Abstract
Green and black tea by-products, obtained from ready-made tea industry, were ensiled at 10°C, 20°C, and 30°C. Green tea by-product silage (GTS) and black tea by-product silage (BTS) were opened at 5, 10, 45 days after ensiling. Fermentation characteristics and nutrient composition, including tannins, were monitored and the silages on day 45 were subjected to in vitro ruminal fermentation to assess anti-nutritive effects of tannins using polyethylene glycol (PEG) as a tannin-binding agent. Results showed that the GTS and BTS silages were stable and fermented slightly when ensiled at 10°C. The GTS stored at 20°C and 30°C showed rapid pH decline and high acetic acid concentration. The BTS was fermented gradually with moderate change of pH and acid concentration. Acetic acid was the main acid product of fermentation in both GTS and BTS. The contents of total extractable phenolics and total extractable tannins in both silages were unaffected by storage temperatures, but condensed tannins in GTS were less when stored at high temperature. The GTS showed no PEG response on in vitro gas production, and revealed only a small increase by PEG on NH3-N concentration. Storage temperature of GTS did not affect the extent of PEG response to both gas production and NH3-N concentration. On the other hand, addition of PEG on BTS markedly increased both the gas production and NH3-N concentration at any ensiled temperature. It can be concluded that tannins in both GTS and BTS suppressed rumen fermentation, and tannins in GTS did more weakly than that in BTS. Ensiling temperature for both tea by-products did not affect the tannin’s activity in the rumen.
Keywords: Tea By-product, Silage Fermentation, Tannin, In vitro Ruminal Degradation
INTRODUCTION
Consumption of ready-made green and black tea in cans, packs or bottles has increased remarkably in recent years in Japan, and East Asian and Southeast Asian countries. Beverage companies that manufacture various tea drinks produce numerous tons of used tea leaves annually as by-products. Previous reports have shown that these kinds of tea by-products generally contained high crude protein (CP), fiber and tannins (e.g., Kondo et al., 2004a; Wang et al., 2011; Ramdani et al., 2013). Tea by-products also contain high moisture (about 800 g/kg); consequently, they deteriorate easily after their release as a by-product. It is therefore necessary to develop the preservation means of tea by-product as a feed resource. Accordingly, ensiling apparently is a suitable means to preserve agricultural or food industrial by-products (Nishino et al., 2003; Wang et al., 2011; Mokhtarpour et al., 2012). In the case of tea by-products, they are produced throughout the year from beverage companies, therefore, the ensiling temperature might affect the fermentation pattern and end products of tea by-product silage.
Due to high content of tannin in tea by-products, attention must be given to tannin’s activity when they are used instead of commercial feed in livestock production. Many investigations into beneficial and problematic effects of tannin on ruminant nutrition have been reported (Waghorn, 2008). Ingestion of moderate level of tannin increase the absorption of amino acids from small intestine, improve animal performance and so on (Waghorn, 2008; Pathak et al., 2013; Hymes-Fecht et al., 2013). On the other hand, animals fed tannin-rich diets showed decreased feed intake, increased fecal N excretion, reduced digestibility, and less ruminal degradability (Waghorn, 2008; Tan et al., 2011; Kozloski et al., 2012). Effects of tannin on feed degradability have been studied in several ways, e.g., in sacco and in vitro. For these methods, polyethylene glycol (PEG) has been used as a tannin binding agent in a bioassay system that shows the effects of tannin on nutrient degradability in the digestive tract, especially in the rumen (Rubanza et al., 2003; Bhatta et al., 2012).
This study examines the fermentation characteristics and changes in phenolics and tannin contents of green tea and black tea by-products ensiled at different temperatures. PEG is also used in this study to bind tannins in order to examine the effects of ensiling on in vitro ruminal fermentation responses to tannins.
MATERIALS AND METHODS
Silage preparation
Green and black tea by-products were acquired from a local ready-made tea drink factory, and moved to Nagoya University Farm in a few hours. Approximately 700 g of tea by-products were packed into polyethylene bags (255 mm×350 mm×0.08 mm) and tying with string after removing air to close the upside of each bag. Bags were stored at three different temperatures, 10°C, 20°C, and 30°C in climate chambers, and opened 5, 10 and 45 days after ensiling. Silages were made in triplicates for each temperature and ensiling period.
In vitro ruminal gas production and ammonia production
The samples of tea by-products before ensiling and their silages on day 45 were incubated in vitro with rumen fluid in calibrated glass syringes (Menke and Steingass, 1988). Each sample was freeze-dried and ground to pass through 1mm screen. Rumen fluid was obtained before morning feeding from three castrated Japanese Shiba goats fed on 720 g hay and 180 g commercial concentrates that were offered in equal proportions twice a day. Prepared buffer solution and ruminal fluid were mixed at a ratio of 2:1. The 100 mL calibrated glass syringes containing 500 mg of samples with or without 1,000 mg of PEG (MW:6,000) and 40 mL of buffered rumen fluid were incubated in a water bath at 39°C in triplicates (Getachew et al., 2000). Gas production was measured at 3, 6, 9, 12 and 24 hours, and at the end of the incubation, the fermentation fluid was collected from the syringes. After centrifugation (1,090×g), the supernatant was collected and frozen at −30°C until ammonia nitrogen (NH3-N) analysis.
Chemical and microbial analyses
Dry matter (DM) was determined by oven drying at 60°C for 48 h. Twenty g of tea by-products and their silages were macerated with 100 mL of distilled water. The macerate was filtered, and the filtrate was subjected to determination of pH, lactic acid, acetic acid, propionic acid, butyric acid, and NH3-N. The pH values and lactic acid concentrations were measured potentiometrically and colorimetrically, respectively (Barnett, 1951). Concentrations of acetic acid, propionic acid, and butyric acid were detected with a gas chromatograph using a FAL-M column (Shimadzu Co., Kyoto, Japan). NH3-N of silage was determined by steam distillation. Silages were weighed before and after ensiling, and the DM loss was calculated. Viable counts of lactic acid bacteria (LAB) associated with original tea by-products and the silages were measured using de Man, Rogosa, Sharpe (MRS) agar plate. The counts were expressed as colony forming unit (cfu) on a fresh matter (FM) base. Samples were freeze-dried and ground to pass through 1 mm screen for the following analysis. Buffering capacity of materials was measured by titration using the dried sample (Playne and McDonald, 1966). Total nitrogen (N) content was determined by the Kjeldahl method and CP content was calculated as 6.25×N. Neutral detergent fiber (NDF), acid detergent fiber (ADF) and acid detergent lignin (ADL) were analyzed as outlined by Van Soest et al. (1991). Cellulose and hemicellulose were calculated by subtracting ADL from ADF and ADF from NDF, respectively. Neutral detergent insoluble CP (NDICP) and acid detergent insoluble CP (ADICP) were determined by the method of Licitra et al. (1996). Water-soluble carbohydrate (WSC) concentrations were determined using the anthrone reaction rate assay (Koehler, 1952). Total extractable phenols (TEPH), total extractable tannins (TET), and condensed tannins (CT) of freeze-dried samples were analyzed by the methods of Makkar and Goodchild (1996). For in vitro gas production test, NH3-N in the culture was determined using the indophenol reaction (Weatherburn, 1967).
Statistical analyses
Fermentation characteristics and chemical composition of silages on day 45 were analyzed by a one-way analysis of variance (ANOVA) and further tested using tukey’s test to compare among various treatment means. Data of in vitro ruminal gas production and NH3-N concentration from materials and silages on day 45 were analyzed by ANOVA, taking into account different batch of rumen fluid for each incubation run, and tested using tukey’s test as above. Effect of PEG on gas production and NH3-N concentration of incubated medium was analyzed by Student’s t-test. All analyses were performed by SAS software version 9.3. (SAS Institute Inc., Cary, NC, USA).
RESULTS AND DISCUSSION
Chemical composition of tea by-products
Both tea by-products contained low DM, WSC, and buffering capacity (Table 1). The numbers of LAB-associated with both kinds of tea by-products were high (about 106 cfu/g FM) as compared with forage (McDonald et al., 1991). CP contents in both tea by-products were close to 300 g/kg DM that were in consistent with Wang et al. (2011). As CP fractions, slowly- and less-degradable protein in black tea by-product was assumed to be higher than in green tea by-product according to NDICP and ADICP contents, respectively (Licitra et al., 1996). Black tea by-product had higher NDF content, which may be affected by the high NDICP content. The ADF and ADL were similar between two kinds of tea by-products. Phenolic compounds were comparable in both tea by-products and these levels were moderate as compared with some tropical browse species (Osuga et al., 2005).
Table 1.
Chemical characteristics and lactic acid bacteria of green and black tea by-product before ensiling
| Attributes | Green tea by-product | Black tea by-product |
|---|---|---|
| Dry matter (DM, g/kg) | 196 ±0.9 | 183 ±1.1 |
| Water soluble carbohydrate (g/kg DM) | 10 ±0.2 | 11 ±0.4 |
| Buffering capacity (meq/kg DM) | 206 ±2.3 | 186 ±2.2 |
| Lactic acid bacteria (log10 cfu/g FM) | 6.28 ±0.08 | 6.28 ±0.12 |
| CP (g/kg DM) | 292 ±1.9 | 290 ±1.3 |
| NDICP (g/kg CP) | 165 ±6.4 | 519 ±6.0 |
| ADICP (g/kg CP) | 70 ±2.1 | 99 ±2.0 |
| NDF (g/kg DM) | 386 ±6.0 | 466 ±9.0 |
| ADF (g/kg DM) | 289 ±4.2 | 277 ±5.1 |
| ADL (g/kg DM) | 96 ±0.9 | 93 ±1.3 |
| Hemicellulose (g/kg DM) | 97 ±3.4 | 189 ±7.3 |
| Cellulose (g/kg DM) | 193 ±4.9 | 184 ±3.5 |
| TEPH (g/kg DM) | 81 ±2.1 | 94 ±0.9 |
| TET (g/kg DM) | 74 ±2.5 | 70 ±1.2 |
| CT (g/kg DM) | 21 ±0.4 | 11 ±0.4 |
cfu, colony forming unit; FM, fresh matter; CP, crude protein; NDICP, neutral detergent insoluble CP; ADICP, acid detergent insoluble CP; NDF, neutral detergent fiber; ADF, acid detergent fiber; ADL, acid detergent lignin; TEPH, total extractable phenolics; TET, total extractable tannins; CT, condensed tannins.
Fermentation characteristics of tea by-products ensiled at different temperatures
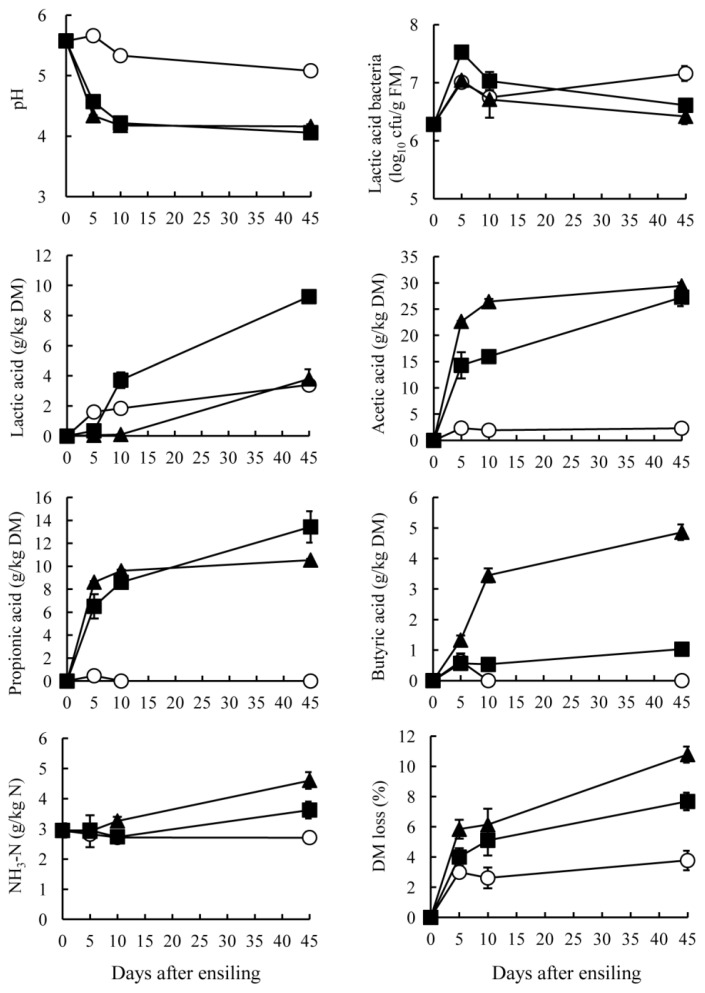
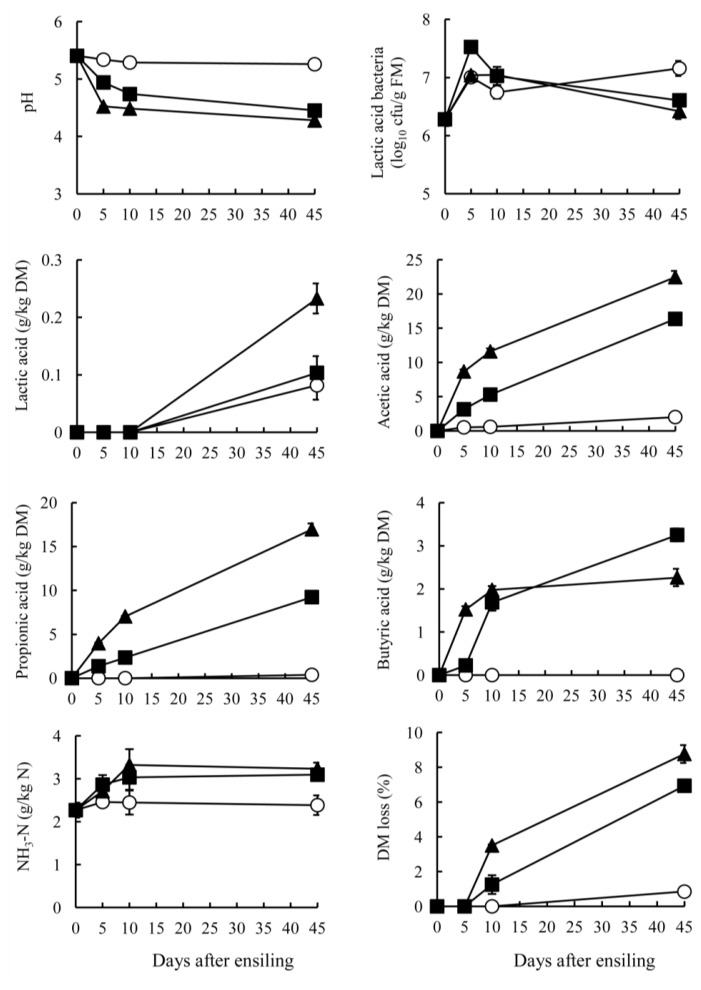
The GTS and BTS stored at 10°C showed small changes in pH, NH3-N and acid production for ensiling periods (Figures 1 and 2). At low temperatures, such as those of winter season, tea by-product silage produced only a small amount of acid with high pH, but the condition could be stabilized if the by-products were packed without air. The GTS stored at 20°C and 30°C showed a rapid decrease of pH around 5 days after ensiling. Subsequently, the pH was maintained below 4.2 during ensiling. On the other hand, the BTS stored at 30°C exhibited the lowest pH (4.28), followed by 20°C (4.48) and 10°C (5.26) (p<0.05). A rapid pH decrease was apparent in the silage stored at 30°C until day 5 and gradual decrease at 20°C through ensiling period. Specific characteristics of tea by-product silages were the organic acid contents. Both GTS and BTS stored at 20°C and 30°C contained acetic acid mainly, resulting in a pH decrease. Acetic and propionic acid in GTS were produced mainly at early stages of ensiling, but they increased gradually in BTS stored at 20°C and 30°C through ensiling periods. In contrast to acetic acid, lactic acid was low in GTS and negligible in BTS. On day 45, the lactic acid content of GTS was the highest in stored at 20°C (9.3 g/kg DM), followed by at 30°C and 10°C (<3.8 g/kg DM). Lactic acid in BTS was not detected during 10 days at any temperature; it was present in small quantities on day 45 (0.2 g/kg DM). Counts of LAB in both silages increased during 5 days of ensiling and they maintained above 106 cfu/g FM at all temperatures. However, these high LAB counts did not effect on lactic acid concentration in GTS and BTS. The pH decrease was almost parallel with the increase of acetic acid. Although acidification by acetic acid is less prevalent than that by lactic acid, GTS and BTS showed low pH, which could be due to the low buffering capacity of the materials compared with forage (200 to 600 meq/kg DM) (McDonald et al., 1991). Butyric acid was not detected in either GTS or BTS at 10°C and was low at 20°C and 30°C (<5 g/kg DM). The sum of lactic acid, acetic acid, propionic acid and butyric acid was high in GTS stored at both 20°C and 30°C (approximately 50 g/kg DM), but it was high only at 30°C in BTS. According to the rate of pH decrease and acid accumulation, green tea by-product can be more fermentable than black tea by-product by ensiling. GTS was preserved well with low pH, but effluent was also found at the bottom of bags when stored at 30°C for 45 days. Therefore, it is expected that lowering moisture contents through addition of high DM materials such as wheat bran and dried beet pulp might be more effective for preservation.
Figure 1.
Changes in pH, numbers of lactic acid bacteria, fermented products, and DM loss of green tea by-product silage ensiled at 10°C (○), 20°C (■) and 30°C (▲). Data were collected on 0, 5, 10, and 45 days after ensiling. Points indicate mean values of triplicates silos with standard errors represented by vertical bars.
Figure 2.
Changes in pH, numbers of lactic acid bacteria, fermented products, and DM loss of black tea by-product silage ensiled at 10°C (○), 20°C (■) and 30°C (▲). Data were collected on 0, 5, 10, and 45 days after ensiling. Points indicate mean values of triplicates silos with standard errors represented by vertical bars.
The NH3-N content was largely unchanged during ensiling and was low (<5 g/kg N) indicating that proteolysis in tea by-product occurred only slightly during ensiling. In general, low pH suppresses the growth of proteolytic bacteria, such as clostridia, thereby engendering low NH3-N contents. In tea by-product silage, it was also presumed that phenolic compounds in tea by-products suppressed clostridial fermentation, which is supported by Ishihara et al. (2001) who reported that tea polyphenols numerically decreased clostridia in bovine intestines. Low NH3-N contents in GTS and BTS might also be attributable to heat-damaged protein when processing tea leaves after harvesting (Graham, 1992). Another mechanism of the low proteolysis might be occurred by the presence of tannin-protein complexes in GTS and BTS. It had been reported that tannins are interacted with plant leaf protein and make insoluble complexes under pH 3.5 to 5.5 (Perez-Maldonado et al., 1995). The pH of GTS and BTS on 45 days after ensiling ranged from 4.1 to 5.3 in the present study that indicated some parts of proteins in GTS and BTS made complexes with tannins. It can also be estimated that these complexes cannot be degraded during ensiling since Albrecht and Muck (1991) reported that a negative correlation between tannin contents and non-protein N concentrations in legume silage. Salawu et al. (1999) also demonstrated that the contents of soluble proteins in grass silage decreased through addition of tannin powder. According to these reports, GTS and BTS could be expected to have several factors including tannins and form of proteins that prevent from proteolysis during ensiling. The DM loss of GTS stored at 30°C was highest, followed by 20°C and 10°C (p<0.05). The loss of GTS occurred mainly during 5 days after ensiling; after 10 days, a greater DM loss was apparent at 20°C and 30°C, but stable at 10°C. On the other hand, the DM loss of BTS occurred mainly 10 days after ensiling. These results also supported the difference of fermentation status between GTS and BTS, as discussed above.
Chemical composition of tea by-products ensiled at different temperatures
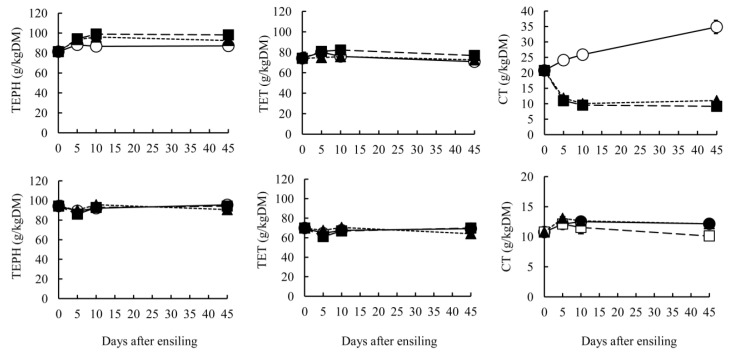
Proteins, fibers and phenolic compounds of GTS and BTS are presented in Table 2 and Figure 3. Protein fractions and fiber content were significantly affected by ensiling temperature (p<0.05). Ensiling temperatures did not affect TEPH and TET contents on day 45 in either GTS or BTS, but temperature did affect the CT contents. In GTS, CT content at 20°C and 30°C was significantly lower than at 10°C (p<0.05). The CT contents in GTS stored at 20°C and 30°C decreased at early stages of ensiling, but increased at 10°C (Figure 3). Our previous reports also showed that CT contents in mixed silage including green tea by-product stored at 30°C was lower than at 15°C (Kondo et al., 2006). These results demonstrate that CT in green tea by-product might be degraded in higher temperatures. Although statistical differences were apparent for CT contents in BTS, the contents were not changed greatly by ensiling temperature. Ott et al. (2005) reported that TEPH, TET, and CT in tannin-containing sorghum grain decreased during ensiling. Osawa et al. (2000) identified lactobacilli producing tannin-degrading enzyme from fermented foods. Therefore, it would be possible to cleave the molecular bond in phenolics during ensiling. On the other hand, some reports have shown the increase of TEPH content during fermentation process, as shown in pearl millet and oat (Khetarpaul and Chauhan, 1991; Kondo et al., 2004b). Nishino et al. (2007) reported that the changes of major polyphneol molecules in green tea leaf during ensiling and showed that TEPH content slightly increased, while the content of (−)-epicatechin gallate (ECG) and (−)-epigallocatechin gallate (EGCG) decreased, but that of (−)-epigallocatechin did not change. Their report implied that gallic acid of B-ring in ECG and EGCG can be liberated. The Folin-Ciocalteu methods to determine TEPH and TET contents are based on the reducing power of hydroxyl function of phenolics to molybdic acid. Therefore, the reaction to Folin solutions would not correspond directly to the amounts of phenolic compounds. It is possible that the reducing power was increased by cleavage of small amount of high molecular phenolic compounds to large amounts of low molecular phenolics. These differences among the studies of tannin contents during ensiling are attributable to various structures of phenolic compounds in plants.
Table 2.
Chemical composition of green and black tea by-product silage on 45 days after ensiling at different temperatures
| Attributes | 10°C | 20°C | 30°C | SEM |
|---|---|---|---|---|
| Green tea by-product silage | ||||
| CP (g/kg DM) | 307 | 316 | 292 | 5.6 |
| NDICP (g/kg CP) | 121b | 115b | 135a | 2.8 |
| ADICP (g/kg CP) | 57b | 66a | 68a | 1.8 |
| NDF (g/kg DM) | 358 | 351 | 368 | 6.7 |
| ADF (g/kg DM) | 277 | 296 | 303 | 6.9 |
| ADL (g/kg DM) | 91b | 101a | 104a | 1.8 |
| Hemicellulose (g/kg DM) | 81a | 55c | 65b | 2.2 |
| Cellulose (g/kg DM) | 187 | 196 | 198 | 5.1 |
| TEPH (g/kg DM) | 87 | 98 | 92 | 4.3 |
| TET (g/kg DM) | 71 | 77 | 73 | 4.0 |
| CT (g/kg DM) | 35a | 9b | 11b | 1.3 |
| Black tea by-product silage | ||||
| CP (g/kg DM) | 287c | 306b | 313a | 1.3 |
| NDICP (g/kg CP) | 539 | 529 | 554 | 6.7 |
| ADICP (g/kg CP) | 102a | 87b | 78c | 2.0 |
| NDF (g/kg DM) | 479b | 493b | 520a | 7.3 |
| ADF (g/kg DM) | 246 | 250 | 255 | 4.9 |
| ADL (g/kg DM) | 83 | 86 | 84 | 5.0 |
| Hemicellulose (g/kg DM) | 234 | 243 | 265 | 8.2 |
| Cellulose (g/kg DM) | 163 | 163 | 171 | 3.8 |
| TEPH (g/kg DM) | 95 | 94 | 91 | 2.2 |
| TET (g/kg DM) | 69 | 70 | 64 | 2.2 |
| CT (g/kg DM) | 12a | 10b | 12a | 0.4 |
SEM, standard error of the mean; CP, crude protein; NDICP, neutral detergent insoluble CP; ADICP, acid detergent insoluble CP; NDF, neutral detergent fiber; ADF, acid detergent fiber; ADL, acid detergent lignin; TEPH, total extractable phenolics; TET, total extractable tannins; CT, condensed tannins.
Means with the different letter (a–c) in a row are significantly different (p<0.05).
Figure 3.
Changes in total extractable phenolics (TEPH), total extractable tannins (TET) and condensed tannins (CT) of green (above) and black (below) tea by-product silage stored at 10°C (○), 20°C (■) and 30°C (▲). Data were collected on 0, 5, 10, and 45 days after ensiling. Points indicate mean values of triplicates silos with standard errors represented by vertical bars.
In vitro ruminal fermentation
Both gas production and ruminal NH3-N concentration were higher in GTS than those in BTS, indicating the higher nutritive potential of GTS as compared to BTS. Higher ensiling temperatures showed significantly lower gas production (p<0.05, Table 3), which would result from the fact that the loss of fermentable carbohydrates during ensiling was higher at 20°C and 30°C than at 10°C. In vitro gas production test with PEG on tannin-rich forage has been a valuable tool to assess effects of tannin on feed degradation in the rumen (Makkar et al., 1995; Getachew et al., 2000). Many researches showed that PEG addition increased in vitro gas production from most of tannin rich feeds (Getachew et al., 2000; Rubanza et al., 2003; Osuga et al., 2007). In the present study, despite the presence of tannins in GTS, gas production was not altered by PEG addition, accorded with some browse reported in Singh et al. (2005) and Osuga et al. (2007). Although it can be expected to have an increase in substrate degradability if tannins activity is reduced by PEG addition, a simultaneous large increase in gas production could simply a result in lower partitioning of nutrients to microbial protein synthesis (Singh et al., 2005). Gas production is a result of feed fermentation in rumen. Proportion of fermentation products depends on feed, especially CP and carbohydrates. But most of CP is degraded to amino acids and NH3, and give less gas. GTS could contain more degradable protein than BTS as expected from lower NDICP content in GTS. Small response of gas production to PEG addition from GTS implies that higher partitioning of fermentable nutrients in GTS to microbial protein synthesis. It can be also inferred that less contribution to gas production from fermentable carbohydrate in GTS due to supplemental tannin binding agents. On the contrary, the amount of gas produced from BTS was increased by PEG addition. The ratio of increase in BTS was unaffected by the ensiling temperature, which means that the ensiling temperature did not influence the extent of suppressive effect of BTS tannin on the ruminal gas production. The NH3-N concentration was increased by PEG addition from both GTS and BTS. These results were similar to those reported using tannin-containing tropical browse (Getachew et al., 2000). Tannins depress ruminal degradation of proteins due to strongly binding with proteins in feed and/or microbial enzymes in rumen (Makkar et al., 1995). Among the tannin-protein complex, several interaction types such as hydrogen bonding, hydrophobic bonding can take place in principle (Oh et al., 1980). The PEG contains more effective cites (oxygen of ether linkage) which bind with hydroxyl group of tannins (Jones, 1965). The PEG as a tannin-binding agent has a high affinity to tannins and deactivate tannin’s binding activity to proteins, and can be expected that PEG makes tannin-bound protein available to microbial degradation (Makkar et al., 1995). The response of improved NH3-N concentration resulting from PEG treatment mainly reflects deactivation of tannin. In the present study, despite similarity in the amounts of tannins in GTS and BTS, relatively lower increase ratio in NH3-N by PEG existed in GTS (38% to 44%) than in BTS (52% to 82%). This indicated that tannins in GTS had a lower anti-nutritional activity than that in BTS when ensiled any temperature, and protein in GTS was relatively degradable than that in BTS.
Table 3.
Effect of polyethylene glycol (PEG) treatment on in vitro gas production and ammonia nitrogen (NH3-N) from green and black tea by-product silage at 24 h after incubation
| Attributes | Day 0 | Day 45 | SEM | ||
|---|---|---|---|---|---|
|
| |||||
| 10°C | 20°C | 30°C | |||
| Gas production (mL/500 mg DM) | |||||
| Green tea by-product silage | |||||
| No PEG | 83.9 | 76.4a | 68.2b | 63.8b | 1.9 |
| With PEG | 85.3 | 77.3a | 69.7b | 64.0c | 1.2 |
| Increase (%) | 1.7 | 1.1 | 2.2 | 0.4 | 1.8 |
| Black tea by-product silage | |||||
| No PEG | 58.4 | 54.8a | 47.7b | 43.9c | 0.6 |
| With PEG | 65.2 | 61.2a,* | 52.8b,* | 48.5c,* | 0.3 |
| Increase (%) | 11.7 | 11.6 | 10.7 | 10.6 | 1.9 |
| NH3-N (mg/40 mL/500 mg DM) | |||||
| Green tea by-product silage | |||||
| No PEG | 6.3 | 7.0b | 8.2a | 8.1a | 0.4 |
| With PEG | 9.0 | 10.0b,* | 11.7a,* | 11.1ab,* | 0.9 |
| Increase (%) | 43.3 | 42.2 | 43.6 | 38.0 | 3.5 |
| Black tea by-product silage | |||||
| No PEG | 5.3 | 4.6b | 5.5a | 5.7a | 0.4 |
| With PEG | 8.3 | 8.3b,* | 9.2a,* | 8.6ab,* | 0.2 |
| Increase (%) | 56.7 | 82.2a | 65.8b | 51.8c | 3.4 |
SEM, standard error of the mean; DM, dry matter.
Means with the different letter (a–c) in a row are significantly different (p<0.05).
Significantly different between No PEG (p<0.05).
CONCLUSION
Ensiling did not affect TEPH and TET contents in either GTS or BTS. The CT contents in GTS could be partly lowered during ensiling at high temperature, but the storage temperature did not affect the suppressive activity of tannins in tea by-products based on in vitro ruminal fermentation test. Lower responses to PEG on gas production and NH3-N concentration from GTS than BTS at any ensiled temperature indicated that tannins in GTS could suppress rumen fermentation more weakly than that in BTS. Further studies are recommended for assessment of the nutritional value of GTS and BTS as protein supplements, compared with commercial protein-rich feedstuffs by in vitro and in vivo digestibility trials.
ACKNOWLEDGMENTS
The authors are grateful to POKKA Corporation (Aichi, Japan) for providing the tea by-products. We wish to thank the members of the Laboratory of Animal Production Sciences in Nagoya University for making GTS and BTS. M. Kondo is indebted to Mrs K. Goto for her helping with chemical analyses.
REFERENCES
- Albrecht KA, Muck RE. Proteolysis in ensiled forage legumes that vary in tannin concentration. Crop Sci. 1991;31:464–469. [Google Scholar]
- Barnett AJG. The colorimetric determination of lactic acid in silage. Biochem J. 1951;49:527–529. doi: 10.1042/bj0490527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatta R, Saravanan M, Luna B, Sampath KT. Phenolic composition, fermentation profile, protozoa population and methane production from sheanut (Butryospermum Parkii) byproducts in vitro. Asian Australas J Anim Sci. 2012;25:1389–1394. doi: 10.5713/ajas.2012.12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getachew G, Makkar HPS, Becker K. Effect of polyethylene glycol on in vitro degradability of nitrogen and microbial protein synthesis from tannin-rich browse and herbaceous legumes. Br J Nutr. 2000;84:73–83. [PubMed] [Google Scholar]
- Graham H. Green tea composition, consumption, and polyphenol chemistry. Prev Med. 1992;21:334–350. doi: 10.1016/0091-7435(92)90041-f. [DOI] [PubMed] [Google Scholar]
- Hymes-Fecht UC, Broderick GA, Muck RE, Grabber JH. Replacing alfalfa or red clover silage with birdsfoot trefoil silage in total mixed rations increases production of lactating dairy cows. J Dairy Sci. 2013;96:460–469. doi: 10.3168/jds.2012-5724. [DOI] [PubMed] [Google Scholar]
- Ishihara N, Chu DC, Akashi S, Juneja LR. Improvement of intestinal microflora balance and prevention of digestive and respiratory organ diseases in calves by green tea extracts. Livest Prod Sci. 2001;68:217–229. [Google Scholar]
- Jones DE. Banana tannin and its reaction with polyethylene glycols. Nature. 1965;206:299–300. doi: 10.1038/206299a0. [DOI] [PubMed] [Google Scholar]
- Khetarpaul N, Chauhan BM. Effect of natural fermentaion on phytate and polyphenol content and in vitro digestibility of starch and protein of pearl millet (Pennisetum typhoideum) J Sci Food Agric. 1991;55:189–195. [Google Scholar]
- Koehler LH. Differentiation of carbohydrates by anthrone reaction rate and color intensity. Anal Chem. 1952;24:1576–1579. [Google Scholar]
- Kondo M, Kita K, Yokota H. Effects of tea leaf waste of green tea, oolong tea, and black tea addition on sudangrass silage quality and in vitro gas production. J Sci Food Agric. 2004a;84:721–727. [Google Scholar]
- Kondo M, Kita K, Yokota H. Feeding value to goats of whole-crop oat ensiled with green tea waste. Anim Feed Sci Technol. 2004b;113:71–81. [Google Scholar]
- Kondo M, Kita K, Yokota H. Evaluation of fermentation characteristics and nutritive value of green tea waste ensiled with byproducts mixture for ruminants. Asian Australas J Anim Sci. 2006;19:533–540. [Google Scholar]
- Kozloski GV, Harter CJ, Hentz F, de Avilar SC, Orlandi T, Stefanello CM. Intake, digestibility and nutrients supply to wethers fed ryegrass and intraruminally infused with levels of Acacia mearnsii tannin extract. Small Rumin Res. 2012;106:125–130. [Google Scholar]
- Licitra G, Hernandez TM, Van Soest PJ. Standarization of procedures for nitrogen fractionation of ruminant feed. Anim Feed Sci Technol. 1996;57:347–358. [Google Scholar]
- Makkar HPS, Blummel M, Becker K. Formation of complexes between polyvinyl pyrrolidones or polyethylene glycols and tannins, and their implication in gas production and true digestibility in in vitro techniques. Br J Nutr. 1995;73:897–913. doi: 10.1079/bjn19950095. [DOI] [PubMed] [Google Scholar]
- Makkar HPS, Goodchild AV. Quantification of Tannins: A Laboratory Manual. ICARDA; Aleppo, Syria: 1996. [Google Scholar]
- McDonald P, Henderson AR, Heron SJ. The Biochemistry of Silage. 2nd Edn. Chalcombe Publications; Aberystwyth, UK: 1991. pp. 31–32. [Google Scholar]
- Menke KH, Stengass H. Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim Res Develop. 1988;28:7–55. [Google Scholar]
- Mokhtarpour A, Naserian A, Tahmasbi AM, Valizadeh R. Effect of feeding pistachio by-products silage supplemented with polyethylene glycol and urea on Holstein dairy cows performance in early lactation. Livest Sci. 2012;148:208–213. [Google Scholar]
- Nishino N, Harada H, Sakaguchi E. Evaluation of fermentation and aerobic stability of wet brewers’s grains ensiled alone or in combination with various feeds as a total mixed ration. J Sci Food Agric. 2003;83:557–563. [Google Scholar]
- Nishino N, Kawai T, Kondo M. Changes during ensilage in fermentation products, tea catechins, antioxidative activity and in vitro gas production of green tea waste stored with or without dried beet pulp. J Sci Food Agric. 2007;87:1639–1644. [Google Scholar]
- Oh HI, Hoff JE, Armstrong GS, Haff LA. Hydrophobic interaction in tannin-protein complexes. J Agric Food Chem. 1980;28:394–398. [Google Scholar]
- Osawa R, Kuroiso K, Goto S, Shimizu A. Isolation of tannin-degrading lactobacilli from humans and fermented foods. Appl Environ Microbiol. 2000;66:3093–3097. doi: 10.1128/aem.66.7.3093-3097.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osuga IM, Abdulrazak1 SA, Ichinohe T, Fujihara T. Chemical composition, degradation characteristics and effect of tannin on digestibility of some browse species from Kenya harvested during the wet season. Asian Australas J Anim Sci. 2005;18:54–60. [Google Scholar]
- Osuga IM, Maindi CN, Abdulrazak SA, Nishino N, Ichinohe T, Fujihara T. Potential nutritive value and tannin bioassay of selected Acacia species from Kenya. J Sci Food Agric. 2007;87:1533–1538. [Google Scholar]
- Ott EM, Aragon M, Gabel M. Ensiling of tannin-containing sorghum grain. Silage production and utilisation; Prceedings of the XIVth International Silage Conference; 2005. p. 178. [Google Scholar]
- Pathak AK, Dutta N, Banerjee PS, Patthanaik AK, Sharma K. Influence of dietary supplementation of condensed tannins through leaf meal mixture on intake, nutrient utilization and performance of Haemonchus contortus infected sheep. Asian Australas J Anim Sci. 2013;26:1446–1458. doi: 10.5713/ajas.2013.13066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Playne MJ, McDonald P. The buffering constituents of herbage and of silage. J Sci Food Agric. 1966;17:264–268. [Google Scholar]
- Perez-Maldonado RA, Norton BW, Kerven GL. Factors affecting in vitro formation of tannin-protein complexes. J Sci Food Agric. 1995;69:291–298. [Google Scholar]
- Ramdani D, Chaudhry AS, Seal CJ. Chemical composition, plant secondary metabolites, and minerals of green and black teas and the effect of different tea-to-water ratios during their extraction on the composition of their spent leaves as potential additives for ruminants. J Agric Food Chem. 2013;61:4961–4967. doi: 10.1021/jf4002439. [DOI] [PubMed] [Google Scholar]
- Rubanza CDK, Shem MN, Otsyina R, Nishino N, Ichinohe T, Fujihara T. Content of phenolics and tannins in leaves and pods of some Acacia and Dichrostachys species and effects on in vitro digestibility. J Anim Feed Sci. 2003;12:645–663. [Google Scholar]
- Salawu MB, Acamovic T, Stewart CS, Hvelplund T, Weisbjerg MR. The use of tannins as silage additives: Effects on silage composition and mobile bag disappearance of dry matter and protein. Anim Feed Sci Technol. 1999;82:243–259. [Google Scholar]
- Singh B, Sahoo A, Sharma R, Bhat TK. Effect of polethylene glycol on gas production parameters and nitrogen disappearance of some tree forages. Anim Feed Sci Technol. 2005;123–124:351–364. [Google Scholar]
- Tan HY, Sieo CC, Abdullah N, Liang JB, Huang XD, Ho YW. Effects of condensed tannins from Leucaena on methane production, rumen fermentation and populations of methanogens and protozoa in vitro. Anim Feed Sci Technol. 2011;169:185–193. [Google Scholar]
- Van Soest PJ, Robertson JD, Lewis BA. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci. 1991;74:3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2. [DOI] [PubMed] [Google Scholar]
- Waghorn GC. Beneficial and detrimental effects of dietary condensed tannins for sustainable sheep and goat production—Progress and challenges. Anim Feed Sci Technol. 2008;147:116–139. [Google Scholar]
- Wang RR, Wang HL, Liu X, Xu CC. Effects of different additives on fermentation characteristics and protein degradation of green tea grounds silage. Asian Australas J Anim Sci. 2011;24:616–622. [Google Scholar]
- Weatherburn MW. Phenol-hypochlorite reaction for determination of ammonia. Anal Chem. 1967;39:971–974. [Google Scholar]