Abstract
Gastroenteric tube feeding plays a major role in the management of patients with poor voluntary intake, chronic neurological or mechanical dysphagia or gut dysfunction, and patients who are critically ill. However, despite the benefits and widespread use of enteral tube feeding, some patients experience complications. This review aims to discuss and compare current knowledge regarding the clinical application of enteral tube feeding, together with associated complications and special aspects. We conducted an extensive literature search on PubMed, Embase and Medline using index terms relating to enteral access, enteral feeding/nutrition, tube feeding, percutaneous endoscopic gastrostomy/jejunostomy, endoscopic nasoenteric tube, nasogastric tube, and refeeding syndrome. The literature showed common routes of enteral access to include nasoenteral tube, gastrostomy and jejunostomy, while complications fall into four major categories: mechanical, e.g., tube blockage or removal; gastrointestinal, e.g., diarrhea; infectious e.g., aspiration pneumonia, tube site infection; and metabolic, e.g., refeeding syndrome, hyperglycemia. Although the type and frequency of complications arising from tube feeding vary considerably according to the chosen access route, gastrointestinal complications are without doubt the most common. Complications associated with enteral tube feeding can be reduced by careful observance of guidelines, including those related to food composition, administration rate, portion size, food temperature and patient supervision.
Keywords: Enteral tube feeding, Percutaneous endoscopic gastrostomy, Refeeding syndrome, Enteral nutrition, Buried bumper syndrome, Nasoenteral tubes, Colocutaneous fistulae
Core tip: Keeping up with new developments in the fast-moving field of enteral nutrition is a challenge for any gastroenterologist. While enteral tube feeding plays a major role in the care of critically ill patients and those with poor voluntary intake, chronic neurological or mechanical dysphagia or gut dysfunction, mechanical, gastrointestinal, infectious and metabolic complications can lead to serious conditions or death. We have undertaken a comprehensive review of current literature assessing the safety and effectiveness of various endoscopic, sonographic, radiologic, electromagnetic and fluoroscopic application techniques. In addition, we address prophylactic measures to prevent complications, problem solutions and special aspects.
INTRODUCTION
Enteral nutritional support plays a very significant part in the management of patients with poor voluntary oral intake[1,2], chronic neurological or mechanical dysphagia[3-5], or intestinal failure[6,7], and in the critically ill[8,9]. Enteral feeding is not only more physiological than parenteral nutrition (PN), but has also been shown to improve patient outcomes, decrease costs[10] and reduce septic complications[11,12] in comparison to PN. In a meta-analysis of 82 randomized controlled trials (RCTs), PN was associated with a significantly increased risk of infectious complications, especially in patients receiving therapy for malignancy[13]. In patients who cannot be fed using routine bedside methods, endoscopically or radiologically-aided methods must be employed to achieve enteral access. Complicated access situations require the additional expertise of a gastroenterologist, who is an integral part of the nutrition support team[14]. While the range and ease of enteral access procedures has been greatly enhanced in recent years by the introduction of new techniques and improved equipment and accessories, many studies have nonetheless demonstrated a high prevalence of tube- and/or feeding-related complications in patients receiving long-term enteral nutrition[15-19].
The purpose of this review is to provide an overview of current knowledge and practice in the rapidly changing and developing field of endoscopic enteral tube feeding (ETF), covering routes of access as well as problems associated with enteral feeding and their solutions.
In principle, tube systems for gastric or jejunal nutrition can be placed by nasal insertion (nasoenteral tubes; NETs), guided percutaneous application, or surgical techniques. Nasal tubes are mainly used for short-term enteral feeding (4-6 wk) and in situations where other methods of enteral feeding are contraindicated. In the long term, however, NETs are often poorly tolerated by the conscious patient, since they not only elicit a foreign body sensation in the pharynx, but may also cause reflux esophagitis and pressure ulcers, and have a tendency to dislocate. NETs can also be a source of psychological stress to the patient, the presence of the tube being a visible sign of his/her illness. Enteral feeding via transnasal tubes is often poorly tolerated by geriatric patients with an acute confusional state, and the need for repeated insertion of tubes following voluntary removal by the patient is demanding and time-consuming for nursing staff. Nasal tubes are not suitable for patients who are to undergo orofacial therapy for potentially reversible dysphagia (in most cases due to cerebrovascular accident). Furthermore, the presence of a nasal tube significantly interferes with swallowing training[20].
Techniques for achieving NET placement include unguided bedside insertion or placement under fluoroscopic, endoscopic, electromagnetic or direct surgical guidance. Depending on experience, the success rate of endoscopic transnasal and transoral NET feeding tube placement has been described to range from 86% to 97%[21].
Blind insertion, the most common technique for nasoenteral intubation, results in malposition in 0.5%-16% of cases, with tracheal, pulmonary, or pleural malposition in 0.3%-15%. This may result in pulmonary or pleural formula infusion, pneumothorax or pulmonary abscess[22]. A study by de Aguilar-Nascimento and Kudsk[23] demonstrated that of 932 blind postpyloric tube placement attempts, 433 (46%) failed and 20 (1.6%) were airway misplacements. Air instillation and auscultation are inaccurate methods for validating the position, and misplacement is often not suspected unless a radiograph is obtained[24,25].
After gastric positioning, spontaneous or prokinetically-assisted transpyloric tube migration occurs only in 5%-15% of patients, compared to 14%-60% using guided assistance. Duodenal intubation can successfully be achieved in 70%-93% of patients using right lateral positioning, gastric insufflation, tube tip angulation, and clockwise rotation during insertion. However, such tube placements require experience and an average of 28-40 min to perform[26]. In a recent study of 616 patients in an intensive care unit (ICU), Rivera et al[27] demonstrated that the use of an electromagnetic tube placement device (ETPD) to monitor tip position of the feeding tube resulted in no adverse events, reduced costs, and earlier initiation of EN.
Failure of bedside NET placement is an indication for the use of fluoroscopy or endoscopy. Enteral intubation under fluoroscopic guidance is successful in 90% of cases, achieving jejunal positioning in 53%, but requires on average 22 min of fluoroscopy room time[28]. While endoscopically-guided (ENET) nasoenteral access techniques have been in use since 1984[29], ENET placement can be very tedious and unrewarding for the endoscopist when compared to other procedures such as polypectomies, PEG or endoscopic retrograde cholangiopancreatography (ERCP). Most endoscopy training programs do not adequately teach techniques for ENET placement. However, a lack of perseverance on the part of the endoscopist may result in unnecessary administration of total parenteral nutrition (TPN) in patients who are in fact suited for enteral feeding. The positioning of feeding tubes distal of the ligament of Treitz may cause some uncertainty even amongst experienced endoscopists[30]. A new, recently-described nasoendoscopical placement method shows promise as a more successful technique for tube placement beyond the duodenal flexure[31-33].
One in ten patients experiences procedure-related complications following NET[34], either at the time of insertion or subsequently. Reported complications of nasal tube feeding include nasopharyngeal lesions, sinusitis, aspiration, diarrhea, intestinal ischemia and metabolic derangements. Aspiration is reported in up to 89% of patients, with no clear advantage of nasoenteric over gastroenteric feeding. Distal duodenal or jejunal feeding may prevent regurgitation of feeding formula[24]. About 2%-5% of patients may present with epistaxis following ENET placement[35,36], a complication shown to be equally common in patients undergoing transnasal vs transoral endoscopy for ENET placement[37]. Tube-related complications (Table 1) depend not only on the route of enteral access, but also on the material and diameter of the feeding tube.
Table 1.
Tube-related complications of enteral tube feeding[203]
| Mechanical complications | Tube obstruction |
| Primary malposition | |
| Perforation of the intestinal tract | |
| Secondary displacement of the feeding tube | |
| Knotting of the tube | |
| Accidental tube removal | |
| Breakage and leakage of the tube Leakage and bleeding from insertion site | |
| Erosion, ulceration and necrosis of skin and mucosa | |
| Intestinal obstruction (ileus) | |
| Hemorrhage | |
| Inadvertent IV infusion of enteral diet | |
| Infectious complications | Infection at the tube insertion site |
| Aspiration pneumonia | |
| Nasopharyngeal and ear infections | |
| Peritonitis | |
| Infective diarrhea | |
| Metabolic complications | Electrolyte disturbances |
| Hyper- and hypoglycemia | |
| Vitamin and trace element deficiency | |
| Tube feeding syndrome (“Refeeding syndrome”) |
A critical review of pulmonary complications associated with the blind placement of narrow-bore nasoenteric tubes (NETs) was recently performed by Sparks and colleagues[38]. Of the 9931 NET placements reviewed, a total of 187 were improper tube placements in the tracheobronchial tree, translating to a 1.9% mean overall malposition rate. These 187 misplacements included 35 (18.7%) reported pneumothoraces, at least 5 of which resulted in patient death. NET malpositioning was reported in 13%-32% of subsequent repositioning attempts[38].
Occlusion of the NET is an underestimated and underreported complication of ETF. It has been reported to occur in 9%-35% of patients[36,39,40], but actual incidence is much higher. The most important underlying cause is ignorance of tube feeding care among nursing staff. The following technical factors predispose to tube occlusion: inadequate irrigation with water, especially after feed or medicine administration; instillation of medications, particularly crushed tablets; narrow lumen; long tubes (for further details, see occlusion of the PEG tube, below).
TECHNICAL ASPECTS OF PERCUTANEOUS FEEDING TUBE PLACEMENT
Percutaneous endoscopic gastrostomy
Percutaneous endoscopic gastrostomy (PEG) is indicated for patients requiring long-term nutritional support (> 30 d) who have a functional gastrointestinal (GI) tract but insufficient oral intake of nutrients. The most common indications include inadequate swallowing as a result of a neurological event, oropharyngeal or esophageal cancer, and severe facial trauma[41,42]. Based on data from 1327 patients, Kurien et al[43] demonstrated in a recently-published trial that patients who underwent gastrostomy had significantly lower mortality compared to those who deferred the procedure.
PEG techniques
In principle, there are three techniques for PEG tube placement; the peroral pull technique, the peroral push technique and the direct percutaneous procedure. The most widely-used technique for PEG placement is the ‘‘pull’’ method introduced by Gauderer et al[44] in 1980, which has replaced surgical gastrostomy as the medium- and long-term solution to enteral nutrition delivery[45], being safer and more cost-effective, with lower procedure-related mortality (0.5%-2%) and lower complication rates[46-48]. Furthermore, tube displacement occurs less frequently than with nasogastric tubes (NGT). The Sack-Vine ‘‘push’’ variant (placing a catheter over a Seldinger wire) yields comparable results[49].
Alternative procedures such as sonographically-controlled PEG are not yet sufficiently technically developed to be adopted on a wide scale[50]. The introducer PEG, using a balloon catheter placed transabdominally into the stomach, was described by Russell et al[51]. The main problem initially associated with this technique was deflection of the stomach wall due to the puncture, combined with the risk of catheter misplacement. However, its safety has since been improved by the use of an intragastrically-positioned T-fastener under fluoroscopic or endoscopic control to fix the stomach to the abdominal wall[52,53]. A new, safe introducer method (Freka Pexact®) has recently become available for patients in whom the standard ‘‘pull’’ PEG cannot be used or would involve an increased risk during passage of the internal bumper. Its main advantage is the combination of a double gastropexy with a peel-away sheath introducer to effect secure fixation of the stomach wall, analogous to surgical gastropexy[54,55].
The success rate of PEG tube placement is as high as 99.5% (range 76%-100%). Reasons for failure include inadequate transillumination, complete oropharyngeal or esophageal obstruction, and gastric resections[21]. The average life span of PEG tubes has been described to be one to two years, with tube degradation being the most common reason for tube replacement[21].
Jejunal tubes through the PEG and direct percutaneous endoscopic jejunostomy
Long-term jejunal feeding can be achieved endoscopically with jejunal tubes through the PEG (JET-PEG) and direct percutaneous endoscopic jejunostomy (DPEJ). Jejunal feeding is appropriate in patients with recurrent vomiting and/or tube feeding-related aspiration, severe gastroesophageal reflux, gastroparesis, gastric outlet obstruction, or total or partial gastrectomy[56]. Though not definitively proven to reduce tube feeding-related aspiration (see below)], the combination of gastric decompression via PEG and simultaneous jejunal nutrition shows clinical benefit in many patients.
JET-PEG placement can be carried out by pushing a jejunal feeding tube through the previously-placed PEG using a ‘‘beneath the scope’’ (BTS) or ‘‘over the wire’’ tube technique. Initial positioning of the tube beyond the ligament of Treitz is essential to reduce the retrograde migration rate. Although feeding tube placement beyond the ligament of Treitz may be considered a technical success, its functional success is largely disappointing because of frequent retrograde tube migration into the stomach and tube dysfunction caused by kinking or obstruction (as the diameter of the jejunal tube is restricted to 12 F). Furthermore, JET-PEG has not been demonstrated to effectively decrease enterorespiratory aspiration compared with PEG tube feeding alone[57,58]. In combined gastric decompression/feeding jejunostomy tubes, the small diameter of the tube often provides inadequate gastric venting and may cause jejunal lumen clogging.
JET-PEG tubes have a high success rate of up to 93%. The mean functional duration of the tubes was found to be 55 d in adults and 39 d in children[21]. Retrograde dislodgment of the jejunal extension tube, tube obstruction and mechanical failure have been described as the most common device-related complications[21]. Endoscopically-placed clips may secure the tube and prevent retrograde migration[59], but this does not overcome the problem of occlusion common to small-caliber tubes.
DPEJ, a modification of the pull PEG technique, appears to be the ideal procedure for long-term jejunal feeding[60]. A colonoscope or an enteroscope is introduced orally into the small bowel, and transillumination is observed on the anterior abdominal wall as an indicator of the scope’s position in the jejunum. A trocar is passed through the anterior abdominal wall directly into the jejunum. As a sounding device for needle puncture, a 21-gauge 1.5 inch needle used for infiltration of local anesthetic may be used. The puncture should be performed with a purposeful, swift stabbing motion of the wrist. Grasping the tip of the sounding needle with a forceps helps to stabilize the jejunal segment and allows proper orientation for insertion of the larger trocar/needle assembly alongside the indwelling sounding needle. A 120-inch insertion wire is advanced through the cannula and grasped by the awaiting forceps or snare, and the procedure is completed as described for a pull-type PEG placement. Though similar to PEG placement, DPEJ is a considerably more difficult technique[60,61]. There are three retrospective studies on DPEJ outcome involving a total of 230 patients from a cancer center, a surgical unit, and a gastroenterological referral center. Successful placement was achieved in 72%-88%[62].
Skin-level gastrostomy
Skin-level gastrostomy was introduced to reduce skin irritation, minimize granulation tissue and improve the patients’ quality of life. It provides easy and convenient access for enteral nutrition and is well established in pediatric patients[63].
The most popular system is the button skin-level nonrefluxing device, first described by Foutch et al[64]. Currently, three button types with two different retaining elements (retention dome and balloon type) are available[65].
Indications for this device are usually peristomal problems and/or the patient’s wish to be independent from the PEG tube. Contraindications are active peristomal infection, a stoma existing less than four weeks after primary PEG insertion, a fistulous stoma channel, and a stomal tract longer than 4.5 cm. Initial application of the button should be carried out under endoscopic control or under fluoroscopic guidance via a guide wire to prevent misplacement and to allow removal of the previously-placed gastrostomy catheter[56]. Despite the potential advantages of a skin-level device, the largest prospective multicenter evaluation of a single-step button (86 patients) reported serious placement problems and a high complication rate[66].
Initiation of feeding
There are various techniques for administering feeds in ETF patients, the most common of which are summarized below (Table 2). The recommendation to delay feeding initiation until 12-24 h after PEG or transabdominal gastrostomy placement was based on a presumption that the GI system would return to normal function during this period of time, allowing a better seal of the enteral opening[46,67]. More recently, however, several prospective randomized studies have clearly demonstrated that, at least in the case of PEG placement, much earlier initiation of feeding after as little as 1-3 h is equally safe[68-71]. This was confirmed in a meta-analysis by Bechtold et al[72].
Table 2.
Techniques for delivery of feeds in enteral tube feeding
| Method of feeding | Indication | Comments |
| Bolus intermittent (by syringe or bulb) | Ambulatory patients | 100-400 mL over 5-10 min multiple times, high risk of aspiration and diarrhea, cheap and convenient for NGT |
| Cyclic intermittent (by gravity or pump) | Partially recumbent | Higher infusion rate for a shorter period (8-16 h); while changing from tube feeds to oral |
| Intermittent drip | Home enteral feeding | 1.5-2 L over 8-12 h overnight, no daytime feeds |
| (by gravity or pump) | ||
| Constant infusion (by gravity or pump) | Bedridden patients ICU patients | Initiate with 20-50 mL/h, altered periodically depending on gastric residual volume, increased chances of aspiration and metabolic abnormalities; incline head end of bed to 45° to reduce aspiration and regurgitation |
NGT: Nasogastric tube; ICU: Intensive care unit.
COMPLICATIONS ASSOCIATED WITH PEG PLACEMENT: INCIDENCE AND MANAGEMENT
About 13%-40% of patients with PEG placement experience minor complications such as maceration due to leakage of gastric contents around the tube, and peristomal pain[46,47,73,74]. Serious complications requiring further intervention have been reported in 0.4%-4.4% of procedures and include peristomal leakage with peritonitis, necrotizing fasciitis of the anterior abdominal wall, gastric bleeding, injury to internal organs, tumor seeding at the PEG site, and death. The 30-d incidence of mortality after PEG has been reported to be in the range of 6.7%-26%[46,47,73]. This high value is due rather to the underlying comorbid factors of these patients than to the procedure itself[21,75,76].
Peristomal wound infections
Incidence and causes: Peristomal wound infections are the most common complication associated with the PEG procedure, with an incidence ranging from 4%-30%[77]. About three quarters of these are minor and resolve when treated with antibiotics. While regular skin and stomal care are crucial for the prevention of local infection, bandaging techniques also play an important role: In a comparative study, Chung et al[78] showed that excessive traction on the gastrostomy tube significantly increases the rate of local infection. Factors predisposing to infection can be (1) technique-related, such as a narrow incision or lack of antibiotic prophylaxis; (2) host factors, e.g., malnutrition, obesity, diabetes, malignancy, drug therapy (immunosuppressive medication, chronic corticosteroid therapy); and (3) nursing care-related, such as improper wound dressing or excessive traction between the internal bumper and the stomach wall.
Prevention and treatment: Two recently-published meta-analyses involving 1100 patients from 10 randomized controlled trials assessing the efficacy of antibiotic prophylaxis in PEG insertion have demonstrated antibiotic prophylaxis to effect a relative risk reduction of 64% and an absolute risk reduction of 15%. Either a first-generation cephalosporin or a penicillin-based prophylaxis should be selected[79,80].
During the last decade, methicillin-resistant Staphylococcus aureus (MRSA) has emerged as an important cause of PEG-related wound infection. Horiuchi et al[81] suggested that, in addition to standard prophylactic antibiotics, MRSA decolonization using oral and nasally-delivered preparations might reduce the risk of MRSA-related peristomal infections in these patients. A recently-published randomized controlled study demonstrated that the use of glycerin hydrogel (GHG) dressings significantly decreases the number of peristomal infections, and that the frequency of dressing changes can be safely extended to 7 d during the first week, making it a less labor-intensive and more cost-effective option for post-PEG wound management.
In the case of transabdominal gastrostomy, where direct transabdominal access of the gastrostomy tube avoids its exposure to oropharyngeal flora, another recent randomized controlled trial demonstrated no statistically significant difference in the rate of peristomal infection with or without preprocedural administration of antibiotics[55].
Clogged feeding tubes
Incidence and causes: The incidence of clogged feeding tubes in PEG is reported to be as high as 23%-35%. Clogging is especially common when thick enteral feeds, bulking agents and medications are delivered through relatively small PEG tubes (i.e., 9 F). Tube occlusion is classified as either obstruction of the internal lumen or mechanical tube failure[39,82].
Prevention and treatment: Since pH values below 4 have been described to promote protein coagulation, repeated gastric residual aspiration should be avoided or minimized[83]. Tubes should also be flushed with 40-50 mL water before and after delivering medications or bulking agents (i.e., psyllium, resins). If possible, all medications should be completely dissolved in water prior to flushing or applied as liquid formulations[84]. Saline should be avoided, since it can crystallize within the tube and promote gradual clogging[85]. Pancreatic enzymes mixed with bicarbonate have been reported to prevent tube clogging effectively[39,82]. Furthermore, they were found superior to carbonated beverages in dissolving clogs[86]. In a recent systematic review, water flushes have been shown to be the most effective method of preventing enteral feeding tube clogging[87]. Finally, clogged tubes can be cleared mechanically using various endoscopic catheters, braided quid wires, or special “declogging” plastic brush devices[25,88].
Peristomal leakage
Incidence and causes: Although its reported incidence appears low (1%-2%), peristomal leakage is in fact a much more common complication[85,88,89]. Several factors contributing to the risk of peristomal leakage have been identified, including excessive cleansing with hydroperoxide, infections, gastric hypersecretion and excessive side torsion along the PEG tube, as well as patient-specific factors that inhibit wound healing (malnutrition, immunodeficiency, diabetes). Furthermore, the risk of peristomal leakage is increased if the tube is not stabilized by means of an external polster[25,85].
Prevention and treatment: Prevention of peristomal leakage must focus on the reduction of risk factors (e.g., antisecretory therapy with PPIs), while barrier creams containing zinc and skin protectants are also recommended[85]. If these fail, the PEG tube can be removed. After waiting 4-6 d to allow the tract to partially close, a new PEG can then be placed through the same tract[85,90]. However, this procedure should only be attempted if sufficient time has passed to ensure scarring of the stomach to the abdominal wall[85,90]. If this is not the case, the PEG tube must be removed and a new PEG tube placed at a different site. On no account should the original PEG tube be replaced by a larger diameter tube, as this may cause enlargement of the tract, resulting in exacerbation of the leakage[85,90]. Conversion of the PEG to a PEG/double-lumen system has been reported to be a successful alternative[25,88], while conversion to a gastrojejunal (GJ) tube can also be considered for feeding distal to the stomach.
Bleeding
Incidence and causes: Acute bleeding is not uncommon after PEG tube placement, with a reported incidence of up to 2.5%[46,91,92]. The most common causes of acute bleeding immediately following PEG tube placement are local vessel injury at skin level, and mucosal tear in the upper GI tract. Risk factors include previous anatomic alteration, anticoagulation, and antiplatelet therapy[41]. In a recent single-center study of 990 patients, Richter et al[93] recently demonstrated the use of serotonin reuptake inhibitors (SRIs) during the 24 h before PEG placement to be a risk factor for increased bleeding. In this cohort, however, no association with aspirin or clopidogrel intake (at any dose) either before or after the procedure was found. The influence of SRI intake on bleeding risk is of particular significance in geriatric patients, since these drugs are administered to a large proportion of this patient population.
Delayed bleeding can be caused by esophagitis, gastritis, gastric erosion, gastric or duodenal ulceration, and the buried bumper syndrome[94]. One study has demonstrated that esophagitis was the cause of delayed GI bleeding in 39% of PEG patients undergoing endoscopy[95].
Prevention and treatment: Current American Society for Gastrointestinal Endoscopy (ASGE) guidelines recommend the continuation of aspirin and non-steroidal anti-inflammatory drugs (NSAIDs), especially in high-risk patients, and the discontinuation of Clopidogrel therapy in low-risk patients, and where appropriate also in individuals with a higher risk of bleeding[92]. Discontinuation of warfarin therapy should also be considered, with the application of unfractionated heparin as bridging therapy[92]. Furthermore, in the light of their aforementioned study, Richter et al[93] recommend the interruption of SRI intake 24 h before the procedure.
Colonic fistulae
Incidence, types and causes: Colonic misplacement of the PEG tube may lead to serious complications, in particular the development of gastrocolic, colocutaneous or gastrocolocutaneous fistulae[96,97]. While these fistulae are rare and cited mostly only as isolated case-reports, Friedmann et al[97] describe 28 cases of colocutaneous fistula resulting from colonic malpositioning of PEG, including 12 cases of gastrocolocutaneous fistula and one of jejunocolocutaneous fistula. In contrast to the gastrocolic fistula, a fistulous passage connecting the stomach with the colon, the gastrocolocutaneous fistula is defined as an epithelial connection between the mucosa of the stomach, the colon, and the skin. Its probable etiology is the penetration of a bowel loop (mostly transverse colon) interposed between the stomach and the abdominal wall, either by inadvertent puncture during tube placement or, more commonly, due to gradual erosion of the tube into the adjacent bowel[98]. Factors predisposing to its occurrence in acute settings are insufficient gastric insufflation, past history of laparotomy causing adhesions and consecutive trapping of bowel loops, and improper transillumination. Up to 45% of colocutaneous fistulae are seen in patients with prior abdominal surgery[99]. These patients should undergo contrast study to rule out an overlap of stomach and colon.
Prevention and treatment: To prevent colonic misplacement, the transillumination of the gastroscope through the abdominal wall, and the endoscopically visible imprint of a finger (or needle) on the skin is still a “conditio sine qua non” before introduction of the needle into the stomach[100]. In the absence of appropriate transillumination, the use of ultrasound and/or computed tomography is recommended to exclude abnormal abdominal anatomy[101], with slow advancement of a small-gauge anesthetic needle (e.g., the needle used to infiltrate local anesthetic into the PEG site) through the abdominal wall into the stomach, aspirating on the attached syringe. A “safe tract” is established by endoscopic visualization of the needle in the gastric lumen and simultaneous return of air into the syringe. Return of fluid or gas into the syringe before the needle is endoscopically visualized within the gastric lumen suggests entry into bowel interposed between the abdominal wall and the stomach[85,97,102].
The most common clinical symptoms associated with fistulae are watery diarrhea containing feed, or the presence of stool around the PEG tube. Rarely, fistulae present acutely with peritonitis, infection, fasciitis, or failure of the formula infusion[97].
Fistulae are diagnosed using contrast agent given via the PEG tube. Several approaches have been suggested for fistula management, ranging from conservative removal of the PEG tubes without laparotomy, hereby allowing the fistula tract to close spontaneously within a few days, to invasive exploration of the colon. Colonic clipping has been reported by Kim et al[103]. While challenging the necessity for such a sophisticated endoscopic intervention in this case (after only 10 d of conservative treatment), Gyökeres et al[104] suggest that the method may be considered an option for the closure of persistent gastrocolic fistulae associated with PEG placement.
Buried bumper syndrome
Incidence and causes: Buried bumper syndrome (BBS) is a rare, mainly long-term complication of PEG, in which the internal bolster migrates from the gastric lumen and lodges in the gastric wall (incomplete BBS) or anywhere along the GI tract outside the gastric lumen (complete BBS)[105-107]. It has a reported prevalence of 1.5%-8.8%[74,88,108-111]. Though normally a late complication (usually presenting not until at least four months post-PEG), BBS has also been reported to occur as early as 21 d after PEG placement[89,94,112]. Common symptoms include immobilization of the PEG tube, feeding difficulties or the need for more pressure when giving feeds, peritubular leakage, complete occlusion of the tube, and the occurrence of abdominal pain.
The main causative factor of BBS is excessive traction between internal and external bumper. Other possible contributing factors include malnutrition, poor wound healing, weight gain due to PEG feeding, and a stiff internal bumper (polyurethane). Diagnosis is mainly clinical but requires endoscopy for confirmation, and may reveal anything from simple ulceration and mucosal overgrowth around the internal bumper to complete outward erosion of the tube with non-visualization of the internal bumper.
Prevention and treatment: To prevent BBS, it is advisable to allow an additional 1.5 cm of space between the external bumper of the PEG tube and the skin in order to minimize the risk of pressure-induced necrosis. Mucosal overgrowth of the inner bumper can be prevented by mobilizing and loosening the PEG from the outside at least every other day. The incidence of BBS is lower in patients with PEG tubes made from Foleys urinary catheter-type silicone tube and in patients with balloon-assisted PEG-introducer devices than in routine PEG patients and those with balloon-tipped replacement tubes (probably because fluid inside the balloon can regulate the pressure more effectively[113]).
Even if it is asymptomatic, buried bumper must be removed once diagnosed, as continued bumper migration may lead to bleeding, perforation, peritonitis and death. Various techniques can be employed to remove the buried device and either reaccess the luminal tract with a new tube or secure an entirely new access site: The ‘‘needle-knife’’ technique can be used in cases of partial or superficial burial[110,114]. Alternatively, the buried tube can be pulled out and simultaneously replaced with a new pull-type feeding tube following insertion of a guide wire through the old tube[109]. Müller-Gerbes et al[115] describe a minimally-invasive technique (push method) where the inner bumper is cut by means of a papillotome brought into the stomach from the outside through the shortened PEG while under constant endoscopic control. Last but not least, it has been reported that the buried tube may be safely removed simply by external traction[111,113,116]. In cases of deep impaction or migration into the abdominal wall, surgical intervention in the form of laparotomy or a laparoscopic approach is required[111,117].
Pneumoperitoneum
Pneumoperitoneum (PNP) following PEG or JET-PEG placement is a known finding occurring after 8%-18%[118-122] of procedures. PNP usually has a benign and self-resolving course which does not warrant any further intervention. However, the recent reported higher incidence of complications requiring intervention in ICU patients with post-PEG PNP suggests the need for more intensive investigation of patients with this finding[119].
Liver injury
Hepatic injury during PEG placement (e.g., transhepatic PEG placement) occurs infrequently, but is a potentially life-threatening and probably underdiagnosed complication[123-125]. Unexplained pain after PEG placement in the absence of wound infection should always raise the suspicion of liver injury. If hepatomegaly is suspected or improper transillumination is present at the puncture site, transabdominal ultrasound must be performed. In addition, the tube insertion site should generally be chosen left of the upper abdominal midline and combined with the “safe tract” technique (see above).
Abdominal wall metastasis at the PEG site
Abdominal wall metastasis as a late complication at the PEG site has been reported with an incidence of < 1%[126,127]. Although it is a rare complication, the malignant seeding of tumor cells is associated with an extremely poor prognosis[126]. Risk factors for abdominal wall metastasis include primary pharyngoesophageal cancer, squamous cell histology, less differentiated and large-sized cancers, and an advanced cancer stage[128]. Since direct mechanical tumor implantation is the most likely mechanism, the pull-string or direct-introducer technique for PEG placement is preferable[55]. If these techniques are not available, an overtube should be used[129] or the PEG placement should be performed after surgical removal of the primary cancer[126-128].
GASTROINTESTINAL COMPLICATIONS
The most common complications observed with ETF involve GI function[31,130-132]. These complications and their possible causes and solutions are listed in Table 3.
Table 3.
Gastrointestinal complications of enteral nutrition; causes, prevention and treatment
| Complication | Cause | Prevention/treatment |
| Diarrhea | Too rapid increase in amount of feed per day | Observe adaptation phase |
| Too rapid infusion rate | Reduce/control infusion rate | |
| Feed temperature too cold | Increase to room temperature | |
| Hyperosmolar feedings (> 300 mOsm) | Use isotonic feeding solution, initially | |
| dilute hyperosmolar feeding solutions | ||
| Lactose intolerance | Use low-lactose or lactose-free diet | |
| Fat malabsorption | Use low-fat or MCT-containing diet | |
| Hypoalbuminemia | Use chemically defined diet and/or feed | |
| Antibiotic therapy or medications | Review medications | |
| Chemotherapy/radiotherapy | Prescribe antidiarrheal medications | |
| Nausea/vomiting | Too rapid infusion rate | Reduce/control infusion rate |
| Bacterial contamination of formula feed/delivery equipment contamination | Handle administration systems hygienically, change delivery equipment every 24 h, keep opened bottles of formula no more than 24 h in refrigerator | |
| Cramps/bloating | Too rapid infusion rate | Reduce/control infusion rate |
| Lactose intolerance | Use low-lactose or lactose-free diet | |
| Fat malabsorption | Use low-fat or MCT-containing diet | |
| Regurgitation/aspiration | Gastric retention | Reduce/control infusion rate, use duodenal tubes, incline patient during food administration |
| Constipation | Inadequate fluid intake | Increase fluid intake, check fluid balance |
| Fiber intake too low | Use fiber-containing formulas | |
| Fecal impaction | Enemas | |
| Electrolyte and hormonal derangement | Osmotic laxatives (lactulose 15-60 mL), | |
| peristaltic agents (e.g., prostigmine 0.25-0.5 mg iv) |
MCT: Medium-chain triglyceride.
Nausea occurs in 10%-20% of patients, while abdominal bloating and cramps from delayed gastric emptying are also common. Additional complications include aspiration and nonocclusive bowel necrosis, which are associated with high mortality[130,133]. In a multicenter observational study of 400 patients, Montejo et al[130] found that 251 patients (63%) experienced one or more GI complications during their feeding course. In a subsequent study, the same group evaluated the incidence of GI complications in gastric- and jejunally-fed patients, and found it to be 57% and 24%, respectively[130,134].
Diarrhea
Incidence and causes: Diarrhea is the most commonly reported GI side effect in patients receiving ETF. Depending on definition[135], diarrhea occurs in up to 30% of patients in medical and surgical wards and more than 80% of patients in the intensive care unit (ICU)[136-139].
The pathogenesis of diarrhea in enterally-fed patients is multi-factorial. Of those factors unrelated to the enteral formula or administration method, the use of antibiotics and/or specific medications is the most common reason for the development of diarrhea[136,139,140]. Diarrhea may be caused either by the medication itself (e.g., oral magnesium or phosphate supplements, antacids, prokinetic agents), or by the formulation in which it is delivered. Medications containing sorbitol can cause diarrhea due to osmotic effects, while antibiotics alter the intestinal flora, favoring the growth of Clostridium difficile (C. difficile), E. coli and Klebsiellae. Thus, courses of antibiotic treatment should be kept as short as possible and the use of prophylactic antibiotics limited. Pseudomembranous colitis is a complication observed with increasing frequency, especially during antibiotic exposure (for reviews see[136,141]). Patients receiving ETF are nine times more likely to develop C. difficile-associated diarrhea than matched non tube-fed patients[142]. Antibiotics can also reduce colonic bacterial production of short chain fatty acids from insoluble carbohydrates and fiber[143].
Hypoalbuminemia (serum albumin level < 2.5 g/dL) has long been implicated as a cause of diarrhea as a result of intestinal edema. However, studies aiming to confirm this have yielded conflicting results[144].
Prevention and treatment: The early identification of diarrhea risk factors and the development of a diarrhea risk management algorithm are recommended. Over the last two decades, several RCTs investigating the use of fibers in the treatment and prevention of ETF-associated diarrhea have been carried out[136]. Results from a meta-analysis suggest fiber to be highly effective in reducing the incidence of diarrhea in patients at increased risk (e.g., postsurgical, critically ill patients)[145]. However, mixed results have been reported regarding the use of different types of fiber (insoluble or soluble) to prevent ETF-induced diarrhea. Schultz et al[146] demonstrated the relative effectiveness of pectin combined with an insoluble fiber formula compared to an insoluble fiber formula alone. The usefulness of soluble fibers for the treatment of diarrhea during enteral nutrition has been demonstrated only in two small studies[147,148]. Partially hydrolyzed guar gum (PHGG) has been shown to reduce the incidence of ETF-induced diarrhea compared with standard, fiber-free formula in both general ward and ICU settings[147,149]. In contrast, supplementation with inulins or fructooligosaccharide (FOS) has been shown to increase flatulence and bowel movement frequency in enterally fed patients[150,151].
The fiber consensus panel of the European Society for Parenteral and Enteral Nutrition (ESPEN) recommends supplementing ETF with PHGG to prevent EN-induced diarrhea in both ICU and postsurgical patients (grade A recommendation)[152]. Guidelines of the American Society for Parenteral and Enteral Nutrition (ASPEN) recommend the use of PHGG in ICU patients (grade D recommendation). However, insoluble fibers should be avoided in all ICU patients (grade C recommendation), as their use may increase the risk of bowel obstruction in the critically ill[9]. A recent systematic review suggests that the use of different fiber mixtures may be the most promising strategy for the prevention of ETF-induced diarrhea[145].
Despite the potential for manipulation of the intestinal microflora in ETF, few studies have investigated the effects of prebiotics or fermentable carbohydrates on the incidence of ETF-associated diarrhea[136,153,154]. To date, eight RCTs have assessed the impact of probiotics in the prevention of ETF-associated diarrhea (Table 4). Of these, five were shown to be beneficial (for review, see[136,155]). The safety of probiotics in critically ill and/or immunocompromised patients has, however, been called into question following an RCT which reported increased mortality in a group of patients with severe acute pancreatitis who took probiotics[156]. Whelan and Myers performed a systematic review focused on adverse events related to probiotics in patients receiving enteral nutrition[157]. Only 3 of 53 trials showed increased complications, which were largely non-infectious in nature and occurred in specific patient groups (e.g., transplant and pancreatitis). The total of 50 trials (> 4000 patients) showed either no effect or a positive effect on outcomes related to safety (e.g., mortality and infections). Thus, in spite of contrasting evidence, it may be concluded that the use of probiotics in enteral formula is a useful tool in preventing ETF-associated diarrhea, but should not be used in transplant patients or the critically ill. A systematic approach to the management of diarrhea in ETF patients is depicted in Figure 1.
Table 4.
Randomized controlled trials measuring the impact of probiotics on enteral nutrition-related diarrhea
| Ref. | Study population | Treatment groups | Sample size (placebo) | Daily dose |
Outcome |
|
| Probiotics | Controls | |||||
| Heimburger et al[204] | Adults starting EN | Lactobacillus acidophilus and L. bulgaricus | 41 (23) | 3000 CFU/d | 31% developed diarrhea | 11% developed diarrhea |
| Alberda et al[205] | Adults startingEN on ICU | VSL#3 - live cells | 10/9 (9) | 9 × 1011 mg/d | 14%/12%1 of days with diarrhea | 23% of days with diarrhea |
| Frohmader et al[206] | Adults startingEN on ICU | VSL#3 | 45 (25) | 9 × 1011 mg/d | 0.5 liquid stools/d | 1.1 liquid stools/d |
| Ferrie et al[207] | Adults with diarrhea during EN on ICU | L. rhamnosus GG | 36 (18) | (2 × 1010 cells/d) and inulin (560 mg/d) | 3.8 d duration of diarrhea | 2.6 d duration of diarrhea |
| Barraud et al[208] | Adults starting EN on ICU | Ergyphilus | 167 (80) | (2 × 1010 CFU/d | 55% developed diarrhea | 53% developed diarrhea |
| Bleichner et al[209] | Adults starting EN on ICU | Saccharomyces boulardii | 128 (64) | 4 × 1010 CFU/d | 7.7% of days with diarrhea | 9.1% of days with diarrhea |
| Schlotterer et al[210] | Burnt adults | Saccharomyces boulardii | 18 (9) | 4 × 1010 CFU/d | 1.5% of days with diarrhea | 14% of days with diarrhea |
| Tempe et al[211] | Adults in ICU | Saccharomyces boulardii | 40 (20) | 1 × 1010 CFU/d | 8.7% of days with diarrhea | 16.9% of days with diarrhea |
Viable probiotics/probiotic sonicates. VSL#3 consists of Lactobacillus casei, Lactobacillus plantarum, Lactobacillus acidophilus, Lactobacillus delbrueckii, Bifidobacterium longum, Bifidobacterium breve, Bifidobacterium infantis, and Streptococcus salivarius. Ergyphilus consists of mainly Lactobacillus rhamnosus GG, Lactobacillus casei, Lactobacillus acidophilus, and Bifidobacterium bifidum.
Figure 1.
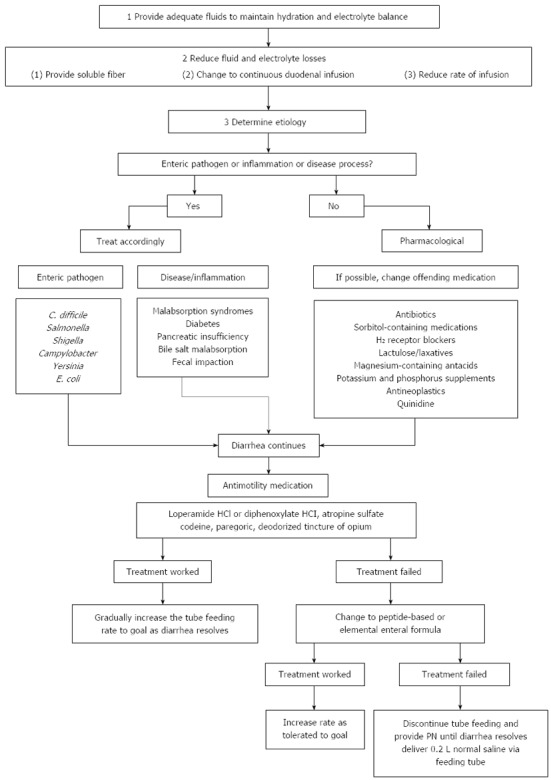
Management of diarrhea in enteral tube-fed patients. Adapted from reference[212].
Constipation
Constipation is less common than diarrhea during ETF, and more prevalent in patients requiring long-term ETF. The primary goal of constipation management in these patients is prevention. Despite the theoretical rationale for fiber supplementation of enteral formulae, there are still no convincing data demonstrating the benefits of fiber supplementation in terms of improved bowel function or prevention of constipation. A meta-analysis from seven RCTs in the acute setting (two ICUs, three surgical and two medical wards) showed that fiber supplementation effected at least a downwards trend in the percentage of patients reporting constipation. Furthermore, a significant reduction in laxative use in patients receiving fiber formula was observed in four studies[145]. In spite of the lack of sufficient data, fiber formulae should therefore be used at least in patients requiring long-term ETF.
PULMONARY COMPLICATIONS
Pneumonia is a potentially life-threatening complication which is usually a consequence of pulmonary aspiration of oral secretions or, less commonly, of gastric and small-bowel contents. It may occur with no obvious evidence of vomiting. Pulmonary aspiration is more common when patients are fed with NGT in a supine position[25,158-160] and is caused by a combination of gravitational back-flow, impairment of the lower esophageal sphincter (LES), lack of swallow-induced LES relaxation, infrequent esophageal body contractions, and the presence of the tube across the gastric cardia[161]. It is very common in patients with impaired consciousness or poor gag reflexes, occurring in up to 30% of those with tracheotomies[162] and 12.5% of neurology patients[163]. However, while feeding via PEG may reduce the risk of aspiration, it will not eliminate it altogether[164], especially in patients in whom GI motility disturbances inhibit this route of feeding[158,165].
Since the incidence of reflux depends on the position of the tip of the feeding tube (6% with the tube tip in the duodenum, 4% near the ligament of Treitz, and 0.4% distal to the ligament of Treitz), several published guidelines favor small bowel over gastric feeding in patients who are at risk for aspiration. This applies in particular to critically ill patients, in whom gastroduodenal atony, caused by increased intracranial pressure, hyperglycemia and stress, can lead to delayed or impaired gastric emptying and decreased transpyloric transport of nutrients (for extended review see Waseem et al[161,166]). However, the results of more than 10 controlled clinical trials[134,167-175] are contrary and inconclusive, particularly with regard to nasoenteral tube feeding[164,168]. Meta-analyses of these studies have also presented conflicting views[164,176,177].
Since it has been shown that even in patients with normal intestinal motility both naso- and gastrojejunal tubes, once correctly positioned, will be moved in a proximal direction by the phase II migrating motor complex, placement of enteral nutrition tubes ≥ 40 cm distal to the ligament of Treitz is considered the optimal method[165,168].
METABOLIC COMPLICATIONS
Artificial feeding may cause a variety of metabolic problems including deficiency or excess of fluids, electrolytes, vitamins and trace elements. Overhydration occurs frequently, particularly when ETF patients are also receiving supplementary intravenous nutrition or fluids. Furthermore, underlying metabolic diseases including diabetes mellitus and renal or hepatic insufficiency must be taken into account while administering ETF.
Refeeding syndrome
Refeeding syndrome (RFS) was first described in malnourished Far East prisoners of war after the Second World War who developed cardiac and neurological symptoms soon after starting eating[178,179], and remains an often forgotten condition. RFS is characterized by electrolyte depletion, fluid shifts and glucose derangements that occur upon oral, enteral or parenteral reinstitution of nutrition in malnourished patients[180].
Incidence: The true incidence of RFS is still not known. The majority of reported cases are prospective and retrospective cohort studies or case series. Patients with anorexia represent the prototypical population of RFS[181], and a recently-published multicenter study from France reported an incidence of 10% in these patients[182]. Other common conditions associated with RFS include hyperemesis, alcoholism, cancer, and malabsorptive syndromes such as short bowel syndrome, inflammatory bowel disease, cystic fibrosis, and various forms of bariatric surgery[181].
Pathophysiology: The pathophysiology of RFS remains poorly understood. It occurs because the body adapts to undernutrition by down-regulating membrane pumping in order to conserve energy. This, in turn, causes leakage of intracellular potassium, magnesium, calcium and phosphate, with subsequent whole-body depletion. Simultaneously, sodium and water also leak into the cells.
Sudden refeeding reverses these processes and, along with insulin, drives electrolytes into the cells, potentially leading to a precipitous fall in circulating levels of the aforementioned electrolytes. This may be accompanied by an acute increase in circulating and extracellular fluid (exogenous administration or endogenous movement of sodium and water out of the cells). To further aggravate the situation, undernourished kidneys have a limited capability to handle salt and water load.
Specific micronutrient deficiencies can compound these problems. Hypophosphatemia is the hallmark of RFS, and is responsible for significant morbidity and even mortality[180]. It can manifest as clinical features of RFS, e.g., rhabdomyolysis, leukocyte dysfunction, respiratory failure, cardiac failure, hypotension, arrhythmia, seizure, coma and sudden death[183].
In adult patients, refeeding hypophosphatemia is more common in enteral than parenteral feeding. This may be due to the incretin effect from absorption of glucose[184]. Cardiac arrest has been reported as a complication of RFS in patients presenting with less than 70% of prior body weight. Prolonged starvation results in a reduction of total cardiac volume, end diastolic volume, and left ventricular mass. During RFS, ventricular volume returns to normal while left ventricular mass remains reduced, leading to fluid retention and congestive cardiac failure. In addition, hypophosphatemia may lead to decreased sarcomere contractility and cause myocardial damage[185,186].
Prevention and treatment: Awareness of RFS and identification of patients at risk are the first steps in preventing refeeding problems[187]. Any patient with no or negligible food intake for more than five days is at risk of developing refeeding problems. High-risk patients include the chronically undernourished and those who have diminished physiological reserves and/or are critically ill[188]. Table 5 summarizes criteria from the National Institute for Health and Clinical Excellence (NICE) for the identification of patients at high risk of RFS. Recommendations for therapy and prevention are shown in Table 6.
Table 5.
Patients at high risk of refeeding syndrome
| Patients with anorexia |
| Patients with chronic alcoholism |
| Oncology patients |
| Postoperative patients |
| Elderly patients (comorbidities, decreased physiological reserves) |
| Patients with uncontrolled diabetes mellitus (electrolyte depletion, diuresis) |
| Patients with chronic malnutrition: |
| Marasmus |
| Prolonged fasting or low energy diet |
| Morbid obesity with profound weight loss |
| High stress unfed for > 7 d |
| Malabsorptive syndromes (inflammatory bowel disease, cystic fibrosis, short bowel syndrome) |
Adapted from reference[188].
Table 6.
Therapy and prevention of refeeding syndrome
| Careful evaluation of cardiovascular system, check for any electrolyte abnormalities before initiating refeeding |
| In severe cases, an initial starting volume of 50%-75% of daily requirements should be used |
| < 7 yr old: 80-100 kcal/kg bw/d |
| 7-10 yr: 75 kcal/kg bw/d |
| 11-14 yr: 60 kcal/kg bw/d |
| 15-18 yr: 50 kcal/kg bw/d |
| > 18 yr: 25 kcal/kg bw/d (or an average 1000 kcal/d initially) |
| If the initial food challenge is tolerated, caloric intake may be increased over the next 3-5 d. Each requirement should be tailored to the individual’s needs, and the above values may need to be adjusted by as much as 30%. Frequent administration of small feeds is recommended. Feeds should provide a minimum of 1 kcal/mL to minimize volume overload |
| Protein |
| Initial regimen for malnourished patients: 0.8-1.0 g/kg bw/d |
| The feed should be rich in essential amino acids, and should gradually be increased, as an intake of 1.2-1.5 g/kg bw/d is needed for anabolism to occur |
| Vitamins/trace elements |
| Thiamine, folic acid, riboflavin, ascorbic acid and pyridoxine should be supplemented, as well as the fat-soluble vitamins A, D, E, and K |
| 300 mg thiamine should be given IV at least 30 min. before refeeding is initiated, and should be continued with 100 mg iv for at least 7 d. Later on, oral thiamine can be supplemented as 100 mg tablets |
| Iron should be supplemented iv according to the Ganzoni formula {iron deficit (mg) = bw (kg) × [(target Hb - actual Hb (g/L )] × 2.4 + depot iron (500 mg)} |
| Minerals |
| Sodium should be restricted (about 1 mmol/kg bw/ or 1.5 g/d), but liberal amounts of phosphorus, potassium and magnesium should be given to patients with normal renal function |
| Magnesium (normal range: 0.8-1.6 mmol/L ) |
| Mild to moderate hypomagnesemia (0.5-0.7 mmol/L ) |
| →Initially 0.5 mmol/kg bw/d over 24 h iv, then 0.25 mmol/kg bw/d for 5 d iv |
| Maintenance requirement |
| →0.2 mmol/kg bw per day iv or 0.4 mmol/kg bw per day orally |
| Phosphate (normal range: 0.85-1.40 mmol/L) |
| Mild hypophosphatemia (0.6-0.85 mmol/L) |
| →0.3-0.6 mmol/kg bw per day orally |
| Moderate hypophosphatemia (0.3-0.6 mmol) |
| →0.3-0.6 mmol/kg bw per day orally |
| Severe hypophosphatemia (< 0.3 mmol/L ) |
| iv supplementation with either potassium phosphate or sodium phosphate (e.g., 0.8 mmol/kg bw monobasic potassium phosphate in half-normal saline by continuous infusion over 8-12 h) |
| Plasma phosphate, calcium, magnesium and potassium should be monitored, and the infusion should be stopped once plasma phosphate concentration exceeds 0.30 mmol/L |
Despite the lack of high level (level A/B) recommendations, overall consensus favors the gradual introduction and advancement of feeding over several days while closely monitoring electrolytes and - to a lesser extent - vitamins and trace elements. Serum phosphate, magnesium, calcium, potassium, urea, and creatinine concentrations should be measured before feeding and repeated daily during the first week after feeding is started.
Caloric intake should generally start with approximately 1000 kcal or 10-15 kcal/kg (25%-50% of estimated requirements) daily, particularly during the first week of refeeding, and be increased by approximately 20% daily until the determined goal is reached. The average weekly weight gain, particularly in extremely undernourished patients (e.g., anorexia nervosa) should not exceed 0.5 kg/wk[189,190]. If hypophosphatemia occurs (< 0.50 mmol/L), it should be corrected with 50 mmol intravenous phosphate over 24 h[185,186,191,192].
In the case of mild to moderate hypomagnesemia (0.5-0.7 mmol/L), 1 g magnesium should be given every 6 h intravenously. Should severe, clinically symptomatic hypomagnesemia occur, 8-12 g magnesium must be given daily in divided doses and serum magnesium monitored every 8-12 h[181,193].
Few data are available regarding optimal vitamin and trace element supplementation. Some authors recommend 300 mg of thiamine (parenteral or enteral) before starting refeeding and 100 mg daily thereafter. In the presence of Wernicke’s encephalopathy, even higher doses of thiamine (500-750 mg) may be warranted[193].
In order to avoid fluid overload, fluid repletion should be carefully controlled. Some authors have recommended initial fluid and sodium restriction to prevent congestive heart failure[189].
ROLE OF NUTRITION SUPPORT TEAMS
Although still limited in number, studies of team vs non-team enteral management provide clear evidence for the positive effects of an organized multidisciplinary approach using protocols and recommendations based on published guidelines[194]. A study in a community hospital showed that management of ETF patients by a nutrition support team (NST) was associated with reductions in mortality rate, length of hospital stay and readmission rate[195]. In a prospective study comparing team vs non-team management of ETF patients at a Veterans Administration Medical Center, Powers and colleagues demonstrated that the former reduced complications and improved patients’ nutrition status[196]. This finding was confirmed at a university teaching hospital[197]. In addition, cost-utility analyses of patients receiving ETF either as hospitalized patients or as outpatients have demonstrated a significant lowering of healthcare costs through the reduction of metabolic and mechanical complications[198-201].
Organization and tasks of nutrition support teams
A well-organized NST should include a physician, a nurse, a nutritionist and a pharmacist[202]. The goal of the NST is to provide high quality nutritional care. This is accomplished through (1) identification of patients who are at risk nutritionally; (2) performance of a comprehensive nutritional assessment to serve as a guide to nutritional therapy; and (3) provision of safe and effective nutritional support. To accomplish these goals, the NST should offer services including inpatient consultations, quality assurance protocols, research programs, home nutrition services, and last but not least, staff educational programs. Proper training of staff on the correct use of nutritional support is crucial, especially with regard to the reduction of complications.
CONCLUSION
Endoscopic methods in particular have facilitated a variety of enteral feeding access options. These should be tried in all patients who are unable to ingest adequate amounts of food but have adequate absorptive capacity of the intestine. Well-trained endoscopists who are extremely proficient in these techniques are indispensable for the successful provision of hospital nutrition support and care. Optimal techniques for tube placement, together with the prompt recognition and immediate management of complications, can significantly reduce overall morbidity and mortality due to ETF-associated complications. To further promote positive patient outcomes and reduce the incidence of complications, ETF should always be managed by multidisciplinary nutrition support teams.
ACKNOWLEDGMENTS
The authors wish to thank Janet Collins (Crohn-Colitis Center Rhein-Main) for proofreading and language support.
Footnotes
P- Reviewers: Campo SMA, Crary MA S- Editor: Zhai HH L- Editor: A E- Editor: Liu XM
References
- 1.Cockfield A, Philpot U. Feeding size 0: the challenges of anorexia nervosa. Managing anorexia from a dietitian’s perspective. Proc Nutr Soc. 2009;68:281–288. doi: 10.1017/S0029665109001281. [DOI] [PubMed] [Google Scholar]
- 2.Zuercher JN, Cumella EJ, Woods BK, Eberly M, Carr JK. Efficacy of voluntary nasogastric tube feeding in female inpatients with anorexia nervosa. JPEN J Parenter Enteral Nutr. 2003;27:268–276. doi: 10.1177/0148607103027004268. [DOI] [PubMed] [Google Scholar]
- 3.Freeman C, Ricevuto A, DeLegge MH. Enteral nutrition in patients with dementia and stroke. Curr Opin Gastroenterol. 2010;26:156–159. doi: 10.1097/MOG.0b013e3283346fae. [DOI] [PubMed] [Google Scholar]
- 4.Gomes CA, Lustosa SA, Matos D, Andriolo RB, Waisberg DR, Waisberg J. Percutaneous endoscopic gastrostomy versus nasogastric tube feeding for adults with swallowing disturbances. Cochrane Database Syst Rev. 2010;(11):CD008096. doi: 10.1002/14651858.CD008096.pub2. [DOI] [PubMed] [Google Scholar]
- 5.Al-Zubeidi D, Rahhal RM. Safety techniques for percutaneous endoscopic gastrostomy tube placement in Pierre Robin Sequence. JPEN J Parenter Enteral Nutr. 2011;35:343–345. doi: 10.1177/0148607110381268. [DOI] [PubMed] [Google Scholar]
- 6.Colomb V, Goulet O. Nutrition support after intestinal transplantation: how important is enteral feeding? Curr Opin Clin Nutr Metab Care. 2009;12:186–189. doi: 10.1097/mco.0b013e328323280f. [DOI] [PubMed] [Google Scholar]
- 7.Barclay AR, Beattie LM, Weaver LT, Wilson DC. Systematic review: medical and nutritional interventions for the management of intestinal failure and its resultant complications in children. Aliment Pharmacol Ther. 2011;33:175–184. doi: 10.1111/j.1365-2036.2010.04514.x. [DOI] [PubMed] [Google Scholar]
- 8.Gramlich L, Kichian K, Pinilla J, Rodych NJ, Dhaliwal R, Heyland DK. Does enteral nutrition compared to parenteral nutrition result in better outcomes in critically ill adult patients? A systematic review of the literature. Nutrition. 2004;20:843–848. doi: 10.1016/j.nut.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 9.McClave SA, Martindale RG, Vanek VW, McCarthy M, Roberts P, Taylor B, Ochoa JB, Napolitano L, Cresci G. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) JPEN J Parenter Enteral Nutr. 2009;33:277–316. doi: 10.1177/0148607109335234. [DOI] [PubMed] [Google Scholar]
- 10.Pritchard C, Duffy S, Edington J, Pang F. Enteral nutrition and oral nutrition supplements: a review of the economics literature. JPEN J Parenter Enteral Nutr. 2006;30:52–59. doi: 10.1177/014860710603000152. [DOI] [PubMed] [Google Scholar]
- 11.Braunschweig CL, Levy P, Sheean PM, Wang X. Enteral compared with parenteral nutrition: a meta-analysis. Am J Clin Nutr. 2001;74:534–542. doi: 10.1093/ajcn/74.4.534. [DOI] [PubMed] [Google Scholar]
- 12.Heyland DK, Stephens KE, Day AG, McClave SA. The success of enteral nutrition and ICU-acquired infections: a multicenter observational study. Clin Nutr. 2011;30:148–155. doi: 10.1016/j.clnu.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 13.McGeer AJ, Detsky AS, O’Rourke K. Parenteral nutrition in cancer patients undergoing chemotherapy: a meta-analysis. Nutrition. 1990;6:233–240. [PubMed] [Google Scholar]
- 14.Fang JC, Delegge MH. Enteral feeding in the critically ill: the role of the gastroenterologist. Am J Gastroenterol. 2011;106:1032–1037; quiz 1038. doi: 10.1038/ajg.2011.77. [DOI] [PubMed] [Google Scholar]
- 15.Crosby J, Duerksen D. A retrospective survey of tube-related complications in patients receiving long-term home enteral nutrition. Dig Dis Sci. 2005;50:1712–1717. doi: 10.1007/s10620-005-2923-z. [DOI] [PubMed] [Google Scholar]
- 16.Crosby J, Duerksen DR. A prospective study of tube- and feeding-related complications in patients receiving long-term home enteral nutrition. JPEN J Parenter Enteral Nutr. 2007;31:274–277. doi: 10.1177/0148607107031004274. [DOI] [PubMed] [Google Scholar]
- 17.Blomberg J, Lagergren J, Martin L, Mattsson F, Lagergren P. Complications after percutaneous endoscopic gastrostomy in a prospective study. Scand J Gastroenterol. 2012;47:737–742. doi: 10.3109/00365521.2012.654404. [DOI] [PubMed] [Google Scholar]
- 18.Richter-Schrag HJ, Richter S, Ruthmann O, Olschewski M, Hopt UT, Fischer A. Risk factors and complications following percutaneous endoscopic gastrostomy: a case series of 1041 patients. Can J Gastroenterol. 2011;25:201–206. doi: 10.1155/2011/609601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee C, Im JP, Kim JW, Kim SE, Ryu DY, Cha JM, Kim EY, Kim ER, Chang DK. Risk factors for complications and mortality of percutaneous endoscopic gastrostomy: a multicenter, retrospective study. Surg Endosc. 2013;27:3806–3815. doi: 10.1007/s00464-013-2979-3. [DOI] [PubMed] [Google Scholar]
- 20.Park RH, Allison MC, Lang J, Spence E, Morris AJ, Danesh BJ, Russell RI, Mills PR. Randomised comparison of percutaneous endoscopic gastrostomy and nasogastric tube feeding in patients with persisting neurological dysphagia. BMJ. 1992;304:1406–1409. doi: 10.1136/bmj.304.6839.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwon RS, Banerjee S, Desilets D, Diehl DL, Farraye FA, Kaul V, Mamula P, Pedrosa MC, Rodriguez SA, Varadarajulu S, et al. Enteral nutrition access devices. Gastrointest Endosc. 2010;72:236–248. doi: 10.1016/j.gie.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 22.Halloran O, Grecu B, Sinha A. Methods and complications of nasoenteral intubation. JPEN J Parenter Enteral Nutr. 2011;35:61–66. doi: 10.1177/0148607110370976. [DOI] [PubMed] [Google Scholar]
- 23.de Aguilar-Nascimento JE, Kudsk KA. Clinical costs of feeding tube placement. JPEN J Parenter Enteral Nutr. 2007;31:269–273. doi: 10.1177/0148607107031004269. [DOI] [PubMed] [Google Scholar]
- 24.Levy H. Nasogastric and nasoenteric feeding tubes. Gastrointest Endosc Clin N Am. 1998;8:529–549. [PubMed] [Google Scholar]
- 25.McClave SA, Chang WK. Complications of enteral access. Gastrointest Endosc. 2003;58:739–751. doi: 10.1016/s0016-5107(03)02147-3. [DOI] [PubMed] [Google Scholar]
- 26.Zaloga GP. Bedside method for placing small bowel feeding tubes in critically ill patients. A prospective study. Chest. 1991;100:1643–1646. doi: 10.1378/chest.100.6.1643. [DOI] [PubMed] [Google Scholar]
- 27.Rivera R, Campana J, Hamilton C, Lopez R, Seidner D. Small bowel feeding tube placement using an electromagnetic tube placement device: accuracy of tip location. JPEN J Parenter Enteral Nutr. 2011;35:636–642. doi: 10.1177/0148607110386047. [DOI] [PubMed] [Google Scholar]
- 28.Ott DJ, Mattox HE, Gelfand DW, Chen MY, Wu WC. Enteral feeding tubes: placement by using fluoroscopy and endoscopy. AJR Am J Roentgenol. 1991;157:769–771. doi: 10.2214/ajr.157.4.1909832. [DOI] [PubMed] [Google Scholar]
- 29.Mann NS, Nair PK, Mann SK, Lehman BH, Harder GL, Knox AL, Howland CC, Reddy AB. Nasoenteral feeding tube insertion via fiberoptic endoscope for enteral hyperalimentation. J Am Coll Nutr. 1984;3:333–339. doi: 10.1080/07315724.1984.10720057. [DOI] [PubMed] [Google Scholar]
- 30.Wiggins TF, DeLegge MH. Evaluation of a new technique for endoscopic nasojejunal feeding-tube placement. Gastrointest Endosc. 2006;63:590–595. doi: 10.1016/j.gie.2005.10.043. [DOI] [PubMed] [Google Scholar]
- 31.O'Keefe SJ, Foody W, Gill S. Transnasal endoscopic placement of feeding tubes in the intensive care unit. JPEN J Parenter Enteral Nutr. 2003;27:349–354. doi: 10.1177/0148607103027005349. [DOI] [PubMed] [Google Scholar]
- 32.Wiegand N, Bauerfeind P, Delco F, Fried M, Wildi SM. Endoscopic position control of nasoenteral feeding tubes by transnasal re-endoscopy: a prospective study in intensive care patients. Am J Gastroenterol. 2009;104:1271–1276. doi: 10.1038/ajg.2009.26. [DOI] [PubMed] [Google Scholar]
- 33.Black H, Yoneda K, Millar J, Allen J, Belafsky P. Endoscopic placement of a novel feeding tube. Chest. 2010;137:1028–1032. doi: 10.1378/chest.09-2229. [DOI] [PubMed] [Google Scholar]
- 34.Brandt CP, Mittendorf EA. Endoscopic placement of nasojejunal feeding tubes in ICU patients. Surg Endosc. 1999;13:1211–1214. doi: 10.1007/pl00009623. [DOI] [PubMed] [Google Scholar]
- 35.Damore LJ, Andrus CH, Herrmann VM, Wade TP, Kaminski DL, Kaiser GC. Prospective evaluation of a new through-the-scope nasoduodenal enteral feeding tube. Surg Endosc. 1997;11:460–463. doi: 10.1007/s004649900390. [DOI] [PubMed] [Google Scholar]
- 36.Patrick PG, Marulendra S, Kirby DF, DeLegge MH. Endoscopic nasogastric-jejunal feeding tube placement in critically ill patients. Gastrointest Endosc. 1997;45:72–76. doi: 10.1016/s0016-5107(97)70305-5. [DOI] [PubMed] [Google Scholar]
- 37.Külling D, Bauerfeind P, Fried M. Transnasal versus transoral endoscopy for the placement of nasoenteral feeding tubes in critically ill patients. Gastrointest Endosc. 2000;52:506–510. doi: 10.1067/mge.2000.107729. [DOI] [PubMed] [Google Scholar]
- 38.Sparks DA, Chase DM, Coughlin LM, Perry E. Pulmonary complications of 9931 narrow-bore nasoenteric tubes during blind placement: a critical review. JPEN J Parenter Enteral Nutr. 2011;35:625–629. doi: 10.1177/0148607111413898. [DOI] [PubMed] [Google Scholar]
- 39.Bourgault AM, Heyland DK, Drover JW, Keefe L, Newman P, Day AG. Prophylactic pancreatic enzymes to reduce feeding tube occlusions. Nutr Clin Pract. 2003;18:398–401. doi: 10.1177/0115426503018005398. [DOI] [PubMed] [Google Scholar]
- 40.Bosco JJ, Gordon F, Zelig MP, Heiss F, Horst DA, Howell DA. A reliable method for the endoscopic placement of a nasoenteric feeding tube. Gastrointest Endosc. 1994;40:740–743. [PubMed] [Google Scholar]
- 41.Itkin M, DeLegge MH, Fang JC, McClave SA, Kundu S, d’Othee BJ, Martinez-Salazar GM, Sacks D, Swan TL, Towbin RB, et al. Multidisciplinary practical guidelines for gastrointestinal access for enteral nutrition and decompression from the Society of Interventional Radiology and American Gastroenterological Association (AGA) Institute, with endorsement by Canadian Interventional Radiological Association (CIRA) and Cardiovascular and Interventional Radiological Society of Europe (CIRSE) Gastroenterology. 2011;141:742–765. doi: 10.1053/j.gastro.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 42.Jain R, Maple JT, Anderson MA, Appalaneni V, Ben-Menachem T, Decker GA, Fanelli RD, Fisher L, Fukami N, Ikenberry SO, et al. The role of endoscopy in enteral feeding. Gastrointest Endosc. 2011;74:7–12. doi: 10.1016/j.gie.2010.10.021. [DOI] [PubMed] [Google Scholar]
- 43.Kurien M, Leeds JS, Delegge MH, Robson HE, Grant J, Lee FK, McAlindon ME, Sanders DS. Mortality among patients who receive or defer gastrostomies. Clin Gastroenterol Hepatol. 2013;11:1445–1450. doi: 10.1016/j.cgh.2013.04.025. [DOI] [PubMed] [Google Scholar]
- 44.Gauderer MW, Ponsky JL, Izant RJ. Gastrostomy without laparotomy: a percutaneous endoscopic technique. J Pediatr Surg. 1980;15:872–875. doi: 10.1016/s0022-3468(80)80296-x. [DOI] [PubMed] [Google Scholar]
- 45.Löser C, Aschl G, Hébuterne X, Mathus-Vliegen EM, Muscaritoli M, Niv Y, Rollins H, Singer P, Skelly RH. ESPEN guidelines on artificial enteral nutrition--percutaneous endoscopic gastrostomy (PEG) Clin Nutr. 2005;24:848–861. doi: 10.1016/j.clnu.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 46.Larson DE, Burton DD, Schroeder KW, DiMagno EP. Percutaneous endoscopic gastrostomy. Indications, success, complications, and mortality in 314 consecutive patients. Gastroenterology. 1987;93:48–52. [PubMed] [Google Scholar]
- 47.Hull MA, Rawlings J, Murray FE, Field J, McIntyre AS, Mahida YR, Hawkey CJ, Allison SP. Audit of outcome of long-term enteral nutrition by percutaneous endoscopic gastrostomy. Lancet. 1993;341:869–872. doi: 10.1016/0140-6736(93)93072-9. [DOI] [PubMed] [Google Scholar]
- 48.Hoepffner N, Schröder O, Stein J. Enteral Nutrition by Endoscopic Means; II. Complications and Management. Z Gastroenterol. 2004;42:1393–1398. doi: 10.1055/s-2004-813807. [DOI] [PubMed] [Google Scholar]
- 49.Hogan RB, DeMarco DC, Hamilton JK, Walker CO, Polter DE. Percutaneous endoscopic gastrostomy--to push or pull. A prospective randomized trial. Gastrointest Endosc. 1986;32:253–258. doi: 10.1016/s0016-5107(86)71841-5. [DOI] [PubMed] [Google Scholar]
- 50.Bleck JS, Reiss B, Gebel M, Wagner S, Strassburg CP, Meier PN, Boozari B, Schneider A, Caselitz M, Westhoff-Bleck M, et al. Percutaneous sonographic gastrostomy: method, indications, and problems. Am J Gastroenterol. 1998;93:941–945. doi: 10.1111/j.1572-0241.1998.00283.x. [DOI] [PubMed] [Google Scholar]
- 51.Russell TR, Brotman M, Norris F. Percutaneous gastrostomy. A new simplified and cost-effective technique. Am J Surg. 1984;148:132–137. doi: 10.1016/0002-9610(84)90300-3. [DOI] [PubMed] [Google Scholar]
- 52.Dewald CL, Hiette PO, Sewall LE, Fredenberg PG, Palestrant AM. Percutaneous gastrostomy and gastrojejunostomy with gastropexy: experience in 701 procedures. Radiology. 1999;211:651–656. doi: 10.1148/radiology.211.3.r99ma04651. [DOI] [PubMed] [Google Scholar]
- 53.Chadha KS, Thatikonda C, Schiff M, Nava H, Sitrin MD. Outcomes of percutaneous endoscopic gastrostomy tube placement using a T-fastener gastropexy device in head and neck and esophageal cancer patients. Nutr Clin Pract. 2010;25:658–662. doi: 10.1177/0884533610385350. [DOI] [PubMed] [Google Scholar]
- 54.Dormann AJ, Glosemeyer R, Leistner U, Deppe H, Roggel R, Wigginghaus B, Huchzermeyer H. Modified percutaneous endoscopic gastrostomy (PEG) with gastropexy--early experience with a new introducer technique. Z Gastroenterol. 2000;38:933–938. doi: 10.1055/s-2000-10025. [DOI] [PubMed] [Google Scholar]
- 55.Shastri YM, Hoepffner N, Tessmer A, Ackermann H, Schroeder O, Stein J. New introducer PEG gastropexy does not require prophylactic antibiotics: multicenter prospective randomized double-blind placebo-controlled study. Gastrointest Endosc. 2008;67:620–628. doi: 10.1016/j.gie.2007.10.044. [DOI] [PubMed] [Google Scholar]
- 56.Dormann AJ, Huchzermeyer H. Endoscopic techniques for enteral nutrition: standards and innovations. Dig Dis. 2002;20:145–153. doi: 10.1159/000067486. [DOI] [PubMed] [Google Scholar]
- 57.DeLegge MH, Patrick P, Gibbs R. Percutaneous endoscopic gastrojejunostomy with a tapered tip, nonweighted jejunal feeding tube: improved placement success. Am J Gastroenterol. 1996;91:1130–1134. [PubMed] [Google Scholar]
- 58.Parasher VK, Abramowicz CJ, Bell C, Delledonne AM, Wright A. Successful placement of percutaneous gastrojejunostomy using steerable glidewire--a modified controlled push technique. Gastrointest Endosc. 1995;41:52–55. doi: 10.1016/s0016-5107(95)70275-x. [DOI] [PubMed] [Google Scholar]
- 59.Udorah MO, Fleischman MW, Bala V, Cai Q. Endoscopic clips prevent displacement of intestinal feeding tubes: a long-term follow-up study. Dig Dis Sci. 2010;55:371–374. doi: 10.1007/s10620-009-0726-3. [DOI] [PubMed] [Google Scholar]
- 60.Mellert J, Naruhn MB, Grund KE, Becker HD. Direct endoscopic percutaneous jejunostomy (EPJ). Clinical results. Surg Endosc. 1994;8:867–869; discussion 869-870. doi: 10.1007/BF00843456. [DOI] [PubMed] [Google Scholar]
- 61.Shike M, Latkany L, Gerdes H, Bloch AS. Direct percutaneous endoscopic jejunostomies for enteral feeding. Gastrointest Endosc. 1996;44:536–540. doi: 10.1016/s0016-5107(96)70005-6. [DOI] [PubMed] [Google Scholar]
- 62.Rumalla A, Baron TH. Results of direct percutaneous endoscopic jejunostomy, an alternative method for providing jejunal feeding. Mayo Clin Proc. 2000;75:807–810. doi: 10.4065/75.8.807. [DOI] [PubMed] [Google Scholar]
- 63.Gauderer MW. Percutaneous endoscopic gastrostomy and the evolution of contemporary long-term enteral access. Clin Nutr. 2002;21:103–110. doi: 10.1054/clnu.2001.0533. [DOI] [PubMed] [Google Scholar]
- 64.Foutch PG, Talbert GA, Gaines JA, Sanowski RA. The gastrostomy button: a prospective assessment of safety, success, and spectrum of use. Gastrointest Endosc. 1989;35:41–44. doi: 10.1016/s0016-5107(89)72684-5. [DOI] [PubMed] [Google Scholar]
- 65.Willwerth BM. Percutaneous endoscopic gastrostomy or skin-level gastrostomy tube replacement. Pediatr Emerg Care. 2001;17:55–58. doi: 10.1097/00006565-200102000-00016. [DOI] [PubMed] [Google Scholar]
- 66.Kozarek RA, Payne M, Barkin J, Goff J, Gostout C. Prospective multicenter evaluation of an initially placed button gastrostomy. Gastrointest Endosc. 1995;41:105–108. doi: 10.1016/s0016-5107(05)80590-5. [DOI] [PubMed] [Google Scholar]
- 67.George J, Crawford D, Lewis T, Shepherd R, Ward M. Percutaneous endoscopic gastrostomy: a two-year experience. Med J Aust. 1990;152:17–19. doi: 10.5694/j.1326-5377.1990.tb124421.x. [DOI] [PubMed] [Google Scholar]
- 68.Brown DN, Miedema BW, King PD, Marshall JB. Safety of early feeding after percutaneous endoscopic gastrostomy. J Clin Gastroenterol. 1995;21:330–331. doi: 10.1097/00004836-199512000-00020. [DOI] [PubMed] [Google Scholar]
- 69.Choudhry U, Barde CJ, Markert R, Gopalswamy N. Percutaneous endoscopic gastrostomy: a randomized prospective comparison of early and delayed feeding. Gastrointest Endosc. 1996;44:164–167. doi: 10.1016/s0016-5107(96)70134-7. [DOI] [PubMed] [Google Scholar]
- 70.McCarter TL, Condon SC, Aguilar RC, Gibson DJ, Chen YK. Randomized prospective trial of early versus delayed feeding after percutaneous endoscopic gastrostomy placement. Am J Gastroenterol. 1998;93:419–421. doi: 10.1111/j.1572-0241.1998.00419.x. [DOI] [PubMed] [Google Scholar]
- 71.Stein J, Schulte-Bockholt A, Sabin M, Keymling M. A randomized prospective trial of immediate vs. next-day feeding after percutaneous endoscopic gastrostomy in intensive care patients. Intensive Care Med. 2002;28:1656–1660. doi: 10.1007/s00134-002-1473-5. [DOI] [PubMed] [Google Scholar]
- 72.Bechtold ML, Matteson ML, Choudhary A, Puli SR, Jiang PP, Roy PK. Early versus delayed feeding after placement of a percutaneous endoscopic gastrostomy: a meta-analysis. Am J Gastroenterol. 2008;103:2919–2924. doi: 10.1111/j.1572-0241.2008.02108.x. [DOI] [PubMed] [Google Scholar]
- 73.Grant MD, Rudberg MA, Brody JA. Gastrostomy placement and mortality among hospitalized Medicare beneficiaries. JAMA. 1998;279:1973–1976. doi: 10.1001/jama.279.24.1973. [DOI] [PubMed] [Google Scholar]
- 74.Mathus-Vliegen LM, Koning H. Percutaneous endoscopic gastrostomy and gastrojejunostomy: a critical reappraisal of patient selection, tube function and the feasibility of nutritional support during extended follow-up. Gastrointest Endosc. 1999;50:746–754. doi: 10.1016/s0016-5107(99)70153-7. [DOI] [PubMed] [Google Scholar]
- 75.DiSario JA. Endoscopic approaches to enteral nutritional support. Best Pract Res Clin Gastroenterol. 2006;20:605–630. doi: 10.1016/j.bpg.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 76.Longcroft-Wheaton G, Marden P, Colleypriest B, Gavin D, Taylor G, Farrant M. Understanding why patients die after gastrostomy tube insertion: a retrospective analysis of mortality. JPEN J Parenter Enteral Nutr. 2009;33:375–379. doi: 10.1177/0148607108327156. [DOI] [PubMed] [Google Scholar]
- 77.Safadi BY, Marks JM, Ponsky JL. Percutaneous endoscopic gastrostomy. Gastrointest Endosc Clin N Am. 1998;8:551–568. [PubMed] [Google Scholar]
- 78.Chung RS, Schertzer M. Pathogenesis of complications of percutaneous endoscopic gastrostomy. A lesson in surgical principles. Am Surg. 1990;56:134–137. [PubMed] [Google Scholar]
- 79.Lipp A, Lusardi G. Systemic antimicrobial prophylaxis for percutaneous endoscopic gastrostomy. Cochrane Database Syst Rev. 2006;(4):CD005571. doi: 10.1002/14651858.CD005571.pub2. [DOI] [PubMed] [Google Scholar]
- 80.Jafri NS, Mahid SS, Minor KS, Idstein SR, Hornung CA, Galandiuk S. Meta-analysis: antibiotic prophylaxis to prevent peristomal infection following percutaneous endoscopic gastrostomy. Aliment Pharmacol Ther. 2007;25:647–656. doi: 10.1111/j.1365-2036.2007.03247.x. [DOI] [PubMed] [Google Scholar]
- 81.Horiuchi A, Nakayama Y, Kajiyama M, Fujii H, Tanaka N. Nasopharyngeal decolonization of methicillin-resistant Staphylococcus aureus can reduce PEG peristomal wound infection. Am J Gastroenterol. 2006;101:274–277. doi: 10.1111/j.1572-0241.2006.00366.x. [DOI] [PubMed] [Google Scholar]
- 82.Sriram K, Jayanthi V, Lakshmi RG, George VS. Prophylactic locking of enteral feeding tubes with pancreatic enzymes. JPEN J Parenter Enteral Nutr. 1997;21:353–356. doi: 10.1177/0148607197021006353. [DOI] [PubMed] [Google Scholar]
- 83.Powell KS, Marcuard SP, Farrior ES, Gallagher ML. Aspirating gastric residuals causes occlusion of small-bore feeding tubes. JPEN J Parenter Enteral Nutr. 1993;17:243–246. doi: 10.1177/0148607193017003243. [DOI] [PubMed] [Google Scholar]
- 84.Behnken I, Gaschott T, Stein J. [Enteral nutrition: drug administration via feeding tube] Z Gastroenterol. 2005;43:1231–1241. doi: 10.1055/s-2005-858737. [DOI] [PubMed] [Google Scholar]
- 85.Schrag SP, Sharma R, Jaik NP, Seamon MJ, Lukaszczyk JJ, Martin ND, Hoey BA, Stawicki SP. Complications related to percutaneous endoscopic gastrostomy (PEG) tubes. A comprehensive clinical review. J Gastrointestin Liver Dis. 2007;16:407–418. [PubMed] [Google Scholar]
- 86.Marcuard SP, Stegall KL, Trogdon S. Clearing obstructed feeding tubes. JPEN J Parenter Enteral Nutr. 1989;13:81–83. doi: 10.1177/014860718901300181. [DOI] [PubMed] [Google Scholar]
- 87.Dandeles LM, Lodolce AE. Efficacy of agents to prevent and treat enteral feeding tube clogs. Ann Pharmacother. 2011;45:676–680. doi: 10.1345/aph.1P487. [DOI] [PubMed] [Google Scholar]
- 88.Baskin WN. Acute complications associated with bedside placement of feeding tubes. Nutr Clin Pract. 2006;21:40–55. doi: 10.1177/011542650602100140. [DOI] [PubMed] [Google Scholar]
- 89.Lin HS, Ibrahim HZ, Kheng JW, Fee WE, Terris DJ. Percutaneous endoscopic gastrostomy: strategies for prevention and management of complications. Laryngoscope. 2001;111:1847–1852. doi: 10.1097/00005537-200110000-00033. [DOI] [PubMed] [Google Scholar]
- 90.Tsang TK, Eaton D, Falconio MA. Percutaneous ostomy dilation: a technique for dilating the closed percutaneous endoscopic gastrostomy sites and reinserting gastrostomies. Gastrointest Endosc. 1989;35:336–337. doi: 10.1016/s0016-5107(89)72805-4. [DOI] [PubMed] [Google Scholar]
- 91.Amann W, Mischinger HJ, Berger A, Rosanelli G, Schweiger W, Werkgartner G, Fruhwirth J, Hauser H. Percutaneous endoscopic gastrostomy (PEG). 8 years of clinical experience in 232 patients. Surg Endosc. 1997;11:741–744. doi: 10.1007/s004649900440. [DOI] [PubMed] [Google Scholar]
- 92.Anderson MA, Ben-Menachem T, Gan SI, Appalaneni V, Banerjee S, Cash BD, Fisher L, Harrison ME, Fanelli RD, Fukami N, et al. Management of antithrombotic agents for endoscopic procedures. Gastrointest Endosc. 2009;70:1060–1070. doi: 10.1016/j.gie.2009.09.040. [DOI] [PubMed] [Google Scholar]
- 93.Richter JA, Patrie JT, Richter RP, Henry ZH, Pop GH, Regan KA, Peura DA, Sawyer RG, Northup PG, Wang AY. Bleeding after percutaneous endoscopic gastrostomy is linked to serotonin reuptake inhibitors, not aspirin or clopidogrel. Gastrointest Endosc. 2011;74:22–34.e1. doi: 10.1016/j.gie.2011.03.1258. [DOI] [PubMed] [Google Scholar]
- 94.Anagnostopoulos GK, Kostopoulos P, Arvanitidis DM. Buried bumper syndrome with a fatal outcome, presenting early as gastrointestinal bleeding after percutaneous endoscopic gastrostomy placement. J Postgrad Med. 2003;49:325–327. [PubMed] [Google Scholar]
- 95.Dharmarajan TS, Yadav D, Adiga GU, Kokkat A, Pitchumoni CS. Gastrostomy, esophagitis, and gastrointestinal bleeding in older adults. J Am Med Dir Assoc. 2004;5:228–232. doi: 10.1097/01.JAM.0000129838.71003.A4. [DOI] [PubMed] [Google Scholar]
- 96.Guloglu R, Taviloglu K, Alimoglu O. Colon injury following percutaneous endoscopic gastrostomy tube insertion. J Laparoendosc Adv Surg Tech A. 2003;13:69–72. doi: 10.1089/109264203321235520. [DOI] [PubMed] [Google Scholar]
- 97.Friedmann R, Feldman H, Sonnenblick M. Misplacement of percutaneously inserted gastrostomy tube into the colon: report of 6 cases and review of the literature. JPEN J Parenter Enteral Nutr. 2007;31:469–476. doi: 10.1177/0148607107031006469. [DOI] [PubMed] [Google Scholar]
- 98.Berger SA, Zarling EJ. Colocutaneous fistula following migration of PEG tube. Gastrointest Endosc. 1991;37:86–88. doi: 10.1016/s0016-5107(91)70634-2. [DOI] [PubMed] [Google Scholar]
- 99.Yamazaki T, Sakai Y, Hatakeyama K, Hoshiyama Y. Colocutaneous fistula after percutaneous endoscopic gastrostomy in a remnant stomach. Surg Endosc. 1999;13:280–282. doi: 10.1007/s004649900964. [DOI] [PubMed] [Google Scholar]
- 100.Ponsky JL, Gauderer MW. Percutaneous endoscopic gastrostomy: indications, limitations, techniques, and results. World J Surg. 1989;13:165–170. doi: 10.1007/BF01658394. [DOI] [PubMed] [Google Scholar]
- 101.Marcy PY, Magné N, Lacroix J, Bailet C. Late presentation of a gastrocolic fistula after percutaneous fluoroscopic gastrostomy. JBR-BTR. 2004;87:17–20. [PubMed] [Google Scholar]
- 102.Strodel WE, Lemmer J, Eckhauser F, Botham M, Dent T. Early experience with endoscopic percutaneous gastrostomy. Arch Surg. 1983;118:449–453. doi: 10.1001/archsurg.1983.01390040059012. [DOI] [PubMed] [Google Scholar]
- 103.Kim HS, Lee DK, Baik SK, Kwon SO. Endoscopic management of colocutaneous fistula after percutaneous endoscopic gastrostomy. Endoscopy. 2002;34:430. doi: 10.1055/s-2002-25284. [DOI] [PubMed] [Google Scholar]
- 104.Gyökeres T, Burai M, Hamvas J, Varsányi M, Mácsai M, Paput L, Köveskuti A, Fekete C, Pap A. Conservative vs. endoscopic closure of colocutaneous fistulas after percutaneous endoscopic gastrostomy complications. Endoscopy. 2003;35:246–247; author reply 248. doi: 10.1055/s-2003-37261. [DOI] [PubMed] [Google Scholar]
- 105.Bumpers HL, Collure DW, Best IM, Butler KL, Weaver WL, Hoover EL. Unusual complications of long-term percutaneous gastrostomy tubes. J Gastrointest Surg. 2003;7:917–920. doi: 10.1007/s11605-003-0040-x. [DOI] [PubMed] [Google Scholar]
- 106.Hussien M, Fawzy M, Carey D. Percutaneous endoscopic gastroscopy tube migration: a rare cause of a common surgical problem. Int J Clin Pract. 2001;55:557–559. [PubMed] [Google Scholar]
- 107.Köhler DM. [The wandering gastrostomy tube] Ugeskr Laeger. 2000;162:3344–3345. [PubMed] [Google Scholar]
- 108.Finocchiaro C, Galletti R, Rovera G, Ferrari A, Todros L, Vuolo A, Balzola F. Percutaneous endoscopic gastrostomy: a long-term follow-up. Nutrition. 1997;13:520–523. doi: 10.1016/s0899-9007(97)00030-0. [DOI] [PubMed] [Google Scholar]
- 109.Venu RP, Brown RD, Pastika BJ, Erikson LW. The buried bumper syndrome: a simple management approach in two patients. Gastrointest Endosc. 2002;56:582–584. doi: 10.1067/mge.2002.128109. [DOI] [PubMed] [Google Scholar]
- 110.Braden B, Brandstaetter M, Caspary WF, Seifert H. Buried bumper syndrome: treatment guided by catheter probe US. Gastrointest Endosc. 2003;57:747–751. doi: 10.1067/mge.2003.184. [DOI] [PubMed] [Google Scholar]
- 111.Lee TH, Lin JT. Clinical manifestations and management of buried bumper syndrome in patients with percutaneous endoscopic gastrostomy. Gastrointest Endosc. 2008;68:580–584. doi: 10.1016/j.gie.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 112.Rino Y, Tokunaga M, Morinaga S, Onodera S, Tomiyama I, Imada T, Takanashi Y. The buried bumper syndrome: an early complication of percutaneous endoscopic gastrostomy. Hepatogastroenterology. 2002;49:1183–1184. [PubMed] [Google Scholar]
- 113.Gençosmanoğlu R, Koç D, Tözün N. The buried bumper syndrome: migration of internal bumper of percutaneous endoscopic gastrostomy tube into the abdominal wall. J Gastroenterol. 2003;38:1077–1080. doi: 10.1007/s00535-003-1199-3. [DOI] [PubMed] [Google Scholar]
- 114.Ma MM, Semlacher EA, Fedorak RN, Lalor EA, Duerksen DR, Sherbaniuk RW, Chalpelsky CE, Sadowski DC. The buried gastrostomy bumper syndrome: prevention and endoscopic approaches to removal. Gastrointest Endosc. 1995;41:505–508. doi: 10.1016/s0016-5107(05)80012-4. [DOI] [PubMed] [Google Scholar]
- 115.Müller-Gerbes D, Aymaz S, Dormann AJ. [Management of the buried bumper syndrome: a new minimally invasive technique--the push method] Z Gastroenterol. 2009;47:1145–1148. doi: 10.1055/s-2008-1027988. [DOI] [PubMed] [Google Scholar]
- 116.Hodges EG, Morano JU, Nowicki MJ. The buried bumper syndrome complicating percutaneous endoscopic gastrostomy in children. J Pediatr Gastroenterol Nutr. 2001;33:326–328. doi: 10.1097/00005176-200109000-00018. [DOI] [PubMed] [Google Scholar]
- 117.Boreham B, Ammori BJ. Laparoscopic percutaneous endoscopic gastrostomy removal in a patient with buried-bumper syndrome: a new approach. Surg Laparosc Endosc Percutan Tech. 2002;12:356–358. doi: 10.1097/00129689-200210000-00010. [DOI] [PubMed] [Google Scholar]
- 118.Wiesen AJ, Sideridis K, Fernandes A, Hines J, Indaram A, Weinstein L, Davidoff S, Bank S. True incidence and clinical significance of pneumoperitoneum after PEG placement: a prospective study. Gastrointest Endosc. 2006;64:886–889. doi: 10.1016/j.gie.2006.06.088. [DOI] [PubMed] [Google Scholar]
- 119.Nazarian A, Cross W, Kowdley GC. Pneumoperitoneum after percutaneous endoscopic gastrostomy among adults in the intensive care unit: incidence, predictive factors, and clinical significance. Am Surg. 2012;78:591–594. [PubMed] [Google Scholar]
- 120.Blum CA, Selander C, Ruddy JM, Leon S. The incidence and clinical significance of pneumoperitoneum after percutaneous endoscopic gastrostomy: a review of 722 cases. Am Surg. 2009;75:39–43. [PubMed] [Google Scholar]
- 121.Alley JB, Corneille MG, Stewart RM, Dent DL. Pneumoperitoneum after percutaneous endoscopic gastrostomy in patients in the intensive care unit. Am Surg. 2007;73:765–767; discussion 768. [PubMed] [Google Scholar]
- 122.Dulabon GR, Abrams JE, Rutherford EJ. The incidence and significance of free air after percutaneous endoscopic gastrostomy. Am Surg. 2002;68:590–593. [PubMed] [Google Scholar]
- 123.Chaer RA, Rekkas D, Trevino J, Brown R, Espat J. Intrahepatic placement of a PEG tube. Gastrointest Endosc. 2003;57:763–765. doi: 10.1067/mge.2003.201. [DOI] [PubMed] [Google Scholar]
- 124.Gubler C, Wildi SM, Bauerfeind P. Liver injury during PEG tube placement: report of two cases. Gastrointest Endosc. 2005;61:346–348. doi: 10.1016/s0016-5107(04)02584-2. [DOI] [PubMed] [Google Scholar]
- 125.Wiggins TF, Kaplan R, DeLegge MH. Acute hemorrhage following transhepatic PEG tube placement. Dig Dis Sci. 2007;52:167–169. doi: 10.1007/s10620-006-9446-0. [DOI] [PubMed] [Google Scholar]
- 126.Cruz I, Mamel JJ, Brady PG, Cass-Garcia M. Incidence of abdominal wall metastasis complicating PEG tube placement in untreated head and neck cancer. Gastrointest Endosc. 2005;62:708–711; quiz 752, 753. doi: 10.1016/j.gie.2005.06.041. [DOI] [PubMed] [Google Scholar]
- 127.Volkmer K, Meyer T, Sailer M, Fein M. [Metastasis of an esophageal carcinoma at a PEG site--case report and review of the literature] Zentralbl Chir. 2009;134:481–485. doi: 10.1055/s-0028-1098769. [DOI] [PubMed] [Google Scholar]
- 128.Cappell MS. Risk factors and risk reduction of malignant seeding of the percutaneous endoscopic gastrostomy track from pharyngoesophageal malignancy: a review of all 44 known reported cases. Am J Gastroenterol. 2007;102:1307–1311. doi: 10.1111/j.1572-0241.2007.01227.x. [DOI] [PubMed] [Google Scholar]
- 129.Couto G. Overtube for preventing abdominal-wall metastasis after PEG-tube placement. Gastrointest Endosc. 2006;63:1087. doi: 10.1016/j.gie.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 130.Montejo JC. Enteral nutrition-related gastrointestinal complications in critically ill patients: a multicenter study. The Nutritional and Metabolic Working Group of the Spanish Society of Intensive Care Medicine and Coronary Units. Crit Care Med. 1999;27:1447–1453. doi: 10.1097/00003246-199908000-00006. [DOI] [PubMed] [Google Scholar]
- 131.Montejo JC, Zarazaga A, López-Martínez J, Urrútia G, Roqué M, Blesa AL, Celaya S, Conejero R, Galbán C, García de Lorenzo A, et al. Immunonutrition in the intensive care unit. A systematic review and consensus statement. Clin Nutr. 2003;22:221–233. doi: 10.1016/s0261-5614(03)00007-4. [DOI] [PubMed] [Google Scholar]
- 132.Schröder O, Hoepffner N, Stein J. Enteral nutrition by endoscopic means; I. Techniques, indications, types of enteral feed. Z Gastroenterol. 2004;42:1385–1392. doi: 10.1055/s-2004-813806. [DOI] [PubMed] [Google Scholar]
- 133.Marvin RG, McKinley BA, McQuiggan M, Cocanour CS, Moore FA. Nonocclusive bowel necrosis occurring in critically ill trauma patients receiving enteral nutrition manifests no reliable clinical signs for early detection. Am J Surg. 2000;179:7–12. doi: 10.1016/s0002-9610(99)00261-5. [DOI] [PubMed] [Google Scholar]
- 134.Montejo JC, Grau T, Acosta J, Ruiz-Santana S, Planas M, García-De-Lorenzo A, Mesejo A, Cervera M, Sánchez-Alvarez C, Núñez-Ruiz R, et al. Multicenter, prospective, randomized, single-blind study comparing the efficacy and gastrointestinal complications of early jejunal feeding with early gastric feeding in critically ill patients. Crit Care Med. 2002;30:796–800. doi: 10.1097/00003246-200204000-00013. [DOI] [PubMed] [Google Scholar]
- 135.Majid HA, Emery PW, Whelan K. Definitions, attitudes, and management practices in relation to diarrhea during enteral nutrition: a survey of patients, nurses, and dietitians. Nutr Clin Pract. 2012;27:252–260. doi: 10.1177/0884533611431986. [DOI] [PubMed] [Google Scholar]
- 136.Whelan K, Schneider SM. Mechanisms, prevention, and management of diarrhea in enteral nutrition. Curr Opin Gastroenterol. 2011;27:152–159. doi: 10.1097/MOG.0b013e32834353cb. [DOI] [PubMed] [Google Scholar]
- 137.Jack L, Coyer F, Courtney M, Venkatesh B. Diarrhoea risk factors in enterally tube fed critically ill patients: a retrospective audit. Intensive Crit Care Nurs. 2010;26:327–334. doi: 10.1016/j.iccn.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 138.Stroud M, Duncan H, Nightingale J. Guidelines for enteral feeding in adult hospital patients. Gut. 2003;52 Suppl 7:vii1–vii12. doi: 10.1136/gut.52.suppl_7.vii1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ritz MA, Fraser R, Tam W, Dent J. Impacts and patterns of disturbed gastrointestinal function in critically ill patients. Am J Gastroenterol. 2000;95:3044–3052. doi: 10.1111/j.1572-0241.2000.03176.x. [DOI] [PubMed] [Google Scholar]
- 140.Btaiche IF, Chan LN, Pleva M, Kraft MD. Critical illness, gastrointestinal complications, and medication therapy during enteral feeding in critically ill adult patients. Nutr Clin Pract. 2010;25:32–49. doi: 10.1177/0884533609357565. [DOI] [PubMed] [Google Scholar]
- 141.Mylonakis E, Ryan ET, Calderwood SB. Clostridium difficile--Associated diarrhea: A review. Arch Intern Med. 2001;161:525–533. doi: 10.1001/archinte.161.4.525. [DOI] [PubMed] [Google Scholar]
- 142.Bliss DZ, Johnson S, Savik K, Clabots CR, Willard K, Gerding DN. Acquisition of Clostridium difficile and Clostridium difficile-associated diarrhea in hospitalized patients receiving tube feeding. Ann Intern Med. 1998;129:1012–1019. doi: 10.7326/0003-4819-129-12-199812150-00004. [DOI] [PubMed] [Google Scholar]
- 143.Stück K, Faul K, Hylla S, Stein J, Breves G. The application of a semi-continuous colon simulation technique (Cositec) for studying the effects of clindamycin on microbial hindgut metabolism. Z Gastroenterol. 1995;33:241–246. [PubMed] [Google Scholar]
- 144.Eisenberg P. An overview of diarrhea in the patient receiving enteral nutrition. Gastroenterol Nurs. 2002;25:95–104. doi: 10.1097/00001610-200205000-00003. [DOI] [PubMed] [Google Scholar]
- 145.Elia M, Engfer MB, Green CJ, Silk DB. Systematic review and meta-analysis: the clinical and physiological effects of fibre-containing enteral formulae. Aliment Pharmacol Ther. 2008;27:120–145. doi: 10.1111/j.1365-2036.2007.03544.x. [DOI] [PubMed] [Google Scholar]
- 146.Schultz AA, Ashby-Hughes B, Taylor R, Gillis DE, Wilkins M. Effects of pectin on diarrhea in critically ill tube-fed patients receiving antibiotics. Am J Crit Care. 2000;9:403–411. [PubMed] [Google Scholar]
- 147.Spapen H, Diltoer M, Van Malderen C, Opdenacker G, Suys E, Huyghens L. Soluble fiber reduces the incidence of diarrhea in septic patients receiving total enteral nutrition: a prospective, double-blind, randomized, and controlled trial. Clin Nutr. 2001;20:301–305. doi: 10.1054/clnu.2001.0399. [DOI] [PubMed] [Google Scholar]
- 148.Nakao M, Ogura Y, Satake S, Ito I, Iguchi A, Takagi K, Nabeshima T. Usefulness of soluble dietary fiber for the treatment of diarrhea during enteral nutrition in elderly patients. Nutrition. 2002;18:35–39. doi: 10.1016/s0899-9007(01)00715-8. [DOI] [PubMed] [Google Scholar]
- 149.Rushdi TA, Pichard C, Khater YH. Control of diarrhea by fiber-enriched diet in ICU patients on enteral nutrition: a prospective randomized controlled trial. Clin Nutr. 2004;23:1344–1352. doi: 10.1016/j.clnu.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 150.Sobotka L, Brátova M, Slemrová M, Manák J, Vizd’a J, Zadák Z. Inulin as the soluble fiber in liquid enteral nutrition. Nutrition. 1997;13:21–25. doi: 10.1016/s0899-9007(97)90874-1. [DOI] [PubMed] [Google Scholar]
- 151.Halmos EP, Muir JG, Barrett JS, Deng M, Shepherd SJ, Gibson PR. Diarrhoea during enteral nutrition is predicted by the poorly absorbed short-chain carbohydrate (FODMAP) content of the formula. Aliment Pharmacol Ther. 2010;32:925–933. doi: 10.1111/j.1365-2036.2010.04416.x. [DOI] [PubMed] [Google Scholar]
- 152.Meier R, Gassull M. Consensus recommendations on the effects and benefits of fibre in clinical practice. Clin Nutr. 2004;10:73–80. [Google Scholar]
- 153.Whelan K. Enteral-tube-feeding diarrhoea: manipulating the colonic microbiota with probiotics and prebiotics. Proc Nutr Soc. 2007;66:299–306. doi: 10.1017/S0029665107005551. [DOI] [PubMed] [Google Scholar]
- 154.Whelan K, Gibson GR, Judd PA, Taylor MA. The role of probiotics and prebiotics in the management of diarrhoea associated with enteral tube feeding. J Hum Nutr Diet. 2001;14:423–433. doi: 10.1046/j.1365-277x.2001.00322.x. [DOI] [PubMed] [Google Scholar]
- 155.McFarland LV. Systematic review and meta-analysis of Saccharomyces boulardii in adult patients. World J Gastroenterol. 2010;16:2202–2222. doi: 10.3748/wjg.v16.i18.2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Besselink MG, van Santvoort HC, Buskens E, Boermeester MA, van Goor H, Timmerman HM, Nieuwenhuijs VB, Bollen TL, van Ramshorst B, Witteman BJ, et al. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;371:651–659. doi: 10.1016/S0140-6736(08)60207-X. [DOI] [PubMed] [Google Scholar]
- 157.Whelan K, Myers CE. Safety of probiotics in patients receiving nutritional support: a systematic review of case reports, randomized controlled trials, and nonrandomized trials. Am J Clin Nutr. 2010;91:687–703. doi: 10.3945/ajcn.2009.28759. [DOI] [PubMed] [Google Scholar]
- 158.McClave SA, DeMeo MT, DeLegge MH, DiSario JA, Heyland DK, Maloney JP, Metheny NA, Moore FA, Scolapio JS, Spain DA, et al. North American Summit on Aspiration in the Critically Ill Patient: consensus statement. JPEN J Parenter Enteral Nutr. 2006;26:S80–S85. doi: 10.1177/014860710202600613. [DOI] [PubMed] [Google Scholar]
- 159.NAGLER R, SPIRO HM. PERSISTENT GASTROESOPHAGEAL REFLUX INDUCED DURING PROLONGED GASTRIC INTUBATION. N Engl J Med. 1963;269:495–500. doi: 10.1056/NEJM196309052691003. [DOI] [PubMed] [Google Scholar]
- 160.Gomes GF, Pisani JC, Macedo ED, Campos AC. The nasogastric feeding tube as a risk factor for aspiration and aspiration pneumonia. Curr Opin Clin Nutr Metab Care. 2003;6:327–333. doi: 10.1097/01.mco.0000068970.34812.8b. [DOI] [PubMed] [Google Scholar]
- 161.Ukleja A. Altered GI motility in critically Ill patients: current understanding of pathophysiology, clinical impact, and diagnostic approach. Nutr Clin Pract. 2010;25:16–25. doi: 10.1177/0884533609357568. [DOI] [PubMed] [Google Scholar]
- 162.Jacobs S, Chang RW, Lee B, Bartlett FW. Continuous enteral feeding: a major cause of pneumonia among ventilated intensive care unit patients. JPEN J Parenter Enteral Nutr. 1990;14:353–356. doi: 10.1177/0148607190014004353. [DOI] [PubMed] [Google Scholar]
- 163.Olivares L, Segovia A, Revuelta R. Tube feeding and lethal aspiration in neurological patients: a review of 720 autopsy cases. Stroke. 1974;5:654–657. doi: 10.1161/01.str.5.5.654. [DOI] [PubMed] [Google Scholar]
- 164.Marik PE, Zaloga GP. Gastric versus post-pyloric feeding: a systematic review. Crit Care. 2003;7:R46–R51. doi: 10.1186/cc2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Silk DB. The evolving role of post-ligament of Trietz nasojejunal feeding in enteral nutrition and the need for improved feeding tube design and placement methods. JPEN J Parenter Enteral Nutr. 2011;35:303–307. doi: 10.1177/0148607110387799. [DOI] [PubMed] [Google Scholar]
- 166.Waseem S, Moshiree B, Draganov PV. Gastroparesis: current diagnostic challenges and management considerations. World J Gastroenterol. 2009;15:25–37. doi: 10.3748/wjg.15.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Strong RM, Condon SC, Solinger MR, Namihas BN, Ito-Wong LA, Leuty JE. Equal aspiration rates from postpylorus and intragastric-placed small-bore nasoenteric feeding tubes: a randomized, prospective study. JPEN J Parenter Enteral Nutr. 1992;16:59–63. doi: 10.1177/014860719201600159. [DOI] [PubMed] [Google Scholar]
- 168.White H, Sosnowski K, Tran K, Reeves A, Jones M. A randomised controlled comparison of early post-pyloric versus early gastric feeding to meet nutritional targets in ventilated intensive care patients. Crit Care. 2009;13:R187. doi: 10.1186/cc8181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Neumann DA, DeLegge MH. Gastric versus small-bowel tube feeding in the intensive care unit: a prospective comparison of efficacy. Crit Care Med. 2002;30:1436–1438. doi: 10.1097/00003246-200207000-00006. [DOI] [PubMed] [Google Scholar]
- 170.Esparza J, Boivin MA, Hartshorne MF, Levy H. Equal aspiration rates in gastrically and transpylorically fed critically ill patients. Intensive Care Med. 2001;27:660–664. doi: 10.1007/s001340100880. [DOI] [PubMed] [Google Scholar]
- 171.Davies AR, Froomes PR, French CJ, Bellomo R, Gutteridge GA, Nyulasi I, Walker R, Sewell RB. Randomized comparison of nasojejunal and nasogastric feeding in critically ill patients. Crit Care Med. 2002;30:586–590. doi: 10.1097/00003246-200203000-00016. [DOI] [PubMed] [Google Scholar]
- 172.Montecalvo MA, Steger KA, Farber HW, Smith BF, Dennis RC, Fitzpatrick GF, Pollack SD, Korsberg TZ, Birkett DH, Hirsch EF. Nutritional outcome and pneumonia in critical care patients randomized to gastric versus jejunal tube feedings. The Critical Care Research Team. Crit Care Med. 1992;20:1377–1387. doi: 10.1097/00003246-199210000-00004. [DOI] [PubMed] [Google Scholar]
- 173.Day L, Stotts NA, Frankfurt A, Stralovich-Romani A, Volz M, Muwaswes M, Fukuoka Y, O’Leary-Kelley C. Gastric versus duodenal feeding in patients with neurological disease: a pilot study. J Neurosci Nurs. 2001;33:148–149, 155-159. doi: 10.1097/01376517-200106000-00007. [DOI] [PubMed] [Google Scholar]
- 174.Heyland DK, Drover JW, MacDonald S, Novak F, Lam M. Effect of postpyloric feeding on gastroesophageal regurgitation and pulmonary microaspiration: results of a randomized controlled trial. Crit Care Med. 2001;29:1495–1501. doi: 10.1097/00003246-200108000-00001. [DOI] [PubMed] [Google Scholar]
- 175.Kortbeek JB, Haigh PI, Doig C. Duodenal versus gastric feeding in ventilated blunt trauma patients: a randomized controlled trial. J Trauma. 1999;46:992–996; discussion 996-998. doi: 10.1097/00005373-199906000-00002. [DOI] [PubMed] [Google Scholar]
- 176.Ho KM, Dobb GJ, Webb SA. A comparison of early gastric and post-pyloric feeding in critically ill patients: a meta-analysis. Intensive Care Med. 2006;32:639–649. doi: 10.1007/s00134-006-0128-3. [DOI] [PubMed] [Google Scholar]
- 177.Gonçalves RR, Leite HP, Nogueira PC. Does small bowel feeding decrease the risk of ventilator-associated pneumonia? JPEN J Parenter Enteral Nutr. 2004;28:60; author reply 61. doi: 10.1177/014860710402800160. [DOI] [PubMed] [Google Scholar]
- 178.Schnitker MA, Mattman PE, Bliss TL. A clinical study of malnutrition in Japanese prisoners of war. Ann Intern Med. 1951;35:69–96. doi: 10.7326/0003-4819-35-1-69. [DOI] [PubMed] [Google Scholar]
- 179.KEYS A. The residues of malnutrition and starvation. Science. 1950;112:371–373. doi: 10.1126/science.112.2909.371. [DOI] [PubMed] [Google Scholar]
- 180.Marinella MA. The refeeding syndrome and hypophosphatemia. Nutr Rev. 2003;61:320–323. doi: 10.1301/nr.2003.sept.320-323. [DOI] [PubMed] [Google Scholar]
- 181.Byrnes MC, Stangenes J. Refeeding in the ICU: an adult and pediatric problem. Curr Opin Clin Nutr Metab Care. 2011;14:186–192. doi: 10.1097/MCO.0b013e328341ed93. [DOI] [PubMed] [Google Scholar]
- 182.Vignaud M, Constantin JM, Ruivard M, Villemeyre-Plane M, Futier E, Bazin JE, Annane D. Refeeding syndrome influences outcome of anorexia nervosa patients in intensive care unit: an observational study. Crit Care. 2010;14:R172. doi: 10.1186/cc9274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Terlevich A, Hearing SD, Woltersdorf WW, Smyth C, Reid D, McCullagh E, Day A, Probert CS. Refeeding syndrome: effective and safe treatment with Phosphates Polyfusor. Aliment Pharmacol Ther. 2003;17:1325–1329. doi: 10.1046/j.1365-2036.2003.01567.x. [DOI] [PubMed] [Google Scholar]
- 184.Zeki S, Culkin A, Gabe SM, Nightingale JM. Refeeding hypophosphataemia is more common in enteral than parenteral feeding in adult in patients. Clin Nutr. 2011;30:365–368. doi: 10.1016/j.clnu.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 185.Marinella MA. Refeeding syndrome in cancer patients. Int J Clin Pract. 2008;62:460–465. doi: 10.1111/j.1742-1241.2007.01674.x. [DOI] [PubMed] [Google Scholar]
- 186.O'Connor LR, Wheeler WS, Bethune JE. Effect of hypophosphatemia on myocardial performance in man. N Engl J Med. 1977;297:901–903. doi: 10.1056/NEJM197710272971702. [DOI] [PubMed] [Google Scholar]
- 187.Sacks GS. Refeeding syndrome: awareness is the first step in preventing complications. J Support Oncol. 2009;7:19–20. [PubMed] [Google Scholar]
- 188.Mehanna HM, Moledina J, Travis J. Refeeding syndrome: what it is, and how to prevent and treat it. BMJ. 2008;336:1495–1498. doi: 10.1136/bmj.a301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Gentile MG, Pastorelli P, Ciceri R, Manna GM, Collimedaglia S. Specialized refeeding treatment for anorexia nervosa patients suffering from extreme undernutrition. Clin Nutr. 2010;29:627–632. doi: 10.1016/j.clnu.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 190.Gentile MG NICE. Eating disorders - core interventions in the treatment and management of anorexia nervosa, bulimia nervosa and related eating disorders. London: NICE; 2009. [PubMed] [Google Scholar]
- 191.Crook MA, Hally V, Panteli JV. The importance of the refeeding syndrome. Nutrition. 2001;17:632–637. doi: 10.1016/s0899-9007(01)00542-1. [DOI] [PubMed] [Google Scholar]
- 192.Havala T, Shronts E. Managing the complications associated with refeeding. Nutr Clin Pract. 1990;5:23–29. doi: 10.1177/011542659000500123. [DOI] [PubMed] [Google Scholar]
- 193.Boateng AA, Sriram K, Meguid MM, Crook M. Refeeding syndrome: treatment considerations based on collective analysis of literature case reports. Nutrition. 2010;26:156–167. doi: 10.1016/j.nut.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 194.Wesley JR. Nutrition support teams: past, present, and future. Nutr Clin Pract. 1995;10:219–228. doi: 10.1177/0115426595010006219. [DOI] [PubMed] [Google Scholar]
- 195.Hassell JT, Games AD, Shaffer B, Harkins LE. Nutrition support team management of enterally fed patients in a community hospital is cost-beneficial. J Am Diet Assoc. 1994;94:993–998. doi: 10.1016/0002-8223(94)92192-x. [DOI] [PubMed] [Google Scholar]
- 196.Powers DA, Brown RO, Cowan GS, Luther RW, Sutherland DA, Drexler PG. Nutritional support team vs nonteam management of enteral nutritional support in a Veterans Administration Medical Center teaching hospital. JPEN J Parenter Enteral Nutr. 1986;10:635–638. doi: 10.1177/0148607186010006635. [DOI] [PubMed] [Google Scholar]
- 197.Brown RO, Carlson SD, Cowan GS, Powers DA, Luther RW. Enteral nutritional support management in a university teaching hospital: team vs nonteam. JPEN J Parenter Enteral Nutr. 1987;11:52–56. doi: 10.1177/014860718701100152. [DOI] [PubMed] [Google Scholar]
- 198.Elia M, Stratton RJ. A cost-utility analysis in patients receiving enteral tube feeding at home and in nursing homes. Clin Nutr. 2008;27:416–423. doi: 10.1016/j.clnu.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 199.Scott F, Beech R, Smedley F, Timmis L, Stokes E, Jones P, Roffe C, Bowling TE. Prospective, randomized, controlled, single-blind trial of the costs and consequences of systematic nutrition team follow-up over 12 mo after percutaneous endoscopic gastrostomy. Nutrition. 2005;21:1071–1077. doi: 10.1016/j.nut.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 200.Schneider PJ. Nutrition support teams: an evidence-based practice. Nutr Clin Pract. 2006;21:62–67. doi: 10.1177/011542650602100162. [DOI] [PubMed] [Google Scholar]
- 201.Klek S, Szybinski P, Sierzega M, Szczepanek K, Sumlet M, Kupiec M, Koczur-Szozda E, Steinhoff-Nowak M, Figula K, Kowalczyk T, et al. Commercial enteral formulas and nutrition support teams improve the outcome of home enteral tube feeding. JPEN J Parenter Enteral Nutr. 2011;35:380–385. doi: 10.1177/0148607110378860. [DOI] [PubMed] [Google Scholar]
- 202.Bischoff SC, Kester L, Meier R, Radziwill R, Schwab D, Thul P. Organisation, regulations, preparation and logistics of parenteral nutrition in hospitals and homes; the role of the nutrition support team - Guidelines on Parenteral Nutrition, Chapter 8. Ger Med Sci. 2009;7:Doc20. doi: 10.3205/000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Mohandas KM, Shastri YM, Shirodkar M. Enteral nutrition by tube feeding in adults. Natl Med J India. 2001;14:285–289. [PubMed] [Google Scholar]
- 204.Heimburger DC, Sockwell DG, Geels WJ. Diarrhea with enteral feeding: prospective reappraisal of putative causes. Nutrition. 1994;10:392–396. [PubMed] [Google Scholar]
- 205.Alberda C, Gramlich L, Meddings J, Field C, McCargar L, Kutsogiannis D, Fedorak R, Madsen K. Effects of probiotic therapy in critically ill patients: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2007;85:816–823. doi: 10.1093/ajcn/85.3.816. [DOI] [PubMed] [Google Scholar]
- 206.Frohmader TJ, Chaboyer WP, Robertson IK, Gowardman J. Decrease in frequency of liquid stool in enterally fed critically ill patients given the multispecies probiotic VSL#3: a pilot trial. Am J Crit Care. 2010;19:e1–11. doi: 10.4037/ajcc2010976. [DOI] [PubMed] [Google Scholar]
- 207.Ferrie S, Daley M. Lactobacillus GG as treatment for diarrhea during enteral feeding in critical illness: randomized controlled trial. JPEN J Parenter Enteral Nutr. 2011;35:43–49. doi: 10.1177/0148607110370705. [DOI] [PubMed] [Google Scholar]
- 208.Barraud D, Blard C, Hein F, Marçon O, Cravoisy A, Nace L, Alla F, Bollaert PE, Gibot S. Probiotics in the critically ill patient: a double blind, randomized, placebo-controlled trial. Intensive Care Med. 2010;36:1540–1547. doi: 10.1007/s00134-010-1927-0. [DOI] [PubMed] [Google Scholar]
- 209.Bleichner G, Bléhaut H, Mentec H, Moyse D. Saccharomyces boulardii prevents diarrhea in critically ill tube-fed patients. A multicenter, randomized, double-blind placebo-controlled trial. Intensive Care Med. 1997;23:517–523. doi: 10.1007/s001340050367. [DOI] [PubMed] [Google Scholar]
- 210.Schlotterer M, Bernasconi P, Lebreton F. Value of Saccharomyces boulardii in the digestive acceptability of continuous-flow enteral nutrition in burnt patients. Nutr Clin Metabol. 1987;1:31–34. [Google Scholar]
- 211.Tempé JD, Steidel AL, Blehaut H, Hasselmann M, Lutun P, Maurier F. [Prevention of diarrhea administering Saccharomyces boulardii during continuous enteral feeding] Sem Hop. 1983;59:1409–1412. [PubMed] [Google Scholar]
- 212.Fuhrman MP. Diarrhea and Tube Feeding. Nutr Clin Pract. 1999;14:83–84. [Google Scholar]


