Abstract
OBJECTIVE: To compare 3 methods of weight determination for medication dose calculations in obese children and to discuss feasibility for use in routine care.
METHODS: This was a patient safety and quality improvement study evaluating patients (2–19 years old) admitted to the pediatric intensive care unit during a 13-month period (July 2010–July 2011). Patients identified as obese (≥95th percentile body mass index [BMI] for age), including severely obese (≥99th percentile BMI for age), were included in the weight method comparison portion of this study. Lean body mass estimations, using equations derived by the Peters and Foster methods, were compared to ideal body weight estimates by using the BMI method. Absolute differences between values generated by the 3 methods, intraclass correlation (ICC), and Bland-Altman plots were calculated.
RESULTS: A total of 1369 patients met initial criteria; 176 met criteria for the dosing weight comparison (age ± SD = 9.28 ± 5 years; actual weight ± SD = 55.5 ± 33.9 kg; 46% female). Sixty were severely obese and 116 were obese. Mean ICC between methods was 0.968 (95% Confidence interval (CI): 0.959, 0.975). The Peters method estimated higher weights than the Foster or BMI method. Bland-Altman plots illustrated good agreement between methods in children with weight below 50 kg, but decreased agreement above 50 kg, which was influenced by sex.
CONCLUSIONS: All methods demonstrated strong correlation and acceptable agreement in children below 50 kg. Systematic biases were identified in children above 50 kg where variance was higher. The BMI method was least complex to calculate and the most feasible method for daily use.
INDEX TERMS: body mass index, lean body mass, ideal body weight, obesity, pediatric
INTRODUCTION
Childhood obesity has become a significant public health problem in the United States. Despite being the leanest state for adults, Colorado is ranked 23rd in the nation for childhood obesity. Between 2003 and 2007, data from the National Survey of Children's Health1 showed the rate of childhood obesity in Colorado grew 23%, the second fastest in the nation. In response to this alarming increase in obesity, Children's Hospital Colorado established an Obesity Safety Task Force with a subcommittee focused on medication dosing.
In pediatric patients, the dose of a medication is routinely individualized by using a measure of body size (kilogram or body surface area) and may be further modified for obese patients. There is currently, however, a lack of consensus among practitioners regarding the best method and definition of a modified dosing weight in obese children. Nevertheless, most researchers agree that obese children have an excess in whole body fat mass, lean mass, and bone mineral content, with the increase in fat mass substantially higher than the increase in lean mass.2–4
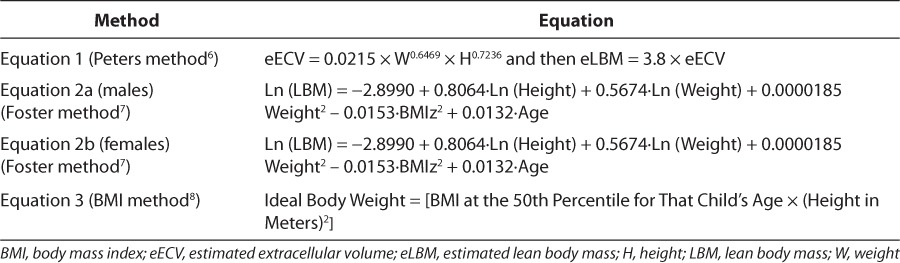
Two methods for calculating lean body mass (LBM), the Foster method and Peters method, have been described in the literature (equations described in Table 1). These methods involve complex mathematical equations to calculate LBM. Given the lack of consensus for dosing weight calculations in obese children, and the need for a method that would be simple and feasible, we questioned if a simple calculation of weight, based on the 50th percentile BMI for age (subsequently referred to as the “BMI method”), would yield similar results. While the weight generated from the BMI method is generally used to identify a child's nutritional risk in relation to height and sex-matched peers, we hypothesize that it could also be used as a scalar for medication dosing.5 Therefore, the specific aim of the study was to compare the BMI method, the Foster method, and Peters method to determine medication dosing in obese critically ill children.5–7
Table 1.
Equations
METHODS
This was a retrospective medical chart review of obese and severely obese children (2–19 years of age) who were admitted to our pediatric intensive care unit (PICU) during a 13-month period (June 2010–July 2011). Obesity and severe obesity were defined as a BMI above the 95th and 99th percentiles, respectively, using growth charts specific for age and sex.8 The PICU is a 26-bed unit, in a freestanding children's hospital, composed of critically ill medical, surgical, and trauma patients. Cardiac patients are admitted to a separate 16-patient-bed unit and were not evaluated in this study. Charts for review were identified with the assistance of a clinical information resource specialist using data extracted from Epic Hyperspace (Epic Rx) (Epic Systems Cooperation, Verona, WI). Patients were excluded from review if they were not obese or severely obese, did not meet the age requirement, or failed to have anthropometric data entered into the electronic medical record within 48 hours of admission. This protocol was reviewed and approved by the Organizational Research Risk and Quality Improvement Panel at our institution and informed parent/patient consent was waived.
Extracted data included age, weight at admission, height at admission, and sex. LBM was predicted by using equations (Table 1) derived by the Peters method (equation 1) and Foster method (equations 2a and 2b).6,7 The Peters method uses a calculation based on estimated extracellular volume (eECV, estimated from weight (kg) and height (cm) of the child, that is extrapolated to estimate LBM in children by using a proportionality constant of 3.8.6 The Foster method is based on linear regression of dual energy x-ray absorptiometry (DXA) and sex-specific equations to estimate LBM from height, weight, age, and BMI-forage z-score, which was calculated for each patient by using QuesGen Systems, Inc, calculator (Burlingame, CA). Equations were validated against the findings from the DXA and using independent samples of children in the National Health and Nutrition Examination Survey (NHANES) database.7 We then compared LBM values from these 2 methods to the ideal body weight (IBW) predicted by using the BMI method (equation 3), an equation often used to determine a patient's nutritional risk.5
Descriptive analyses were first performed on each variable in the data set. Results are presented as mean ± standard deviation and range, or percentage where appropriate. Medians are reported if significant skewness was observed. Intraclass correlation (ICC) analyses were performed on the continuous data to assess the absolute differences between the values generated by the 3 methods. Bland-Altman plots were generated to illustrate agreement and any systematic bias in the values produced by the methods.
RESULTS
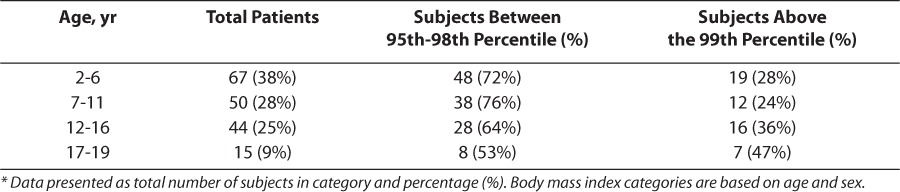
During the 13-month study period, a total of 1369 patients, 2 to 19 years of age, were admitted to the PICU. One hundred seventy-six patients met criteria for the comparison of dosing weight methods. Patient demographics are reported in Table 2. One hundred sixteen patients were defined as obese and 60 were defined as severely obese. This corresponds to an overall obesity rate among admissions with weight and height measures of approximately 13% and a severe obesity rate of 4.4%. When stratified by age, those children between 2 to 6 years of age had the highest overall rate of obesity and children aged 17 to 19 years had the highest overall rate of severe obesity, as described in Table 3.
Table 2.
Characteristics of Study Population (n=176 Patients)
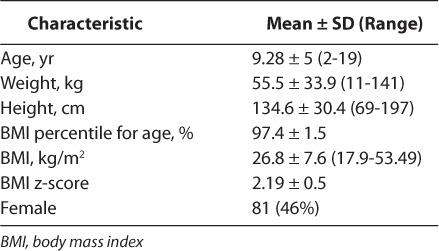
Table 3.
Detail of Study Subjects by Age Category and Obesity Status (Total N=176) *
The mean ICC between the 3 methods was high at 0.968 (95% CI: 0.959, 0.975) (Figure 1). The Peters method tended to estimate higher weights than the Foster or BMI method, a trend that is shown in the comparison of the Foster and Peters methods to IBW in Figure 1A and B. All methods demonstrated stronger correlation at weights below 50 kg and exhibited flare at the highest weights. Additionally, the Foster and Peters methods demonstrated significantly differing ICC between the male subjects and female subjects above the 50-kg weight mark, shown in Figure 1C.
Figure 1.
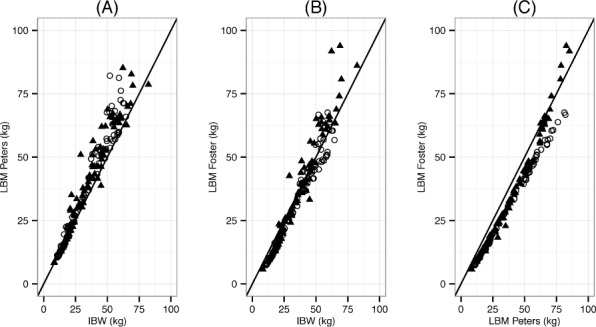
Intraclass correlation between 3 methods of calculating a dosing weight in obese and severely obese critically ill children (age: 2–19 years, n=176).*
(A) LBM as predicted by the Peters method is plotted against IBW predicted by the 50th percentile BMI method. (B) LBM as predicted by the Foster method is plotted against IBW predicted by the 50th percentile BMI method. (C) LBM as predicted by the Foster and Peters methods.
BMI, body mass index; IBW, ideal body weight; ICC, intraclass correlation; LBM, lean body mass; ○, females; ▴, males
* Mean ICC = 0.968. The high ICC value suggests the 3 methods perform well at assigning comparable weight to the patients. Highest agreement is illustrated when LBM or IBW of a child is below 50 kg. Once the predicted LBM or IBW exceeds 50 kg, overall agreement between methods is less reliable.
Bland-Altman plots illustrated good agreement between methods in children weighing less than 50 kg. However, method agreement begins to diverge above an ideal body weight of 50 kg and is influenced by sex (Figure 2). The Peters method systematically estimated LBM 4.3 kg higher than the BMI or Foster method (Figure 2 panel A and C, respectively). Agreement appears highest between the Foster and BMI methods with a mean difference in the 2 methods of −1.17 kg, with BMI method estimating lower weights (Figure 2 panel B). Variance was higher among all 3 methods in children with an IBW above 50 kg.
Figure 2.
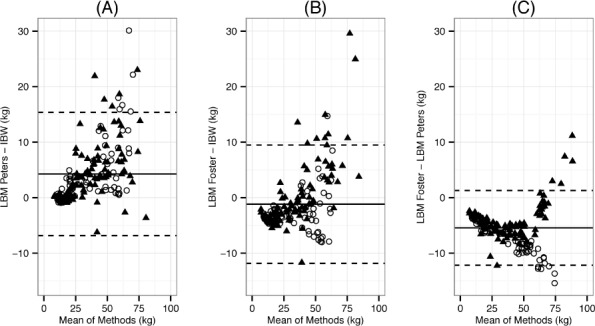
Bland-Altman plots depicting agreement between LBM and IBW in children 2 to 19 years of age (n=176).*
(A) The difference between LBM using the Peters method and IBW using the 50th percentile BMI method. (B) The difference between LBM using the Foster method and IBW using the 50th percentile BMI method. Bias was lowest in B. (C) The difference between predicted LBM using the Peters method and Foster method to predict LBM.
BMI, body mass index; IBW, ideal body weight; LBM, lean body mass; ○, females; ▴, males
* Solid line represents mean and dashed lines denote 1.96 standard deviations.
Differences in weight calculation based on sex are evident when examining the Bland-Altman plots between the Foster and Peters methods. At ideal weights below 50 kg, there is a systematic bias in the results between the 2 methods and the behavior is similar for both males and females. Above 50 kg, however, there is a clear change in the pattern between males and females, with the Foster method yielding higher LBM in males and lower LBM in females than the Peters method. This trend is evident, although less pronounced, when the Foster method is compared to the BMI method. Sex agreement biases were not identified when comparing the Peters method and the BMI method.
DISCUSSION
There is a distinct lack of consensus among practitioners regarding the best method and definition of a modified dosing weight in obese children. The results of this study support the use of a simple equation to calculate a dosing weight for obese children in a clinical setting. We report correlation and agreement results between 3 methods used to determine a modified body weight in critically ill obese and severely obese children between 2 and 19 years of age. The Foster and Peters methods use mathematical equations to calculate a weight derived from age, sex, height, proportionality constant, and/or z-score attained from a secondary source. The BMI method uses age, sex, and BMI. The Foster and Peters methods are complex and thus may be less feasible and more difficult to apply than the BMI method. The BMI method had strong agreement with both mathematical equations (strongest with Foster), but at patient weights above 50 kg, the agreement becomes weaker. Nevertheless, the BMI method was simple, quick, and could easily be incorporated into the daily workflow of clinical pharmacists involved with medication dosing.
This evaluation was triggered by the rapid rise in obesity rates at our institution and across the country in combination with the known reliance of medication dosing on body size in children. It is well recognized that variables such as age, weight, body composition, and disease state can affect the clinical effect of a drug and thus the design of a medication dosing regimen. In fact, published dosing recommendations for infants and children are based on the foreseeable changes in protein binding, water and fat stores, cytochrome p450 isoenzyme capacity, and renal function that occur during normal childhood growth and development.
Recent evaluations have suggested that total body weight (TBW) and LBM are the preferred size descriptors to use for maintenance medication dosing in obese patients because these descriptors correlate better with parameters responsible for predicting drug dose and interval, namely, volume of distribution and clearance.9–11 It has been suggested that loading doses of hydrophilic medications in obese children should be based on ideal body weight, whereas partially hydrophilic medications should be based on a percentage of total body weight (e.g., adjusted body weight) and lipophilic medications should be based simply on total body weight. Since maintenance doses rely more on intrinsic metabolic capacity of the liver and kidney, LBM is considered the best scalar to use.2,3 This study identified a practical and safe method of determining a modified dosing weight in obese children, which can represent LBM predicted by more complex methods.
Calculation of LBM and IBW in Children
DXA, magnetic resonance imaging, skinfold measurements, and mathematical equations are some methods that have been used; however, none are considered gold standards for determining LBM and IBW in children.12,13 Predictive mathematical equations for LBM offer the benefits of ease of use and low cost in the clinical environment. Two such equations, the Peters and Foster equations, have recently been published; they describe LBM estimations in the pediatric population and were assessed in this study.
The Peters method of estimating LBM (equation 1) is based on the assumption that ECV is proportional to LBM in both adults and children. Peters evaluated a total of 296 patients, including 69 children. They measured glomerular filtration rate and ECV of the patients and compared values to estimated LBM (eLBM) by using an equation described by Boer et al.14 The ECV in the pediatric cohort was estimated from the Boer equation on the basis of height and weight and converted to eLBM by using the relationship between eLBM and ECV determined in the adults from the other centers.6
The Peters equation incorporates the simple measurements of height and weight to assess the eLBM of patients but has several limitations. First, while the equation was evaluated in children, most subjects were not obese. Second, the equation assumes that LBM is directly proportional to ECV, which is analogous to estimating LBM from TBW. Third, the accuracy of this equation depends on the accuracy of the adult data evaluated, with the assumption that the ECV-LBM relationship is the same in children as in adults, and the accuracy with which ECV can be estimated from height and weight in children. Lastly, the resultant equations require multiple calculations, complex coefficients, and may be difficult to perform clinically.
The Foster method of estimating LBM (equations 2a and 2b) was developed and validated in a large sample size of pediatric patients. DXA was used to measure LBM in 836 healthy children and linear regression was used to develop sex-specific equations to estimate LBM from height, weight, age, and BMI for age z-score to serve as an index of adiposity. Equations were validated in a local independent sample of 332 children and in data collected for 4190 children by NHANES, which provided DXA-derived estimates of LBM from a nationally representative sample of children aged 8 to 19 years. This method demonstrated LBM within 4% of the LBM measured by DXA.7
The Foster method considered a wide variety of potential variables and adjusted for sex and spectrum of adiposity. It was validated in several large population samples of children, following equation derivation, and appears to reflect LBM of children accurately when compared to DXA scanning methods. The agreement between methods was strongest when assessing the BMI method compared to the Foster method. The large number of pediatric patients assessed with the Foster method reinforces the recommendation to use the BMI method for approximating LBM in children in a clinical setting. While this method has many advantages, the equations were validated by using DXA imaging, a tool that has not been authenticated across all ages included in the current study. It also requires the additional calculation of a sex-specific BMI z-score, which is used to account for excess adiposity and to normalize the contribution of the excess weight to the predicted LBM. BMI z-score is a measure of weight relative to height, age, and sex and is not a direct measure of adiposity. Foster et al7 use 2 different equations for calculation of male and female LBM, potentially adding systematic bias to the method at higher weights. This may explain the sex differences between methods in our evaluation of the Foster in relation to the Peters method. Finally, the equations derived are complex and may be difficult to perform consistently and accurately.
The BMI method is a simple process that provides a rapid estimation of IBW by using height, weight, sex, and age. A child's 50th percentile BMI-forage is determined by using a Centers for Disease Control (CDC) growth chart and is then multiplied by the patient's height (m2) to achieve an IBW. One criticism of the BMI method is that it may not reflect adiposity as accurately in children as it does in adults owing to differing levels of fat and fat-free mass that normally occur during periods of growth.14–16 BMI among children appears to vary according to degree of fatness but appears to reflect fat mass index best when BMI-forage is greater than or equal to the 85th percentile.17,18 Other investigators have shown that accuracy of BMI as an indicator of obesity in children increases as BMI increases.19–21 This is an acceptable feature of the BMI method of weight determination for the purposes of our study because we used this technique for dose adjustments only in children with a BMI above the 95th percentile for age.
Limitations and Recommendations
There are limitations to this study that need to be acknowledged. While the World Health Organization publishes BMI standards for all ages, the current CDC BMI charts are not designed for children younger than 2 years and thus these young children were excluded from our analysis. In addition, our sample size of 176 patients, while representing a wide variety of age ranges and BMI values, was extracted from a single center and may not completely represent features of all obese children. Lastly, it is possible that patients in our cohort may have exhibited alterations in body composition secondary to critical illness that may be absent or less pronounced in healthier children. In particular, the weight of acutely ill children may be higher (owing to capillary leak and peripheral edema) or lower (owing to prolonged illness and reduced caloric intake before intensive care unit [ICU] admittance) than their weight during health. The measured weight of any child during an acute illness should be carefully considered, and clinicians must use their clinical judgment when deciding if the admission weight is accurate. The impact of capillary leak and fluid retention on body weight in our study should have been minimal because we used admission weights, rather than weights collected after an extended period of ICU stay. Nevertheless, neither the Foster nor the Peters method incorporated critically ill patients into their equations.
The clinical implications of overdosing and underdosing specific medications in obese patients are real and require action.22 Our results support the use of the BMI method for medication dosing in children when ideal weights generated from the method are less than 50 kg. However, because our results demonstrated weaker agreement between the 3 methods at higher weights, we cannot make a sweeping recommendation for the BMI method in children with ideal weights above 50 kg.
Since the BMI method tended to estimate lower weights (compared to the Foster and Peters methods) in children with ideal weights greater than 50 kg, we believe this feature may offer a safety advantage by producing more conservative doses for medications in which toxicity is a concern. However, for medications with low risk for toxicity and high risk for treatment failure (e.g., many antimicrobials), the danger of underdosing may be too great if the BMI method is used. Therefore, in children with an IBW above 50 kg, we recommend using the maximum adult dose when the concern for underdosing exists. This dosing practice is also supported by the Pediatric Pharmacy Advocacy Group position statement.23 It goes without saying that doses of medication that can be adjusted by serum concentration analysis should continue to be incorporated into daily practice of the obese child.
Conclusions and Future Directions
Weights generated with the BMI method demonstrate strong agreement and correlation with the Peters and Foster methods of calculating LBM in patients with ideal weights below 50 kg. Superiority of one method for calculation of IBW or LBM was not demonstrated, and therefore ease of clinical use becomes the driving factor for daily application. Of the 3 methods, the BMI method is the most feasible, allowing for seamless integration into the daily workflow of the clinical pharmacist. Clinical caution and judgment should be used when using the BMI method in children with ideal weights above 50 kg. Collaboration between pharmacists and clinician experts in childhood obesity assessment and care is critical for further progress. Continued research into pharmacokinetic and pharmacodynamic alterations in obese children is necessary to support pharmaceutical intervention. In the future, development of standards of practice, medication dosing guidelines, and trials evaluating the impact of medication dose adjustments in obese patients will be necessary to optimize patient safety and outcomes.
ACKNOWLEDGMENT
This work was presented at the 22nd Annual PPAG Conference, Indianapolis, Indiana, May 2013. We would like to thank Amy Poppy, PharmD, Heather Skillman, MS, RD, and Lynn Schultz for their useful comments and review of this work.
ABBREVIATIONS
- BMI
body mass index
- CDC
Center for Disease Control
- CI
Confidence interval
- DXA
dual energy x-ray absorptiometry
- ECV
extracellular volume
- eECV
extracellular volume
- eLBM
estimated lean body mass
- IBW
ideal body weight
- ICC
intraclass correlation
- ICU
intensive care unit
- LBM
lean body mass
- NHANES
National Health and Nutrition Examination Survey
- PICU
pediatric intensive care unit
- TBW
total body weight
Footnotes
DISCLOSURE The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
REFERENCES
- 1.Data source: 2007 National Survey of Children's Health. Data analysis provided by the Child and Adolescent Health Measurement Initiative, Data Resource Center. http://www.childhealthdata.org/. Accessed August 31, 2012.
- 2.Kendrick JG, Carr RR, Ensom MH. Pharmacokinetics and drug dosing in obese children. J Pediatr Pharmacol Ther. 2010;15(2):94–109. [PMC free article] [PubMed] [Google Scholar]
- 3.Mulla H, Johnson TN. Dosing dilemmas in obese children. Arch Dis Child Educ Pract Ed. 2010;95(4):112–117. doi: 10.1136/adc.2009.163055. [DOI] [PubMed] [Google Scholar]
- 4.Wells JC, Fewtrell MS. Measuring body composition. Arch Dis Child. 2006;91(7):612–617. doi: 10.1136/adc.2005.085522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phillips S, Edlbeck A, Kirby M, Goday P. Ideal body weight in children. Nutr Clin Pract. 2007;22(2):240–245. doi: 10.1177/0115426507022002240. [DOI] [PubMed] [Google Scholar]
- 6.Peters AM, Snelling HL, Glass DM, Bird NJ. Estimation of lean body mass in children. Br J Anaesth. 2011;106(5):719–723. doi: 10.1093/bja/aer057. [DOI] [PubMed] [Google Scholar]
- 7.Foster BJ, Platt RW, Zemel BS. Development and validation of a predictive equation for lean body mass in children and adolescents. Ann Hum Biol. 2012;39(3):171–182. doi: 10.3109/03014460.2012.681800. [DOI] [PubMed] [Google Scholar]
- 8.Barlow SE. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120(suppl 4):S164–S192. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 9.Green B, Duffull SB. What is the best size descriptor to use for pharmacokinetic studies in the obese? Br J Clin Pharmacol. 2004;58(2):119–133. doi: 10.1111/j.1365-2125.2004.02157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robinson JD, Lupkiewicz SM, Palenik L. Determination of ideal body weight for drug dosage calculations. Am J Hosp Pharm. 1983;40(6):1016–1019. [PubMed] [Google Scholar]
- 11.McLeay SC, Morrish GA, Kirkpatrick CM, Green B. The relationship between drug clearance and body size: systematic review and meta-analysis of the literature published from 2000 to 2007. Clin Pharmacokinet. 2012;51(5):319–330. doi: 10.2165/11598930-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 12.Ellis KJ, Shypailo RJ. Bone mineral and body composition measurements: cross-calibration of pencil-beam and fan-beam dual-energy X-ray absorptiometers. J Bone Miner Res. 1998;13(10):1613–1618. doi: 10.1359/jbmr.1998.13.10.1613. [DOI] [PubMed] [Google Scholar]
- 13.Shypailo RJ, Butte NF, Ellis KJ. DXA: can it be used as a criterion reference for body fat measurements in children? Obesity (Silver Spring) 2008;16(2):457–462. doi: 10.1038/oby.2007.81. [DOI] [PubMed] [Google Scholar]
- 14.Boer P. Estimated lean body mass as an index for normalization of body fluid volumes in humans. Am J Physiol. 1984;247:F632–F636. doi: 10.1152/ajprenal.1984.247.4.F632. [DOI] [PubMed] [Google Scholar]
- 15.Pai MP, Paloucek FP. The origin of the “ideal” body weight equations. Ann Pharmacother. 2000;34(9):1066–1069. doi: 10.1345/aph.19381. [DOI] [PubMed] [Google Scholar]
- 16.Franklin MF. Comparison of weight and height relations in boys from 4 countries. Am J Clin Nutr. 1999;70(1):157S–162S. [PubMed] [Google Scholar]
- 17.Horlick M. Body mass index in childhood—measuring a moving target. J Clin Endocrinol. 2001;86(9):4059–4060. doi: 10.1210/jcem.86.9.7948. [DOI] [PubMed] [Google Scholar]
- 18.Taylor RW, Jones IE, Williams SM, Goulding A. Body fat percentages measured by dual-energy X-ray absorptiometry corresponding to recently recommended body mass index cutoffs for overweight and obesity in children and adolescents aged 3–18 y. Am J Clin Nutr. 2002;76(6):1416–1421. doi: 10.1093/ajcn/76.6.1416. [DOI] [PubMed] [Google Scholar]
- 19.Freedman DS, Wang J, Maynard LM et al. Relation of BMI to fat and fat-free mass among children and adolescents. Int J Obes (Lond) 2005;29(1):1–8. doi: 10.1038/sj.ijo.0802735. [DOI] [PubMed] [Google Scholar]
- 20.Schaefer F, Georgi M, Wühl E, Schärer K. Body mass index and percentage fat mass in healthy German schoolchildren and adolescents. Int J Obes Relat Metab Disord. 1998;22(5):461–469. doi: 10.1038/sj.ijo.0800608. [DOI] [PubMed] [Google Scholar]
- 21.Bray GA, DeLany JP, Harsha DW et al. Evaluation of body fat in fatter and leaner 10-y-old African American and white children: the Baton Rouge Children's Study. Am J Clin Nutr. 2001;73(4):687–702. doi: 10.1093/ajcn/73.4.687. [DOI] [PubMed] [Google Scholar]
- 22.Longo C, Bartlett G, Macgibbon B et al. The effect of obesity on antibiotic treatment failure: a historical cohort study. Pharmacoepidemiol Drug Saf. 2013;22(9):970–976. doi: 10.1002/pds.3461. [DOI] [PubMed] [Google Scholar]
- 23.Johnson PN, Miller JL, Boucher EA Medication dosing in overweight and obese children. http://www.ppag.org/obesedose/. Accessed September 20, 2013.


