Abstract
A study was conducted to evaluate the effects of resveratrol and essential oils from medicinal plants on the growth performance, immunity, digestibility, and fecal microbial shedding of weaned piglets. A total of 48 weaned piglets (8 kg initial weight, 28-d-old) were randomly allotted to four dietary treatments with 3 replications of 4 piglets each. The dietary treatments were NC (negative control; basal diet), PC (positive control; basal diet+0.002% apramycin), T1 (basal diet+0.2% resveratrol), and T2 (basal diet+0.0125% essential oil blend). All piglets were orally challenged with 5 ml culture fluid containing 2.3×108 cfu/ml of Escherichia coli KCTC 2571 and 5.9×108 cfu/ml Salmonella enterica serover Typhimurium. The PC group (p<0.05) showed the highest average daily gain (ADG) and average daily feed intake (ADFI) throughout the experimental period, although feed conversion ratio (FCR) was improved in the T1 group (p>0.05). Serum IgG level was increased in the T1 group, whereas TNF-α levels was reduced in the supplemented groups compared to control (p<0.05). The PC diet improved the dry matter (DM) digestibility, whereas PC and T2 diets improved nitrogen (N) digestibility compared to NC and T1 diets (p<0.05). Fecal Salmonella and E. coli counts were reduced in all treatment groups compared to control (p<0.05). Fecal Lactobacillus spp. count was increased in the T2 group compared to others (p<0.05). Dietary treatments had no significant effect on fecal Bacillus spp. count throughout the entire experimental period. Based on these results, resveratrol showed strong potential as antibiotic alternatives for reversing the adverse effects of weaning stress on growth performance, immunity and microbial environment in E. coli and Salmonella-challenged piglets.
Keywords: Resveratrol, Essential Oil Blend, Growth, Immunity, Microorganism, Challenged Piglets
INTRODUCTION
Salmonella typhimurium and Escherichia coli are two of the most frequently isolated pathogens from pig farms, inducing poor performance in the post-weaning period (Bhandari et al., 2008) and food-borne illnesses in humans (Rugbjerg et al., 2004). Generally, antibiotics are used at sub-therapeutic levels to treat Salmonella and E. coli infection as well as to protect host animals from weaning stress such as poor growth, reduced immunity, and an imbalanced microbial ecosystem. However, due to the appearance of resistant bacteria and residues in animal products, the use of antibiotics has been prohibited by the European Union (Regulation (EC) No. 1831/2003). This ban is expected to result in reduced livestock performance as well as higher incidence of intestinal disorders due to increased proliferation of gut pathogens (Hughes et al., 2005). Therefore, a natural, safe, and inexpensive alternative with activities similar to antibiotics should be developed in order to improve the health and performance of animals.
Phytogenics, a relatively new class of feed additives, comprise a wide variety of herbs, spices, and products derived thereof, such as essential oils, polyphenols (resveratrol), etc. The possible mechanisms of action of herbal extracts in animals are related to alteration of the intestinal microbiota, increased digestibility and absorbance of nutrients (Hernandez et al., 2004), as well as antioxidative and immunomodulatory activities (Chang et al., 1995). The rapid metabolization and short half-life of bioactive compounds suggest a minimum risk of accumulation and cross-resistance in tissues (Kohlert et al., 2000). However, experimental research on the addition of synthetic antimicrobials and vegetable extracts to swine diets has shown conflicting results. In this study, two commercial products, ResPig® (containing resveratrol from mulberry and Japanese knotweed) and Biomin® PEP (containing oregano, anise, orange peel, and chicory essential oils), were used as phytogenic feed additives. Resveratrol (trans-3,5,4′-trihydroxystilbene) is a stilbenoid, a type of natural polyphenol and aromatic phytoalexin found predominantly in grapes, berries (mulberries), and Japanese knotweed. Trans-resveratrol is found to be stable under 75% humidity and 40°C in the presence of air (Baur et al., 2006). It is thought to possess strong antimicrobial activity (Paulo et al., 2010) and has gained wide attention due to its ability to inhibit various animal diseases (Baur et al., 2006). On the other hand, essential oils are volatile, oily liquids that have been used to replace antibiotic supplements in weanling pigs without affecting performance (Cho et al., 2006). Some essential oils display strong antimicrobial activity, selectivity against Gram-negative over Gram-positive bacteria (Lin et al., 2000). De Souza et al. (2008) found no significant interference on the essential oil antimicrobial activity and chemical composition under different heating treatments (room temp. to 120°C). Further, most essential oils are classified as Generally Recognized as Safe (GRAS; FDA, 2004). Both of the phytogenics assumed to improve livestock performance as well as may be added to the set of non-antibiotic growth promoters. Therefore, this study evaluated the effects of resveratrol and an essential oil blend made from medicinal plants on growth performance, immunity, nutrient digestibility, and fecal microbial shedding in E. coli and Salmonella enterica serover Typhimurium challenged piglets.
MATERIAL AND METHODS
Animal and experimental design
A 4-wk experiment was conducted with a total of 48 newly weaned piglets (crossbred ((Landrace×Yorkshire)× Duroc), mean body weight 8 kg, 28 d of age) in order to evaluate the effects of the resveratrol and an essential oil blend as alternatives to antimicrobial feed additives such as apramycin. The piglets were allotted to one of the four dietary treatments in a completely randomized design based on their initial body weight. Each treatment had three replicate pens with four pigs per pen. The dietary treatments included NC (negative control; basal diet), PC (positive control; basal diet+0.002% apramycin), T1 (basal diet+0.2% Respig®; containing resveratrol), and T2 (basal diet+0.0125% Biomin® PEP; containing essential oil blend). A commercial pellet pig starter diet (Table 1) was used as a basal diet formulated to meet the nutrient requirements recommended by the NRC (1998). Molasses was added at 4.3% level to help in the manufacture of pellets. The piglets were allowed ad libitum access to feed and water. All other managements were carried out in accordance with the general practice.
Table 1.
Ingredients and chemical compositions of the basal diets (starter)
| Item | |
|---|---|
| Ingredients (%, as-fed basis) | |
| Yellow corn | 45.15 |
| Wheat | 23.00 |
| Wheat bran | 4.00 |
| Soybean meal | 18.00 |
| Limestone | 0.98 |
| Calcium phosphate | 1.10 |
| Salt | 0.25 |
| Vitamin and mineral premix1 | 0.55 |
| Animal fat | 2.50 |
| Molasses | 4.30 |
| L-lysine | 0.17 |
| Chemical composition (as-fed basis)2 | |
| ME (kcal/kg) | 3,265.00 |
| Crude protein (%) | 18.00 |
| Ca (%) | 0.70 |
| Available phosphorus (%) | 0.55 |
| Lysine (%) | 0.95 |
| Methionine (%) | 0.30 |
Vitamin and mineral premix provided the following nutrients per kg of complete diet: vitamin A, 6,000 IU; vitamin D3, 800 IU; vitamin E, 20 IU; vitamin K3, 2 mg; thiamin, 2 mg; riboflavin, 4 mg; vitamin B6, 2 mg; vitamin B12, 1 mg; pantothenicacid, 11 mg; niacin, 10 mg; biotin, 0.02 mg; Cu (copper sulfate), 21 mg; Fe (ferrous sulfate), 100 mg; Zn (zinc sulfate), 60 mg; Mn (manganese sulfate), 90 mg; I (calcium iodate), 1.0 mg; Co (cobalt nitrate), 0.3 mg; Se (sodium selenite), 0.3 mg.
Calculated values.
Oral challenge
All piglets (32 d of age) were orally challenged with 5 ml of culture fluid containing 2.3×108 cfu/ml of E. coli KCTC 2571 and 5.9×108 cfu/ml of Salmonella enterica serover Typhimurium at the back of the oral cavity using a micropipette tip. The bacteria-rich solution was slowly dribbled into each piglet’s throat in order to trigger the swallowing reflex and minimize the passage of inoculants into the lungs.
Test materials
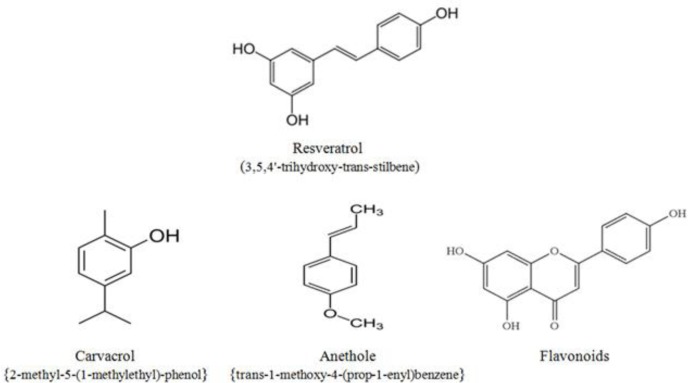
The plant extract Respig® (Merck Animal Health, Korea) used in this study was composed of resveratrol from mulberry and Japanese knotweed. The essential oil blend Biomin® PEP (Biomin Inc., San Antonio, TX) was composed of oregano (Origanum vulgare), anise (Pimpinella anisum), orange peel (Citrus sinensis), and chicory (Cichorium intybus) essential oils. As main ingredients, the essential oil blend contained the phenolic monoterpenes carvacrol, anethol, and flavonoids. The structural formula of resveratrol and main bioactive components of the essential oils blend are shown in Figure 1. Apramycin (Advacare Pharma) is an aminoglycoside-type antibiotic containing the active ingredient apramycin sulfate.
Figure 1.
Basic structural formula of resveratrol and the main bioactive components of essential oils blend
Measurement and analysis
Body weight and feed intake were measured at 0, 2, and 4 wks, and ADG, ADFI and FCR (feed/gain) were also calculated. Chromic oxide (Cr2O3) was added at a concentration of 0.2% as an indigestible index, and fresh samples of feed and feces were collected from each pen at the end of the experiment. The samples were then dried in a hot oven dryer at 70°C for 72 h, ground, and passed through a 1-mm seive. Feed and feces samples were analyzed for dry matter (DM) and nitrogen (N) concentrations (AOAC, 2000). Chromium content was determined by atomic absorption spectrophotometry (AA-6200, Shimadzu Corp., Japan), and apparent digestibilities of DM and N were calculated by indirect methods. For immunological analysis blood samples were collected from individual pigs from the jugular vein at the end of the experiment and sera were separated from blood samples by centrifugation (4°C, 1,610 g). Immunoglobulins (IgG, IgA, and IgM) and TNF-α were determined using a commercial ELISA kit (BETHYL Laboratories Inc., USA) and an ELISA reader (Thermo Lab Systems, Finland).
For microbiological analysis, fecal samples were collected from each group on the 1st, 2nd, 3rd, and 4th week post-challenge. Samples were then serially diluted in sterile saline (0.9%) in a 1:10 dilution and cultured on agar media in duplicate. The culture media for S. typhimurium, E. coli, Lactobacillus spp., and Bacillus spp. were Salmonella-Shigella (SS), MacConkey (MAC), Mann, Rogosa and Sharpe (MRS), and Mannitol Egg Yolk Polymyxin (MYP) agar, respectively. After incubation for 24 h at 37°C, colonies were counted. Microflora enumerations were expressed as log10 cfu/ml.
Statistical analysis
For all of the statistical analysis for this experiment, the pen served as the experimental unit. All data were analyzed as CRD using the GLM Procedure of SAS (SAS, 2003). Duncan’s multiple range tests (Duncan, 1955) were used to test the significant differences between treatments. Variability in the data was expressed as standard error (SE) and a probability level of p<0.05 was considered as statistically significant, whereas a trend was expressed when p<0.10.
RESULTS
Growth performance parameters
In this study average daily gain (ADG), average daily feed intake (ADFI) and feed conversion ratio (FCR) were considered as growth performance parameters. Piglets fed PC diet showed numerically higher ADG, ADFI (p<0.05), as well as a slightly reduced FCR (p>0.05) compared to control throughout the experimental period (Table 2). Over the entire experimental period, the T1 group showed increased ADG and ADFI compared to the NC and T2 groups (p<0.05). However, FCR was improved in the T1 group compared to the other groups during the overall experimental period (p>0.05).
Table 2.
Effects of resveratrol and essential oils blend on growth performance and nutrient digestibility of challenged piglets1
| Items | Treatments2
|
SE3 | p-value | |||
|---|---|---|---|---|---|---|
| NC | PC | T1 | T2 | |||
| Initial body weight (kg) | 8.03 | 8.09 | 7.96 | 8.02 | 0.54 | 0.99 |
| Average daily gain (g/d) | ||||||
| 0 to 2 wk | 291b | 400a | 295b | 246b | 62.8 | 0.03 |
| 2 to 4 wk | 176b | 317a | 294a | 206b | 41.6 | <0.01 |
| 0 to 4 wk | 234c | 358a | 294b | 226c | 35.1 | <0.01 |
| Average daily feed intake (g/d) | ||||||
| 0 to 2 wk | 440b | 521a | 410c | 499a | 17.8 | <0.0001 |
| 2 to 4 wk | 516c | 866a | 714b | 470d | 9.02 | <0.0001 |
| 0 to 4 wk | 478c | 694a | 562b | 484c | 10.8 | <0.0001 |
| Feed conversion ratio (feed/gain) | ||||||
| 0 to 2 wk | 1.52b | 1.35b | 1.45b | 2.10a | 0.32 | 0.03 |
| 2 to 4 wk | 2.94 | 2.81 | 2.44 | 2.42 | 0.48 | 0.34 |
| 0 to 4 wk | 2.05 | 1.97 | 1.92 | 2.17 | 0.22 | 0.41 |
| Digestibility of nutrients (%) | ||||||
| Dry matter | 74.7c | 88.0a | 77.4bc | 78.7b | 1.55 | <0.0001 |
| Nitrogen | 67.6b | 78.4a | 70.6b | 77.6a | 2.83 | <0.01 |
Means in a row with no common superscripts significantly differ (p<0.05).
Values represent the means of three pens with four pigs per pen.
NC (Negative control), basal diet; PC (Positive control), basal diet+0.002% Apramycin; T1, basal diet+0.2% Respig (containing resveratrol); T2, basal diet+0.0125% Biomin PEP; (essential oils blend).
Pooled standard error.
Digestibility of nutrients
In our study we measured the digestibility of dry matter (DM) and nitrogen (N) to evaluate the effect of dietary treatments. Digestibility of DM were improved by PC, T2 and T1 compared to NC diet (p<0.05). On the other hand, PC and T2 diets showed the strongest effects on N digestibility (p<0.05) compared to NC and T1 diets (Table 2).
Serum immunoglobulin and cytokine profile
Among the different immunoglobulins and cytokines IgG, IgM, IgA and TNF-α are the most important profile for immunity. Table 3 shows that the serum IgG level of pigs fed T1 diet increased compared to those fed NC diet, whereas it remained intermediate in the T2 and PC groups (p<0.05). Although there were no statistical differences in serum IgM and IgA levels among the treatments (p>0.05), concentrations were slightly higher in the T2 group. The TNF-α level was highest in the NC group, lowest in the PC group and intermediate in the T1 and T2 groups (p<0.10).
Table 3.
Effects of resveratrol and essential oils blend on serum levels of immunoglobulins (mg/mL) and TNF-α (pg/mL) of challenged piglets1
| Items | Treatments2
|
SE3 | p-value | |||
|---|---|---|---|---|---|---|
| NC | PC | T1 | T2 | |||
| IgG (mg/ml) | 304c | 366b | 466a | 409b | 27.7 | <0.01 |
| IgM (mg/ml) | 30.2 | 29.9 | 30.0 | 30.3 | 0.64 | 0.90 |
| IgA (mg/ml) | 6.33 | 5.80 | 6.85 | 6.87 | 0.83 | 0.04 |
| TNF-α (pg/ml) | 136a | 99.0b | 115ab | 108ab | 17.7 | 0.09 |
Means in a row with no common superscripts significantly differ (p<0.05) or tend to differ (p<0.10).
Values represent the means of three pens with four pigs per pen.
NC (Negative control), basal diet; PC (Positive control), basal diet+0.002% Apramycin; T1, basal diet+0.2% Respig (containing resveratrol); T2, basal diet+0.0125% Biomin PEP; (essential oils blend).
Pooled standard error.
Fecal microbial shedding
S. typhimurium and E. coli are the most abundant pathogenic microorganisms, whereas Bacillus spp. and Lactobacillus spp. are most important beneficial microorganisms in pig’s GI tract. Table 4 shows that the dietary treatments had no significant effects on fecal shedding of S. typhimurium, E. coli, and Bacillus spp. at 1st week (p>0.05). Lactobacillus spp. count was higher in the T1 and NC groups (p<0.05) compared to the PC group, whereas it did not significantly differ from that of the T2 group (p>0.05). During 2nd week pigs fed PC diet showed lower fecal S. typhimurium (p<0.05) and E. coli (p>0.05) counts than any other dietary treatment. Accordingly, there were no significant differences in Lactobacillus spp. and Bacillus spp. counts among the treatments (p>0.05).
Table 4.
Effects of dietary treatments on fecal microbial concentrations in challenged piglets (cfu/ml)1
| Week | Treatments2
|
SE3 | p-value | |||
|---|---|---|---|---|---|---|
| NC | PC | T1 | T2 | |||
| S. typhimurium | ||||||
| 1 | 4.09 | 3.54 | 3.68 | 4.04 | 0.37 | 0.26 |
| 2 | 4.09a | 1.88b | 3.82a | 4.37a | 0.52 | <0.01 |
| 3 | 4.26a | 1.33b | 3.66a | 3.99a | 0.42 | <0.01 |
| 4 | 4.34a | 0.80c | 3.34b | 3.39b | 0.44 | <0.0001 |
| E. coli | ||||||
| 1 | 4.88 | 5.09 | 5.46 | 5.45 | 0.33 | 0.16 |
| 2 | 5.64 | 5.19 | 5.58 | 5.75 | 0.35 | 0.29 |
| 3 | 6.08a | 5.06b | 5.34b | 5.25b | 0.27 | 0.01 |
| 4 | 6.23a | 5.07b | 5.26b | 5.13b | 0.18 | <0.01 |
| Lactobacillus spp. | ||||||
| 1 | 7.04a | 6.23b | 7.21a | 6.62ab | 0.39 | 0.05 |
| 2 | 7.24 | 6.73 | 7.33 | 7.29 | 0.32 | 0.15 |
| 3 | 7.12ab | 6.55b | 7.08ab | 7.50a | 0.36 | 0.07 |
| 4 | 6.95b | 7.18b | 6.79b | 7.71a | 0.27 | 0.01 |
| Bacillus spp. | ||||||
| 1 | 5.91 | 6.01 | 6.17 | 6.21 | 0.25 | 0.46 |
| 2 | 6.33 | 6.53 | 6.37 | 6.64 | 0.39 | 0.76 |
| 3 | 6.38 | 6.43 | 6.29 | 6.56 | 0.44 | 0.90 |
| 4 | 6.22 | 6.23 | 6.10 | 6.75 | 0.54 | 0.51 |
Means in a row with no common superscripts significantly differ (p<0.05) or tend to differ (p<0.10).
Values represent the means of three pens with four pigs per pen.
NC (Negative control), basal diet; PC (Positive control), basal diet+0.002% Apramycin; T1, basal diet+0.2% Respig (containing resveratrol); T2, basal diet+0.0125% Biomin PEP; (essential oils blend).
Pooled standard error.
At 3rd week post challenge, the PC group showed the lowest shedding of S. typhimurium compared to control (p<0.05), whereas E. coli shedding was reduced by all dietary treatments (p<0.05). Lactobacillus spp. (p<0.10) and Bacillus spp. (p>0.05) counts both increased upon administration of T2 diet compared to others. At 4th week post challenge, the PC group showed the lowest S. typhimurium counts, the NC diet group the highest counts, and the T1 and T2 groups intermediate counts (p<0.05). The NC group also showed the highest E. coli shedding compared to other treatments (p<0.05). Both Lactobacillus spp. and Bacillus spp. counts increased in the T2 group, although Bacillus spp. count did not significantly differ among the groups (p>0.05).
DISCUSSION
In the present study, we evaluated whether or not resveratrol and an essential oils blend can influence growth performance, humoral immune function, nutrient digestibility, as well as fecal shedding of microorganisms as alternatives to antibiotics in challenged weaned piglets. The results of our study showed that PC diet significantly improved ADG and ADFI during the post-weaning period in piglets, consistent with the findings of Cromwell (1991) and Oetting et al. (2006). An improved FCR was found in T1 group, whereas the growth performance of T2 group was poor. Antibiotic growth promoters exert positive effects on animal growth performance by reducing the pathogenic microbial load, thereby increasing nutrient utilization in the gastrointestinal tract (Cromwell, 2001). The observed decrease in feed intake in T1 group during 0 to 2 wks period may be due to the aversive palatability of resveratrol leading to an immediate reduction in feed intake (Alexandre et al., 2010). The improved FCR in piglets fed T1 diet could be attributed to the effects of resveratrol, which mediated reduction of pathogenic microbes (E. coli and Salmonella) and improved utilization of nutrients in the digestive tract. Similar improvements in FCR have been observed upon treatment with resveratrol (Paulo et al., 2010) to pigs or concentrated grape pomace containing resveratrol (Viveros et al., 2011) to broilers. The poor growth performance in T2 group can be explained as low feed intake by the effect of strong flavor of essential oil which reduced the palatability of the diet. Jugl-Chizzola et al. (2006) previously found dose-related depressions of palatability in pigs fed essential oils from thyme and oregano. Stress induced by pathogenic challenge could be another reason and more positive results can be obtained under normal conditions. Kroismayr et al. (2008) previously found no significant effects of essential oils on growth performance parameters of weaned piglets compared to antibiotic and negative control. The oral challenge of pigs with pathogenic E. coli 2571 and Salmonella has been used widely as a model of postweaning lag phase (Wellock et al., 2008). The feed intake and growth rate responses have proven to be useful measures of the clinical manifestations in pigs with enteric disease (Balaji et al., 2000). The poor growth performance in this experiment compared to the target performance of pigs of the same age (ADG: 234 g vs 460 g; ADFI: 478 g vs 620 g; FCR: 2.05 vs 1.35) can be explained as the effect of challenging with pathogenic E. coli and Salmonella (Close, 2002).
Further, we observed improved DM and N digestibility in the PC and T2 groups compared to the NC and T1 groups. Similar improvement in digestibility has been shown in pigs fed an essential oils blend (Huang et al., 2010). The antibiotic effects of essential oil supplementation may influence the formation of intestinal bacterial colonies as well as promote production of digestive enzymes, which could influence DM and N digestibility (Stoni et al., 2006). However, Cho et al. (2006) found negative effect of essential oils on N digestibility.
Plasma IgG is the major serum immunoglobulin that protects extra vascular compartments against pathogenic microorganisms, resulting in long-lasting immunity. In this study, resveratrol and essential oils blend showed the ability to boost IgG levels in weaned piglets under challenged conditions (p<0.05). Similar increases in IgG concentration upon treatment with resveratrol or different plant extracts such as cinnamon, thyme, and oregano have been confirmed in mice and pigs (Liu et al., 2003; Namkung et al., 2004). In contrast, the IgG level in pigs remained the same as control following treatment with oregano oil mixed with anis and citrus oils (Kommera et al., 2006). The mechanism by which phytogenic feed additives increase IgG levels is largely unknown and should be elucidated. It is possible that active molecules from phytogenics (polyphenols, essential oils, etc.) act as additional ligands of Fc receptors, which bind to IgG and stimulate the immune response (Nimmerjahn and Ravetch, 2010). Cytokines such as IL-1β and TNF-α, which are known to mediate the inflammatory response (Dinarello, 1988), have been linked to alterations in nutrient uptake and utilization (Spurlock, 1997). In our results, the TNF-α level was higher in the NC group compared to the PC group, whereas it was intermediate in the T1 and T2 groups, consistent with the findings of Trevisi et al. (2007).
Pathogenic microorganisms reduce the growth rate and health status of pigs by producing toxins, utilizing the nutrients within host animals (Gaggia et al., 2010), and suppressing microbes that synthesize vitamins or other growth factors (Falaki et al., 2010). To evaluate the effects of various dietary treatments on fecal shedding of microorganisms (S. typhimurium, E. coli, Lactobacillus spp., and Bacillus spp.), bacterial concentrations were determined at 1st, 2nd, 3rd, and 4th week post challenge. The order of inhibitory effects of the dietary treatments on fecal shedding of microorganisms was: S. typhimurium; PC>T1>T2>NC, E. coli; PC>T2>T1>NC, Lactobacillus spp.; T1>NC>PC>T2, Bacillus spp.; T1>NC>PC>T2.
The PC diet showed the highest inhibitory effect on S. typhimurium and E. coli which is likely due to the bactericidal action of Apramycin. The T1 diet showed the highest inhibitory effect on Lactobacillus spp. and Bacillus spp., in contrast T2 diet increased their number. Manzanilla et al. (2004) and Castillo et al. (2006) also found an increase in the number of Lactobacillus spp. upon supplementation with mixed essential oils. The main bioactive compound of the essential oil blend used in this study was the phenolic carvacrol. Si et al. (2006) reported that carvacrol is less active against Lactobacilli and Bifidobacteria in comparison to pathogenic bacteria such as E. coli and S. typhimurium, which is partially consistent with our observations. Resveratrol is a phytoalexin of low molecular weight that has been shown to possess bacteriostatic activity specifically against Gram-positive bacteria such as Bacillus spp. (Paulo et al., 2010) rather than Gram-negative bacteria such as S. typhimurium and E. coli.
Although it is generally accepted that antibiotics administered at growth-promoting doses reduce the number of bacteria in the gut, the response in the literature is not always consistent, depending on the type of antibiotic and dosage. Indeed, resistance against aminoglycoside type antibiotics may be responsible for the increased fecal shedding of Lactobacillus spp. observed in our study (Zhou et al., 2005; Castillo et al., 2006).
IMPLICATIONS
In total, the above findings suggest that supplementing resveratrol to weaned piglets could have beneficial effects on growth performance, immune function and the microbial ecosystem following challenge. The growth performance parameters investigated in this study were not positively affected by the essential oil blend. Further feeding trials using resveratrol and essential oils at different inclusion rates are required in order to better understand the effects of such additives as antibiotic alternatives as well as to elucidate their mechanisms of action.
Acknowledgments
The study was supported by the Cooperative Research Program for Agriculture Science and Technology Development, Rural Development Administration, Republic of Korea.
REFERENCES
- Alexandre DP, Stéphane B, Fabienne A. Resveratrol suppresses body mass gain in a seasonal non-human primate model of obesity. BMC Physiol. 2010;10:11. doi: 10.1186/1472-6793-10-11. Available at: http://www.biomedcentral.com/1472-6793/10/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AOAC . Official methods of analysis. 17th edn. Association of Official Analytical Chemists; Washington, DC: 2000. [Google Scholar]
- Balaji R, Wright KJ, Hill CM, Dritz SS, Knoppel EL, Minton JE. Acute phase responses of pigs challenged orally with Salmonella typhimurium. J Anim Sci. 2000;78:1885–1891. doi: 10.2527/2000.7871885x. [DOI] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, et al. Resveratrol improves health and survival of mice on a high calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandari SK, Xu B, Nyachoti CM, Giesting DW, Krause DO. Evaluation of alternatives to antibiotics using an Escherichia coli K88+ model of piglet diarrhea: effects on gut microbial ecology. J Anim Sci. 2008;86:836–847. doi: 10.2527/jas.2006-822. [DOI] [PubMed] [Google Scholar]
- Castillo M, Martín-Orúe SM, Roca M, Manzanilla EG, Badiola I, Perez JF, Gasa J. The response of gastrointestinal microbiota to avilamycin, butyrate, and plant extracts in early-weaned pigs. J Anim Sci. 2006;84:2725–2734. doi: 10.2527/jas.2004-556. [DOI] [PubMed] [Google Scholar]
- Chang CP, Chang JY, Wang FY, Chang JG. The effect of Chinese medicinal herb Zingiberis rhizoma extract on cytokine secretion by human peripheral blood mononuclear cells. J Ethnopharmacol. 1995;48:13–19. doi: 10.1016/0378-8741(95)01275-i. [DOI] [PubMed] [Google Scholar]
- Cho JH, Chen YJ, Min BJ, Kim HJ, Kwon OS, Shon KS, Kim IH, Kim SJ, Asamer A. Effect of essential oils supplementation on growth performance, IgG concentration and fecal noxious gas concentration of weaned pigs. Asian Australas J Anim Sci. 2006;19:80–85. [Google Scholar]
- Close WH. Premier Pig Program Manual. Alltech Inc.; Melbourne, Australia: 2002. pp. 6.4–1. [Google Scholar]
- Cromwell GL. Antimicrobial agents. In: Miller ER, Ullrey DE, Lewis AJ, editors. Swine Nutrition. Butterworth-Heinemann; Stoneham, Massachusetts: 1991. pp. 297–314. [Google Scholar]
- Cromwell GL. Antimicrobial and promicrobial agents. In: Lewis AJ, Southern LL, editors. Swine Nutrition. Butterworth-Heinemann; Stoneham, Massachusetts: 2001. pp. 405–412. [Google Scholar]
- De Souza EL, Stamford TLM, Lima EDO, Barbosa Filho JM, Marques MOM. Interference of heating on the antimicrobial activity and chemical composition of Origanum vulgare L. (Lamiaceae) essential oil. Ciênc Tecnol Aliment. 2008;28:418–422. [Google Scholar]
- Dinarello CA. Biology of interleukin 1. FASEB J. 1988;2:108–115. [PubMed] [Google Scholar]
- Duncan DB. Multiple range and multiple F test. Biometrics. 1955;11:1–42. [Google Scholar]
- Falaki M, Shams Shargh M, Dastar B, Zerehdaran S, Khomairi M. The investigation of intestinal microflora and growth response of young broilers given feed supplemented with different levels of probiotic and prebiotic. J Anim Vet Adv. 2010;9:2685–2690. [Google Scholar]
- FDA 2004 Food and Drug Administration of the US, 21 CFR 184. Online. Available at: http://www.cfsan.fda.gov/eafus.html.
- Gaggia F, Mattarelli P, Biavati B. Probiotics and prebiotics in animal feeding for safe food production. Int J. Food Microbiol. 2010;141(Suppl 1):S15–S28. doi: 10.1016/j.ijfoodmicro.2010.02.031. [DOI] [PubMed] [Google Scholar]
- Hernandez F, Madrid J, Garcia V, Orengo J, Megias MD. Influence of two plant extract on broiler performance, digestibility, and digestive organ size. Poult Sci. 2004;83:169–174. doi: 10.1093/ps/83.2.169. [DOI] [PubMed] [Google Scholar]
- Huang Y, Yoo JS, Kim HJ, Wang Y, Chen YJ, Cho JH, Kim IH. Effects of dietary supplementation with blended essential oils on growth performance, nutrient digestibility, blood profiles and fecal characteristics in weanling pigs. Asian Australas J Anim Sci. 2010;23:607–613. [Google Scholar]
- Hughes RJ, Brooker JD, Smyl C. Growth rate of broiler chickens given condensed tannins extracted from grape seed. Proceedings of the 17th Annual Australian Poultry Science Symphosium, Poultry Research Foundation, University of Sidney; Australia. 2005. pp. 65–68. [Google Scholar]
- Jugl-Chizzola M, Ungerhofer E, Gabler C, Hagmuller W, Chiz-zola R, Zitterl-Eglseer K, Franz C. Testing of the palatability of Thymus vulgaris L. and Origanum vulgare L. as flavouring feed additive for weaner pigs on the basis of a choice experiment. Berl Munch Tierarztl Wochenschr. 2006;119:238–243. [PubMed] [Google Scholar]
- Kroismayr A, Schedle K, Sehm J, Pfaffl MW, Plitzner C, Foissy H, Ettle T, Mayer H, Schreiner M, Windisch W. Effects of antimicrobial feed additives on gut microbiology and blood parameters of weaned piglets. Die Bodenkultur. 2008;59:111–120. [Google Scholar]
- Kommera SK, Mateo RD, Neher FJ, Kim SW. Phytobiotics and organic acids as potential alternatives to the use of antibiotics in nursery pig diets. Asian Australas J Anim Sci. 2006;19:1784–1789. [Google Scholar]
- Kohlert C, Van Rensen I, Marz R, Schindler G, Graefe EU, Veit M. Bioavailability and pharmokinetics of natural volatile terpenes in animal and humans. Planta Medica. 2000;66:495–505. doi: 10.1055/s-2000-8616. [DOI] [PubMed] [Google Scholar]
- Lin CM, Preston JF, Wei C. Antibacterial mechanism of allyl isothiocyanate. J Food Prot. 2000;63:727–734. doi: 10.4315/0362-028x-63.6.727. [DOI] [PubMed] [Google Scholar]
- Liu HS, Pan CE, Yang W, Liu XM. Antitumor and immunomodulatory activity of resveratrol on experimentally implanted tumor of H22 in Balb/c mice. World J Gastroenterol. 2003;9:1474–1476. doi: 10.3748/wjg.v9.i7.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzanilla EG, Perez JF, Martin M, Kamel C, Baucells F, Gasa J. Effect of plant extracts and formic acid on the intestinal equilibrium of early-weaned pigs. J Anim Sci. 2004;82:3210–3218. doi: 10.2527/2004.82113210x. [DOI] [PubMed] [Google Scholar]
- Namkung H, Li M, Gong J, Yu H, Cottrill M, de Lange CFM. Impact of feeding blends of organic acids and herbal extracts on growth performance, gut microbiota and digestive function in newly weaned pigs. Can J Anim Sci. 2004;84:697–704. [Google Scholar]
- NRC (National Research Council) Nutrient requirement of Swine. 10th Ed. National Academy Press; Washington, DC, USA: 1998. [Google Scholar]
- Nimmerjahn F, Ravetch JV. Antibody-mediated modulation of immune responses. Immunol Rev. 2010;236:265–275. doi: 10.1111/j.1600-065X.2010.00910.x. [DOI] [PubMed] [Google Scholar]
- Oetting LL, Utiyama CE, Giani PA, Ruiz UD, Miyada VS. Effects of herbal extracts and antimicrobials on apparent digestibility, performance, organs morphometry and intestinal histology of weanling pigs. Braz J Anim Sci. 2006;35:1389–1397. [Google Scholar]
- Paulo L, Ferreira S, Gallardo E, Queiroz JA, Domingues F. Antimicrobial activity and effects of resveratrol on human pathogenic bacteria. World J Microbiol Biotechnol. 2010;26:1533–1538. [Google Scholar]
- Regulation (EC) No 1831/2003. Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on additives for use in animal nutrition. Official Journal of the European Union. 2003;L268:29–43. [Google Scholar]
- Rugbjerg H, Wingstrand A, Hald T, Andersen JS, Lo DM, Wong Fo, Korsgaard H. Estimating the number of undetected multi-resistant Salmonella Typhimurium DT104 infected pig herds in Denmark. Prev Vet Med. 2004;65:147–171. doi: 10.1016/j.prevetmed.2004.07.001. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc . SAS/STAT User’s Guide. 9.1 Edition. SAS Institute Inc; Cary, North Carolina, USA: 2003. [Google Scholar]
- Si W, Gong J, Tsao R, Zhou T, Yu H, Poppe C, Johnson R, Du Z. Antimicrobial activity of essential oils and structurally related food additives towards selected pathogenic and beneficial gut bacteria. J Appl Microbiol. 2006;100:296–305. doi: 10.1111/j.1365-2672.2005.02789.x. [DOI] [PubMed] [Google Scholar]
- Spurlock ME. Regulation of metabolism and growth during immune challenge: an overview of cytokine function. J Anim Sci. 1997;75:1773–1783. doi: 10.2527/1997.7571773x. [DOI] [PubMed] [Google Scholar]
- Stoni A, Zitterl-Egelseer K, Kroismayr A, Wetscherek W, Windisch W. Tissue recovery of essential oils used as feed additive in piglet feeding and impact on nutrient digestibility. Proc Soc Nutr Physiol. 2006;15:60. [Google Scholar]
- Trevisi P, Merialdi G, Mazzoni M, Casini L, Tittarelli C, De Filippi S, Minieri L, Lalatta-Costerbosa G, Bosi P. Effect of dietary addition of thymol on growth, salivary and gastric function, immune response, and excretion of Salmonella enteric serovar Typhimurium, in weaning pigs challenged with this microbe strain. Ital J Anim Sci. 2007;6:374–376. [Google Scholar]
- Viveros A, Chamorro S, Pizarro M, Arija I, Centeno C, Brenes A. Effects of dietary polyphenol-rich grape products on intestinal microflora and gut morphology in broiler chicks. Poult Sci. 2011;90:566–578. doi: 10.3382/ps.2010-00889. [DOI] [PubMed] [Google Scholar]
- Wellock IJ, Fortomaris PD, Houdijk JG, Kyriazakis I. Effects of dietary protein supply, weaning age and experimental enterotoxigenic Escherichia coli infection on newly weaned pigs: health. Animal. 2008;2:834–842. doi: 10.1017/S1751731108002048. [DOI] [PubMed] [Google Scholar]
- Zhou JS, Pillidge CJ, Gopal PK, Gill HS. Antibiotic susceptibility profiles of new probiotic Lactobacillus and Bifidobacterium strains. Int J Food Microbiol. 2005;98:211–217. doi: 10.1016/j.ijfoodmicro.2004.05.011. [DOI] [PubMed] [Google Scholar]