Abstract
NLR family pyrin domain containing 3 (NLRP3) is a key component of the inflammasome, whose assembly is a crucial part of the innate immune response. The aim of the present study was to evaluate the association between exon 3 polymorphisms of NLRP3 and the susceptibility to digestive disorders in rabbits. In total, five coding single-nucleotide polymorphisms (cSNPs) were identified; all of which are synonymous. Among them, c.456 C> and c.594 G> were further genotyped for association analysis based on case-control design (n =162 vs n =102). Meanwhile, growing rabbits were experimentally induced to digestive disorders by feeding a fiber-deficient diet, subsequently they were subjected to mRNA expression analysis. Association analysis revealed that haplotype H1 (the two cSNPs: GT) played a potential protective role against digestive disorders (p<0.001). The expression of NLRP3 in the group H1HX1 (H1HX1 is composed of H1H1, H1H3 and H1H4) was the lowest among four groups which were classified by different types of diplotypes. Those results suggested that the NLRP3 gene was significantly associated with susceptibility to digestive disorders in rabbit.
Keywords: Digestive Disorders, NLRP3, SNP, Rabbit
INTRODUCTION
Pyrin domain containing 3 (NLRP3), coding NLRP3 protein, is a member of the NALP subfamily in the CATERPILLER family (Ting et al., 2006). Several members of the cytosolic NLR family have been identified as the key regulators of cytokine production (Kanneganti et al., 2007). NLRP3 interacts with the adaptor protein Apoptosis-associated speck-like protein to activate caspase-1 in inflammasomes, which are protein complexes responsible for the maturation and secretion of interleukin-1β (IL-1β) and IL-1 (Agostini et al., 2004; Dowds et al., 2004; Lamkanfi et al., 2007). NACHT domain (domain present in NAIP CIITA HET-E and TP1), also known as NBS (nucleotide-binding site domain) coded by exon 3 of the rabbit NLRP3 gene, plays a pivotal role in molecular recognition of host innate immunity (Royet and Reichhart, 2003). The variations and functions of NLRP3 gene, especially the exon 3 of the NLRP3 gene of mouse and human, have been widely investigated (Bauer et al., 2010; Roberts et al., 2010). However, the association between genetic polymorphisms and expression of NLRP3 and in domestic rabbit digestive disorders has not been reported.
In recent decades, the incidence of disease has increased along with the dramatic genetic improvement of rabbit fertility. Among them, digestive disorders are one of the major common diseases (Rosell, 2003). Resistance to digestive disorders has been proposed to be genetically determined in experimental populations (Eady et al., 2007; Garreau et al., 2008). In this study, exon 3 of the rabbit NLRP3 gene was resequenced for mutational analysis; subsequently the association between the NLRP3 mutation and the susceptibility to digestive disorders was estimated in a control-case population. Meantime, we also experimentally induced digestive disorders by feeding a fiber-deficient diet to growing rabbits, which were then studied for an association between mRNA expression and NLRP3 gene genotype.
MATERIAL AND METHODS
Ethics statement
Animal care and tissue collection procedures involved in the present study were approved by the Institutional Animal Care and Use Committee in College of Animal Science and Technology, Sichuan Agricultural University, Sichuan, China (DKY-B20090908).
Recruitment of control-case group
In the experimental rabbit farm of Sichuan Agricultural University, the recruitment process of case and control rabbits has been specified in our previous study (Zhang et al., 2011). In brief, after being weaned at 28 d of age, New Zealand White rabbits were fed with pelleted food (16% protein, 10.8 MJ/kg) until 84 d of age. The food was restricted to approximately 80% of average ad libitum intake and water ad libitum. During the experimental period from the 28th to 84th d, the dead individuals were clinically autopsied, and only symptoms of watery content, dilatation, impaction, congestion and mucus in the intestinal tract were finally recruited into the case group. In the same rabbit population, the healthy rabbits without clinical records of any disease were collected into the control group. A total of 264 New Zealand White (NZW) rabbit ear tissues were collected from 162 cases and 102 controls in order to determine that a genetic relationship within three generations didn’t exist in the control-case group.
Experimentally induced digestive disorders
Fiber deficient diet as one of the most important risk factors can induce the incidence of digestive disorders in growing rabbit (Gidenne et al., 2000; Bennegadi et al., 2001). Thus in this study, rabbits were artificially induced to a status of digestive disorders by feeding the fiber-deficiency diet (CF = 9%, in contrast to the standard diet of CF = 15%). Briefly, a total of 60 healthy growing rabbits at 49 d of age were randomly selected. Following a 7-d dietary transition period, all rabbits were fed with the fiber-deficient diet (CF = 9%).
Animals were carefully observed twice a day with precise records for all clinical signs of digestive disorders, such as reduced feed intake, diarrhea, constipation (caecal impaction), and presence of mucus in excreta. On the 70th day, all rabbits were slaughtered by intravenous administration of sodium pentobarbital and subsequently subjected to histopathological examinations and an autopsy. In the end, 41 rabbits were successfully sampled and classified into three groups according to different severity status of digestive disorders based on clinical signs and gastrointestinal tract symptoms. The individuals that could not be clearly classified into any group were excluded. In brief, there were 8 in the healthy group, 9 in the mild inflammation group and 24 the in severe inflammation group. Colons were collected for RNA isolation and immediately frozen in liquid nitrogen and stored at −80°C. Ear tissues of 41 rabbits were also collected for DNA isolation.
DNA and RNA isolation
The ear tissues from induced digestive disorders group and control-case group were used for genomic DNA extraction by AxyPrep Genomic DNA Miniprep Kit (Axygen, USA). The frozen colon tissues from experimentally induced digestive disorders group were used for RNA extraction by RNAiso Pure RNA Isolation Kit (TaKaRa, China). A NanoVue Plus (GE, American) was used to assess the concentration and quality of DNA and RNA on the basis of absorbance of UV light at 260 (A260) and 280 nm (A280). The value range from 1.6 to 1.8 means the qualification for next study.
Screening mutation site in NLRP3
The genetic polymorphisms in exon 3 of NLRP3 gene were scanned in 24 rabbits from control-case group by Sanger direct sequencing method. According to the reference sequence of rabbit NLRP3 gene (GenBank ID: NW_003159516), 2 primer pairs were used for mutation screening (Table 1). The PCR reaction condition was performed as follows: one denaturation cycle at 94°C for 5 min, followed by 39 cycles at 94°C for 30 s, 62 to 62.2°C for 30 s and 72°C for 65 s, then an extension cycle at 72°C for 10 min. The 30 μl reaction volume included 15 μl 2×Taq PCR MasterMix (Aidlab, China), 1.2 μl of each primer (10 pmol/μl), 3 μl DNA template (20 ng/μl), 9.6 μl ddH2O. PCR products were sequenced as previously described (Zhang et al., 2011).
Table 1.
Information of the primers used for PCR, HRM analysis and RT-PCR
| Primers | Primer F (5′-3′) | Primer R (5′-3′) | Amplicon size (bp) | Annealing temperature(°C) |
|---|---|---|---|---|
| SNP scanning | ||||
| Seq 1 | ACAGGAAGCAGGTTGAAGAAAGG | GGTGGTGATGAGCAGTGAGGC | 871 | 62 |
| Seq 2 | CCACCACCTCCAAGACCAC | CAGCAGACGCTTACTCTACCC | 922 | 62.2 |
| Genotyping | ||||
| HRM456 | GCTGTGCCCGCAGTCCGATT | AGGCGAAGCCGTGTGTAGCG | 151 | 60.5 |
| HRM594 | GCGGGAGCAGGAGTTGGTGG | GCTGCCCTGAAGCCCAGTCC | 197 | 63.2 |
| Quantification | ||||
| HPRT | CGAGGACTTGGAAAGGGTG | CAGCAGGTCAGCAAAGAACT | 151 | 63 |
| NLRP3 | TGAGGAAGAGGACACGGGACG | GAGCCTGGTGGACCTGATTGC | 92 | 58.8 |
Genotyping using high-resolution melting (HRM)
Primer pairs of HRM456 F/R and HRM594 F/R (Table 1) were designed to amplify the small fragment containing the target SNPs of c.456 C>G and c.594 G>T, which was further subjected to HRM analysis in control-case group. PCR reactions were performed on Bio-Rad CFX96 real-time PCR detection system (Bio-Rad, Inc., Hercules, CA, USA). All samples were amplified in duplicate, and each run contained a non-template control (NTC) and three types of individuals as the standard including sequencing-verified reference sequence-type, heterozygous and homozygous genotypes. The PCR reaction used SsoFast EvaGreen Supermix (Bio-Rad, CA, USA), the volume and condition were similar as previously described (Zhang et al., 2011). HRM curve data was analyzed using the manufacturer’s software.
Gene mRNA expression in colon tissue using real-time RT-PCR
RNA was used to synthesize cDNA applying the PrimeScript RT reagent kit (TaKaRa, China). Two pair primers (Table 1) were designed to amplify the short fragment including reference gene hypoxanthine phosphoribosyltransferase (HPRT, GenBank ID: EF062857.1) and the target gene NLRP3 (GenBank ID: NW_003159516). PCR reactions were performed on Bio-Rad CFX96 real-time PCR detection system (Bio-Rad, Inc., Hercules, CA, USA). The total reaction volume of 10 μl/well included 5 μl of iQ SYBR Green Super mix (Bio-Rad, CA, USA), 0.4 μl of each primer (10 pmol/μl), 1 μl cDNA template (20 ng/μl), and 3.2 μl ddH2O. Reaction condition was 98.0°C for 3 min, 39 cycles of 98.0°C for 5 s and 56 to 63.0°C for 25 s, subsequently 95.0°C for 10 s, then Melt Curve from 65°C to 95°C per increments of 0.2°C for 10 s to read plate. The standard curves were diluted 10-fold gradient from 10−3 to 10−9 to ensure an amplification efficiency of 100%±5%. Then 41 experimentally induced digestive disorders samples were run in triplicate for the three genes, each run containing a NTC.
Data analysis
The DNA sequences were assembled and analyzed using the DNAstar program (DNAS Inc, Madison, WI, USA). The relative synonymous codon usage (RSCU) value (SNc/Na) was associated with a change in local translation elongation rates (Sharp and Li, 1986), as previously described to assess the synonymous mutation (Sauna and Kimchi-Sarfaty, 2011). The alleles and genotypes frequency differences between control-case groups were tested with the Fisher’s exact test. Odds ratios (ORs) for the allelic association were estimated by unconditional logistic regression analysis.
The individual gene expressions were quantified via real-time RT-PCR and normalised by the vandasompele method (Vandesompele et al., 2002). Gene expression data was assessed for normal Gaussian distribution and then analyzed by ANOVA test and LSD test. Haplotypes were constructed with PHASE version 2.1 with the default parameters (Stephens et al., 2001; Zheng et al., 2004). The frequency differences of alleles and genotypes between the case group and control group were tested with the Fisher’s exact test. Hardy-Weinberg equilibrium (HWE) and pairwise LD tests were performed in SHEsis web-based platform (Li et al., 2009). All statistical analyses were performed using SAS v8.0 statistical package (SAS Institute, NC, USA). p<0.05 was considered statistically significant.
RESULTS
Mutation analysis of rabbit NLRP3 gene
Exon 3 of rabbit NLRP3 gene was detected in 24 individuals (12 individuals were randomly selected from case group and control group, respectively). The sequencing result showed that five coding single nucleotide polymorphisms (cSNPs) (c.456 C>G, c.594 G>T, c.1224 G>T, c.1248 G>A, c.1627 G>A) were all synonymous mutations. In order to represent the effect of synonymous mutations on codon usage bias, the RSCU value was analyzed (Table 2). ΔRSCU values in c.456 C>G (−24.7), c.594 G>T (−8.2) and c.1627 (−1.4) were negative (ΔRSCU <0), which means the variation introduces a rarer codon associated with a slower local rate of translation elongation compared with the reference type. While the ΔRSCU values in c.1224 G>T (3.9) and c.1248 G>A (2.6) were positive (ΔRSCU>0). Moreover, the higher ΔRSCU value is more suitable for “optimal codon” (Comeron, 2004). Among the five mutations, the ΔRSCU value of c.456 C>G (−24.7) and c.594 G>T (−8.2) were obviously higher than others. Thus, synonymous mutations c.456 C>G and c.594 G>T were chosen for HRM analysis.
Table 2.
Codon usage of synonymous mutations in the coding NLRP3 gene
| mRNA position | Codon change
|
RSCU change
|
ΔRSCU | Amino acid | ||
|---|---|---|---|---|---|---|
| From | To | From | To | |||
| 456 | GCC | GCG | 34.2 | 9.5 | −24.7 | A |
| 594 | GGG | GGT | 17 | 8.8 | −8.2 | G |
| 1224 | CCG | CCT | 8.7 | 12.6 | 3.9 | P |
| 1248 | ACG | ACA | 9.1 | 11.7 | 2.6 | T |
| 1627 | AGG | AGA | 10.6 | 9.2 | −1.4 | R |
RSCU = Relative synonymous codon usage.
Case-control association of c.456 C>G and c.594 G>T mutations
Total of 264 NZW rabbits including 162 cases and 102 controls were genotyped using HRM technology in c.456 C>G and c.594 G>T site. The frequencies in case vs control group were significantly different in allele C (c.456 C>G, 48% vs 30%, p<0.01) and allele G (c.594 G>T 62% vs 53%, p<0.01). Similarly, the frequencies of GG genotype (16.7% vs 50%, p<0.01) and TT genotype (8% vs 24.5%, p<0.01) were significantly different. The case-control association analysis revealed that both allele G and T carried a potential protective role for digestive disorders with odds ratio (OR) value of 0.4537 (case vs control, 95% confidence interval 0.3132 to 0.6573, p<0.01) and 0.6975 (case vs control, 95% confidence interval 0.4892 to 0.9944, p<0.05) respectively. Under these models, association analysis also suggested GG genotype decreased risk of digestive disorders with an odds ratio (OR) value of 0.20 (case vs control, 95% confidence interval 0.11 to 0.35, p<0.01) while G/G-G/T genotype increased risk of digestive disorders with an odds ratio (OR) value of 3.72 (case vs control, 95% confidence interval 1.80 to 7.68, p<0.01) (Table 3).
Table 3.
NLRP3 alleles and genotypes associated with digestive disorders in rabbit
| Models | Genotypes/allele | Cases (%) n = 162 | Controls (%) n = 102 | OR (95% CI) | p values |
|---|---|---|---|---|---|
| c.456C | 157 (48%) | 61 (30%) | 1.00 | <0.0001 | |
| c.456G | 167 (52%) | 143 (70%) | 0.4537(0.3132–0.6573) | ||
| Co-dominant | G/G | 27 (16.7%) | 51 (50%) | 1 | <0.0001 |
| G/C | 113 (69.8%) | 41 (40.2%) | 0.19 (0.11–0.35) | ||
| C/C | 22 (13.6%) | 10 (9.8%) | 0.24 (0.10–0.58) | ||
| Dominant | G/G | 27 (16.7%) | 51 (50%) | 1 | <0.0001 |
| G/C-C/C | 135 (83.3%) | 51 (50%) | 0.20 (0.11–0.35) | ||
| Recessive | G/G-G/C | 140 (86.4%) | 92 (90.2%) | 1 | 0.35 |
| C/C | 22 (13.6%) | 10 (9.8%) | 0.69 (0.31–1.53) | ||
| Over-dominant | G/G-C/C | 49 (30.2%) | 61 (59.8%) | 1 | <0.0001 |
| G/C | 113 (69.8%) | 41 (40.2%) | 0.29 (0.17–0.49) | ||
| Log-additive | - | - | - | 0.35 (0.23–0.55) | <0.0001 |
| c.594G | 200 (0.62) | 108 (0.53) | 1.00 | 0.0463 | |
| c.594T | 124 (0.38) | 96 (0.47) | 0.6975(0.4892–0.9944) | ||
| Co-dominant | G/G | 51 (31.5%) | 51 (31.5%) | 1 | 0.0008 |
| G/T | 98 (60.5%) | 98 (60.5%) | 0.77 (0.44–1.36) | ||
| T/T | 13 (8%) | 13 (8%) | 3.16 (1.41–7.08) | ||
| Dominant | G/G | 51 (31.5%) | 51 (31.5%) | 1 | 0.85 |
| G/T-T/T | 111 (68.5%) | 111 (68.5%) | 1.05 (0.62–1.80) | ||
| Recessive | G/G-G/T | 149 (92%) | 149 (92%) | 1 | 0.0002 |
| T/T | 13 (8%) | 13 (8%) | 3.72 (1.80–7.68) | ||
| Over-dominant | G/G-T/T | 64 (39.5%) | 64 (39.5%) | 1 | 0.014 |
| G/T | 98 (60.5%) | 98 (60.5%) | 0.54 (0.32–0.89) | ||
| Log-additive | - | - | - | 1.51 (1.03–2.23) | 0.033 |
OR = Odds ratio, CI = Confidence interval.
The haplotype analysis showed that two cSNPs had formed four haplotypes among the case and control groups composed of 264 individuals. The four haplotypes were conveniently defined as allele H1 (the two cSNPs: GT), allele H2 (the two cSNPs: CG), allele H3 (the two cSNPs: GG) and allele H4 (the two cSNPs: CT) to simplify the term usage. The case-control association analysis revealed that haplotype H1 played a potential protective role against digestive disorders (Table 4).
Table 4.
Haplotype association with digestive disorders in the rabbit (n = 264)
| Haplotype | Cases/total | OR (95% CI) | p-value |
|---|---|---|---|
| H1 | 23/81 | 1.00 | - |
| H2 | 57/80 | 6.24 (3.15–12.38) | <0.001 |
| H3 | 144/229 | 4.27 (2.46–7.42) | <0.001 |
| H4 | 100/138 | 6.63 (3.60–012.22) | <0.001 |
OR = Odds ratio, CI = Confidence interval.
Among the 264 individuals of case and control groups, the average frequencies of haplotypes H1, H2, H3 and H4 were 0.3568, 0.3530, 0.2303 and 0.0599 respectively (Table 4), which further determined the diplotypes frequencies of 4.9% (H1H1), 33.7% (H1H2), 13.6% (H1H3), 7.2% (H1H4), 2.7% (H2H2), 17.4% (H2H3), 7.6% (H2H4), 11.0% (H3H3) and 1.9% (H4H4). The HWE test did not reject the null hypothesis (p>0.05). Significant deviations from Hardy-Weinberg equilibrium (HWE) were observed only in control group (c.456:p = 4.53×10−7, c.594:p = 0.00036).
NLRP3 gene mRNA expression in digestive disorders
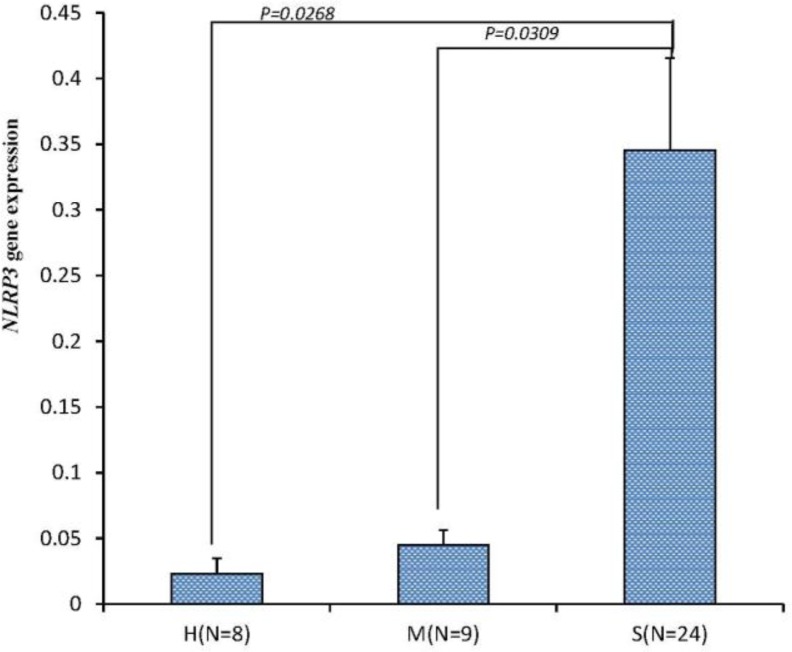
Forty-one digestive disorders individuals consisted of 8 in healthy group (H), 9 in mild digestive disorders group (M) and 24 in severe digestive disorders group (S). The mRNA expression of NLRP3 gene was detected using real-time RT-PCR. The mRNA expression of the NLRP3 gene in different states of digestive disorders is exhibited in Figure 1. The expression level of the NLRP3 gene in group S (0.3455±0.0440) was significantly higher than that in group M (0.0452±0.0314, p<0.05), and dramatically higher than that in group H (0.2256±0.0193 p<0.01).
Figure 1.
The expression of NLRP3 in different severity states of digestive disorders. H, M, S and N respectively represent healthy group, mild digestive disorders, severe digestive disorders group and the number of each group. The histogram represents the results as mean±standard error of the mean (SEM). Only the significant level (p<0.05) can be marked out.
NLRP3 gene mRNA expression in different genotypes of NLRP3 gene
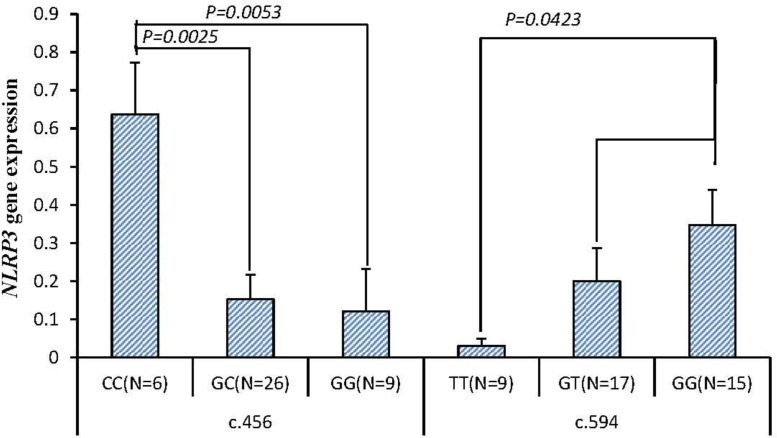
The NLRP3 gene was genotyped by HRM analysis in 41 rabbits which were experimentally induced to digestive disorders. The different expression in six genotypes of NLRP3 genes are shown in Figure 2. In SNP c.456 C>G the NLRP3 gene expression level of CC genotype (0.6379±0.1354) was significantly higher than GC genotype (0.1526±0.0650) and GG genotype (0.1211±0.1105, p< 0.01). In SNP c.594 G>T the NLRP3 gene expression level of TT genotype (0.0302±0.0191) was significantly higher than GG genotype (0.3470±0.0923, p<0.05).
Figure 2.
The expression differences of NLRP3 in different allies for NLRP3 genotypes. N represents the number of each allies. Values are presented as mean±standard error of the mean (SEM). Only the significant level (p<0.05) can be marked out.
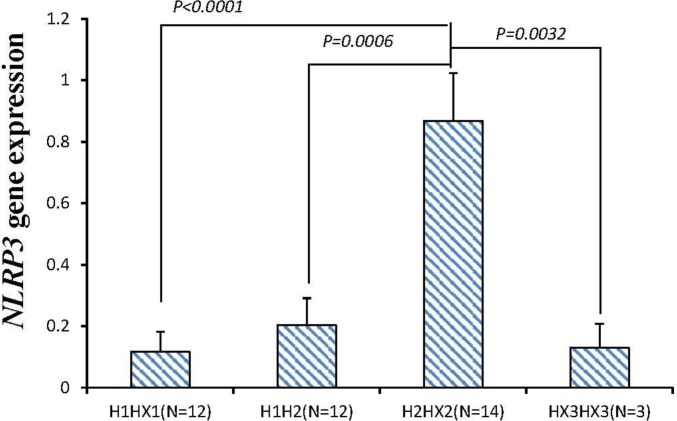
The mRNA expressions of the NLRP3 gene were divided into four groups according to the different diplotypes of the 41 rabbits. Group H1HX1 was composed of H1H1, H1H3 and H1H4. Group H2HX2 was composed of H2H2, H2H3 and H2H4. Group HX3HX3 was composed of H3H3 and H4H4 (Figure 3). The result showed that the NLRP3 expression in group H1HX was the lowest among four groups and significantly lower than that in group H2HX (p<0.0001).
Figure 3.
The expressions of NLRP3 gene in different diplotypes of rabbits. Group H1HX1 is composed of H1H1, H1H3 and H1H4. Group H2HX2 is composed of H2H2, H2H3 and H2H4. Group HX3HX3 is composed of H3H3 and H4H4. N represents the number of each group. The histogram represents the results as mean±standard error of the mean (SEM). Only the significant level (p<0.05) is marked out.
DISCUSSION
Digestive disorder is a complex multifactorial disease, the pathogenesis of which is still not well understood. NLRP3 is a member of the CATERPILLER family of genes encoding proteins that comprise a nucleotide-binding domain and a leucine-rich repeat domain. Cryopyrin, the protein encoded by NLRP3, controls the inflammasome, a crucial molecular platform that regulates activation of caspase-1 and processing of interleukin (IL)-1β, two key mediators of inflammation (Martinon and Tschopp, 2006; Mariathasan and Monack, 2007; Pétrilli et al., 2007). Mutations in NLRP3 are known to be responsible for three rare autoinflammatory disorders. The combination of the polymorphisms CARDS (C10X) and NLRP3 (Q705K) was shown to be associated with rheumatoid arthritis (Kastbom et al., 2008). Men who have both the C10X and Q705K alleles in CARDS and NLRP3, and who express wild-type alleles of Nod2 are at an increased risk of developing Crohn’s disease (CD) (Schoultz et al., 2009). CD is an idiopathic inflammatory bowel disease in humans comparable in many features to bovine paratuberculosis involving an abnormal mucosal immune response. Cummings et al. (2010) found four SNPs in NLRP3 are in high linkage disequilibrium (LD) with each other. Rs4925648 and rs10925019 are the most strongly associated with CD susceptibility. Therefore, NLRP3 is considered as one of the most plausible candidate genes associated with digestive disorders in rabbits.
The degeneracy of the genetic code means that many mutations in coding sequences, especially at the third base of codons, do not affect protein sequence and are therefore considered silent. However, there is increasing evidence that they could still have effects on transcription, splicing, mRNA transport or translation, any of which could alter the phenotype (Goymer, 2007) and cause diseases. In the present study, we, for the first time, found the NLRP3 polymorphisms was significantly correlated with the genetic resistance of rabbits digestive disorders, which expands our knowledge about the important role of NLRP3 polymorphisms in determining the genetic resistance to infectious diseases in domestic animal based on case-control study. Although these coding SNPs of c.456 C>G and c.594 G>T did not result in amino acid change, the association analysis also revealed that both allele c.456 G and allele c.594 T were significantly associated with decreased risk of developing digestive disorders in rabbits (p<0.05). In addition, the haplotype analysis also supported this point of view. However, the potential biological effects or causative variants linked to this synonymous SNP should be further investigated.
Among the 162 case subjects recruited from the general commercial population, we guaranteed their homogeneity by performing the same selection criteria according to the clinical manifestations and pathological symptoms, which has been described in detail in our former study (Zhang et al., 2011). However, we could not absolutely exclude the possibility that these case individuals could be further divided into different subtypes of digestive disorders by subjecting them to intestinal microbiota analysis with cultivation-based and nucleotide sequence-based methods (Andoh et al., 2009). Furthermore, we were also unable to confirm whether an individual death was caused by primary digestive disorders due to the underdeveloped clinical diagnosis in farm animals. This potential heterogeneity among case subjects would more or less reduce the detection power for our case-control association study. Even so, mRNA expression analysis also revealed the practical roles of NLRP3 involved in the development of digestive disorders, which was experimentally induced by feeding a fiber-deficient diet. In rabbits, the dietary fiber level has been considered as an important environmental factor associated with digestive disorders. However, the direct influence of dietary fibre component on NLRP3 mRNA expression has not been reported.
Strong deviations from HWE are believed to most likely associate with genotyping errors (Ott, 2004). However, the significant deviations from HWE for c.456 C>G and c.594 G>T variants observed in the present study could not be explained by potential genotyping errors because we performed strict quality control for HRM analysis (see Materials and Methods for details). Because there is selective pressure (Digestive disorders) in the case group, it is reasonable that non-random mating would be the main factor accounting for the failure in fitting assumption of HWE (Liu and Hu, 2010).
In our study, the expression of NLRP3 significantly up-regulated following the aggravation of intestinal inflammation (Figure 1), this result is similar to an earlier study that found the expression of the NLRP3 gene is low in colons of healthy mice but elevated in colons of mice with colitis (Villani et al., 2008). Dupaul-Chicoine et al. (2010) also found that loss of NLRP3 resulted in more severe DSS colitis and Hirota et al. (2011) found that Nlrp3−/−mice were more susceptible to experimental colitis. At the same time, we found that the NLRP3 expression level of genotype GG (c.456) and TT (c.594) in severe digestive disorders status were significantly and respectively lower than that in genotype CC (c.456) and GG (c.594) (Figure 2). The expression of NLRP3 in the group H1HX1 was the lowest in the four groups and highly significantly lower than that of the group H2HX2 (p<0.0001) (Figure 3). Of course, the potential biological effects on cytokines expression or causative variants linked to this synonymous SNP should be further investigated.
CONCLUSION
Both the genetic association study and the gene expression analysis suggest that the NLRP3 gene plays an important role in the susceptibility of digestive disorders. Thus, NLRP3 has a potential as a candidate gene for digestive disorders in rabbit. However, further research is required to confirm this conclusion and explore the potential biological implications.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Grant No: 31172197) and Earmarked Fund for China Agriculture Research 284 System (Grant No: CARS-44-A-2).
REFERENCES
- Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. NALP3 forms an IL-1 [beta]-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004;20:319–325. doi: 10.1016/s1074-7613(04)00046-9. [DOI] [PubMed] [Google Scholar]
- Andoh A, Benno Y, Kanauchi O, Fujiyama Y. Recent advances in molecular approaches to gut microbiota in inflammatory bowel disease. Curr Pharm Des. 2009;15:2066–2073. doi: 10.2174/138161209788489186. [DOI] [PubMed] [Google Scholar]
- Bauer C, Duewell P, Mayer C, Lehr HA, Fitzgerald KA, Dauer M, Tschopp J, Endres S, Latz E, Schnurr M. Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut. 2010;59:1192–1199. doi: 10.1136/gut.2009.197822. [DOI] [PubMed] [Google Scholar]
- Bennegadi N, Gidenne T, Licois D. Impact of fibre deficiency and sanitary status on non-specific enteropathy of the growing rabbit. Anim Res. 2001;50:401–413. [Google Scholar]
- Comeron JM. Selective and mutational patterns associated with gene expression in humans: Influences on synonymous composition and intron presence. Genetics. 2004;167:1293–1304. doi: 10.1534/genetics.104.026351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings J, Cooney R, Clarke G, Beckly J, Geremia A, Pathan S, Hancock L, Guo C, Cardon L, Jewell D. The genetics of NOD like receptors in Crohn’s disease. Tissue Antigens. 2010;76:48–56. doi: 10.1111/j.1399-0039.2010.01470.x. [DOI] [PubMed] [Google Scholar]
- Dowds TA, Masumoto J, Zhu L, Inohara N, Núñez G. Cryopyrin-induced interleukin 1β secretion in monocytic cells. J Biol Chem. 2004;279:21924–21928. doi: 10.1074/jbc.M401178200. [DOI] [PubMed] [Google Scholar]
- Dupaul-Chicoine J, Yeretssian G, Doiron K, Bergstrom KSB, McIntire CR, LeBlanc PM, Meunier C, Turbide C, Gros P, Beauchemin N. Control of intestinal homeostasis, colitis, and colitis-associated colorectal cancer by the inflammatory caspases. Immunity. 2010;32:367–378. doi: 10.1016/j.immuni.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Eady S, Garreau H, Gilmour A. Heritability of resistance to bacterial infection in meat rabbits. Livest Sci. 2007;112:90–98. [Google Scholar]
- Garreau H, Eady S, Hurtaud J, Legarra A. Genetic parameters of production traits and resistance to digestive disorders in a commercial rabbit population. Proc. 9th World Rabbit Congr; 2008. pp. 10–13. [Google Scholar]
- Gidenne T, Pinheiro V, Falcao e Cunha L. A comprehensive approach of the rabbit digestion: consequences of a reduction in dietary fibre supply. Livest Prod Sci. 2000;64:225–237. [Google Scholar]
- Goymer P. Synonymous mutations break their silence. Nat Rev Genet. 2007;8:92. [Google Scholar]
- Hirota SA, Ng J, Lueng A, Khajah M, Parhar K, Li Y, Lam V, Potentier MS, Ng K, Bawa M. NLRP3 inflammasome plays a key role in the regulation of intestinal homeostasis. Inflamm Bowel Dis. 2011;17:1359–1372. doi: 10.1002/ibd.21478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanneganti TD, Lamkanfi M, Núñez G. Intracellular NOD-like receptors in host defense and disease. Immunity. 2007;27:549–559. doi: 10.1016/j.immuni.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Kastbom A, Verma D, Eriksson P, Skogh T, Wingren G, Söderkvist P. Genetic variation in proteins of the cryopyrin inflammasome influences susceptibility and severity of rheumatoid arthritis (the Swedish TIRA project) Rheumatology. 2008;47:415–417. doi: 10.1093/rheumatology/kem372. [DOI] [PubMed] [Google Scholar]
- Lamkanfi M, Kanneganti TD, Franchi L, Núñez G. Caspase-1 inflammasomes in infection and inflammation. J Leukoc Biol. 2007;82:220–225. doi: 10.1189/jlb.1206756. [DOI] [PubMed] [Google Scholar]
- Li Z, Zhang Z, He Z, Tang W, Li T, Zeng Z, He L, Shi Y. A partition-ligation-combination-subdivision EM algorithm for haplotype inference with multiallelic markers: update of the SHEsis ( http://analysis.bio-x.cn) Cell Res. 2009;19:519–523. doi: 10.1038/cr.2009.33. [DOI] [PubMed] [Google Scholar]
- Liu H, Hu Y. Hardy-Weinberg equilibrium in genetic epidemiology. Zhong nan da xue xue bao. Yi xue ban= Journal of Central South University. Med Sci. 2010;35:90–93. doi: 10.3969/j.issn.1672-7347.2010.01.013. [DOI] [PubMed] [Google Scholar]
- Mariathasan S, Monack DM. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat Rev Immunol. 2007;7:31–40. doi: 10.1038/nri1997. [DOI] [PubMed] [Google Scholar]
- Martinon F, Tschopp J. Inflammatory caspases and inflammasomes: master switches of inflammation. Cell Death Differ. 2006;14:10–22. doi: 10.1038/sj.cdd.4402038. [DOI] [PubMed] [Google Scholar]
- Ott J. Association of genetic loci. Neurology. 2004;63:955–958. doi: 10.1212/wnl.63.6.955. [DOI] [PubMed] [Google Scholar]
- Pétrilli V, Dostert C, Muruve DA, Tschopp J. The inflammasome: a danger sensing complex triggering innate immunity. Curr Opin Immunol. 2007;19:615–622. doi: 10.1016/j.coi.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Roberts R, Topless R, Phipps-Green A, Gearry R, Barclay M, Merriman T. Evidence of interaction of CARD8 rs2043211 with NALP3 rs35829419 in Crohn’s disease. Genes Immun. 2010;11:351–356. doi: 10.1038/gene.2010.11. [DOI] [PubMed] [Google Scholar]
- Rosell J. Health status of commercial rabbitries in the Iberian peninsula. A practitioner’s study during 2002. World Rabbit Sci. 2003;11:157–169. [Google Scholar]
- Royet J, Reichhart JM. Detection of peptidoglycans by NOD proteins. Trends Cell Biol. 2003;13(12):610–614. doi: 10.1016/j.tcb.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Sauna ZE, Kimchi-Sarfaty C. Understanding the contribution of synonymous mutations to human disease. Nat Rev Genet. 2011;12:683–691. doi: 10.1038/nrg3051. [DOI] [PubMed] [Google Scholar]
- Schoultz I, Verma D, Halfvarsson J, Törkvist L, Fredrikson M, Sjöqvist U, Lördal M, Tysk C, Lerm M, Söderkvist P. Combined polymorphisms in genes encoding the inflammasome components NALP3 and CARD8 confer susceptibility to Crohn’s disease in Swedish men. Am J Gastroenterol. 2009;104:1180–1188. doi: 10.1038/ajg.2009.29. [DOI] [PubMed] [Google Scholar]
- Sharp PM, Li WH. Codon usage in regulatory genes in Escherichia coli does not reflect selection for ‘rare’codons. Nucleic Acids Res. 1986;14:7737–7749. doi: 10.1093/nar/14.19.7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens M, Smith NJ, Donnelly P. A new statistical method for haplotype reconstruction from population data. Am J Hum Genet. 2001;68:978–989. doi: 10.1086/319501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting JPY, Kastner DL, Hoffman HM. CATERPILLERs, pyrin and hereditary immunological disorders. Nat Rev Immunol. 2006;6:183–195. doi: 10.1038/nri1788. [DOI] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:research0034. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villani AC, Lemire M, Fortin G, Louis E, Silverberg MS, Collette C, Baba N, Libioulle C, Belaiche J, Bitton A. Common variants in the NLRP3 region contribute to Crohn’s disease susceptibility. Nat Genet. 2008;41:71–76. doi: 10.1038/ng285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang GW, Wang HZ, Chen SY, Li ZC, Zhang WX, Lai SJ. A reduced incidence of digestive disorders in rabbits is associated with allelic diversity at the TLR4 locus. Vet Immunol Immunopathol. 2011;144:482–486. doi: 10.1016/j.vetimm.2011.08.009. [DOI] [PubMed] [Google Scholar]
- Zheng SL, Augustsson-Bälter K, Chang B, Hedelin M, Li L, Adami HO, Bensen J, Li G, Johnasson JE, Turner AR. Sequence variants of toll-Like receptor 4 are associated with prostate cancer risk. Cancer Res. 2004;64:2918. doi: 10.1158/0008-5472.can-03-3280. [DOI] [PubMed] [Google Scholar]