Abstract
Nonalcoholic fatty liver disease (NAFLD) and cardiovascular disease (CVD) are two diseases that are common in the general population. To date, many studies have been conducted and demonstrate a direct link between NAFLD and CVD, but the exact mechanisms for this complex relationship are not well established. A systematic search of the PubMed database revealed that several common mechanisms are involved in many of the local and systemic manifestations of NAFLD and lead to an increased cardiovascular risk. The possible mechanisms linking NAFLD and CVD include inflammation, oxidative stress, insulin resistance, ectopic adipose tissue distribution, dyslipidemia, endothelial dysfunction, and adiponectin, among others. The clinical implication is that patients with NAFLD are at an increased risk of CVD and should undergo periodic cardiovascular risk assessment.
Keywords: Nonalcoholic fatty liver disease, Cardiovascular disease, Metabolic syndrome, Risk assessment
Core tip: The link between nonalcoholic fatty liver disease (NAFLD) and cardiovascular disease (CVD) should be carefully evaluated in future research, which represents an intriguing field of investigation. A better understanding of the role of inflammation, oxidative stress, and insulin resistance, among other mechanisms, may be necessary to clarify the pathogenesis of NAFLD and CVD, and thereby contribute to the development of new therapies.
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is a common liver disease seen in clinical practice. It comprises a wide disease spectrum ranging from simple steatosis to nonalcoholic steatohepatitis (NASH), which can progress to end stage liver disease, cirrhosis and hepatocellular carcinoma[1]. Initially, NAFLD was considered a benign liver disease, but it is now regarded as the liver manifestation of metabolic syndrome (MetS), a highly atherogenic condition. Most patients are overweight or obese with insulin resistance (IR), hypertension, and dyslipidemia. When compared with control subjects who do not have NAFLD, patients with NAFLD have a higher prevalence of atherosclerosis, which is independent of obesity and other established risk factors[2]. The role of NAFLD as a potential independent cardiovascular disease (CVD) risk factor has recently gained considerable prominence. The purpose of this review is to discuss the pathogenesis of NAFLD, the relationship between NAFLD and CVD, the mechanisms that link both conditions, and the clinical implications that may influence NAFLD and risk of CVD.
NAFLD, METS AND DIABETES
In general, NAFLD is diagnosed based on the following criteria: liver biopsy showing steatosis in at least 5% of hepatocytes or imaging study confirmation; exclusion of liver disease of other etiology, including alcohol-induced liver disease (history of excessive alcohol consumption greater than 20 g/d), drug-induced liver disease, autoimmune or viral hepatitis, as well as cholestatic or metabolic/genetic liver disease[3]. A joint interim statement of the International Diabetes Federation defines MetS with three of the five criteria, including elevated waist circumference, elevated triglycerides, reduced high-density lipoprotein (HDL), elevated blood pressure, and elevated fasting-glucose levels[4]. Approximately 90% of patients with NAFLD have at least one of the features of MetS, and approximately 33% meet the complete diagnosis, placing NAFLD as the hepatic representation of MetS[5]. The prevalence of metabolic abnormalities such as diabetes and hypertension were increased up to 15-fold in patients with NASH compared to steatosis independent of age or body mass index (BMI). The relationship between diabetes and NAFLD remains poorly understood; thus, further prospective study of the association and outcomes should be performed. NAFLD predicts the development of type 2 diabetes mellitus (T2 DM) and vice versa, and each condition may serve as a progression factor for the other. In a recent study of T2 DM patients with an average BMI of 36 kg/m2, over 60% of patients who underwent weight reduction surgery (n = 64 of 92) had moderate to severe NAFLD on liver biopsy[6]. A study by Leite et al[7] in patients with T2 DM found that three of the 92 patients had histological evidence of cirrhosis secondary to NAFLD without clinical evidence of liver disease. To approach the relationship between NAFLD and diabetes from another perspective, multiple studies supported by a recent meta-analysis have shown that NAFLD was associated with IR and diabetes and that NAFLD presence predicted the development of diabetes[8]. In a Korean study of 11091 individuals without diabetes at baseline, where fasting insulin levels were also measured and divided into quartiles, the 5-year (crude) OR for developing T2 DM in the presence of ultrasound evidence of fatty liver at baseline was 5.05 (95%CI: 2.08-12.29) for the lowest insulin quartile and was 6.34 (95%CI: 3.58-11.21) for the highest insulin quartile[9]. After multivariate adjustment, including for baseline glucose level, the OR for the highest insulin quartile remained significant at 2.42 (95%CI: 1.23-4.75)[10].
NAFLD AND CARDIOVASCULAR DISEASE PREVALENCE
The clinical manifestations of NAFLD, such as steatosis and inflammation, are additional risk factors of CVD, although the precise mechanisms by which NAFLD contributes to CVD are still the subject of ongoing research. The age of onset of CVD events in NAFLD patients ranged from 45 to 65 years[11]. All had significantly higher estimated CVD risk at 10 years (17% vs 10%) by the Framingham risk score (FRS) than NAFLD patients without new CVD events[12]. The mortality rate among patients with NAFLD followed for 8 years was higher than that in the general population. In another study consisting of biopsy-diagnosed NAFLD patients who were followed for 18 years, CVD was among the common causes of death after all of the cancers combined[13].
Clinical studies
Table 1 summarized several latest clinical studies on the relationship between NAFLD and CVD. Targher et al[9] showed a significant increase of atrial fibrillation (AF) in the presence of NAFLD (retrospective, n = 702, Caucasians). Lu et al[8] suggested that NAFLD was a strong independent predictor of CVD and may play a central role in the cardiovascular risk of MetS. DeFilippis et al[10] showed that CT-diagnosed NAFLD was associated with the atherogenic dyslipidemia phenotype in a dose dependent fashion. These relationships persisted after adjustment for several metabolic risk factors and HOMA-IR, suggesting a possible independent pathophysiologic role between NAFLD and dyslipidemia (prospective, n = 3362, Caucasian, Chinese, African American, Hispanic). Feitosa et al[11] showed that ALT (≥ 40 U/L) was a predictor of prevalent coronary heart disease (CHD) in men but not in women, while CT measured liver fat (FL) was not significant in either sex (prospective, n = 2756, European-American). Akın et al[12] showed that obese children and adolescents with NAFLD are at an risk of early atherosclerotic changes. As liver function tests are not sufficient to identify patients with fatty liver, ultrasonographic evaluation of NAFLD might be considered in all obese children and adolescents (cross-sectional, n = 157, Turkey). Catena et al[14] showed that in essential hypertensive patients without additional cardiovascular risk factors, NAFLD is associated with IR but not with increased arterial stiffness (observational, cross-sectional, n = 68, Italy). Colak et al[15] showed that NAFLD is associated with earlier endothelial dysfunction in patients with atherosclerosis compared to control subjects (observational case-control, n = 51, Turkey). Based on the evidence enumerated above, NAFLD patients have higher incidence of AF, atherosclerotic changes, dyslipidemia and coronary heart disease. Therefore, our primary goal was to systematically evaluate the possible mechanisms linking NAFLD and CVD, their implication in clinical practice and various treatment modalities.
Table 1.
Published studies on the association between nonalcoholic fatty liver disease and cardiovascular disease
| Ref. | Population sample size | Diagnosis methods | Outcomes | Main results |
| Targher et al[9] | 702 patients with T2 DM | Liver ultrasound | AF | NAFLD is strongly associated with an increased prevalence of persistent and permanent AF in patients with T2 DM |
| Lu et al[8] | 7042 participants | Liver ultrasound and CT | c-IMT and CAD | NAFLD was significantly associated with cardiovascular outcomes independent of conventional risk factors |
| Defilippis et al[10] | 3362 subjects aged 45-84 yr | CT | Atherogenic dyslipidemia | CT-diagnosed NAFLD was associated with atherogenic dyslipidemia even after adjustment for several metabolic risk factors |
| Feitosa et al[11] | 2756 subjects | CT and elevated ALT | CHD | FL and ALT (> 40 U/L) were each individually associated with prevalent CHD. However, when accounting for traditional metabolic risk factors in a multivariate model FL and no predictive value for CHD |
| Akin et al[12] | 157 obese patients | Liver ultrasound | c-IMT | Obese patients with NAFLD had markedly increased c-IMT than those without NAFLD |
| Catena et al[14] | 68 patients with essential hypertension | Liver ultrasound | AASI | In hypertensive patients, AASI and symmetric AASI were higher than in normotensive subjects (P < 0.001), but both indices of vascular stiffness were comparable in patients with and without NAFLD |
| Colak et al[15] | 51 patients in study group and 21 in control group | Liver biopsy | c-IMT | C-IMT was significantly higher in patients with NAFLD group(P < 0.001) |
T2 DM: Type 2 diabetes mellitus; AF: Atrial fibrillation; c-IMT: Carotid intima media thickness; FT: Fatty liver; CHD: Coronary heart disease; CAD: Coronary atherogenic dyslipidemia; AASI: Ambulatory arterial stiffness index.
Classical and new emerging risk factors
The new risk factors for CVD include markers such as inflammation (e.g., C-reactive protein, lipoprotein A), homocystine, and markers of fibrinolytic and homeostatic function [e.g., fibrinogen, tissue plasminogen activator, and plasminogen activator inhibitor-1 (PAI-1)]. These markers are also associated with NAFLD[13]. A case-control study of 35 patients diagnosed with NAFLD by liver biopsy and 45 healthy controls showed that the plasma homocysteine level was higher in NAFLD patients compared to the control group (P = 0.0341)[16]. The classic common risk factors for NAFLD and CVD are age and gender, physical inactivity, T2DM, hyperlipidemia, obesity, and hypertension[16].
POSSIBLE MECHANISMS LINKING NAFLD AND CVD
The association of NAFLD with MetS and diabetes maybe partially explain the increased risk of CVD with NAFLD. Additionally, several studies showed that NAFLD in itself may contribute to the increased risk of CVD[17-19]. However, the exact mechanisms for this complex relationship are not clear. It is likely that several highly interrelated factors contribute to the enhanced risk of diabetes and metabolic syndrome in persons with NAFLD. The following factors are possible explanations of underlying mechanisms of the association between CVD and NAFLD.
Oxidative stress and inflammation
It has recently been shown that NAFLD is an independent risk factor for death from CVD. Although the precise mechanism linking NAFLD and CVD is unclear, a systematic review and meta-analysis have shown that a marker of NAFLD (and oxidative stress) may be the key link between NAFLD and CVD[20]. These data suggest that some component of oxidative stress, perhaps induced by the disease process in NAFLD, may be involved in the pathogenesis of CVD. Oxidative stress plays an important role in the progression from simple steatosis to steatohepatitis[15]. The association between oxidative stress and NAFLD in humans has been demonstrated by the immunohistochemical detection of lipid peroxidation products and 8-hydroxy-deoxyguanosine in the plasma and liver biopsies from patients with NAFLD[21]. Inflammation is crucial in the pathogenesis of NAFLD, and adipose tissue is now considered a metabolically active endocrine organ that produces pro-inflammatory cytokines, including TNF-α, IL-6, C-reactive protein (CRP) and IL-8. There is also evidence to support the activation of other inflammatory pathways, oxidative stress, as well as de novo pathways by TNF-α. A cross-sectional survey of 360 people indicated that an increase in CRP (OR = 1.37; 95%CI: 1.06-1.77) per 1 SD (1.48 mg/L) was an independent risk factor for NAFLD[21].
IR
Increased IR is an undisputed major contributor to NAFLD, MetS, and atherosclerosis. In fact, liver fat content appears to be the best independent predictor of IR in skeletal muscle, adipose tissue, and the liver[12]. Similarly, adverse CV outcome is likely to be associated with liver fat/inflammation in a monotonic relationship, progressively increasing with more advanced stages of NAFLD. While IR promotes fatty acid accumulation in the liver, the latter causes hepatic IR characterized by a lack of suppression of endogenous liver glucose production[22]. Therefore, NAFLD might act as a stimulus for further increased whole-body IR and dyslipidemia (with a characteristic overproduction of triglyceride- and cholesterol-rich remnant particles), leading to accelerated atherosclerosis[1].
Visceral fat
Individuals with obesity have large amounts of visceral adipose tissue (VAT). VAT is a metabolically active endocrine organ that can secrete pro-inflammatory cytokines, adipokines and hormones that mediate inflammation and IR, which, in turn, affect CV risk factors[23]. VAT is defined as intra-abdominal fat bounded by parietal peritoneum or transversalis fascia. Recently, increased VAT assessed by CT showed a significant association with CVD, which was defined by the presence of plaque calcification[24,25]. However, the mechanisms linking visceral fat or obesity to CV disease are strongly related to IR, which itself is robustly associated with CV risk and atherosclerosis, already reviewed in detail[26]. It is therefore unclear whether VAT actually confers direct CV risk through secreted factors, or indirectly via IR-related processes, or both[16]. Further studies to identify the association between NAFLD and CVD through VAT should be considered.
Ectopic adipose tissue distribution
In keeping with the adverse cardiovascular effects of ectopic hepatic fat, recent studies have also described the effects of ectopic fat accumulation in the heart, which correlates with visceral fat, IR and MetS parameters[27]. Additionally, ectopic hepatic fat has been shown to be independently associated with NAFLD[28]. In a recent meta-analysis of 16 studies (n = 2872), epicardial fat thickness or volume was significantly associated with the presence of coronary artery disease[2,29].
Adiponectin
Mature adipocytes act as an active endocrine and paracrine organ, secreting an increasing number of growth factors that participate in diverse metabolic processes, particularly IR[4,30,31]. Patients with NAFLD exhibit reduced levels of adiponectin, which are inversely correlated with the severity of NAFLD histology[32]. The reduced production of adiponectin associated with obesity may contribute to the progression of NAFLD[33].
Dyslipidaemia
Dyslipidaemia causes upregulation of the transcription factor sterol regulatory element binding protein-1c (SREBP-1c), and both insulin and SREBP-1c synergistically stimulate genes involved in de-novo lipogenesis. SREBP-1c also causes inhibition of FFA oxidation, leading to increased hepatic lipid content[34-36]. To compensate for the increased hepatic triglycerides, the liver forms an atherogenic lipid profile, consisting of high TG levels, low high-density lipoprotein (HDL) cholesterol, increased small, dense low-density lipoprotein (LDL) particles, increased very low-density lipoprotein (VLDL) cholesterol levels and elevated apolipoprotein B100 concentration; all of which are strongly associated with adverse cardiovascular outcomes[37-39].
Postprandial hyperlipidemia
NAFLD may be linked to accelerated atherogenesis through the presence of abnormal lipoprotein metabolism, especially during the post-prandial phase[11]. Postprandial hyperlipidemia is a risk factor for both NAFLD and CVD[40-42]. The atherosclerotic risk of postprandial hyperlipidemia is derived from an increase of remnant lipoproteins (RLPs)[15]. In patients with IR, an increase of postprandial RLP values usually occurs and becomes a risk factor for coronary heart disease[43-46]. Swarbrick et al[47] showed that consumption of fructose-sweetened, but not glucose-sweetened, beverages for 10 wk increases de novo lipid synthesis as well as the 24-h postprandial TG, which includes increased levels of apoB, LDL, oxidized LDL, RLP triglyceride, and the apoB/apoA1 ratio (all biomarkers were increased for CVD). Therefore, postprandial hyperlipidaemia may explain, at least in part, why some lean, overweight, and obese individuals with NAFLD may encounter CVD despite normal fasting lipid profiles or taking lipid-lowering medication[48-51].
Endothelial dysfunction
Endothelial dysfunction is now recognized as the earliest detectable component in the development of atherosclerosis. In both diabetic and non-diabetic cohorts, studies have shown an independent association between impaired endothelium-dependent flow-mediated dilation (FMD) and NAFLD. In addition, lower FMD was observed in NASH compared with simple steatosis, again confirming the graded association of CV risk with severity of NAFLD[52-54].
Chronic kidney disease
NAFLD may also be associated with a detrimental effect on other organs that may have a direct or indirect influence on CVD or organs that may accelerate the presentation of CVD[55]. For example, NAFLD has been shown to be associated with the development of chronic kidney disease (CKD) in Korean individuals. Therefore, NAFLD may indirectly modulate the risk of CVD through CKD[56].
Obstructive sleep apnea
Obstructive sleep apnea (OSA) is characterized by loud and frequent snoring, periods of apnea during sleep, and excessive day somnolence[9,12,57]. Interestingly, OSA is also regarded as one of the factors that accelerate the progression of NAFLD to NASH. Importantly, a considerable number of studies have shown an increase in the incidence of CVD in people with OSA. Animal studies have shown that OSA can lead to an increase in IR and alterations in lipid metabolism in the presence of NAFLD[14]. The conclusion of that study was that OSA is associated with an increased risk of CVD, which has also been demonstrated in epidemiological, clinical and physiological studies[22].
Pro-coagulation and hypofibrinolysis
The prothrombotic state in the atherosclerosis process encompasses platelet hyper-aggregability, hypercoagulability and hyper-fibrinolysis[14,58,59]. Markers of fibrinolytic and hemostatic function (e.g., fibrinogen, tissue plasminogen activator, and PAI-1-antigens) are strongly associated with NAFLD. PAI-1 is expressed in visceral adipose tissue. Plasma PAI-1 levels are more closely related to fat accumulation and PAI-1 expression in the liver than in adipose tissue, suggesting that, among insulin-resistant individuals, fatty liver is an important site of PAI-1 production. Fibrinogen, von Willebrand factor (vWF) and PAI-1 are also considered markers of the acute-phase reaction of inflammation and thrombosis, and have been closely linked to CVD[7,59-62].
CLINICAL IMPLICATIONS
It is evident that patients with NASH are more prone to developing CVD (increased mortality by 86%) than patients with simple steatosis (increased mortality by 55%). We suggest adding a new modality of approaching patients with NAFLD[23]. The primary objective of any NAFLD therapy is to improve steatohepatitis and fibrosis, with the ultimate goal of preventing CVD and liver-related death.
Once the diagnosis of NAFLD is made, the first step will be a lifestyle intervention using a combination of diet, active walking, and behavior modification, with a goal of a 10% weight reduction[10]. Recently, Koskinen et al[63] showed that modest wine drinking (20-30 g/daily) offers protection against suspected NAFLD. Lifestyle modification remains the cornerstone of management. Weight loss and increased physical activity are effective mediators of NAFLD, and their role in CVD risk reduction is well established. However, before initiating any significant increase in exercise level, patients at risk should be evaluated for underlying CVD.
The second step is to assess the risk of hepatic fibrosis. The noninvasive methods for fibrosis evaluation include plasma cytokeratine 18 fragments and Angulo score[9]. The invasive method (liver biopsy) remains the only reliable means to determine prognosis based on the severity of fibrosis.
The third step will include the assessment of cardiovascular risk stratification. We suggest the use of measurements of the carotid arteries (IMT) in non-diabetic NAFLD patients and/or the Framingham score with an effort test, as well as biomarkers of inflammation (C-reactive protein, fibrinogen), oxidative stress, (MDA, Paraoxonase), IR (HOMA), lipotoxicity (TG, HDL, LDL, TC), OGTT, and microalbumin/creatinine ratio[13].
The final step is to initiate appropriate therapy according to the comorbidities and the clinical status of each patient[21] (Figure 1).
Figure 1.
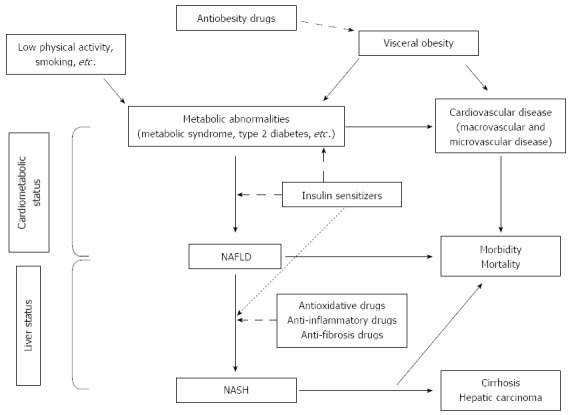
Evaluated treatments for nonalcoholic fatty liver disease. NAFLD: Nonalcoholic fatty liver disease; NASH: Nonalcoholic steatohepatitis.
Insulin sensitizers
Metformin was associated with improvement in hepatocellular ballooning, but not fibrosis, steatosis, inflammation or NAFLD activity score (NAS). TZDs should be reserved for second-line treatment in the majority of patients. One exception may be patients with T2DM and NAFLD in whom TZDM therapy may rectify both conditions. TZDs improved insulin sensitivity and steatohepatitis in a large, multicenter randomized controlled trial Pioglitazone or Vitamin E for NASH Study (PIVENS). However, we should notice its drawback of lower extremity edema and weight gain (average 2 to 5 kg).
Incretin-based therapies
A direct relationship between the gastrointestinal and endocrine systems has recently been appreciated with the discovery of neuroendocrine hormones known as incretins. The two primary incretins are GLP-1 and glucose-dependent insulinotropic polypeptide (GIP). There is accumulating and convincing pre-clinical evidence that GLP-1 and its analogues have the ability to decrease hepatic steatosis in animal models, suggesting that there is potential for incretin-based medications to reverse or delay progression through the stages of NAFLD to cirrhosis[64]. There is an urgent need for prospective clinical trials designed to scrutinize the potential of these agents for treatment of NAFLD beyond improvements in metabolic parameters, such as weight loss.
Cytoprotective and antioxidant agents
Two randomized trials[65,66] showed that high-dose bile acids are unlikely to provide significant benefit, although their use was routinely advocated.
Two recently published, large, randomized controlled trials, PIVENS and TONIC[67,68], assessed the effect of vitamin E on adult and pediatric NAFLD populations, respectively. Vitamin E treatment resulted in improvements in hepatocellular ballooning and NAS in both trials. These results for vitamin E are quite promising and suggest that patients with biopsy-proven steatohepatitis associated with hepatocellular ballooning (NAS ≥ 4) may benefit from its use.
Antitumour necrosis factor-alpha agents
This type of therapy has been studied in a number of small NAFLD trials, two of which have assessed histological response and demonstrated improvement in steatosis, inflammation and ballooning[69,70].
Lipid-lowering agents
One recently published large study[71] provided compelling evidence that lipid-lowering agents, such as statins, are safe and efficacious in patients with NAFLD/NASH and that the agents can induce a reduction in the extent of the hepatic steatosis.
Phlebotomy
One prospective phase II study[72,73] of phlebotomy with paired liver biopsies that evaluated phlebotomy therapy in NAFLD patients suggested that iron reduction may improve liver histology.
Surgical intervention and anti-obesity drugs
Bariatric surgery is an increasingly popular therapeutic option among morbidly obese patients. One recently published study[71] reported that surgery should complement treatment of obesity-related comorbidity, but not replace established therapy.
Theoretically, improving NAFLD via weight loss is an ideal approach in obese or overweight people because other complications are simultaneously ameliorated with weight loss. Of several commonly used antiobesity medications, orlistat and sibutramine are available for long-term prescription. Harrison[74] reported that subjects who lost ≥ 5% of their body weight over 9 mo experienced improvements in IR and steatosis, while subjects who lost ≥ 9% of their body weight also experienced improvements in hepatic histology[75,76]. Both orlistat and sibutramine have been shown to have beneficial effects on body weight, lipid profiles, glucose metabolism, and inflammatory markers in several trials[77]. However, insufficient safety data are available regarding the long-term outcomes of antiobesity therapy. Indeed, sibutramine was reported to increase blood pressure and heart rate, which may limit its use in clinical practice[76].
CONCLUSION
NAFLD is regarded as the hepatic component of metabolic syndrome and is associated with a high risk of developing CVD. Oxidative stress, inflammation dyslipidemia, IR, visceral fat, low adiponectin, ectopic adipose tissue distribution, endothelial dysfunction and postprandial dyslipidemia are the main factors that lead to and further aggravate the course of NAFLD, as well as accelerate the progress of atherosclerosis and development of CVD. NAFLD patients should be considered candidates not only for aggressive treatment of their liver disease but also for careful monitoring and potential treatment of underlying CVD risk factors because many patients with NAFLD will have major CVD events and die prior to the development of advanced liver disease.
Footnotes
P- Reviewers: Kim SH, Xu Y S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Ma S
References
- 1.Hashizume H, Sato K, Yamazaki Y, Horiguchi N, Kakizaki S, Mori M. A prospective study of long-term outcomes in female patients with nonalcoholic steatohepatitis using age- and body mass index-matched cohorts. Acta Med Okayama. 2013;67:45–53. doi: 10.18926/AMO/49256. [DOI] [PubMed] [Google Scholar]
- 2.Blackett PR, Sanghera DK. Genetic determinants of cardiometabolic risk: a proposed model for phenotype association and interaction. J Clin Lipidol. 2013;7:65–81. doi: 10.1016/j.jacl.2012.04.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nestel PJ, Mensink RP. Perspective: nonalcoholic fatty liver disease and cardiovascular risk. Curr Opin Lipidol. 2013;24:1–3. doi: 10.1097/MOL.0b013e32835c0834. [DOI] [PubMed] [Google Scholar]
- 4.Williams KH, Shackel NA, Gorrell MD, McLennan SV, Twigg SM. Diabetes and nonalcoholic Fatty liver disease: a pathogenic duo. Endocr Rev. 2013;34:84–129. doi: 10.1210/er.2012-1009. [DOI] [PubMed] [Google Scholar]
- 5.Kucukazman M, Ata N, Yavuz B, Dal K, Sen O, Deveci OS, Agladioglu K, Yeniova AO, Nazligul Y, Ertugrul DT. Evaluation of early atherosclerosis markers in patients with nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2013;25:147–151. doi: 10.1097/MEG.0b013e32835a58b1. [DOI] [PubMed] [Google Scholar]
- 6.Gökçe S, Atbinici Z, Aycan Z, Cınar HG, Zorlu P. The relationship between pediatric nonalcoholic fatty liver disease and cardiovascular risk factors and increased risk of atherosclerosis in obese children. Pediatr Cardiol. 2013;34:308–315. doi: 10.1007/s00246-012-0447-9. [DOI] [PubMed] [Google Scholar]
- 7.Leite NC, Villela-Nogueira CA, Pannain VL, Bottino AC, Rezende GF, Cardoso CR, Salles GF. Histopathological stages of nonalcoholic fatty liver disease in type 2 diabetes: prevalences and correlated factors. Liver Int. 2011;31:700–706. doi: 10.1111/j.1478-3231.2011.02482.x. [DOI] [PubMed] [Google Scholar]
- 8.Lu H, Liu H, Hu F, Zou L, Luo S, Sun L. Independent Association between Nonalcoholic Fatty Liver Disease and Cardiovascular Disease: A Systematic Review and Meta-Analysis. Int J Endocrinol. 2013;2013:124958. doi: 10.1155/2013/124958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Targher G, Mantovani A, Pichiri I, Rigolon R, Dauriz M, Zoppini G, Morani G, Vassanelli C, Bonora E. Non-alcoholic fatty liver disease is associated with an increased prevalence of atrial fibrillation in hospitalized patients with type 2 diabetes. Clin Sci (Lond) 2013;125:301–309. doi: 10.1042/CS20130036. [DOI] [PubMed] [Google Scholar]
- 10.DeFilippis AP, Blaha MJ, Martin SS, Reed RM, Jones SR, Nasir K, Blumenthal RS, Budoff MJ. Nonalcoholic fatty liver disease and serum lipoproteins: the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2013;227:429–436. doi: 10.1016/j.atherosclerosis.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feitosa MF, Reiner AP, Wojczynski MK, Graff M, North KE, Carr JJ, Borecki IB. Sex-influenced association of nonalcoholic fatty liver disease with coronary heart disease. Atherosclerosis. 2013;227:420–424. doi: 10.1016/j.atherosclerosis.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akın L, Kurtoglu S, Yikilmaz A, Kendirci M, Elmalı F, Mazicioglu M. Fatty liver is a good indicator of subclinical atherosclerosis risk in obese children and adolescents regardless of liver enzyme elevation. Acta Paediatr. 2013;102:e107–e113. doi: 10.1111/apa.12099. [DOI] [PubMed] [Google Scholar]
- 13.Huang Y, Bi Y, Xu M, Ma Z, Xu Y, Wang T, Li M, Liu Y, Lu J, Chen Y, et al. Nonalcoholic fatty liver disease is associated with atherosclerosis in middle-aged and elderly Chinese. Arterioscler Thromb Vasc Biol. 2012;32:2321–2326. doi: 10.1161/ATVBAHA.112.252957. [DOI] [PubMed] [Google Scholar]
- 14.Catena C, Bernardi S, Sabato N, Grillo A, Ermani M, Sechi LA, Fabris B, Carretta R, Fallo F. Ambulatory arterial stiffness indices and non-alcoholic fatty liver disease in essential hypertension. Nutr Metab Cardiovasc Dis. 2013;23:389–393. doi: 10.1016/j.numecd.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Colak Y, Senates E, Yesil A, Yilmaz Y, Ozturk O, Doganay L, Coskunpinar E, Kahraman OT, Mesci B, Ulasoglu C, et al. Assessment of endothelial function in patients with nonalcoholic fatty liver disease. Endocrine. 2013;43:100–107. doi: 10.1007/s12020-012-9712-1. [DOI] [PubMed] [Google Scholar]
- 16.de Carvalho SC, Muniz MT, Siqueira MD, Siqueira ER, Gomes AV, Silva KA, Bezerra LC, D’Almeida V, de Oliveira CP, Pereira LM. Plasmatic higher levels of homocysteine in non-alcoholic fatty liver disease (NAFLD) Nutr J. 2013;12:37. doi: 10.1186/1475-2891-12-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thakur ML, Sharma S, Kumar A, Bhatt SP, Luthra K, Guleria R, Pandey RM, Vikram NK. Nonalcoholic fatty liver disease is associated with subclinical atherosclerosis independent of obesity and metabolic syndrome in Asian Indians. Atherosclerosis. 2012;223:507–511. doi: 10.1016/j.atherosclerosis.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 18.Arslan U, Kocaoğlu I, Balcı M, Duyuler S, Korkmaz A. The association between impaired collateral circulation and non-alcoholic fatty liver in patients with severe coronary artery disease. J Cardiol. 2012;60:210–214. doi: 10.1016/j.jjcc.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Beaton MD. Current treatment options for nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Can J Gastroenterol. 2012;26:353–357. doi: 10.1155/2012/725468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cakır E, Ozbek M, Colak N, Cakal E, Delıbaşi T. Is NAFLD an independent risk factor for increased IMT in T2DM? Minerva Endocrinol. 2012;37:187–193. [PubMed] [Google Scholar]
- 21.Bhatia LS, Curzen NP, Byrne CD. Nonalcoholic fatty liver disease and vascular risk. Curr Opin Cardiol. 2012;27:420–428. doi: 10.1097/HCO.0b013e328354829c. [DOI] [PubMed] [Google Scholar]
- 22.Severova MM, Saginova EA, Galliamov MG, Ermakov NV, Rodina AV, Fomin VV, Mukhin NA. [Clinicopathogenetic characteristics of cardiorenal syndrome in non-alcoholic fatty liver disease] Ter Arkh. 2012;84:15–20. [PubMed] [Google Scholar]
- 23.Heuer M, Kaiser GM, Kahraman A, Banysch M, Saner FH, Mathé Z, Gerken G, Paul A, Canbay A, Treckmann JW. Liver transplantation in nonalcoholic steatohepatitis is associated with high mortality and post-transplant complications: a single-center experience. Digestion. 2012;86:107–113. doi: 10.1159/000339344. [DOI] [PubMed] [Google Scholar]
- 24.Wang CC, Tseng TC, Hsieh TC, Hsu CS, Wang PC, Lin HH, Kao JH. Severity of fatty liver on ultrasound correlates with metabolic and cardiovascular risk. Kaohsiung J Med Sci. 2012;28:151–160. doi: 10.1016/j.kjms.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown TM. Nonalcoholic fatty liver disease and cardiovascular disease risk. Clin Gastroenterol Hepatol. 2012;10:568–569. doi: 10.1016/j.cgh.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 26.Coracina A, Gaiani S, Cosma A, Pellizzari P, Pizzi C, de Kreutzenberg S, Cecchet D, Sacerdoti D, Tessari P. No association between the degree of liver steatosis and early signs of vasculopathy in T2DM. Nutr Metab Cardiovasc Dis. 2012;22:e11–e12. doi: 10.1016/j.numecd.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 27.Bhatia LS, Curzen NP, Calder PC, Byrne CD. Non-alcoholic fatty liver disease: a new and important cardiovascular risk factor? Eur Heart J. 2012;33:1190–1200. doi: 10.1093/eurheartj/ehr453. [DOI] [PubMed] [Google Scholar]
- 28.Chatrath H, Vuppalanchi R, Chalasani N. Dyslipidemia in patients with nonalcoholic fatty liver disease. Semin Liver Dis. 2012;32:22–29. doi: 10.1055/s-0032-1306423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Treeprasertsuk S, Leverage S, Adams LA, Lindor KD, St Sauver J, Angulo P. The Framingham risk score and heart disease in nonalcoholic fatty liver disease. Liver Int. 2012;32:945–950. doi: 10.1111/j.1478-3231.2011.02753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim D, Choi SY, Park EH, Lee W, Kang JH, Kim W, Kim YJ, Yoon JH, Jeong SH, Lee DH, et al. Nonalcoholic fatty liver disease is associated with coronary artery calcification. Hepatology. 2012;56:605–613. doi: 10.1002/hep.25593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stepanova M, Younossi ZM. Independent association between nonalcoholic fatty liver disease and cardiovascular disease in the US population. Clin Gastroenterol Hepatol. 2012;10:646–650. doi: 10.1016/j.cgh.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 32.Li G, Hu H, Shi W, Li Y, Liu L, Chen Y, Hu X, Wang J, Gao J, Yin D. Elevated hematocrit in nonalcoholic fatty liver disease: a potential cause for the increased risk of cardiovascular disease? Clin Hemorheol Microcirc. 2012;51:59–68. doi: 10.3233/CH-2011-1509. [DOI] [PubMed] [Google Scholar]
- 33.Bonapace S, Perseghin G, Molon G, Canali G, Bertolini L, Zoppini G, Barbieri E, Targher G. Nonalcoholic fatty liver disease is associated with left ventricular diastolic dysfunction in patients with type 2 diabetes. Diabetes Care. 2012;35:389–395. doi: 10.2337/dc11-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahmed MH, Abu EO, Byrne CD. Non-Alcoholic Fatty Liver Disease (NAFLD): new challenge for general practitioners and important burden for health authorities? Prim Care Diabetes. 2010;4:129–137. doi: 10.1016/j.pcd.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 35.Hacihamdioğlu B, Okutan V, Yozgat Y, Yildirim D, Kocaoğlu M, Lenk MK, Ozcan O. Abdominal obesity is an independent risk factor for increased carotid intima- media thickness in obese children. Turk J Pediatr. 2011;53:48–54. [PubMed] [Google Scholar]
- 36.Dogru T, Genc H, Tapan S, Ercin CN, Ors F, Aslan F, Kara M, Sertoglu E, Bagci S, Kurt I, et al. Elevated asymmetric dimethylarginine in plasma: an early marker for endothelial dysfunction in non-alcoholic fatty liver disease? Diabetes Res Clin Pract. 2012;96:47–52. doi: 10.1016/j.diabres.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 37.Alkhouri N, Kistangari G, Campbell C, Lopez R, Zein NN, Feldstein AE. Mean platelet volume as a marker of increased cardiovascular risk in patients with nonalcoholic steatohepatitis. Hepatology. 2012;55:331. doi: 10.1002/hep.24721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Motta AB. Report of the international symposium: polycystic ovary syndrome: first Latin-American consensus. Int J Clin Pract. 2010;64:544–557. doi: 10.1111/j.1742-1241.2009.02259.x. [DOI] [PubMed] [Google Scholar]
- 39.Gawrieh S, Baye TM, Carless M, Wallace J, Komorowski R, Kleiner DE, Andris D, Makladi B, Cole R, Charlton M, et al. Hepatic gene networks in morbidly obese patients with nonalcoholic fatty liver disease. Obes Surg. 2010;20:1698–1709. doi: 10.1007/s11695-010-0171-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hessheimer AJ, Forner A, Varela M, Bruix J. Metabolic risk factors are a major comorbidity in patients with cirrhosis independent of the presence of hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 2010;22:1239–1244. doi: 10.1097/MEG.0b013e32833aa19b. [DOI] [PubMed] [Google Scholar]
- 41.Lee YJ, Shim JY, Moon BS, Shin YH, Jung DH, Lee JH, Lee HR. The relationship between arterial stiffness and nonalcoholic fatty liver disease. Dig Dis Sci. 2012;57:196–203. doi: 10.1007/s10620-011-1819-3. [DOI] [PubMed] [Google Scholar]
- 42.Ahmed MH, Barakat S, Almobarak AO. Nonalcoholic fatty liver disease and cardiovascular disease: has the time come for cardiologists to be hepatologists? J Obes. 2012;2012:483135. doi: 10.1155/2012/483135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hurjui DM, Niţă O, Graur LI, Mihalache L, Popescu DS, Huţanaşu IC, Ungureanu D, Graur M. Non-alcoholic fatty liver disease is associated with cardiovascular risk factors of metabolic syndrome. Rev Med Chir Soc Med Nat Iasi. 2012;116:692–699. [PubMed] [Google Scholar]
- 44.Cameron I, Alam MA, Wang J, Brown L. Endurance exercise in a rat model of metabolic syndrome. Can J Physiol Pharmacol. 2012;90:1490–1497. doi: 10.1139/y2012-097. [DOI] [PubMed] [Google Scholar]
- 45.Santos RD, Agewall S. Non-alcoholic fatty liver disease and cardiovascular disease. Atherosclerosis. 2012;224:324–325. doi: 10.1016/j.atherosclerosis.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 46.Hurjui DM, Niţă O, Graur LI, Mihalache L, Popescu DS, Graur M. The central role of the non alcoholic fatty liver disease in metabolic syndrome. Rev Med Chir Soc Med Nat Iasi. 2012;116:425–431. [PubMed] [Google Scholar]
- 47.Swarbrick MM, Stanhope KL, Elliott SS, Graham JL, Krauss RM, Christiansen MP, Griffen SC, Keim NL, Havel PJ. Consumption of fructose-sweetened beverages for 10 weeks increases postprandial triacylglycerol and apolipoprotein-B concentrations in overweight and obese women. Br J Nutr. 2008;100:947–952. doi: 10.1017/S0007114508968252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Senateş E, Colak Y, Yeşil A, Coşkunpinar E, Sahin O, Kahraman OT, Erkalma Şenateş B, Tuncer I. Circulating resistin is elevated in patients with non-alcoholic fatty liver disease and is associated with steatosis, portal inflammation, insulin resistance and nonalcoholic steatohepatitis scores. Minerva Med. 2012;103:369–376. [PubMed] [Google Scholar]
- 49.Vlachopoulos C, Manesis E, Baou K, Papatheodoridis G, Koskinas J, Tiniakos D, Aznaouridis K, Archimandritis A, Stefanadis C. Increased arterial stiffness and impaired endothelial function in nonalcoholic Fatty liver disease: a pilot study. Am J Hypertens. 2010;23:1183–1189. doi: 10.1038/ajh.2010.144. [DOI] [PubMed] [Google Scholar]
- 50.Brandt ML, Harmon CM, Helmrath MA, Inge TH, McKay SV, Michalsky MP. Morbid obesity in pediatric diabetes mellitus: surgical options and outcomes. Nat Rev Endocrinol. 2010;6:637–645. doi: 10.1038/nrendo.2010.167. [DOI] [PubMed] [Google Scholar]
- 51.Fan JG, Zhou Q, Wo QH. [Effect of body weight mass and its change on the incidence of nonalcoholic fatty liver disease] Zhonghua Gan Zang Bing Zazhi. 2010;18:676–679. doi: 10.3760/cma.j.issn.1007-3418.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 52.Rivera R, Vanaclocha F. [Nonalcoholic fatty liver disease and psoriasis] Actas Dermosifiliogr. 2010;101:657–658. [PubMed] [Google Scholar]
- 53.Ashburn DD, Reed MJ. Gastrointestinal system and obesity. Crit Care Clin. 2010;26:625–627. doi: 10.1016/j.ccc.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 54.Roĭtberg GE, Sharkhun OO, Ushakova TI. [Non-alcoholic fatty liver disease as an atherosclerosis risk factor] Eksp Klin Gastroenterol. 2010;(7):20–24. [PubMed] [Google Scholar]
- 55.Kwon YM, Oh SW, Hwang SS, Lee C, Kwon H, Chung GE. Association of nonalcoholic fatty liver disease with components of metabolic syndrome according to body mass index in Korean adults. Am J Gastroenterol. 2012;107:1852–1858. doi: 10.1038/ajg.2012.314. [DOI] [PubMed] [Google Scholar]
- 56.Băloşeanu CL, Streba CT, Vere CC, Comănescu V, Rogoveanu I. Association between liver histology, carotid ultrasonography and retinal vascular changes in patients with nonalcoholic fatty liver disease (NAFLD) Rom J Morphol Embryol. 2012;53:609–614. [PubMed] [Google Scholar]
- 57.Kubes P, Mehal WZ. Sterile inflammation in the liver. Gastroenterology. 2012;143:1158–1172. doi: 10.1053/j.gastro.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 58.Li X, Xia M, Ma H, Hofman A, Hu Y, Yan H, He W, Lin H, Jeekel J, Zhao N, et al. Liver fat content is associated with increased carotid atherosclerosis in a Chinese middle-aged and elderly population: the Shanghai Changfeng study. Atherosclerosis. 2012;224:480–485. doi: 10.1016/j.atherosclerosis.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 59.Yang YY, Huang YT, Lee TY, Chan CC, Yeh YC, Lee KC, Lin HC. Rho-kinase-dependent pathway mediates the hepatoprotective effects of sorafenib against ischemia/reperfusion liver injury in rats with nonalcoholic steatohepatitis. Liver Transpl. 2012;18:1371–1383. doi: 10.1002/lt.23520. [DOI] [PubMed] [Google Scholar]
- 60.Saleh DA, Ismail MA, Ibrahim AM. Non alcoholic fatty liver disease, insulin resistance, dyslipidemia and atherogenic ratios in epileptic children and adolescents on long term antiepileptic drug therapy. Pak J Biol Sci. 2012;15:68–77. doi: 10.3923/pjbs.2012.68.77. [DOI] [PubMed] [Google Scholar]
- 61.Ampuero J, Romero-Gómez M. [Influence of non-alcoholic fatty liver disease on cardiovascular disease] Gastroenterol Hepatol. 2012;35:585–593. doi: 10.1016/j.gastrohep.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 62.Mantovani A, Zoppini G, Targher G, Golia G, Bonora E. Non-alcoholic fatty liver disease is independently associated with left ventricular hypertrophy in hypertensive Type 2 diabetic individuals. J Endocrinol Invest. 2012;35:215–218. doi: 10.1007/BF03345421. [DOI] [PubMed] [Google Scholar]
- 63.Koskinen J, Magnussen CG, Kähönen M, Loo BM, Marniemi J, Jula A, Saarikoski LA, Huupponen R, Viikari JS, Raitakari OT, et al. Association of liver enzymes with metabolic syndrome and carotid atherosclerosis in young adults. The Cardiovascular Risk in Young Finns Study. Ann Med. 2012;44:187–195. doi: 10.3109/07853890.2010.532152. [DOI] [PubMed] [Google Scholar]
- 64.Samson SL, Bajaj M. Potential of incretin-based therapies for non-alcoholic fatty liver disease. J Diabetes Complications. 2013;27:401–406. doi: 10.1016/j.jdiacomp.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 65.Leuschner UF, Lindenthal B, Herrmann G, Arnold JC, Rössle M, Cordes HJ, Zeuzem S, Hein J, Berg T. High-dose ursodeoxycholic acid therapy for nonalcoholic steatohepatitis: a double-blind, randomized, placebo-controlled trial. Hepatology. 2010;52:472–479. doi: 10.1002/hep.23727. [DOI] [PubMed] [Google Scholar]
- 66.Ratziu V, de Ledinghen V, Oberti F, Mathurin P, Wartelle-Bladou C, Renou C, Sogni P, Maynard M, Larrey D, Serfaty L, et al. A randomized controlled trial of high-dose ursodesoxycholic acid for nonalcoholic steatohepatitis. J Hepatol. 2011;54:1011–1019. doi: 10.1016/j.jhep.2010.08.030. [DOI] [PubMed] [Google Scholar]
- 67.Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lavine JE, Schwimmer JB, Van Natta ML, Molleston JP, Murray KF, Rosenthal P, Abrams SH, Scheimann AO, Sanyal AJ, Chalasani N, et al. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA. 2011;305:1659–1668. doi: 10.1001/jama.2011.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Adams LA, Zein CO, Angulo P, Lindor KD. A pilot trial of pentoxifylline in nonalcoholic steatohepatitis. Am J Gastroenterol. 2004;99:2365–2368. doi: 10.1111/j.1572-0241.2004.40064.x. [DOI] [PubMed] [Google Scholar]
- 70.Satapathy SK, Sakhuja P, Malhotra V, Sharma BC, Sarin SK. Beneficial effects of pentoxifylline on hepatic steatosis, fibrosis and necroinflammation in patients with non-alcoholic steatohepatitis. J Gastroenterol Hepatol. 2007;22:634–638. doi: 10.1111/j.1440-1746.2006.04756.x. [DOI] [PubMed] [Google Scholar]
- 71.Nseir W, Mograbi J, Ghali M. Lipid-lowering agents in nonalcoholic fatty liver disease and steatohepatitis: human studies. Dig Dis Sci. 2012;57:1773–1781. doi: 10.1007/s10620-012-2118-3. [DOI] [PubMed] [Google Scholar]
- 72.Dixon JB. Surgical management of obesity in patients with morbid obesity and nonalcoholic fatty liver disease. Clin Liver Dis. 2014;18:129–146. doi: 10.1016/j.cld.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 73.Beaton MD, Chakrabarti S, Levstik M, Speechley M, Marotta P, Adams P. Phase II clinical trial of phlebotomy for non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2013;37:720–729. doi: 10.1111/apt.12255. [DOI] [PubMed] [Google Scholar]
- 74.Harrison AG. Fragmentation reactions of methionine-containing protonated octapeptides and fragment ions therefrom: an energy-resolved study. J Am Soc Mass Spectrom. 2013;24:1555–1564. doi: 10.1007/s13361-013-0706-x. [DOI] [PubMed] [Google Scholar]
- 75.Harrison SA, Fecht W, Brunt EM, Neuschwander-Tetri BA. Orlistat for overweight subjects with nonalcoholic steatohepatitis: A randomized, prospective trial. Hepatology. 2009;49:80–86. doi: 10.1002/hep.22575. [DOI] [PubMed] [Google Scholar]
- 76.Tziomalos K, Krassas GE, Tzotzas T. The use of sibutramine in the management of obesity and related disorders: an update. Vasc Health Risk Manag. 2009;5:441–452. doi: 10.2147/vhrm.s4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Filippatos TD, Derdemezis CS, Gazi IF, Nakou ES, Mikhailidis DP, Elisaf MS. Orlistat-associated adverse effects and drug interactions: a critical review. Drug Saf. 2008;31:53–65. doi: 10.2165/00002018-200831010-00005. [DOI] [PubMed] [Google Scholar]


