Abstract
OBJECTIVES: To determine the proportion of infections caused by extended-spectrum ß-lactamase (ESBL)–producing Klebsiella or Escherichia coli Gram-negative organisms in the pediatric intensive care unit (PICU), and to identify risk factors for these infections.
METHODS: A retrospective, single-center chart review of patients admitted to a PICU in a 5-year period with infections caused by Klebsiella species or E coli was completed.
Data collected include demographics, length of stay, outcome, and relevant risk factors previously defined in the literature.
RESULTS: A total of 110 isolates were cultured from 94 patients. A total of 53% of the isolates were E coli, and the remainder were Klebsiella subspecies. Of the 110 isolates, 13 isolates (11.8%) in 7 patients were ESBL positive. The ESBL-producing isolates were equally distributed amongE coli and Klebsiella and were primarily cultured from tracheal aspirates. Most of the ESBL-positive isolates (9 of 13; 69%) were cultured from patients who received ceftazidime and/or cefotaxime in the preceding 30 days. Patients infected with E coli had higher PRISM 1 scores and were more likely to have a Foley catheter, whereas infections with Klebsiella were more common in mechanically ventilated males. Although not statistically significant, 80% of patients who were infected with non–ESBL-producing organisms survived to hospital discharge versus 57% of those infected with ESBL-producing E coli and Klebsiella.
CONCLUSIONS: Almost 12% of E coli and Klebsiella isolates in this patient population tested positive for ESBL production. ESBL production was equally distributed between E coli and Klebsiella species. These organisms were cultured from 7% of the study patients. As reported in previous studies, patients infected with ESBL-producing organisms most often had received prior cephalosporins and had a longer length of stay in the PICU.
INDEX TERMS: extended spectrum beta-lactamase, antibiotic resistance, pediatric intensive care unit
INTRODUCTION
Infection is a major problem among children in pediatric intensive care units (PICUs) worldwide.1 Gram-negative organisms are responsible for a significant proportion of nosocomial infections in adults and children.1–3 The antibiotic susceptibility pattern of Gram-negative organisms has been changing during the last several years, with a shift toward organisms being more resistant to antibiotics previously considered the treatment of choice. This is due, at least in part, to the widespread use of broad-spectrum antibiotics, such as cephalosporins, for the treatment of infections caused by β-lactamase–producing organisms that rendered previously used antibiotics, such as penicillin, ineffective.3
The mid-1980s saw the advent of Gram-negative organisms, particularly Enterobacteriaceae, such as Escherichia coli and Klebsiella species, that produced β-lactamases with an extended spectrum of activity.3 The extended-spectrum β-lactamases (ESBLs) produced by these organisms are capable of hydrolyzing the β-lactam ring of several antibiotics. This renders a wide variety of antibiotics, including third-generation cephalosporins, monobactams, and penicillins, ineffective in the treatment of infections caused by these organisms. Organisms that produce ESBLs typically retain in vitro susceptibility to cefoxitin, cefotetan, and carbapenems, although treatment failures have been seen.4–6
Several studies, mostly in adult patients, have demonstrated that infections due to ESBL-producing organisms are associated with increased treatment failure, higher mortality, longer hospital stays, and higher health care costs.2,7 Although population-based estimates of the prevalence and incidence of this burden exist, the statistics vary widely from continent to continent, and even from center to center, with ranges as low as 6% and as high as even 70%.3,4,7 Prolonged hospital stay, prolonged stay in an intensive care unit (ICU), residence in a long-term care facility, recent exposure to multiple antibiotics (specifically third-generation cephalosporins), and indwelling invasive devices are some of the risk factors that have been identified for the acquisition of infections with ESBL-producing organisms in adults.8–11 Pediatric risk factors have not been clearly elicited as of yet. Zaoutis et al12 identified female sex, corticosteroid use within 60 days of acquiring the infection, and infection with Klebsiella species as being independently predictive of bloodstream infections due to ESBL-producing organisms in children. The authors also identified recent exposure to third-generation cephalosporins as a pertinent risk factor. Kim et al13 identified prior hospitalization, ICU admission within the preceding 30 days, mechanical ventilation, presence of a central venous catheter, development of breakthrough bacteremia during antibiotic therapy, and exposure to extended-spectrum cephalosporins within 30 days of infection as risk factors for acquiring infections caused by ESBL-producing E coli or Klebsiella pneumoniae in a cohort of neonates with bacteremia. Although these data are important, the widespread applicability of this information in pediatric subpopulations is limited because both of the above-mentioned studies only included children with bloodstream infections. This study sought primarily to determine the prevalence of infections caused by ESBL-producing E coli and Klebsiella species in children admitted to this PICU, and secondarily to identify risk factors for infections with such organisms in these patients.
MATERIALS AND METHODS
Approval of this study was obtained from the Institutional Review Board of the University of Alabama at Birmingham. Patients who had been admitted to the Children's of Alabama 19-bed PICU during a 5-year period between January 2000 and December 2004 and had documented cultures with E coli or Klebsiella were identified. Eligible patients were identified from the institution's microbiology database. Patient records were analyzed to determine whether the microbiologic data represented a true infection in the opinion of the treating physician; those thought to be colonized with E coli or Klebsiella were not included in the study.
Patients with a tracheostomy were also excluded because many of these children are chronically colonized, making it difficult to interpret the clinical relevance of positive cultures, especially from tracheal aspirates.
Patient data collected included age, sex, primary diagnosis, length of hospital stay prior to identification of the infection, and length of stay in the PICU prior to positive cultures. A PRISM 1 score was collected on each patient. The PRISM 1 score, defined as the pediatric risk of mortality, is considered a predictor of mortality for critically ill children where the higher score is more related to poor outcome. The presence or absence of potentially important risk factors was noted, including immunosuppression, current or recent corticosteroid use (in prior 30 days), malignancy, chronic renal or hepatic disease, need for renal replacement therapy, and use of parenteral nutrition. Invasive devices, such as central venous catheters, arterial lines, endotracheal tubes, nasogastric/transpyloric feeding tubes, dialysis catheters, Foley catheters, and ventriculoperitoneal shunts or external ventricular drains, were also recorded, as was prior antibiotic exposure in the previous 30 days. Information regarding the organism isolated, site of isolation, and antibiotic susceptibility pattern was obtained from the microbiology database. Patient outcomes at the end of hospitalization were recorded as survived or died.
For this study E coli and Klebsiella isolates as ESBL-producing organisms were investigated because of the frequency of their occurrence in the institution and in the literature supporting these two common ESBL-producing organisms.11–13 All isolates of E coli and Klebsiella species were tested by disk diffusion with 30 mcg of cefotaxime, ceftazidime, ceftriaxone, or aztreonam. Those isolates that showed a zone of inhibition less than 27, 22, 25, and 27 mm, respectively, to the antibiotic tested were considered to be potential ESBL-producing organisms. The isolates were subjected to phenotypic testing to confirm ESBL-producing activity. Confirmatory testing was performed by incubating the isolates with cefotaxime (30 mcg) and ceftriaxone (30 mcg), either alone or in combination with clavulanic acid. The zone of inhibition was measured after an incubation period of 16 to 18 hours. A ≥5-mm increase in a zone diameter for either antimicrobial agent tested in combination with clavulanic acid versus its zone when tested alone was confirmatory of ESBL production. Isolates confirmed to be ESBL positive were reported as being resistant to all penicillins, cephalosporins, and aztreonam. The method used in this series is standard and recommended by the 2007 National Committee for Clinical Laboratory Standards.14
Descriptive analyses generated mean ± SD, median, and interquartile ranges where appropriate. Forward stepwise regression analysis was performed using available variables (excluding length of stay and antibiotic use) to identify those variables that were risk factors for acquisition of infections with ESBL-producing E coli or Klebsiella species. The variables that emerged as significant in the stepwise regression analysis at p<0.1 were subsequently used for logistic regression analysis. Odds ratios were also determined. SigmaStat 3.1 (Systat software) was used for the analysis.
RESULTS
Approximately 6017 patients were admitted to the PICU during the study period. A total of 161 patients were identified from whom either E coli or Klebsiella species were isolated in this same study period. Of these, 62 patients were excluded because of the presence of a tracheostomy. Medical records were incomplete for 5 patients. Therefore, 94 patients were included in this analysis. The median age of patients in this series was 17.5 months (range, 1 week to 21 years); 32 patients (34%) were male. The median PRISM 1 score of patients with infections caused by ESBL-producing organisms was 14, indicating a moderate level of acuity.
A total of 110 isolates were cultured from 94 patients. A total of 58 of the 110 isolates (53%) were E coli, with the remainder being Klebsiella species. K pneumoniae represented most of the Klebsiella species; 6 of the 7 ESBL-producing Klebsiella isolates were K pneumoniae, whereas the remaining one was Klebsiella oxytoca. Of the 110 isolates, 13 samples (11.8%) from 7 patients were determined to be positive for ESBL production, which did include reviewing duplicate isolates from individual patients.
These isolates were cultured from 7 patients. Table 1 shows the breakdown of all organisms cultured, whereas Table 2 reveals the sites of organism isolation, including the number of study patients with such isolates.
Table 1.
Prevalence of Escherichia coli and Klebsiella isolates
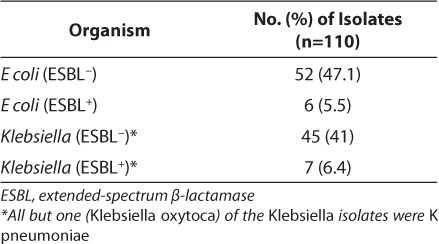
Table 2.
Sites of Isolation
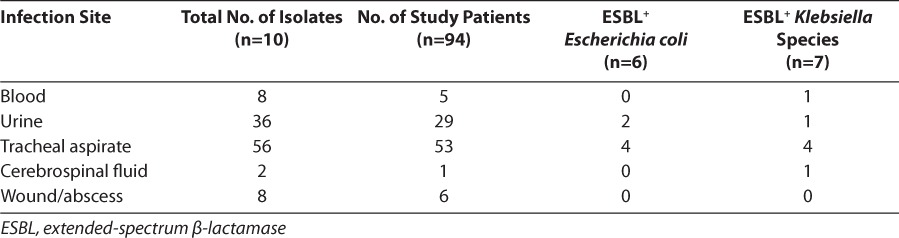
An analysis of possible risk factors reveals 9 of 13 ESBL-positive isolates (69%) were cultured from patients who had been exposed to either ceftazidime and/or cefotaxime in the preceding 30 days, in comparison with only 3.5% in the ESBL-negative comparator group. Infections with ESBL-producing E coli were associated with higher PRISM 1 scores (p<0.05) and the presence of a Foley catheter (p<0.05). Infections with Klebsiella were more common in mechanically ventilated males (p<0.05), with a trend toward increased incidence among patients with a central venous line.
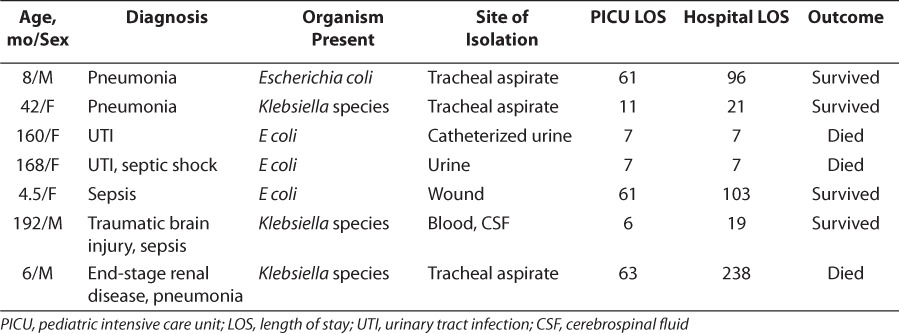
The lengths of stay in the hospital and PICU were analyzed among patients with infections caused by ESBL-producing organisms, were compared with those caused by non–ESBL-producing organisms, and are presented in Table 3. Although patients who were infected with organisms that produced ESBLs had longer stays in the PICU and hospital than patients with ESBL-negative organisms, only the difference in the length of stay in the PICU attained statistical significance (p=0.02). A total of 80% of patients who were infected with organisms that did not produce ESBLs survived to hospital discharge, whereas 57% of those infected with ESBL-producing organisms survived to discharge. This difference did not attain statistical significance. Table 4 describes relevant patient information for patients infected by ESBL-producing organisms.
Table 3.
Median Length of Pediatric Intensive Care Unit (PICU) and Hospital Stay in Days, and Interquartile Ranges (IQRs)

Table 4.
Relevant Information on Patients Infected by Extended-Spectrum β-Lactamase–Positive Organisms
DISCUSSION
There has been an increasing prevalence of ESBL-producing organisms during the past decade. Recent estimates for ESBL-producing organisms are reported to be around 12% in ICUs in the United States.12 In one series of 728 neonates with Gram-negative sepsis, ESBLs were detected in 86.6% of Klebsiella isolates and 63.65% of E coli isolates.15 Blaschke et al16 showed a rising incidence of ESBL-producing E coli and Klebsiella species during a 5-year period between 2004 and 2007 in a pediatric cohort. In this series, 7% of patients with positive cultures for either of the two organisms studied were infected with ESBL-producing organisms, whereas almost 12% of the 110 total isolates were documented to be ESBL producing. This study focused on children admitted to the ICU because they represent a high-risk group for the outcome being studied. Although there is some information about the rising prevalence of ESBL-producing organisms in adult ICUs, the applicability of this information to the pediatric setting is limited. This is because children admitted to PICUs are a diverse population, and often present with combinations of medical and surgical diagnoses. Knowledge of the local prevalence of Gram-negative organisms and associated risk factors is important in optimizing therapy in critically ill children, who constitute an especially vulnerable patient population.
ESBL production is a bacterial mechanism of resistance most commonly found in the Enterobacteriaceae family. The most frequent ESBL-producing organisms are E coli and Klebsiella species,11–13 thus the reason for studying these organisms in this retrospective chart review. Enterobacter, Serratia, Salmonella, Pseudomonas aeruginosa,11,13,15 and Proteus species also produce ESBLs.7
Outbreaks of infections due to ESBL-producing Klebsiella have been reported to occur in high-risk areas, such as ICUs and nursing homes.17–19 Historically, most of these outbreaks have been caused by Temoniera (TEM)–producing or sulfhydryl (SHV)–producing organisms. In this case series, ESBL-producing organisms were identified using phenotypic methods alone, and further genotyping was not performed. This information would be beneficial in studying the epidemiology of these infections in a more thorough manner.
A multitude of risk factors have been described for acquiring an infection with ESBL-producing Gram-negative bacteria. Recurrent in the literature is the relationship of exposure to third-generation cephalosporins and acquisition of infections caused by ESBL-producing organisms.8,15,20 Consistent with this, in this patient series, 9 of the 13 isolates (69%) were identified as ESBL-producing organisms in patients who had been treated with third-generation cephalosporins (cefotaxime or ceftazidime) within the 30 days prior to positive culture; however, this was not statistically significant.
Unique to this study is the association between specific organisms and additional risk factors. The data revealed that E coli was more likely to infect patients with higher PRISM 1 scores and the presence of a Foley catheter. Infections with Klebsiella were more common in males who were mechanically ventilated. A statistically insignificant trend toward an increased incidence of Klebsiella was seen among patients with a central venous catheter.
Treatment options for infections caused by ESBL-producing organisms are limited because of the multidrug resistance that is often exhibited by these bacteria. Patients infected with ESBL-producing organisms are often initially treated with inappropriate therapy, and this has been conclusively linked to poor clinical outcomes.21 It is noteworthy that treatment failure can occur despite the use of antibiotics that appear to be effective against the ESBL-producing organism in vitro. In a review of outcomes of bacteremia caused by ESBL-producing organisms in 36 patients, Patterson and Bonomo22 showed treatment failure in all cases in which the minimum inhibitory concentration (MIC) values of the cephalosporin used were in the intermediate range. Treatment failure also occurred in 54% of cases in which the MIC of the cephalosporin used for therapy was in the susceptible range. The inoculum effect, whereby the MIC increases with increasing inoculums of the organism in question,23 is a possible explanation for the mismatch between in vitro and in vivo susceptibility. Treatment failure, which has variably been defined as continued fever >48 hours after instituting therapy or death within 2 weeks,13,17,23,24 was not specifically analyzed in this study.
A 2007 review of the Gram-negative “health care crisis” recognized a general consensus that resistance is associated with negative clinical outcomes.25 Ramphal and Ambrose23 examined numerous studies in an attempt to determine the effect of infections with ESBL-producing organisms on clinical outcomes. The investigators discussed several comparative studies where ESBL status had no effect on outcomes, but also some studies that did find an association with poor outcome. They concluded that many of the studies were underpowered. Several other studies have demonstrated that patients with infections due to ESBL-producing bacteria had worse clinical outcomes than patients with infections caused by non–ESBL-producing organisms.13,26,27
Infections due to these multidrug-resistant Gram-negative organisms result in increased costs of medical care, primarily driven by increased length of stay in this population.13,27–29 In this study, lengths of hospital and PICU stay, as well as survival to discharge, were used as markers of outcome. Patients with infections due to ESBL-producing E coli or Klebsiella had a longer duration of hospital stay as well as PICU stay. The median length of PICU stay among patients who were infected with ESBL-producing organisms was 25 days, as opposed to 12 days in patients who were infected with non–ESBL-producing organisms. The median length of hospital stay in patients who were infected with ESBL-producing organisms was 56 days, as opposed to 25 days in patients who were infected by non–ESBL-producing organisms. Only the difference in length of stay in the PICU attained statistical significance. Some studies found no association between initial inappropriate antimicrobial therapy and outcome in patients infected with ESBL-producing organisms; however, they did demonstrate that patients infected with ESBL-producing organisms had a longer average hospital stay.7,13 Although this study did not specifically look at hospital costs, it is plausible that patients with infections caused by ESBL-producing organisms had higher hospital-related costs directly related to prolonged duration of resource use in the ICU.
In much of the literature seeking a relationship between infections caused by ESBL-producing organisms and clinical outcomes, mortality specifically was assessed. Although Zaoutis et al12 found no significant difference in all-cause mortality in patients with ESBL-producing organisms, Kim et al13 reported a higher mortality rate in ESBL-infected patients that was statistically significant. Ariffin et al27 studied pediatric patients with Klebsiella infection and reported an overall sepsis-related mortality rate of 50% in patients infected with ESBL-producing organisms, as opposed to 13% in those infected with organisms that did not produce ESBLs. In this patient series, the overall survival rate was 80%, whereas 4 of the 7 patients (57%) infected with ESBL-producing organisms survived to discharge. The corresponding mortality in patients who had infections caused by non–ESBL-producing organisms was 18% (16 of 87). Although this was not found to be statistically significant, it should be interpreted in light of the small number of patients and relatively low prevalence of ESBL-producing organisms in this population; this may be a clinically important difference between the two groups.
There were some limitations to this study, including its retrospective design, small sample size, lack of genotypic analysis, and use of multiple isolates from some patients; these limit the widespread extrapolation of these data. Lastly, new data have emerged on MIC breakpoints for these organisms since the completion of this study.30,31 This study found that 7% of all children who were infected with E coli or Klebsiella species were infected by ESBL-producing strains, a rate lower than what was seen in previous studies. Of these, a high percentage of patients had been exposed to third-generation cephalosporins in the preceding 30 days and also had a longer length of PICU stay, also similar to what has been reported previously. Further prospective studies with phenotypic characterization of the ESBL-producing organisms, as well as analysis of initial therapy, treatment failure incidence, and hospital costs in addition to outcome and length of stay, are needed to determine the burden of ESBL-producing organisms in the pediatric population.
ACKNOWLEDGMENT
All work was performed at Children's of Alabama, Birmingham, Alabama. A portion of this research was presented at the Society of Critical Care Medicine's 38th Critical Care Congress in Nashville, Tennessee, February 2009. Dr Lowros was a Doctor of Pharmacy candidate at Samford University McWhorter School of Pharmacy, Birmingham, Alabama, at the time of this research.
ABBREVIATIONS
- ESBL
extended-spectrum ß-lactamase
- ICU
intensive care unit
- MIC
minimum inhibitory concentration
- PICU
pediatric intensive care unit
- SHV
sulfhydryl
- TEM
Temoniera
Footnotes
DISCLOSURE The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
REFERENCES
- 1.Richards MJ, Edwards JR, Culver DH et al. Nosocomial infections in pediatric intensive care units in the United States. Pediatrics. 1999;103(4):e39. doi: 10.1542/peds.103.4.e39. [DOI] [PubMed] [Google Scholar]
- 2.Peleg AY, Hooper DC. Hospital-acquired infections due to gram-negative bacteria. N Engl J Med. 2010;362(19):1804–1813. doi: 10.1056/NEJMra0904124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong-Beringer A. Therapeutic challenges associated with extended-spectrum, B-lactamase-producing Escherichia coli and Klebsiella pneumoniae. Pharmacotherapy. 2001;21(5):583–592. doi: 10.1592/phco.21.6.583.34537. [DOI] [PubMed] [Google Scholar]
- 4.Gupta V. An update on newer beta-lactamases. Indian J Med Res. 2007;126(5):417–427. [PubMed] [Google Scholar]
- 5.Bonomo RA, Rudin SA, Shlaes DM. Tazobactam is a potent inactivator of selected inhibitor-resistant class A β-lactamases. FEMS Microbiol Lett. 1997;148(1):59–62. doi: 10.1111/j.1574-6968.1997.tb10267.x. [DOI] [PubMed] [Google Scholar]
- 6.Chaibi EB, Sirot D, Paul G et al. Inhibitor-resistant TEM β-lactamases: phenotypic, genetic, and biochemical characteristics. J Antimicrob Chemother. 1999;43(4):447–458. doi: 10.1093/jac/43.4.447. [DOI] [PubMed] [Google Scholar]
- 7.Rodriquez-Bano J, Pascual A. Clinical significance of extended-spectrum B-lactamases. Expert Rev Anti Infect Ther. 2008;6(5):671–683. doi: 10.1586/14787210.6.5.671. [DOI] [PubMed] [Google Scholar]
- 8.Superti SV, Augusti G, Zavascki AP. Risk factors for and mortality of extended-spectrum-β-lactamase-producing Klebsiella pneumoniae and Escherichia coli nosocomial bloodstream infections. Rev Inst Med Trop Sao Paulo. 2009;51(4):211–216. doi: 10.1590/s0036-46652009000400006. [DOI] [PubMed] [Google Scholar]
- 9.Graffunder EM, Preston KE, Evans AM et al. Risk factors associated with extended-spectrum β-lactamase-producing organisms at a tertiary care hospital. J Antimicrob Chemother. 2005;56(1):139–145. doi: 10.1093/jac/dki180. [DOI] [PubMed] [Google Scholar]
- 10.Goyal A, Prasad KN, Prasad A et al. Extended spectrum β-lactamases in Escherichia coli and Klebsiella pneumoniae and associated risk factors. Indian J Med Res. 2009;129(6):695–700. [PubMed] [Google Scholar]
- 11.Pfaller MA, Segreti J. Overview of the epidemiological profile and laboratory detection of extended-spectrum β-lactamases. Clin Infect Dis. 2006;42(suppl 4):S153–S163. doi: 10.1086/500662. [DOI] [PubMed] [Google Scholar]
- 12.Zaoutis TE, Goyal M, Chu JH et al. Risk factors for and outcomes of bloodstream infection caused by extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella species in children. Pediatrics. 2005;115(4):942–949. doi: 10.1542/peds.2004-1289. [DOI] [PubMed] [Google Scholar]
- 13.Kim Y, Pai H, Lee H et al. Bloodstream infections by extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae in children: epidemiology and clinical outcome. Antimicrob Agents Chemother. 2002;46(5):1481–1491. doi: 10.1128/AAC.46.5.1481-1491.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clinical Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Seventeenth Informational Supplement. Wayne, PA: CLSI; 2007. Publication no. M100–S17. [Google Scholar]
- 15.Jain A, Roy I, Gupta MK et al. Prevalence of extended-spectrum β-lactamase-producing Gram-negative bacteria in septicaemic neonates in a tertiary care hospital. J Med Microbiol. 2003;52(5):421–425. doi: 10.1099/jmm.0.04966-0. [DOI] [PubMed] [Google Scholar]
- 16.Blaschke AJ, Korgenski EK, Daly JA et al. Extended spectrum β lactamase-producing pathogens in a children's hospital: a 5-year experience. Am J Infect Control. 2009;37(6):435–441. doi: 10.1016/j.ajic.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foglia EE, Fraser VJ, Elward AM. Effect of nosocomial infections due to antibiotic-resistant organisms on length of stay and mortality in the pediatric intensive care unit. Infect Control Hosp Epidemiol. 2007;28(3):299–306. doi: 10.1086/512628. [DOI] [PubMed] [Google Scholar]
- 18.Ndugulile F, Jureen R, Harthug S et al. Extended spectrum B-lactamase among gram-negative bacteria of nosocomial origin from an intensive care unit of a tertiary health facility in Tanzania. BMC Infect Dis. 2005;5:86–91. doi: 10.1186/1471-2334-5-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaynes R, Edwards JR; National Nosocomial Infections Surveillance System. Overview of nosocomial infections caused by gram-negative bacilli. Clin Infect Dis. 2005;41(6):848–854. doi: 10.1086/432803. [DOI] [PubMed] [Google Scholar]
- 20.Waterer GW, Wunderlink RG. Increasing threat of gram-negative bacteria. Crit Care Med. 2001;29(4):N75–N81. doi: 10.1097/00003246-200104001-00004. [DOI] [PubMed] [Google Scholar]
- 21.Schiappa DA, Hayden MG, Matushek FN et al. Ceftazidime-resistant Klebsiella pneumoniae and Escherichia coli bloodstream infection: a case-control and molecular epidemiologic investigation. J Infect Dis. 1996;174(3):529–536. doi: 10.1093/infdis/174.3.529. [DOI] [PubMed] [Google Scholar]
- 22.Patterson DL, Bonomo R. Extended-spectrum beta-lactamases: a clinical update. Clin Microbiol Rev. 2005;18(4):657–686. doi: 10.1128/CMR.18.4.657-686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramphal R, Ambrose PG. Extended-spectrum β-lactamases and clinical outcomes: current data. Clin Infect Dis. 2006;42(suppl 4):S164–S172. doi: 10.1086/500663. [DOI] [PubMed] [Google Scholar]
- 24.Lee SY, Kotapati S, Kuti J et al. Impact of extended spectrum B-lactamase-producing Escherichia coli and Klebsiella species on clinical outcomes and hospital costs: a matched cohort study. Infect Control Hosp Epidemiol. 2006;27(11):1226–1232. doi: 10.1086/507962. [DOI] [PubMed] [Google Scholar]
- 25.Lautenbach E, Polk R. Resistant gram-negative bacilli: a neglected healthcare crisis? Am J Health Syst Pharm. 2007;64(suppl 14):S3–S21. doi: 10.2146/ajhp070477. [DOI] [PubMed] [Google Scholar]
- 26.Ho PL, Chan WM, Tsang KW et al. Bacteremia caused by Escherichia coli producing extended spectrum beta lactamase: a case control study of risk factors and outcomes. Scand J Infect Dis. 2002;34(8):567–573. doi: 10.1080/00365540210147516. [DOI] [PubMed] [Google Scholar]
- 27.Ariffin H, Navaratnam P, Mohamed M et al. Ceftazidime resistant bloodstream infections with Klebsiella pneumoniae in children with febrile neutropenia. Int J Infect Dis. 2000;4(1):21–25. doi: 10.1016/s1201-9712(00)90061-4. [DOI] [PubMed] [Google Scholar]
- 28.Lautenbach E, Patel JB, Bilker WB et al. Extended-spectrum B-lactamase-producing Escherichia coli and Klebsiella pneumoniae: risk factors for infection and impact of resistance on outcomes. Clin Infect Dis. 2001;32(8):1162–1171. doi: 10.1086/319757. [DOI] [PubMed] [Google Scholar]
- 29.Cosgrove SE. The relationship between antimicrobial resistance and patient outcomes: mortality, length of hospital stay, and health care costs. Clin Infect Dis. 2006;42(suppl 2):S82–S89. doi: 10.1086/499406. [DOI] [PubMed] [Google Scholar]
- 30.Roberts JA, Kwa A, Montakantikul P et al. Pharmacodynamic profiling of intravenous antibiotics against prevalent gram-negative organisms across the globe: the PASSPORT Program–Asia-Pacific Region. Int J Antimicrob Agent. 2011;37(3):225–229. doi: 10.1016/j.ijantimicag.2010.10.024. [DOI] [PubMed] [Google Scholar]
- 31.Livermore DM. Fourteen years in resistance. Int J Antimicrob Agent. 2012;39(4):283–294. doi: 10.1016/j.ijantimicag.2011.12.012. [DOI] [PubMed] [Google Scholar]


