Abstract
OBJECTIVES: The standard of care for treatment of an asthma exacerbation includes oxygen, inhaled short-acting bronchodilators, and systemic corticosteroids; adjunctive therapies, such as intravenous magnesium sulfate, can be used for patients who are having life-threatening exacerbations. The purpose of this study was to analyze the prescribing patterns as well as the safety of intravenous magnesium sulfate for the treatment of acute asthma exacerbations in pediatric patients across multiple hospitals in New Jersey.
METHODS: This retrospective chart review was conducted at 4 medical centers in New Jersey on patients who presented to the emergency department between January 1, 2010, and December 31, 2010.
RESULTS: Fifty-three patients were included in the study. In the emergency department, 98% of patients received inhaled albuterol plus ipratropium and 85% received systemic corticosteroids before intravenous magnesium sulfate administration. The median dose of magnesium sulfate was 40 mg/kg with a median time of administration of 20 minutes. One patient experienced hypotension that was thought to be related to magnesium sulfate administration.
CONCLUSIONS: This study demonstrates that weight-based dosage, as well as time of administration of magnesium sulfate for pediatric patients with an acute asthma exacerbation, varies across different institutions in New Jersey. Magnesium sulfate use was safe in this patient population.
INDEX TERMS: asthma, children, magnesium, pediatric
INTRODUCTION
Asthma is a chronic inflammatory disorder of the airways that is characterized by changing and persistent symptoms, airway obstruction, inflammation, and hyperresponsiveness.1 An asthma exacerbation occurs when symptoms are uncontrolled, causing shortness of breath, coughing, and wheezing. Less severe exacerbations can be managed at home; however, more severe exacerbations warrant an emergency department visit and possibly a hospital admission. Exacerbations may be life-threatening, as evidenced by the 185 children in the United States that died from asthma in 2007.2
Upon presentation to the emergency department, it is the standard of care to treat asthmatic patients with oxygen, inhaled short-acting bronchodilators, and often a short course of systemic corticosteroids.1 Furthermore, the 2007 National Institutes of Health (NIH) Guidelines for the Diagnosis and Management of Asthma list adjunctive therapies, such as intravenous magnesium, to be considered for patients who have life-threatening exacerbations, especially if they remain in the severe category after 1 hour of first-line therapy.1 These patients have dyspnea at rest with a loud wheeze that is heard throughout inspiration and expiration and their oxygen saturation is less than 90%.1 Past studies have looked at a variety of dosing schedules including bolus regimens,3–7 as well as bolus doses followed by continuous infusions.8 At the present time, the asthma guidelines do not provide a specific recommendation regarding dosing and frequency of intravenous magnesium sulfate administration in this patient population; however, the Pediatric & Neonatal Dosage Handbook9 does provide a dosing recommendation of 25 to 75 mg/kg/dose up to 2 g for the indication of bronchodilation, but does not suggest what the infusion time should be.
The exact mechanism of how magnesium sulfate benefits patients experiencing an asthma exacerbation is still largely unknown; however, several different mechanisms have been proposed. One of these includes its ability to inhibit the cellular uptake of calcium across smooth muscle membranes, which leads to bronchial smooth muscle relaxation.10 Others propose that magnesium helps to decrease the release of histamine by inhibiting the degranulation of mast cells,11 or that it decreases the amount of acetylcholine released at motor nerve terminals.12
We have observed that the practice among hospitals in New Jersey differs on the administration of magnesium sulfate. This study was conducted to further investigate the differences in practice as well as the safety and efficacy of magnesium sulfate administration for an acute asthma exacerbation in pediatric patients.
MATERIALS AND METHODS
This retrospective chart review was conducted across 4 different medical centers in New Jersey. These medical centers will be identified as A, B, C, or D. Medical centers A, B, and D are children's hospitals within adult hospitals and medical center C has pediatric units within the main hospital. The study was approved by the institutional review boards at each respective medical center in addition to the Institutional Review Board at Rutgers University. Patients were identified by billing reports at each specific institution for the dates of January 1, 2010, to December 31, 2010. Owing to a change in the computer system, information from medical center B was unable to be obtained for the dates of January 1, 2010, to April 30, 2010. All patients aged 18 years or younger who were treated with intravenous magnesium sulfate for an acute asthma exacerbation in the emergency department were included in the study. Patients who were admitted to the hospital before receiving intravenous magnesium sulfate were excluded.
Data were collected by using a data collection form and included age, race, sex, height, weight, home medications, and the number of previous hospitalizations for an asthma exacerbation. Data collected from the emergency department included medications that were administered before giving intravenous magnesium sulfate; time until intravenous magnesium sulfate administration; and dose, frequency, length of intravenous infusion and adverse reactions of magnesium sulfate. Serum magnesium concentrations were recorded, when available, and reflect levels that were drawn after magnesium sulfate administration. To evaluate for safety, the length of the infusion time was assessed to see if it was extended owing to possible adverse drug reactions.
The primary purpose of this study was to analyze the prescribing patterns as well as safety of intravenous magnesium sulfate for the treatment of an acute asthma exacerbation in pediatric patients across multiple hospital institutions throughout New Jersey. Secondary outcomes included the number of patients requiring intubation and/or a pediatric intensive care unit (PICU) admission after receiving intravenous magnesium sulfate, magnesium dosage regimens, the number of patients who required a hospital admission, as well as mean magnesium serum concentrations, when reported.
RESULTS
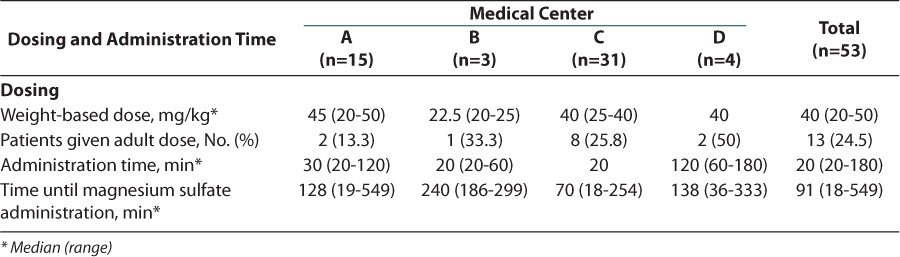
Across the 4 medical centers, a total of 53 pediatric patients received intravenous magnesium sulfate for the treatment of an acute asthma exacerbation in the emergency department. The baseline characteristics for each individual medical center can be found in Table 1. Before the current admission, most patients had a history of asthma and were receiving treatment with maintenance and/or rescue medications at home. In addition, approximately 95% of patients received standard therapy for an asthma exacerbation before receiving treatment with intravenous magnesium sulfate (Figure 1). The weight-based dose of intravenous magnesium sulfate differed among the 4 medical centers, with medical center B administering a smaller milligram-per-kilogram dose (Table 2). Medical center D had the longest infusion time with a mean of 120 minutes compared to the other 3 medical centers, all of which had mean administration times that were approximately 30 minutes (Table 2).
Table 1.
Baseline Characteristics
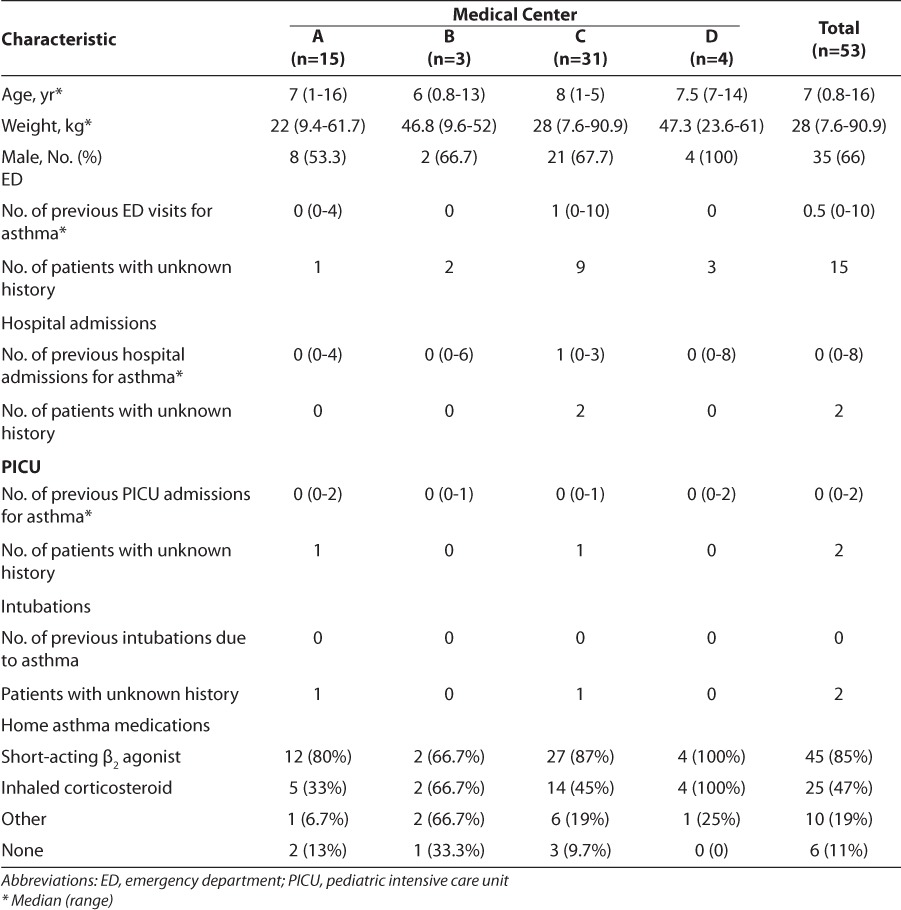
Figure 1.
Emergency department management.
 Medical Center A (N = 15);
Medical Center A (N = 15);  Medical Center B (N = 3);
Medical Center B (N = 3);  Medical Center C (N = 31);
Medical Center C (N = 31);  Medical Center D (N = 4)
Medical Center D (N = 4)
Table 2.
Magnesium Sulfate Administration
Only 1 patient, from medical center A, experienced an adverse drug reaction related to the infusion of intravenous magnesium sulfate. This patient experienced hypotension, which resolved after decreasing the infusion rate (time of infusion was increased from 1 hour to 5 hours). No deaths were reported.
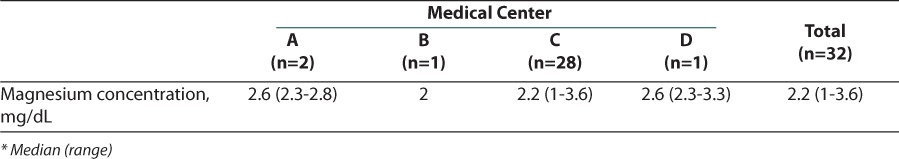
The results of the secondary outcome looking at disposition of patients can be found in Figure 2. Two patients, both from medical center C, required intubation after receiving intravenous magnesium sulfate, and a total of 39 patients across the 4 medical centers required a PICU admission. Figure 2 also shows that, when combining all 4 medical centers, a total of 94% of patients required hospital admission. Only 3 patients were discharged directly from the emergency department and all of these patients were from medical center A. Not every patient had a magnesium serum concentration drawn (Table 3), but for those who did, the serum concentrations were similar among the 4 medical centers and most were slightly above the normal range of 1.5 to 2.5 mg/dL. These values reflect serum concentrations that were drawn after magnesium sulfate administration.
Figure 2.
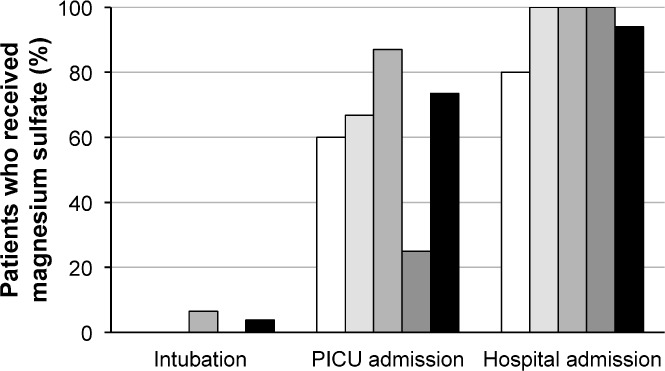
Primary and secondary outcomes.
 Hospital A;
Hospital A;  Hospital B;
Hospital B;  Hospital C;
Hospital C;  Hospital D;
Hospital D;  Total
Total
Abbreviation: PICU, pediatric intensive care unit
Table 3.
Serum Magnesium Concentrations
DISCUSSION
Our study results demonstrated that most of the New Jersey medical centers that were evaluated currently use the bolus method of magnesium sulfate administration. The weight-based doses differed within institutions depending on the ordering emergency department physician; however, dosages were within the previously published range of 25 to 100 mg/kg/dose3–7 and also reflect what is currently recommended within the Pediatric & Neonatal Dosage Handbook.9 Medical center D has adopted a policy of using continuous infusion magnesium sulfate in its PICU, while not routinely using a bolus dose in the emergency department.
Despite the lack of concrete data regarding its mechanism of action, intravenous magnesium sulfate has been used to treat patients with asthma for more than 25 years, with the first study being published in 1987 by Okayama and colleagues.13 They evaluated the bronchodilating effects of magnesium sulfate in 10 adult asthmatic patients by measuring lung function after the administration of 250 mg of intravenous magnesium sulfate. Their results led them to conclude that intravenous magnesium sulfate produces rapid and marked bronchodilation when given to patients with mild asthma. This was followed by the first randomized, double-blind, placebo-controlled study by Skobeloff and colleagues,14 which was published in 1989. The researchers randomized β-agonist–resistant patients to receive either a normal saline infusion or an infusion of 1.2 g of magnesium sulfate over a 20-minute period. Similar to their colleagues before, they too demonstrated an improvement in lung function in patients treated with the magnesium sulfate therapy, with minimal adverse drug reactions. Since these first publications in adult patients, there have been a number of studies conducted in pediatric patients that have evaluated a variety of weight-based doses as well as frequencies of magnesium sulfate administration.3–7 Patients in these studies were classified as having moderate to severe asthma, and magnesium sulfate doses ranged from 25 to 100 mg/kg/dose.3–7 Following the practice of the earlier studies, the magnesium sulfate for these patients was given as a single dose over a period of either 20 or 35 minutes. While most studies have demonstrated a clinical benefit when using magnesium sulfate for the treatment of an acute asthma exacerbation, 1 study7 did not reach the same conclusion. This study was unique in that all patients with a moderate to severe asthma exacerbation were randomized to receive either 75 mg/kg/dose of intravenous magnesium sulfate or placebo instead of administering it only to patients who were β2-agonist unresponsive. They found no statistically significant difference in their primary outcome, which was the degree of improvement as assessed by pulmonary index scores over a 120-minute period.
The practice of continuous infusion is supported by 1 retrospective study in children8 as well as some case reports in adult patients15,16 and focuses on maintaining constant magnesium concentrations instead of peaks and troughs. The retrospective chart review, conducted by Glover and colleagues,8 evaluated a total of 40 patients who were treated with intravenous magnesium sulfate for an acute asthma exacerbation. They found that patients who weighed less than or equal to 30 kg received a mean magnesium dose of 21.6 ± 6 mg/kg/hr after receiving a mean bolus dose of 35.3 ± 12.7 mg/kg and patients who weighed more than 30 kg received a mean magnesium dose of 14.6 ± 4.2 mg/kg/hr after receiving a mean bolus dose of 21.9 ± 9.9 mg/kg. Major cardiovascular adverse drug reactions were not reported during magnesium sulfate infusion. Although target magnesium serum concentrations during a continuous infusion have not yet been established, the authors of this study targeted a magnesium serum concentration between 3 and 5 mg/dL. Similarly, medical center D targets magnesium serum concentrations of 3.5 to 5.5 mg/dL.
The chief safety concern during the administration of intravenous magnesium sulfate is hemodynamic instability, mainly hypotension.17 Only 1 patient in our study experienced hypotension during a bolus dose of magnesium sulfate. The patient's 40 mg/kg dose was being infused over a 1-hour period when the patient became hypotensive. This 1-hour infusion time is twice as long as what has been reported in the literature for this indication3–7 as well as the median administration time of the patients evaluated in this study. However, it is still faster than what is recommended in the package insert17 and in the Pediatric & Neonatal Dosage Handbook9 for general intravenous magnesium sulfate administration. This patient's hypotension resolved after slowing the infusion rate and no further intervention was necessary.
Figure 1 demonstrates that pediatric patients in this study received the standard-of-care treatment for an asthma exacerbation, including a short-acting bronchodilator in combination with ipratropium as well as systemic corticosteroids as outlined in the 2007 NIH asthma guidelines.1 Greater than 90% of patients received albuterol plus ipratropium. One patient did not receive the combination and was immediately given continuous albuterol upon presentation to the emergency department and was later admitted to the PICU. In addition, 8 patients received systemic corticosteroids before emergency department admission. All of these patients were eventually admitted to the PICU and 1 patient required intubation. These data indicate that intravenous magnesium sulfate was appropriately used in these 4 medical centers as an adjunctive therapy in patients who did not respond to the standard-of-care treatment options.
Forty of 53 patients required intubation and/or a PICU admission. Since this retrospective chart review did not contain a control group, we were unable to determine if the use of intravenous magnesium sulfate prevented hospital or PICU admissions. We were also limited with data collection from medical center B owing to a change in the computer system. In addition, owing to the lack of patients from medical center B as well as D, 87% of patients included were from medical centers A and C and could be a source of bias in the results. Other limitations include determining the accurate timing of magnesium sulfate administration and the decision regarding disposition of the patient. Many times the decision to hospitalize a patient is made before the administration of intravenous magnesium sulfate. This decision process may also be reflected in the results of the secondary outcome that looked at the total number of patients hospitalized. In this study, only 3 patients (5.6%) were discharged home after receiving intravenous magnesium sulfate.
Another secondary outcome evaluated was mean magnesium serum concentrations. When comparing the magnesium serum concentrations that were obtained, it is evident that this practice also differs among the medical centers. Medical centers A and C both administer intravenous magnesium sulfate as a bolus dose; however, medical center A does not routinely check magnesium serum concentrations, while medical center C does. Across all 4 medical centers, most patients had a measured serum concentration above the normal value of 1.5 to 2.5 mg/dL. The significance of these higher serum concentrations, as well as what the target serum concentration should be, still requires further investigation.
Prescribing practices and monitoring of intravenous magnesium sulfate varies among institutions in New Jersey. Regardless of the regimen, administration of intravenous magnesium sulfate for the treatment of an asthma exacerbation in pediatric patients was generally well tolerated. Further prospective studies are needed to determine the optimal dosage regimen of magnesium sulfate by using objective measures and disposition of patients, based on dose and regimen of magnesium sulfate that was administered.
ABBREVIATIONS
- NIH
National Institutes of Health
- PICU
pediatric intensive care unit
Footnotes
DISCLOSURE The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
REFERENCES
- 1.Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Bethesda, MD: National Heart, Lung, and Blood Institute, National Asthma Education and Prevention Program; 2007. NIH publication 07-4051. [Google Scholar]
- 2.Asthma in the US: growing every year. Centers for Disease Control and Prevention. 2011. http://www.cdc.gov/vitalsigns/Asthma/. Accessed January 22, 2014.
- 3.Ciarallo L, Sauer AH, Shannon MW. Intravenous magnesium therapy for moderate to severe pediatric asthma: results of a randomized, placebo-controlled trial. J Pediatr. 1996;129(6):809–814. doi: 10.1016/s0022-3476(96)70023-9. [DOI] [PubMed] [Google Scholar]
- 4.Devi PR, Kumar L, Singhi SC et al. Intravenous magnesium sulfate in acute severe asthma not responding to conventional therapy. Indian Pediatr. 1997;34(5):389–397. [PubMed] [Google Scholar]
- 5.Gurkan F, Haspolat K, Bosnak M et al. Intravenous magnesium sulphate in the management of moderate to severe acute asthmatic children nonresponding to conventional therapy. Eur J Emerg Med. 1999;6(3):201–205. doi: 10.1097/00063110-199909000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Ciarallo L, Brousseau D, Reinert S. Higher-dose intravenous magnesium therapy for children with moderate to severe acute asthma. Arch Pediatr Adolesc Med. 2000;154(10):979–983. doi: 10.1001/archpedi.154.10.979. [DOI] [PubMed] [Google Scholar]
- 7.Scarfone RJ, Loiselle JM, Joffe MD et al. A randomized trial of magnesium in the emergency department treatment of children with asthma. Ann Emerg Med. 2000;36(3):572–578. doi: 10.1067/mem.2000.111060. [DOI] [PubMed] [Google Scholar]
- 8.Glover ML, Machado C, Totapally BR. Magnesium sulfate administered via continuous intravenous infusion in pediatric patients with refractory wheezing. J Crit Care. 2002;17(4):255–258. doi: 10.1053/jcrc.2002.36759. [DOI] [PubMed] [Google Scholar]
- 9.Taketomo CK, Hodding JH, Kraus DM, editors. Pediatric & Neonatal Dosage Handbook. 19th ed. Hudson, OH: Wolters Kluwer Health; 2012. Magnesium sulfate; pp. 1059–1061. [Google Scholar]
- 10.Skobeloff EM. An ion for the lungs. Acad Emerg Med. 1996;3(12):1082–1084. doi: 10.1111/j.1553-2712.1996.tb03363.x. [DOI] [PubMed] [Google Scholar]
- 11.Rolla G, Bucca C, Bugiani M et al. Reduction of histamine-induced bronchoconstriction by magnesium in asthmatic subjects. Allergy. 1987;42(3):186–188. doi: 10.1111/j.1398-9995.1987.tb02198.x. [DOI] [PubMed] [Google Scholar]
- 12.Del Castillo J, Engbaek L. The natures of the neuromuscular block produced by magnesium. J Physiol. 1954;124(2):370–384. doi: 10.1113/jphysiol.1954.sp005114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okayama H, Aikawa T, Okayama M et al. Bronchodilating effect of intravenous magnesium sulfate in bronchial asthma. JAMA. 1987;257(8):1076–1078. [PubMed] [Google Scholar]
- 14.Skobeloff EM, Spivey WH, McNamara RM, Greenspon L. Intravenous magnesium sulfate for the treatment of acute asthma in the emergency department. JAMA. 1989;262(9):1210–1213. [PubMed] [Google Scholar]
- 15.Mills R, Leadbeater M, Ravalia A. Intravenous magnesium sulphate in the management of refractory bronchospasm in a ventilated asthmatic. Anaesthesia. 1997;52(8):776–785. doi: 10.1111/j.1365-2044.1997.176-az0312.x. [DOI] [PubMed] [Google Scholar]
- 16.Sydow M, Crozier TA, Zielmann S. High-dose intravenous magnesium sulfate in the management of life-threatening status asthmaticus. Intensive Care Med. 1993;19(8):467–471. doi: 10.1007/BF01711089. [DOI] [PubMed] [Google Scholar]
- 17.Schaumburg, IL: APP Pharmaceuticals, LLC; 2008. Magnesium Sulfate [package insert] [Google Scholar]


