Abstract
Leptin plays an important role in energy homeostasis and reproductive function in fish, especially in reproduction. Migrating fish, such as salmonoids, are affected by external environmental factors, and salinity changes are a particularly important influence on spawning migrations. The aim of this study was to test whether changes in salinity affect the expression of leptin, estrogen receptors (ERs), and vitellogenin (VTG) in chum salmon (Oncorhynchus keta). The expression and activity of leptin, the expression of ERs and VTG, and the levels of estradiol-17β and cortisol increased after the fish were transferred to FW, demonstrating that changes in salinity stimulate the HPG axis in migrating female chum salmon. These findings reveal details about the role of elevated leptin levels and sex steroid hormones in stimulating sexual maturation and reproduction in response to salinity changes in chum salmon.
Keywords: Chum Salmon, Estrogen Receptors, Leptin, Maturation, Salinity Change, Vitellogenin
INTRODUCTION
The spawning migration of chum salmon (Oncorhynchus keta) consists of several phases: the initiation of homing behavior, approach to the coast, migration from seawater (SW) to fresh water (FW) and upstream migration (Ueda, 2011). Maturing chum salmon are regulated by the hypothalamic-pituitary-gonadal (HPG) axis, which controls not only this migration but also the associated process of sexual maturation. It has been found that the exogenous application of gonadotropin-releasing hormones (GnRHs) can activate the HPG axis, accelerating maturation and the timing of FW entry and upstream movement in many species of salmonoids. In fish and other vertebrates, the control of reproduction via GnRHs is a complex process that involves the interaction of a number of factors, including the gonadal steroid hormones known as gonadotropin hormones (GTHs) (Urano et al., 1999; Makino et al., 2007).
Additionally, chum salmon are semelparous and cease feeding activity before the initiation of upriver migration for spawning. Thus, each individual has only one opportunity to reproduce, and all reproductive activities must be supported by the fish’s stored energy. This energy reserve is obtained during SW life and carried to the FW spawning site during migration (Hendry and Berg, 1999; Crossin et al., 2004).
Recently, several studies have investigated salmon spawning behaviors in view of the absence of food intake during migration. Of interest in this context is leptin, known as the appetite-suppressing hormone (Rønnestad et al., 2010; Trombley and Schmitz, 2013). As also shown in mammals, leptin is a peptide hormone that plays important roles in energy homeostasis and reproductive function (Zhang et al., 1994). Leptin is produced and secreted by adipocytes, and circulating leptin levels are proportional to the size of the fat store, thus functioning as a peripheral adiposity signal to the brain (Zhang et al., 1994; Maffei et al., 1995). Many peptide hormones are involved in the interplay among appetite, metabolic rate, and energy storage. Especially, leptin is most important for its role in the neuroendocrine signaling system (Rayner and Trayhurn, 2001). Additionally, leptin has demonstrated effects on the regulation of the energy balance through appetite reduction and increased energy consumption (Sahu, 2004). Leptin is known to maintain energy homeostasis by controlling feeding behavior through complex interactions between neurotransmitters, neuropeptides and other hormones present in the hypothalamus of fish (de Pedro and Björnsson, 2001; Volkoff et al., 2005).
Most studies on the physiological role of leptin in teleosts address its function in appetite, body weight regulation, and metabolism. However, the possible role of leptin in teleost reproduction remains largely unexplored (Denver et al., 2011; Trombley and Schmitz, 2013).
A previous study, confined to the role of leptin in sex maturation and reproduction in fish, has reported that human leptin protein, acting at the level of the pituitary, directly stimulated follicle-stimulating hormone (FSH) and luteinizing hormone (LH) release in female rainbow trout (Oncorhynchus mykiss) (Weil et al., 2003).
Additionally, recent studies have reported that sex steroid hormones, e.g., estradiol-17β (E2) play an important role in determining the plasma concentration of leptin in mammals (Cella et al., 2000; Kikuchi et al., 2001). To date, however, few studies have addressed the mechanisms of action of leptin and E2 in fish.
The steroid hormone estrogen is essential in reproduction and plays important roles in sexual maturation and differentiation, including oogenesis, vitellogenesis, and testicular development. In addition, estrogen influences growth, gonad sex differentiation, and the reproductive cycle and lipid and bone metabolism through mechanisms mediated primarily by nuclear estrogen receptors (ERs) (Ryffel, 1978; Ishibashi and Kawashima, 2001).
The induction of vitellogenin (VTG), a precursor yolk protein, in response to estrogens by an ER-mediated pathway is well documented in several oviparous (egg-laying) fish species (Ryffel, 1978). In largemouth bass (Micropterus salmoides) and Atlantic salmon (Salmo salar), ERα is highly correlated with VTG mRNA levels in the liver during the spawning phase (Sabo-Attwood et al., 2004; Meucci and Arukwe, 2006).
Additionally, another hormone, cortisol, is commonly known as the SW adaptation hormone. However, in species such as salmon, whose life cycles include both SW and FW phases, cortisol is involved in FW adaptation via interaction with prolactin (PRL) in conjunction with ionic regulation in FW as well as SW (Laurent and Perry, 1990; Zhou et al., 2004). However, cortisol is known to increase the concentration of leptin. It also has effects on appetite and energy balance prior to sexual maturity (Reid and Weber, 2000; Rayner and Trayhum, 2001).
In addition, the timing of sexual maturation and spawning in fish may be affected by artificial endocrine regulation and environmental parameters such as water temperature, photoperiod, and salinity (i.e., FW and/or SW adaptation) (Hirano et al., 1990; Pankhurst and Thomas, 1998; Duncan et al., 2000). Furthermore, the behavior and physiology of migrating fish such as salmonoids are also affected by external environmental factors such as photoperiod and water temperature (Duncan et al., 2000). Changing salinity is one of the most important environmental factors that directly influence spawning migration. However, little information is available about the effects of changes in environmental salinity on sex steroid hormones and maturation (Hirano et al., 1990). Previous studies have shown that the maturation that occurs as fish move from SW to FW can be regulated by GnRHa treatment (Cooperman et al., 2010), the HPG axis, and PRL, all of which act as FW adaptation hormones during spawning migration (Onuma et al., 2005; Kim et al., 2013).
This study conducted experiments on adult female chum salmon. Three experimental groups were investigated (SW, 50% SW, and FW), corresponding to the changes in salinity from coastal areas (SW) upstream to the hatchery area (FW). Fish were progressively transferred/adapted from the coastal area through a series of hypo-osmotic environments (SW→50% SW→FW). We investigated the possible effects of these changes on the maturity and sex maturation hormones. In adult female chum salmon transferred from coastal SW to an artificially hypo-osmotic environment (SW→50% SW→FW), we examined the expression of leptin; the appetite-suppressing ER hormones ERα, ERβ1, and ERβ2; the sex steroid hormone receptor; VTG; precursor yolk protein mRNA; and the levels of plasma leptin, E2, and cortisol. These effects were investigated in the context of the relationship between physiological changes and salinity changes.
MATERIALS AND METHODS
Experimental fish
Mature female chum salmon (average length, 62.4±7.4 cm; weight, 2.62±0.57 kg; gondosomatic index [GSI, gonad weight/body weight], 18.3±3.8; hepatosomatic index [HSI, liver weight/body weight], 2.3±0.7) were collected from Ishikari Bay, Hokkaido, Japan (a coastal marine area) on October 15, 2011 and transported to Hanazono, Hokkaido, Japan. The fish were maintained in four 200-L tanks for the duration of the experiment (3 days).
The transfer of the chum salmon from SW (35 psu) to FW (0 psu) followed a specific procedure. The salmon were acclimated in a square tank filled with SW. Ground water was then added to the tank to achieve a concentration of 50% SW (17.5 psu). The fish were maintained in this water for 24 h, after which more ground water was added to completely dilute the tank water to FW. The fish were then held for an additional 24 h. The water temperature was maintained at 20±0.5°C. Fish were not fed during the experimental period.
Sampling
The liver and gonad were collected from 5 randomly selected fish from each salinity group (SW, 50% SW, and FW) 24 h after transfer. The organs were immediately frozen in liquid nitrogen and stored at −80°C until total RNA extraction was performed. Additionally, blood was collected from the caudal vasculature using a 3-mL heparinized syringe and centrifuged (10,000×g, 4°C, 5 min). The plasma was then stored at −80°C until analysis.
Quantitative PCR (QPCR)
Quantitative PCR (QPCR) was conducted on the total RNA extracted from the liver and gonad of chum salmon to determine the relative expression of leptin, ERs (ERα, ERβ1, and ERβ2), and VTG mRNA using a TRIzol kit (Gibco/BRL, USA). Primers for QPCR were designed with reference to the known sequences of chum salmon, which are shown in Table 1. QPCR amplification was conducted using a BIO-RAD CFX96 Real-Time System (Bio-Rad, Hercules, CA, USA). QPCR was performed as follows: 1 cycle of denaturation at 95°C for 5 min, 35 cycles of denaturation at 95°C for 20 s, and annealing at 55°C for 20 s. For each experimental group, QPCR was run in triplicate to confirm consistency. Additionally, as an internal control, the experiments were repeated using β-actin, which gave a reaction efficiency of 95.7%. All data were expressed as change with respect to the corresponding β-actin-calculated cycle threshold (ΔCt) levels. The calibrated ΔCt value (ΔΔCt) for each sample and the internal control (β-actin) was calculated as ΔΔCt = 2^−(ΔCtsample−ΔCtinternal control). In addition, to ensure that the primers amplified a specific product, we performed a melting curve analysis, which showed that the products of each primer pair had a single melting point.
Table 1.
Primers used for amplification of QPCR
| Genes | Primer | DNA sequences |
|---|---|---|
| Leptin (KF040053) | Forward | 5′-GCT GGA GAA CTG GAT GAT AT-3′ |
| Reverse | 5′-CAT ACT TCC TGG TAG TGT CTT-3′ | |
| ERα (KF278947) | Forward | 5′-GGA CTG TGT GGA GGG TAT-3′ |
| Reverse | 5′-AGA GTT GAG CAA GAT GAT GG-3′ | |
| ERβ1 (KF278948) | Forward | 5′-GCA GGG TTT CGT GGA TAT-3′ |
| Reverse | 5′-TGT TGG AGT TGA GGA GGA T-3′ | |
| ERβ2 (KF278949) | Forward | 5′-GAC AAG AAC CGC CGT AAA-3′ |
| Reverse | 5′-CTC ACA CCA CAC TTC ATC AT-3′ | |
| VTG (AB474573) | Forward | 5′-AAT CTC TGA CAT TGA TGT TGA CCT-3′ |
| Reverse | 5′-GCC TTC ACC CTT CCG ACC-3′ | |
| β-actin (JX183093) | Forward | 5′-ATT TGG CAT CAC ACC TTC T-3′ |
| Reverse | 5′-TTC TCC CTG TTG GCT TTG-3′ |
ER = Estrogen receptor, VTG = Vitellogenin.
Western blot analysis
Total protein was extracted from the gonad and liver of female chum salmon using a protein extraction buffer (5.6 mM Tris, 0.55 mM EDTA, 0.55 mM EGTA, 0.1% SDS, 0.15 mg/mL PMSF, and 0.15 mg/mL leupeptin). It was then sonicated and quantified using the Bradford method (Bio-Rad). Total protein (30 μg per lane) was loaded onto a 4% acrylamide stacking gel and a 12% acrylamide resolving gel, and a protein ladder (Bio-Rad) was used for reference. Samples were electrophoresed at 80 V through the stacking gel and at 150 V through the resolving gel until the bromophenol blue dye front had run off of the gel. The gels were then immediately transferred to a 0.2-μm polyvinylidene difluoride membrane (Bio-Rad) at 85 V for 1.5 h at 4°C. The membranes were then blocked with 5% milk in Tris-buffered saline (TBS) (pH 7.4) for 45 min and subsequently washed in TBS. The membranes were incubated with ER antibodies (ERα, dilution 1:1,000, Sigma, St Louis, MO, USA; ERβ, dilution 1:4,000, Santa Cruz Biotech, CA, USA), followed by horseradish peroxidase-conjugated anti-rabbit (ERα) and mouse (ERβ) IgG secondary antibodies (dilution 1:5,000; Bio-Rad) for 60 min. In addition, the membranes were incubated with VTG antibodies (dilution 1:4,000), followed by horseradish peroxidase-conjugated anti-mouse IgG secondary antibodies (dilution 1:5,000; Bio-Rad) for 60 min. The internal control was β-tubulin (dilution 1:5,000; ab6046, Abcam, Cambridge, UK), followed by horseradish peroxidase-conjugated anti-rabbit IgG secondary antibodies (dilution 1:5,000; Bio-Rad) for 60 min. Bands were detected using Western Bright ECL (Advansta, Menlo Park, CA, USA) with a 30 s exposure, using a Molecular Imager ChemiDoc XRS+ Systems (Bio-Rad). The membrane images were scanned by a high resolution scanner and the band density estimated using a computer program (Image Lab Software, version 3.0, Bio-Rad). The ratio of internal control (β-tubulin)/ERs (ERα and ERβ) or VTG for each concentration was calculated and plotted against the concentration of the internal control.
Plasma parameter analysis
Plasma leptin (EL012870FI; Cusabio Biotech, Hubei, China), E2 (E13017Fh; Cusabio Biotech), and cortisol (E08487f: Cusabio Biotech) levels were analyzed using the enzyme-linked immunosorbent assay (ELISA) kit.
An anti-antibody specific to the antibody and antigen of the hormones (leptin, E2, and cortisol) was pre-coated onto a microplate, followed by 50 μL of plasma and 50 μL of HRP-conjugate. These were mixed well and then incubated for 1 h at 37°C. Following the last wash, any remaining Wash Buffer was aspirated or decanted off, and 50 μL of substrate solution was added to each well. The substrate solutions were then incubated for 15 min at 37°C in the dark, during which they changed from colorless or light blue to darker shades of blue. Following incubation, 50 μL of stop solution was added to each well, resulting in a color change from blue to yellow. The optical density of the solution in each well was then determined within 10 min using a microplate reader set to 450 nm.
Statistical analysis
All data were analyzed using the SPSS statistical package (version 10.0; SPSS Inc., Chicago, IL, USA). A one-way analysis of variance followed by a Tukey post hoc test was used to test for significant differences in the data (p<0.05). Values are expressed as the mean±SE.
RESULTS
Expression of leptin mRNA during salinity change
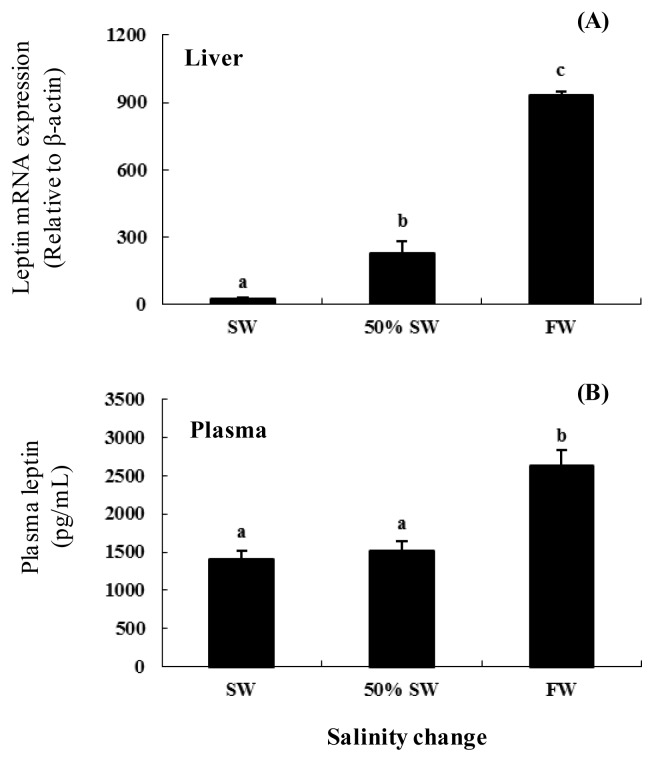
The expression of leptin mRNA in the liver was significantly higher (approximately 1.8-fold) following transfer to FW than in 50% SW and SW. The plasma leptin concentration was 1,401.9±112.1 pg/mL in the SW control group. Following the transfer to 50% SW, the plasma leptin level was maintained at 1,517.3±121.0 pg/mL. The plasma leptin level increased significantly to 2,617.7±200.2 pg/mL following transfer to FW (approximately 1.7- and 1.9-fold higher than in 50% SW and SW, respectively) (Figure 1).
Figure 1.
Expression and activity of leptin in the liver of female chum salmon after transfer from seawater (SW, 35 psu) to freshwater (FW, 0 psu). (A) Leptin mRNA levels relative to β-actin mRNA levels in the liver of female chum salmon during the salinity change, based on quantitative real-time PCR. We reverse-transcribed 3 μg of total RNA prepared from liver and amplified the sample using gene-specific primers. The results are expressed as normalized fold expression (relative to control) with respect to β-actin levels for the same sample. (B) Plasma leptin levels during salinity change in chum salmon were analyzed with a plate reader. Values are means±SE (n = 5). Letters shown with means indicate significant differences between SW, 50% SW, and FW within the same time period after salinity change (p<0.05).
Expression of ER mRNA during salinity change
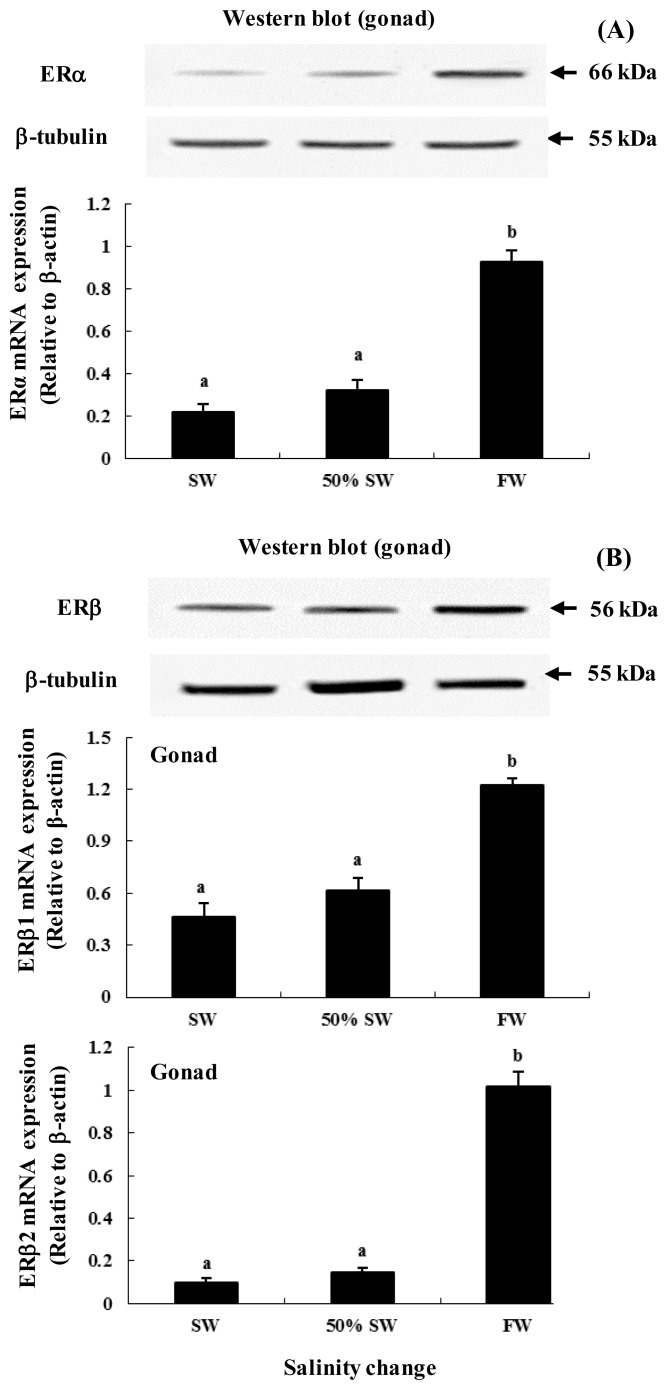
The Western blot analysis detected ER proteins whose sizes corresponded to the predicted sizes for chum salmon (approximately ERα, 66 kDa; ERβ, 56 kDa) and exhibited similar patterns of mRNA expression across the 3 salinity groups (Figure 2). In addition, ERα and ERβs mRNA expression in gonad were significantly higher following the transfer to FW (approximately 2.2-, 1.4-, and 2.57-fold higher for ERα, ERβ1, and ERβ2, respectively) than in 50% SW and SW (Figures 2A and 2B).
Figure 2.
Expression and protein levels of ERs in gonad of female chum salmon after salinity transfer from seawater (SW, 35 psu) to freshwater (FW, 0 psu). Western blot of (A) ERα (66 kDa) and ERβ (56 kDa) in gonad of chum salmon during the salinity change, with β-tubulin (55 kDa) as the internal control. Additionally, expression of (A) ERα, (B) ERβ1, and ERβ2 in gonad of female chum salmon during salinity change based on quantitative real-time PCR. Values are means±SE (n = 5). Letters shown with means indicate significant differences between SW, 50% SW, and FW within the same time period after salinity change (p<0.05).
Plasma E2 assay
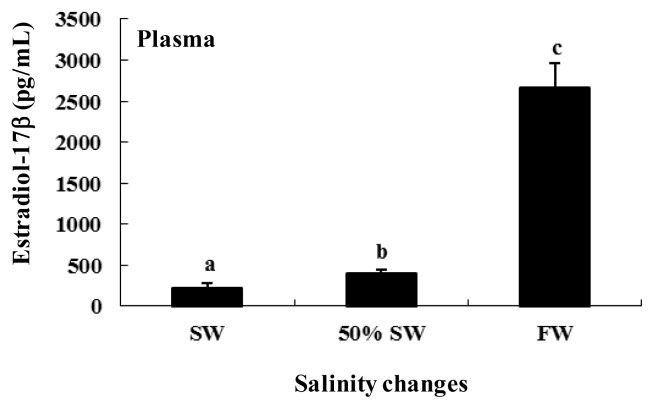
The plasma E2 levels were 221.2±48.0 pg/mL in SW and 406.6±46.0 pg/mL following the transfer to 50% SW. Following the transfer to FW, the plasma E2 levels increased significantly to 2,659.9±300.2 pg/mL (approximately 6.5- and 11.9-fold greater than the corresponding levels for 50% SW and SW, respectively) (Figure 3).
Figure 3.
Plasma estradiol-17β (E2) levels during salinity change in female chum salmon. Values are means±SE (n = 5). Letters shown with means indicate significant differences between SW, 50% SW, and FW within the same time period after salinity change (p<0.05).
Expression of VTG mRNA during salinity change
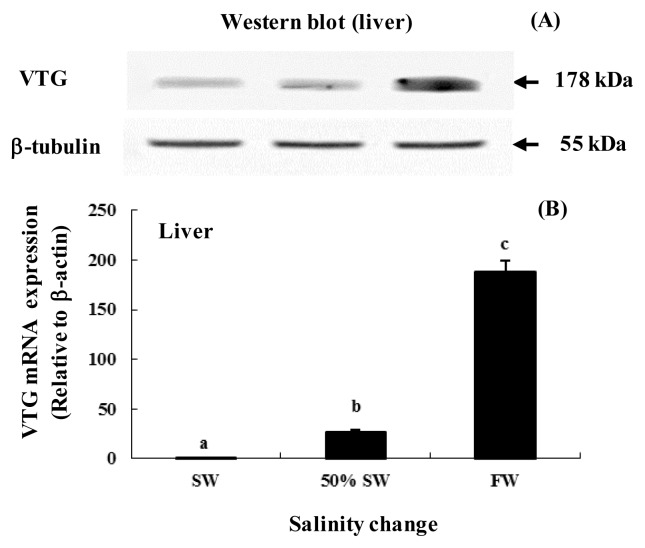
The expression of VTG mRNA and VTG protein in the liver is shown in Figure 4. The Western blot analysis detected a VTG protein of a size that corresponded to the predicted size for chum salmon (approximately 178 kDa). This protein exhibited similar mRNA expression across all 3 salinity groups (Figure 4A). In addition, VTG mRNA expression in liver was significantly higher following transfer to FW (approximately 7.3- and 152.5-fold higher, respectively) than in 50% SW and SW (Figure 4B).
Figure 4.
VTG mRNA expression levels in the liver of female chum salmon after transfer from seawater (SW, 35 psu) to freshwater (FW, 0 psu). (A) Western blot of VTG (monoclonal rabbit antiserum; dilution 1:4,000; 178 kDa) protein expression in liver of chum salmon during the salinity change, with β-tubulin (178 kDa) as the internal control. (B) Expression of VTG mRNA level in the liver of female chum salmon during salinity change based on quantitative real-time PCR. Values are means±SE (n = 5). Letters shown with means indicate significant differences between SW, 50% SW, and FW within the same time period after salinity change (p<0.05).
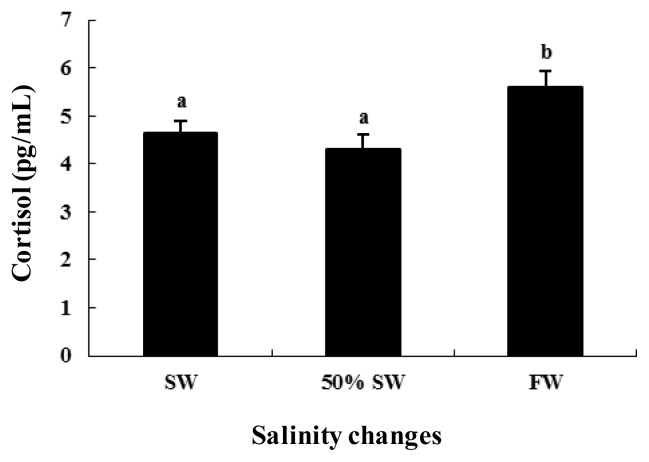
Changes in plasma cortisol levels
The plasma cortisol levels were 4.6±0.3 pg/mL in the SW control group. The plasma cortisol levels were maintained at 4.3±0.3 pg/mL following transfer to 50% SW but increased significantly to 5.6±0.3 pg/mL following transfer to FW.
DISCUSSION
This study was conducted to address the effects of the transition to the parent stream in adult female chum salmon. The study only controlled the salinity change during the transition from the coastal SW environment to the hatchery FW environment (upstream). The expression and concentration of leptin in relationship to appetite-suppressing hormones (ERs) and VTG mRNA were compared following the transfer of migrating adult female chum salmon from SW to an artificially hypo-osmotic environment (SW→50% SW→FW). In addition, changes in salmon plasma leptin and E2 levels in response to these salinity changes were examined. Our findings suggest that the process of sexual maturation is affected by salinity changes during migration in chum salmon.
The expression of mRNA in liver and the plasma concentration of leptin increased in FW as a result of the artificial change in environmental salinity (Figure 1). In a similar result found by a previous study, the leptin mRNA expression pattern of Arctic charr (Salvelinus alpinus) increased seasonally in autumn. This effect was interpreted as a result of changes in adiposity. Alternatively, however, the effect could be related to maturation, as all fish matured during the study (Frøiland et al., 2010). Additionally, in another salmonoid species, the ayu (Plecoglossus altivelis), higher levels of circulating leptin were detected during and after spawning in both males and females, and the appetite decreased during sexual maturation (Nagasaka et al., 2006). The results of the current study suggest that maturation-related hormones increased as a result of the artificial change in environmental salinity from FW to SW and that the amount of leptin also increased.
ERs and VTG genes are involved in the development and maturity of oocytes in teleosts, is E2 (Nagahama et al., 1995; Tyler and Sumpter, 1996). They are also involved in the maturation of oocytes after the synthesis of VTG through the binding of E2 and ERs in the liver (Flouriot et al., 1996; Bowman et al., 2002; Davis et al., 2009).
In this study, the transition from SW to FW was associated with increases in the expression of ERα, ERβ1, ERβ2, and VTG mRNA in the gonad and liver. It was also associated with increases in plasma E2 and VTG (Figures 2, 3, and 4).
Previous studies have shown that gonadal maturation in chum salmon is primarily regulated by elevated sex steroid hormones such as E2, testosterone (T) and 11-ketotestosterone (11-KT) during the spawning migration (Swanson et al., 2003; Onuma et al., 2009). Additionally, Makino et al. (2007) have reported that elevated levels of sex steroid hormones, such as E2 and T, are associated with FW adaptation in pre-spawning chum salmon.
Furthermore, the increase in GnRH stimulates the synthesis and secretion of PRL in the pituitary of FW-adapted masu salmon (Hirano, 1987; Onuma et al., 2009). Nagasaka et al. (2006) have reported that leptin levels, PRL, and E2 also increase during spawning season and that PRL and E2 are also involved in leptin secretion, suggesting that PRL and E2 may directly or indirectly stimulate leptin production. The production of leptin could be responsible for the anorexigenic effect. The plasma E2 levels increased in FW (Figure 3), suggesting that increased E2 levels are associated with increased concentrations of leptin. This mechanism is associated with ecological processes because the salmon do not eat when they migrate upstream.
The levels of cortisol, ERs, and VTG increased in FW (Figure 5), supporting the findings of previous studies (Rayner and Trayhum, 2001; Nagasaka et al., 2006) that showed that increased concentrations of estrogen and cortisol directly increase leptin, are involved in appetite, energy balance, and growth, and accelerate the secretion of GnRH to induce sexual maturation.
Figure 5.
Plasma cortisol levels during salinity change in female chum salmon. Values are means±SE (n = 5). Letters shown with means indicate significant differences between SW, 50% SW, and FW within the same time period after salinity change (p<0.05).
Cortisol is involved in FW adaptation in fish through interaction with PRL adaptation in conjunction with ionic regulation in both FW and SW (Laurent and Perry, 1990; Zhou et al., 2004).
Slater et al. (1995) have reported that in the sockeye salmon (O. nerka), the percentage of spawning in a FW environment was found to be higher than that in SW during the same time period. As noted above, the acceleration of final maturation and ovulation by hormonal induction combined with an SW to FW transition may shorten the period for maturation and ovulation (Slater et al., 1995). In this context, as shown in the current study, the activity of the HPG axis is an effected by the endocrine system. For example, homeostatic mechanisms maintain the salinity and water balance of migrating salmon, even if the salinity of the environment is changed artificially (hyper-osmotic environment → hypo-osmotic environment). Therefore, environment of salinity change affects the secretion of sex steroid hormones.
In brief, we found that i) leptin acts in chum salmon to influence the development of ovarian hormones and to store the energy reserves required for the synthesis of sex hormones during a change in salinity and ii) the salinity change also produces changes in ERs that affect maturation and spawning by stimulating VTG subunits and plasma E2. These findings suggest that an artificial hypo-osmotic environment induced the synthesis of leptin, which was directly involved in the feeding inhibition and homeostasis of the salmon. In turn, leptin affects the ERs, VTG, and cortisol, allowing chum salmon to achieve sexual maturation and reproduction through the activation of the HPG axis.
ACKNOWLEDGEMENTS
This work was supported by the Basic Science Research Program through the Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2011-0007095), and by was supported by Korea Maritime and Ocean University Research Fund, 2012.
REFERENCES
- Bowman CJ, Kroll KJ, Gross TG, Denslow ND. Estradiol-induced gene expression in largemouth bass (Micropterus salmoides) Mol Cell Endocrinol. 2002;196:67–77. doi: 10.1016/s0303-7207(02)00224-1. [DOI] [PubMed] [Google Scholar]
- Cella F, Giordano G, Cordera R. Serum leptin concentrations during the menstrual cycle in normal-weight women: effects of an oral triphasic estrogen-progestin medication. Eur J Endocrinol. 2000;142:174–178. doi: 10.1530/eje.0.1420174. [DOI] [PubMed] [Google Scholar]
- Cooperman MC, Hinch SG, Crossin GT, Cooke SJ, Patterson DA, Olsson I, Lotto A, Welch D, Shrimpton JM, Farrell AP, van der Kraak G. Effects of experimental manipulations of salinity and maturation status on the physiological condition and mortality of homing adult sockeye salmon held in a laboratory. Physiol Biochem Zool. 2010;83:459–472. doi: 10.1086/650473. [DOI] [PubMed] [Google Scholar]
- Copeland DL, Duff RJ, Liu Q, Prokop J, Londraville RL. Leptin in teleost fishes: an argument for comparative study. Front Physiol. 2011;2:26. doi: 10.3389/fphys.2011.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossin GT, Hinch SG, Farrell AP, Higgs DA, Lotto AG, Oakes JD, Healey MC. Energetics and morphology of sockeye salmon: effects of upriver migratory distance and elevation. J Fish Biol. 2004;65:788–810. [Google Scholar]
- Davis LK, Visitacion N, Riley L, Hiramatsu N, Sullivan C, Hirano T, Gordon Grau E. Effects of o,p’-DDE, heptachlor, and 17β-estradiol on vitellogenin gene expression and the growth hormone/insulin-like growth factor-I axis in the tilapia, Oreochromis mossambicus. Comp Biochem Physiol C. 2009;149:507–514. doi: 10.1016/j.cbpc.2008.11.007. [DOI] [PubMed] [Google Scholar]
- De Pedro N, Björnsson BT. Regulation of food intake by neuropeptides and hormones. In: Houlihan D, Boujard T, Jobling M, editors. Food intake in fish. Blackwell Science; Oxford, UK: 2001. pp. 269–296. [Google Scholar]
- Denver RJ, Bonnett RM, Boorse GC. Evolution of leptin structure and function. Neuroendocrinology. 2011;94:21–38. doi: 10.1159/000328435. [DOI] [PubMed] [Google Scholar]
- Duncan NJ, Selkirk C, Porter M, Magwood S, Bromage N. In: Norberg B, Kjesbu OS, Taranger GL, Andersson E, Stefansson SO, editors. The effect of altered photoperiods on maturation of male and female Atlantic salmon (Salmo salar), observations of different responses and mechanisms; Proceedings of the 6th International Symposium on the Reproductive Physiology of Fish; Bergen, Norway. 2000. p. 344. [Google Scholar]
- Frøiland E, Murashita K, Jørgensen EH, Kurokawa T. Leptin and ghrelin in anadromous Arctic charr: cloning and change in expressions during a seasonal feeding cycle. Gen Comp Endocrinol. 2010;165:136–143. doi: 10.1016/j.ygcen.2009.06.010. [DOI] [PubMed] [Google Scholar]
- Hendry AP, Berg OK. Secondary sexual characteristics, energy use, senescence, and the cost of reproduction in sockeye salmon. Can J Zool. 1999;77:1663–1675. [Google Scholar]
- Hirano T, Ogasawara T, Bolton JP, Collie NL, Hasegawa S, Iwata M. Osmoregulatory role of prolactin in lower vertebrates. In: Kirsch R, Lahlou B, editors. Comparative Physiology and Environmental Adaptations. Vol. 1. Karger; Basel, Swiss: 1987. pp. 112–124. [Google Scholar]
- Hirano T, Ogasawara T, Hasegawa S, Iwata M, Nagahama Y. Changes in plasma hormone levels during loss of hypoosmoregulatory capacity in mature chum salmon (Oncorhynchus keta) kept in seawater. Gen Comp Endocrinol. 1990;78:254–262. doi: 10.1016/0016-6480(90)90012-b. [DOI] [PubMed] [Google Scholar]
- Ishibashi O, Kawashima H. Cloning and characterization of the functional promoter of mouse estrogen receptor beta gene. Biochim. Biophys Acta. 2001;1519:223–229. doi: 10.1016/s0167-4781(01)00232-9. [DOI] [PubMed] [Google Scholar]
- Kikuchi N, Andoh K, Abe Y, Yamada K, Mizunuma H, Ibuki Y. Inhibitory action of leptin on early follicular growth differs in immature and adult female mice. Biol Reprod. 2001;65:66–71. doi: 10.1095/biolreprod65.1.66. [DOI] [PubMed] [Google Scholar]
- Kim NN, Shin HS, Choi YJ, Yamamoto Y, Fukaya K, Ueda H, Choi CY. Effect of hypo-osmotic environmental changes on the expression of gonadotropin-releasing hormone, its receptor, and gonadotropin hormone subunit mRNA in adult chum salmon (Oncorhynchus keta) Mar Freshw Behav Physiol. 2013;45:397–410. [Google Scholar]
- Laurent P, Perry SF. The effects of cortisol on gill chloride cell morphology and ionic uptake on the freshwater trout, Salmo gairdneri. Cell Tissue Res. 1990;259:429–442. [Google Scholar]
- Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, Fei H, Kim S, Lallone R, Ranganathan S. Leptin levels in human and rodent: measurement of plasma leptin and obRNA in obese and weight-reduced subjects. Nat Med. 1995;1:1155–1161. doi: 10.1038/nm1195-1155. [DOI] [PubMed] [Google Scholar]
- Makino K, Onuma TA, Kitahashi T, Ando H, Ban M, Urano A. Expression of hormone genes and osmoregulation in homing chum salmon: a mini review. Gen Comp Endocrinol. 2007;152:304–309. doi: 10.1016/j.ygcen.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Meucci V, Arukwe A. Transcriptional modulation of brain and hepatic estrogen receptor and P450-arom isotypes in juvenile Atlantic salmon (Salmo salar) after waterborne exposure to the xenoestrogen, 4-nonylphenol. Aquat Toxicol. 2006;77:167–177. doi: 10.1016/j.aquatox.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Nagasaka R, Okamoto N, Ushio H. Increased leptin may be involved in the short life span of ayu (Plecoglossus altivelis) J Experimen Zool Part A. 2006;305:507–512. doi: 10.1002/jez.a.279. [DOI] [PubMed] [Google Scholar]
- Nagahama Y, Yoshikuni M, Yamashita M, Tokumoto T, Katsu Y. Regulation of oocyte growth and maturation in fish. Curr Top Dev Biol. 1995;30:103–145. doi: 10.1016/s0070-2153(08)60565-7. [DOI] [PubMed] [Google Scholar]
- Onuma TA, Higa M, Ando H, Ban M, Urano A. Elevation of gene expression for salmon gonadotropin-releasing hormone in discrete brain loci of prespawning chum salmon during upstream migration. J Neurobiol. 2005;63:126–145. doi: 10.1002/neu.20125. [DOI] [PubMed] [Google Scholar]
- Onuma TA, Sato S, Katsumata H, Makino K, Hu W, Jodo A, Davis ND, Dickey JT, Ban M, Ando H, Fukuwaka M, Azumaya T, Swanson P, Urano A. Activity of the pituitary-gonadal axis is increased prior to the onset of spawning migration of chum salmon. J Exp Biol. 2009;212:56–70. doi: 10.1242/jeb.021352. [DOI] [PubMed] [Google Scholar]
- Pankhurst NW, Thomas PM. Maintenance at elevated temperature delays the steroidogenic and ovulatory responsiveness of rainbow trout Oncorhynchus mykiss to luteinizing hormone releasing hormone analogue. Aquaculture. 1998;166:163–177. [Google Scholar]
- Rayner DV, Trayhurn P. Regulation of leptin production: sympathetic nervous system interactions. J Mol Med. 2001;79:8–20. doi: 10.1007/s001090100198. [DOI] [PubMed] [Google Scholar]
- Rønnestad I, Nilsen TO, Murashita K, Angotzi AR, Gamst Moen AG, Stefansson SO, Kling P, Thrandur Björnsson B, Kurokawa T. Leptin and leptin receptor genes in Atlantic salmon: cloning, phylogeny, tissue distribution and expression correlated to long-term feeding status. Gen Comp Endocrinol. 2013;168:55–70. doi: 10.1016/j.ygcen.2010.04.010. [DOI] [PubMed] [Google Scholar]
- Ryffel GU. Synthesis of vitellogenin, an attractive model for investigating hormone-induced gene activation. Mol Cell Endocrinol. 1978;12:237–246. doi: 10.1016/0303-7207(78)90082-5. [DOI] [PubMed] [Google Scholar]
- Sabo-Attwood T, Kroll KJ, Denslow ND. Differential expression of largemouth bass (Micropterus salmoides) estrogen receptor isotypes alpha, beta, and gamma by estradiol. Mol Cell Endocrinol. 2004;218:107–118. doi: 10.1016/j.mce.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Sahu A. Leptin signalling in the hypothalamus: emphasis on energy homeostasis and leptin resistance. Front Neuroendocrinol. 2004;24:225–253. doi: 10.1016/j.yfrne.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Slater CH, Schreck CB, Amend DF. GnRHa injection accelerates final maturation and ovulation/spermiation of sockeye salmon (Oncorhynchus nerka) in both fresh and salt water. Aquaculture. 1995;130:279–285. [Google Scholar]
- Swanson P, Dickey JT, Campbell B. Biochemistry and physiology of fish gonadotropins. Fish Physiol Biochem. 2003;28:53–59. [Google Scholar]
- Trombley S, Schmitz N. Leptin in fish: possible role in sexual maturation in male Atlantic salmon. Fish Physiol Biochem. 2013;39:103–106. doi: 10.1007/s10695-012-9731-0. [DOI] [PubMed] [Google Scholar]
- Tyler CR, Sumpter JP. Oocyte growth and development in teleosts. Rev Fish Biol Fish. 1996;6:287–318. [Google Scholar]
- Ueda H. Physiological mechanism of homing migration in Pacific salmon from behavioral to molecular biological approaches. Gen Comp Endocrinol. 2011;170:222–232. doi: 10.1016/j.ygcen.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Urano A, Ando H, Ueda H. Molecular neuroendocrine basis of spawning migration in salmon. In: Kwon HB, Joss JMP, Ishi S, editors. Recent Progress in Molecular and Comparative Endocrinology. Horm. Res. Center; Kwangju, Korea: 1999. pp. 46–56. [Google Scholar]
- Volkoff H, Canosa LF, Unniappan S, Cerdá-Reverter JM, Bernier NJ, Kelly SP, Peter RE. Neuropeptides and the control of food intake in fish. Gen Comp Endocrinol. 2005;142:3–19. doi: 10.1016/j.ygcen.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Weil C, Le Bail PY, Sabin N, Le Gac F. In vitro action of leptin on FSH and LH production in rainbow trout (Onchorynchus mykiss) at different stages of the sexual cycle. Gen Comp Endocrinol. 2003;130:2–12. doi: 10.1016/s0016-6480(02)00504-x. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- Zhou B, Kelly SP, Wood CM. Response of developing cultured freshwater gill epithelia to gradual apical media dilution and hormone supplementation. J Exp Zool A Comp Exp Biol. 2004;301:867–881. doi: 10.1002/jez.a.108. [DOI] [PubMed] [Google Scholar]