returns to late pregnancy value by day +56 (Figure 3). The expression pattern for IGF-I mRNA is similar to GHR1A. The hepatic abundance of GHR protein is also reduced at parturition when GHR1A expression declines, but recovers when GHR1A is normalized. However, plasma IGF-I concentration remain low even though hepatic abundance of the GHR and IGF-I mRNA return to pre-calving value. This might be caused by alternation in IGFBPs and ALS genes, which consequently might affect the plasma IGF-I stability. The abundance profile of ALS mRNA and protein is similar to GHR1A and IGF-I mRNA (Figure 4) (Kim et al., 2006). These results suggest that ALS plays little or no role in the persisting plasma IGF-I depression. Thus, IGFBP3 might be the other possible candidate behind the reduced plasma IGF-I level. When analyzed by ligand blotting, IGFBP3 remains low until day +56 after parturition (unpublished data). The regulation of IGFBP3 during the periparturient period needs to be addressed in future studies. As for insulin, plasma insulin concentration declines in a parallel manner with the decrease in plasma IGF-I after parturition. Insulin infusion into early lactating cows increases GHR1A, IGF-I mRNA in liver, GHR in adipose tissues and plasma IGF-I concentration (Butler et al., 2003; Rhoads et al., 2004). Thus, insulin plays as a recoupling factor for the somatotropic axis. Furthermore, the abundance of GHR protein in adipose tissue decreases in low-insulin states such as postparturition and undernutrition (Rhoads et al., 2004; Rhoads et al., 2007). The recent studies have been indicated that the single nucleotide polymorphisms (SNPs) in relation with genes of the somatotrophic axis are associated with performance traits in dairy cattle. On the GHR sequences, the allelic substitution of GHR4.2 in the 5′ non-coding region and F279Y in exon 8 was associated with milk yield (Waters et al., 2010). Further extensive study showed that F279Y on the GHR was significantly associated with milk fat and protein as well (Waters et al., 2012). Variation in the GH (one GH SNP, GH33) sequence was associated with milk yield, fat and protein contents. A single IGF-I SNP, IGFi3 was significantly associated with milk yield (Mullen et al., 2011).
Figure 3.
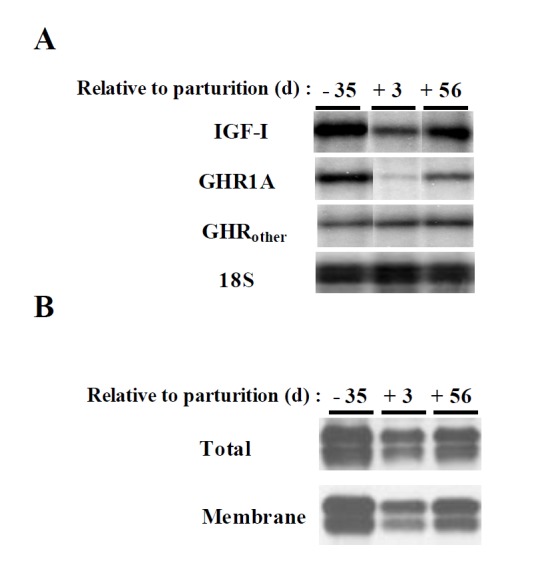
The abundance of hepatic IGF-I mRNA, GHR1A mRNA and GHR protein during the periparturient period. (A) IGF-I, GHR1A and GHRother mRNA were analyzed by RPA. (B) Total cellular proteins (total) and membrane protein (membrane) were analyzed by western blotting for the abundance of the GHR (adapted from Kim et al., 2004).
Figure 4.
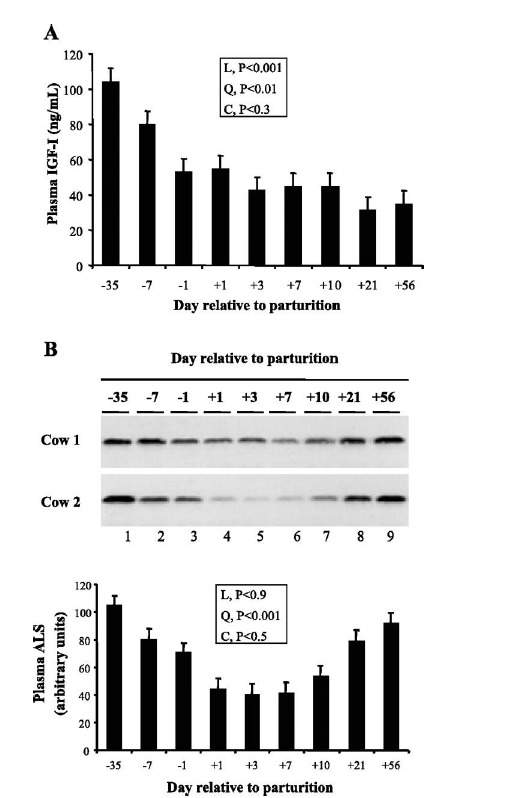
Profile of plasma ALS abundance during the periparturient period. Four multiparous dairy cows were studied during the period between day −35 and +56. (A) Plasma IGF-I concentration was measured by RIA. (B) Plasma ALs was measured by western blotting. The effect of time was partitioned into linear (L), quadratic (Q) and cubic (C) contrasts (adapted from Kim et al., 2006).
CONCLUSIONS
The somatotropic axis plays a pivotal role in growth and metabolism. GH, IGF-I complexes and insulin are key hormones that coordinate physiological adaptations to preserve metabolic homeostasis. The actions of GH are mediated by the activity and abundance of GHR and its intracellular signaling. In ruminant, catabolic states (undernutrition and parturition) modulate the abundance of hepatic GHR and its downstream target genes such as IGF-I and ALS. However, the abundance of hepatic JAK2 and STAT5 proteins are almost invariant during the periparturient period (unpublished data). Future studies will be needed to measure activation/phosphorylation of hepatic JAK2, STAT5 or other intracellular protein as an index for GH responsiveness. Overall, understanding the mechanisms of somatotropic axis modulation during the periparturient period by key hormones may lead to improvement for dairy production.
REFERENCES
- Bauman DE. Bovine somatotropin and lactation: from basic science to commercial application. Domest Anim Endocrinol. 1999;17:101–116. doi: 10.1016/s0739-7240(99)00028-4. [DOI] [PubMed] [Google Scholar]
- Bauman DE. Ruminant Physiology digestion, metabolism, growth and reproduction. Vol. 18. CABI Publishing; New York: 2000. p. 331. [Google Scholar]
- Bauman DE, Vernon RG. Effects of exogenous bovine somatotropin on lactation. Annu Rev Nutr. 1993;13:437–461. doi: 10.1146/annurev.nu.13.070193.002253. [DOI] [PubMed] [Google Scholar]
- Baxter RC. Characterization of the acid-labile subunit of the growth hormone-dependent insulin-like growth factor binding protein complex. J Clin Endocrinol Metab. 1988;67:265–272. doi: 10.1210/jcem-67-2-265. [DOI] [PubMed] [Google Scholar]
- Baxter RC, Martin JL. Structure of the Mr 140,000 growth hormone-dependent insulin-like growth factor binding protein complex: determination by reconstitution and affinity-labeling. Proc. Natl. Acad. Sci USA. 1989;86:6898–6902. doi: 10.1073/pnas.86.18.6898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter RC, Martin JL, Beniac VA. High molecular weight insulin-like growth factor binding protein complex. Purification and properties of the acid-labile subunit from human serum. J Biol Chem. 1989;264:11843–11848. [PubMed] [Google Scholar]
- Bell AW. Regulation of organic nutrient metabolism during transition from late pregnancy to early lactation. J Anim Sci. 1995;73:2804–2819. doi: 10.2527/1995.7392804x. [DOI] [PubMed] [Google Scholar]
- Binoux M, Hossenlopp P. Insulin-like growth factor (IGF) and IGF-binding proteins: comparison of human serum and lymph. J Clin Endocrinol Metab. 1988;67:509–514. doi: 10.1210/jcem-67-3-509. [DOI] [PubMed] [Google Scholar]
- Boisclair YR, Seto D, Hsieh S, Hurst KR, Ooi GT. Organization and chromosomal localization of the gene encoding the mouse acid labile subunit of the insulin-like growth factor binding complex. Proc. Natl. Acad. Sci USA. 1996;93:10028–10033. doi: 10.1073/pnas.93.19.10028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers CY. Growth hormone-releasing peptide (GHRP) Cell Mol Life Sci. 1998;54:1316–1329. doi: 10.1007/s000180050257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazeau P, Vale W, Burgus R, Ling N, Butcher M, Rivier J, Guillemin R. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science. 1973;179:77–79. doi: 10.1126/science.179.4068.77. [DOI] [PubMed] [Google Scholar]
- Butler JH, Gluckman PD. Circulating insulin-like growth factor-binding proteins in fetal, neonatal and adult sheep. J Endocrinol. 1986;109:333–338. doi: 10.1677/joe.0.1090333. [DOI] [PubMed] [Google Scholar]
- Butler ST, Marr AL, Pelton SH, Radcliff RP, Lucy MC, Butler WR. Insulin restores GH responsiveness during lactation-induced negative energy balance in dairy cattle: effects on expression of IGF-I and GH receptor 1A. J Endocrinol. 2003;176:205–217. doi: 10.1677/joe.0.1760205. [DOI] [PubMed] [Google Scholar]
- Cohick WS, McGuire MA, Clemmons DR, Bauman DE. Regulation of insulin-like growth factor-binding proteins in serum and lymph of lactating cows by somatotropin. Endocrinology. 1992;130:1508–1514. doi: 10.1210/endo.130.3.1371453. [DOI] [PubMed] [Google Scholar]
- Dai J, Baxter RC. Molecular cloning of the acid-labile subunit of the rat insulin-like growth factor binding protein complex. Biochem Biophys Res Commun. 1992;188:304–309. doi: 10.1016/0006-291x(92)92385-b. [DOI] [PubMed] [Google Scholar]
- Daughaday WH, Rotwein P. Insulin-like growth factors I and II. Peptide, messenger ribonucleic acid and gene structures, serum, and tissue concentrations. Endocr Rev. 1989;10:68–91. doi: 10.1210/edrv-10-1-68. [DOI] [PubMed] [Google Scholar]
- Davey HW, Xie T, McLachlan MJ, Wilkins RJ, Waxman DJ, Grattan DR. STAT5b is required for GH-induced liver IGF-I gene expression. Endocrinology. 2001;142:3836–3841. doi: 10.1210/endo.142.9.8400. [DOI] [PubMed] [Google Scholar]
- Edens A, Talamantes F. Alternative processing of growth hormone receptor transcripts. Endocr Rev. 1998;19:559–582. doi: 10.1210/edrv.19.5.0347. [DOI] [PubMed] [Google Scholar]
- Etherton TD, Bauman DE. Biology of somatotropin in growth and lactation of domestic animals. Physiol Rev. 1998;78:745–761. doi: 10.1152/physrev.1998.78.3.745. [DOI] [PubMed] [Google Scholar]
- Firth SM, Baxter RC. Cellular actions of the insulin-like growth factor binding proteins. Endocr Rev. 2002;23:824–854. doi: 10.1210/er.2001-0033. [DOI] [PubMed] [Google Scholar]
- Firth SM, Ganeshprasad U, Baxter RC. Structural determinants of ligand and cell surface binding of insulin-like growth factor-binding protein-3. J Biol Chem. 1998;273:2631–2638. doi: 10.1074/jbc.273.5.2631. [DOI] [PubMed] [Google Scholar]
- Gatford KL, Egan AR, Clarke IJ, Owens PC. Sexual dimorphism of the somatotrophic axis. J Endocrinol. 1998;157:373–389. doi: 10.1677/joe.0.1570373. [DOI] [PubMed] [Google Scholar]
- Hayirli A. The role of exogenous insulin in the complex of hepatic lipidosis and ketosis associated with insulin resistance phenomenon in postpartum dairy cattle. Vet Res Commun. 2006;30:749–774. doi: 10.1007/s11259-006-3320-6. [DOI] [PubMed] [Google Scholar]
- Heap D, Collier RJ, Boyd CK, Lucy MC. Expression of alternate growth hormone receptor messenger RNA in ovary and uterus of cattle. Domest Anim Endocrinol. 1996;13:421–430. doi: 10.1016/0739-7240(96)00072-0. [DOI] [PubMed] [Google Scholar]
- Heap D, Lucy MC, Collier RJ, Boyd CK, Warren WC. Rapid communication: nucleotide sequence of the promoter and first exon of the somatotropin receptor gene in cattle. J Anim Sci. 1995;73:1529. doi: 10.2527/1995.7351529x. [DOI] [PubMed] [Google Scholar]
- Herrington J, Carter-Su C. Signaling pathways activated by the growth hormone receptor. Trends Endocrinol Metab. 2001;12:252–257. doi: 10.1016/s1043-2760(01)00423-4. [DOI] [PubMed] [Google Scholar]
- Houseknecht KL, Dwyer DA, Lanna DP, Bauman DE. Effect of somatotropin on adipose tissue metabolism: ontogeny of the enhanced response to adrenergic challenge in the lactating cow. Domest Anim Endocrinol. 1995;12:105–113. doi: 10.1016/0739-7240(94)00013-q. [DOI] [PubMed] [Google Scholar]
- Janosi JB, Ramsland PA, Mott MR, Firth SM, Baxter RC, Delhanty PJ. The acid-labile subunit of the serum insulin-like growth factor-binding protein complexes. Structural determination by molecular modeling and electron microscopy. J Biol Chem. 1999;274:23328–23332. doi: 10.1074/jbc.274.33.23328. [DOI] [PubMed] [Google Scholar]
- Jiang H, Lucy MC. Involvement of hepatocyte nuclear factor-4 in the expression of the growth hormone receptor 1A messenger ribonucleic acid in bovine liver. Mol Endocrinol. 2001a;15:1023–1034. doi: 10.1210/mend.15.6.0652. [DOI] [PubMed] [Google Scholar]
- Jiang H, Lucy MC. Variants of the 5′-untranslated region of the bovine growth hormone receptor mRNA: isolation, expression and effects on translational efficiency. Gene. 2001b;265:45–53. doi: 10.1016/s0378-1119(01)00356-0. [DOI] [PubMed] [Google Scholar]
- Jiang H, Okamura CS, Lucy MC. Isolation and characterization of a novel promoter for the bovine growth hormone receptor gene. J Biol Chem. 1999;274:7893–7900. doi: 10.1074/jbc.274.12.7893. [DOI] [PubMed] [Google Scholar]
- Jiang H, Okamura CS, Boyd CK, Lucy MC. Identification of Sp1 as the transcription factor for the alternative promoter P2 of the bovine growth hormone receptor gene. J Mol Endocrinol. 2000;24:203–214. doi: 10.1677/jme.0.0240203. [DOI] [PubMed] [Google Scholar]
- Jiang H, Wang Y, Wu M, Gu Z, Frank SJ, Torres-Diaz R. Growth hormone stimulates hepatic expression of bovine growth hormone receptor mRNA through STAT-activation of a major growth hormone receptor gene promoter. Endocrinology. 2007;148:3307–3315. doi: 10.1210/en.2006-1738. [DOI] [PubMed] [Google Scholar]
- Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- Kim JW, Rhoads RP, Block SS, Overton TR, Frank SJ, Boisclair YR. Dairy cows experience selective reduction of the hepatic growth hormone receptor during the periparturient period. J Endocrinol. 2004;181:281–290. doi: 10.1677/joe.0.1810281. [DOI] [PubMed] [Google Scholar]
- Kim JW, Rhoads RP, Segoale N, Kristensen NB, Bauman DE, Boisclair YR. Isolation of the cDNA encoding the acid labile subunit (ALS) of the 150 kDa IGF-binding protein complex in cattle and ALS regulation during the transition from pregnancy to lactation. J Endocrinol. 2006;189:583–593. doi: 10.1677/joe.1.06824. [DOI] [PubMed] [Google Scholar]
- Kim JW, Boislciar YR. Growth hormone signaling in the regulation of acid laile subunit. Asian-Aust J Anim Sci. 2008;21:754–768. [Google Scholar]
- Knapp JR, Freetly HC, Reis BL, Calvert CC, Baldwin RL. Effects of somatotropin and substrates on patterns of liver metabolism in lactating dairy cattle. J Dairy Sci. 1992;75:1025–1035. doi: 10.3168/jds.S0022-0302(92)77846-1. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Boyd CK, Bracken CJ, Lamberson WR, Keisler DH, Lucy MC. Reduced growth hormone receptor (GHR) messenger ribonucleic acid in liver of periparturient cattle is caused by a specific down-regulation of GHR 1A that is associated with decreased insulin-like growth factor I. Endocrinology. 1999;140:3947–3954. doi: 10.1210/endo.140.9.7000. [DOI] [PubMed] [Google Scholar]
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- Kopchick JJ, Andry JM. Growth hormone (GH), GH receptor, and signal transduction. Mol Genet Metab. 2000;71:293–314. doi: 10.1006/mgme.2000.3068. [DOI] [PubMed] [Google Scholar]
- Le Roith D, Bondy C, Yakar S, Liu JL, Butler A. The somatomedin hypothesis: 2001. Endocr Rev. 2001;22:53–74. doi: 10.1210/edrv.22.1.0419. [DOI] [PubMed] [Google Scholar]
- Lee CY, Kwak I, Chung CS, Choi WS, Simmen RC, Simmen FA. Molecular cloning of the porcine acid-labile subunit (ALS) of the insulin-like growth factor-binding protein complex and detection of ALS gene expression in hepatic and non-hepatic tissues. J Mol Endocrinol. 2001;26:135–144. doi: 10.1677/jme.0.0260135. [DOI] [PubMed] [Google Scholar]
- Leong SR, Baxter RC, Camerato T, Dai J, Wood WI. Structure and functional expression of the acid-labile subunit of the insulin-like growth factor-binding protein complex. Mol Endocrinol. 1992;6:870–876. doi: 10.1210/mend.6.6.1379671. [DOI] [PubMed] [Google Scholar]
- Lucy MC, Jiang H, Kobayashi Y. Changes in the somatotrophic axis associated with the initiation of lactation. J. Dairy Sci. 2001;84(E. Suppl.):E113–E119. [The paper is available at http://www.adsa.org/jds/abs/2001/jds_es113.htm] [Google Scholar]
- Lupu F, Terwilliger JD, Lee K, Segre GV, Efstratiadis A. Roles of growth hormone and insulin-like growth factor 1 in mouse postnatal growth. Dev Biol. 2001;229:141–162. doi: 10.1006/dbio.2000.9975. [DOI] [PubMed] [Google Scholar]
- Mayo KE. Molecular cloning and expression of a pituitary-specific receptor for growth hormone-releasing hormone. Mol Endocrinol. 1992;6:1734–1744. doi: 10.1210/mend.6.10.1333056. [DOI] [PubMed] [Google Scholar]
- McGuire MA, Vicini JL, Bauman DE, Veenhuizen JJ. Insulin-like growth factors and binding proteins in ruminants and their nutritional regulation. J Anim Sci. 1992;70:2901–2910. doi: 10.2527/1992.7092901x. [DOI] [PubMed] [Google Scholar]
- Mullen MP, Lynch CO, Waters SM, Howard DJ, Boyle PO, Kenny DA, Buckley F, Horan B, Diskin MG. Single nucleotide polymorphisms in the growth hormone and insulin-like growth factor-1 genes are associated with milk production, body condition score and fertility traits in dairy cows. Gen Mol Res. 2011;10:1819–1830. doi: 10.4238/vol10-3gmr1173. [DOI] [PubMed] [Google Scholar]
- Ooi GT, Boisclair YR. Molecular biology of the IGF binding proteins. In: Rosenfeld R, Roberts C Jr, editors. Contemporary Endocrinology. The IGF system Humans Press, Inc; Totowa, NJ: 1999. pp. 111–139. [Google Scholar]
- Ooi GT, Hurst KR, Poy MN, Rechler MM, Boisclair YR. Binding of STAT5a and STAT5b to a single element resembling a gamma- interferon-activated sequence mediates the growth hormone induction of the mouse acid-labile subunit promoter in liver cells. Mol Endocrinol. 1998;12:675–687. doi: 10.1210/mend.12.5.0115. [DOI] [PubMed] [Google Scholar]
- Rajaram S, Baylink DJ, Mohan S. Insulin-like growth factor-binding proteins in serum and other biological fluids: regulation and functions. Endocr Rev. 1997;18:801–831. doi: 10.1210/edrv.18.6.0321. [DOI] [PubMed] [Google Scholar]
- Rechler MM. Insulin-like growth factor binding proteins. Vitam Horm. 1993;47:1–114. doi: 10.1016/s0083-6729(08)60444-6. [DOI] [PubMed] [Google Scholar]
- Renaville R, Hammadi M, Portetelle D. Role of the somatotropic axis in the mammalian metabolism. Domest Anim Endocrinol. 2002;23:351–360. doi: 10.1016/s0739-7240(02)00170-4. [DOI] [PubMed] [Google Scholar]
- Rhoads RP, Kim JW, Leury BJ, Baumgard LH, Segoale N, Frank SJ, Bauman DE, Boisclair YR. Insulin increases the abundance of the growth hormone receptor in liver and adipose tissue of periparturient dairy cows. J Nutr. 2004;134:1020–1027. doi: 10.1093/jn/134.5.1020. [DOI] [PubMed] [Google Scholar]
- Rhoads RP, Kim JW, Van Amburgh ME, Ehrhardt RA, Frank SJ, Boisclair YR. Effect of nutrition on the GH responsiveness of liver and adipose tissue in dairy cows. J Endocrinol. 2007;195:49–58. doi: 10.1677/JOE-07-0068. [DOI] [PubMed] [Google Scholar]
- Schwartzbauer G, Menon RK. Regulation of growth hormone receptor gene expression. Mol Genet Metab. 1998;63:243–253. doi: 10.1006/mgme.1998.2685. [DOI] [PubMed] [Google Scholar]
- Twigg SM, Baxter RC. Insulin-like growth factor (IGF)-binding protein 5 forms an alternative ternary complex with IGFs and the acid-labile subunit. J Biol Chem. 1998;273:6074–6079. doi: 10.1074/jbc.273.11.6074. [DOI] [PubMed] [Google Scholar]
- Twigg SM, Kiefer MC, Zapf J, Baxter RC. Insulin-like growth factor-binding protein 5 complexes with the acid- labile subunit. Role of the carboxyl-terminal domain. J Biol Chem. 1998;273:28791–28798. doi: 10.1074/jbc.273.44.28791. [DOI] [PubMed] [Google Scholar]
- Ueki I, Ooi GT, Tremblay ML, Hurst KR, Bach LA, Boisclair YR. Inactivation of the acid labile subunit gene in mice results in mild retardation of postnatal growth despite profound disruptions in the circulating insulin-like growth factor system. Proc. Natl. Acad. Sci USA. 2000;97:6868–6873. doi: 10.1073/pnas.120172697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters MJ, Shang CA, Behncken SN, Tam SP, Li H, Shen B, Lobie PE. Growth hormone as a cytokine. Clin Exp Pharmacol Physiol. 1999;26:760–764. doi: 10.1046/j.1440-1681.1999.03129.x. [DOI] [PubMed] [Google Scholar]
- Waters SM, Berry DP, Mullen MP. Polymorphisms in genes of the somatotrophic axis are independently associated with milk production, udder health, survival and animal size in Holstein-Friesian dairy cattle. J Anim Breed Genet. 2012;129:70–78. doi: 10.1111/j.1439-0388.2011.00938.x. [DOI] [PubMed] [Google Scholar]
- Waters SM, McCabe MS, Howard DJ, Giblin L, Magee DA, MacHugh DE, Berry DP. Associations between newly discovered polymorphisms in the Bos taurus growth hormone receptor gene and performance traits in Holstein-Friesian dairy cattle. Anim Genet. 2011;42:39–49. doi: 10.1111/j.1365-2052.2010.02087.x. [DOI] [PubMed] [Google Scholar]
- Woelfle J, Rotwein P. In vivo regulation of growth hormone-stimulated gene transcription by STAT5b. Am J Physiol Endocrinol Metab. 2004;286:E393–401. doi: 10.1152/ajpendo.00389.2003. [DOI] [PubMed] [Google Scholar]
- Woelfle J, Chia DJ, Rotwein P. Mechanisms of growth hormone action: identification of conserved Stat5 binding sites that mediate GH-induced insulin-like growth factor-I gene activation. J Biol Chem. 2003;278:51261–51266. doi: 10.1074/jbc.M309486200. [DOI] [PubMed] [Google Scholar]
- Zapf J, Hauri C, Waldvogel M, Froesch ER. Acute metabolic effects and half-lives of intravenously administered insulinlike growth factors I and II in normal and hypophysectomized rats. J Clin Invest. 1986;77:1768–1775. doi: 10.1172/JCI112500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu T, Goh EL, Graichen R, Ling L, Lobie PE. Signal transduction via the growth hormone receptor. Cell Signal. 2001;13:599–616. doi: 10.1016/s0898-6568(01)00186-3. [DOI] [PubMed] [Google Scholar]


