Abstract
AIM: To determine an appropriate compartmentalization of endoscopic submucosal dissection (ESD) or endoscopic mucosal resection (EMR) for duodenal tumors.
METHODS: Forty-six duodenal lesions (excluding papillary lesions) from 44 patients with duodenal tumors treated endoscopically between 2005 and 2013 were divided into the ESD and EMR groups for retrospective comparison and analysis.
RESULTS: The mean age was 65 ± 9 years (35-79 years). There were 24 lesions from men and 22 from women. The lesions consisted of 6 early cancers, 31 adenomas and 9 neuroendocrine tumors. Lesion location was the duodenal bulb in 15 cases and the descending part of the duodenum in 31 cases. The most common macroscopic morphology was elevated type in 21 cases (45.6%). Mean tumor diameter was 11.9 ± 9.7 mm (3-60 mm). Treatment procedure was ESD (15 cases) vs EMR (31 cases). The examined parameters in the ESD vs EMR groups were as follows: mean tumor diameter, 12.9 ± 14.3 mm (3-60 mm) vs 11.4 ± 6.7 mm (4-25 mm); en bloc resection rate, 86.7% vs 83.9%; complete resection rate, 86.7% vs 74.2%; procedure time, 86.5 ± 63.1 min (15-217 min) vs 13.2 ± 17.0 min (2-89 min) (P < 0.0001); intraprocedural perforation, 3 cases vs none (P = 0.0300); delayed perforation, none in either group; postprocedural bleeding, 1 case vs none; mean postoperative length of hospitalization, 8.2 ± 2.9 d (5-16 d) vs 6.1 ± 2.0 d (2-12 d) (P = 0.0067); recurrence, none vs 1 case (occurring at 7 mo postoperatively).
CONCLUSION: ESD was associated with a longer procedure time and a higher incidence of intraprocedural perforation; EMR was associated with a lower rate of complete resection.
Keywords: Duodenal tumor, Endoscopic submucosal dissection, Endoscopic mucosal resection, Cancer, Adenoma, Neuroendocrine tumor
Core tip: Endoscopic treatment of duodenal lesions is associated with a high incidence of complications. In particular, duodenal endoscopic submucosal dissection (ESD) is technically difficult. Therefore, the indications for duodenal ESD are not yet to be established. This study aimed to determine an appropriate compartmentalization of duodenal ESD or endoscopic mucosal resection (EMR). ESD was associated with a longer procedure time and a higher incidence of intraprocedural perforation; EMR was associated with a lower rate of complete resection. For early duodenal cancer and neuroendocrine tumors, which require en bloc resection, ESD is preferable if en bloc resection by EMR is difficult, while EMR is sufficient for endoscopic treatment of adenomas.
INTRODUCTION
Endoscopic submucosal dissection (ESD) is widely recognized as a useful treatment procedure for early gastric cancer[1,2]. In recent years, the indications for ESD have been expanded to include esophageal and colorectal cancer[3-5]. There have also been some reports of the application of ESD for duodenal tumors[6-9]. However, the technical difficulty of this procedure for lesions located in the duodenum is extremely high, because of the poor operability of endoscopes in this location, the thin duodenal wall, high degree of fibrillization of the submucosal layer. The risk of accidental complications such as delayed bleeding and perforation due to exposure to bile and duodenal juice is also high. Therefore, the indications for ESD remain controversial[1].
There have been no reports of comparison between ESD and endoscopic mucosal resection (EMR) for the treatment of duodenal tumors. In this study, we retrospectively compared and analyzed the data of patients with duodenal tumors, excluding papillary lesions, treated by ESD or EMR.
MATERIALS AND METHODS
Study population
This study included 46 duodenal lesions (excluding papillary lesions) from 44 patients who underwent endoscopic treatment for duodenal tumors at the Saitama Medical Center between 2005 and 2013. Clinical background, including tumor size, location, macroscopic type and histology, was analyzed in all the cases. The duodenal lesions were then divided into two groups, the ESD and EMR groups, for retrospective comparison and analysis of the treatment outcomes, including the procedure time, en bloc resection rate, complete resection rate, complication rate and postoperative length of hospitalization, as well as the clinical background.
Patient selection criteria
We discussed the choice of therapy between ESD and EMR at the preoperative conference. We usually choose EMR for duodenal adenomas. On the other hand, we choose ESD for (1) well-differentiated intramucosal carcinoma; (2) neuroendocrine tumors (NET) measuring less than 1 cm diameter and invading deeper than submucosal layer; and (3) adenomas suspected of being cancerous in endoscopic findings, which are unlikely to be amenable to en bloc resection by EMR. We made our definitive decision in consideration of the background factors of the patients in addition to the above.
Histopathological evaluation
All patients were examined by gastrointestinal endoscopy and biopsy prior to the endoscopic treatment. The diagnoses of all the duodenal lesions were confirmed by histopathologic examination. Histologically, complete resection was defined as the absence of tumor cells at the resection margin of the specimen plus endoscopic en-bloc resection.
EMR procedure
We usually used a single-channel upper GI endoscope with a water-jet system, the GFI-Q260J (Olympus, Japan). For the treatment of lesions located near the duodenal papilla, the duodenal endoscope TJF-260V (Olympus) was used. Physiological saline was used for local injection into the submucosal layer, and a Snare Master (Olympus) and a captivator (Boston Scientific, Japan) were used for the resection of the lesions (Figure 1).
Figure 1.
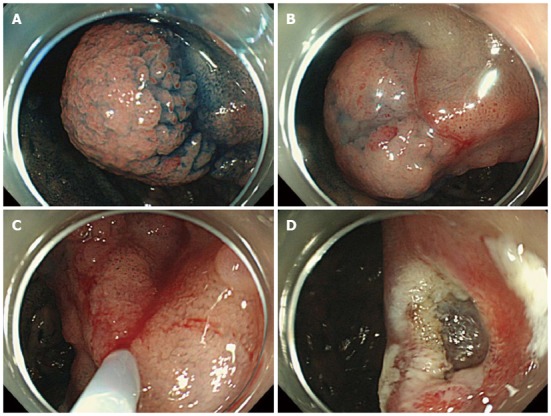
Endoscopic mucosal resection of a duodenal cancer. A: A protruded-type tumor 2.5 cm × 2.5 cm in size was identified; B: We performed a submucosal injection; C: The tumor was grasped with a snare; D: Complete mucosal resection was finished.
ESD procedure
The ESD procedures were carried out by operators who had at least 5 years of experience in performing ESD. The GIF-Q260J (Olympus) endoscope was used. Hyaluronic acid solution is essential as the submucosal injection solution; a 1% sodium hyaluronate solution (Suvenyl; Chugai Pharmaceutical, Japan) (used until 2007) or 0.4% sodium hyaluronate solution for submucosal injection (Mucoup; Johnson and Johnson, Japan) (used from 2008) was mixed in 10% glycerin containing 5% fructose and 0.9% NaCl (Glyceol; Chugai Pharmaceutical). A mucosal incision around the lesion and submucosal dissection for complete removal of the lesion were performed using the Flex knife (Olympus) or Dual knife (Olympus). The Hook knife (Olympus) was also used, particularly in cases where dissection of the submucosa was difficult. A high-frequency generator (VIO 300D; ERBE, Germany) was used during incision of the mucosa. We have used carbon dioxide insufflation during the ESD since 2009 (Figure 2).
Figure 2.
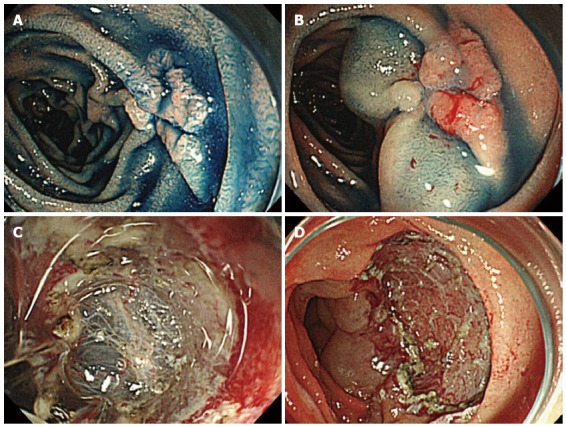
Endoscopic submucosal dissection of a duodenal adenoma. A: A depressed type tumor 1.5 cm × 1.5 cm in size was identified; B: We performed a submucosal injection. The lifting of the lesion after submucosal injection was poor; C: Then, submucosal dissection was done; D: Complete submucosal dissection was finished.
Statistical analysis
Data are expressed as mean ± SD or percentage. Statistical analysis was performed using the Student’s t-test and Fisher’s exact test. The macroscopic type, histology, and complications were compared by the χ2 test. All the data analyses were performed using the StatView software (version 5.0, SAS Institute Inc., United States). Differences at P values of less than 0.05 were regarded as significant.
RESULTS
The mean age of the patients was 65 ± 9 years (range: 35-79 years). There were 24 lesions from men and 22 from women. The 46 duodenal tumors included 6 early cancers (13.0%), 31 adenomas (67.4%) and 9 NETs (19.6%). The lesions were located in the duodenal bulb in 15 cases (32.6%) and the descending part of the duodenum in 31 cases (67.4%). The most common macroscopic morphology was the elevated type in 21 cases (45.6%), followed by the protruded-type and submucosal tumor (SMT) in 9 cases each (19.6%). The mean tumor diameter was 11.9 ± 9.7 mm (3-60 mm). As for the treatment procedure, ESD was performed in 15 cases, and EMR in 31 cases. The mean tumor diameter was 12.9 ± 14.3 mm (3-60 mm) in the ESD group and 11.4 ± 6.7 mm (4-25 mm) in the EMR group. There were many cases of depressed-type and SMT lesions in the ESD group, while all the protruded-type lesions were included in the EMR group. According to the tumor type, NET accounted for a half of the cases in the ESD group, while the most common tumor type in the EMR group was adenoma, accounting for 80.6% of the tumors in this group. The proportion of cases of adenocarcinoma was similar between the two groups, although the preoperative pathological diagnosis was adenoma in 3 of 4 cases of adenocarcinoma in the EMR group (Table 1).
Table 1.
Clinical characteristics of 15 endoscopic submucosal dissection and 31 endoscopic mucosal resection performed in 44 patients n (%)
| Total | ESD (n = 15) | EMR (n = 31) | P value | |
| Age, yr, mean ± SD (range) | 65 ± 9 (35-79) | 68 ± 5 (57-79) | 64 ± 10 (35-77) | 0.0775 |
| Male/female | 24/22 | 6/9 | 18/13 | 0.3480 |
| Location in duodenum | 0.0457 | |||
| Bulb | 15 (32.6) | 8 (53.3) | 7 (22.6) | |
| Second portion | 31 (67.4) | 7 (46.7) | 24 (77.4) | |
| Size, mm, mean ± SD (range) | 11.9 ± 9.7 (3-60) | 12.9 ± 14.3 (3-60) | 11.4 ± 6.7 (4-25) | 0.6474 |
| ≥ 20 mm | 8 (17.4) | 2 (13.3) | 6 (19.4) | |
| < 20 mm | 38 (82.6) | 13 (86.7) | 25 (80.6) | |
| Macroscopic type | 0.0009 | |||
| Protruded | 9 (19.6) | 0 | 9 (29.0) | |
| Elevated | 21 (45.6) | 4 (26.7) | 17 (54.8) | |
| Depressed | 7 (15.2) | 4 (26.7) | 3 (9.7) | |
| SMT | 9 (19.6) | 7 (46.6) | 2 (6.5) | |
| Histology | 0.0044 | |||
| Adenocarcinoma | 6 (13.0) | 2 (13.3) | 4 (12.9) | |
| Adenoma | 31 (67.4) | 6 (40.0) | 25 (80.6) | |
| NET | 9 (19.6) | 7 (46.7) | 2 (6.5) |
ESD: Endoscopic submucosal dissection; EMR: Endoscopic mucosal resection; SMT: Submucosal tumor; NET: Neuroendocrine tumor.
The en bloc resection rate was 86.7% in the ESD group and 83.9% in the EMR group, and the complete resection rate was 86.7% in the ESD group and 74.2% in the EMR group. En bloc resection of the lesions was not achieved in 2 cases of the ESD group and 5 cases of the EMR group. To describe in further detail, the 2 cases of the ESD group in which en bloc resection was not achieved included one elevated-type adenoma measuring 60 mm in diameter and one NET measuring 8 mm in diameter showing invasion beyond the submucosal layer. Both the lesions were located in the duodenal bulb. For the latter case, treatment was discontinued because of the difficulty in detachment of the tumor. In the EMR group, there were a total of 7 cases in which en bloc resection was not achieved, consisting of 3 protruded-type lesions, 3 elevated-type lesions, and 1 depressed-type lesion. The cases in which complete resection was not achieved included the 2 above-described cases of the ESD group in which en bloc resection was not achieved, and 8 cases of the EMR group. Of these 8 cases from the EMR group, the lesion was located in the duodenal bulb in 4 cases and in the descending part of the duodenum in 4 cases; the macroscopic type was the protruded type in 5 cases, the elevated type in 2 cases, and the depressed type in 1 case. The procedure time was 86.5 ± 63.1 min (range: 15-217 min) in the ESD group and 13.2 ± 17.0 min (range: 2-89 min) in the EMR group (P < 0.0001). As for complications, intraprocedural perforation occurred in 3 cases of the ESD group, but in none of the cases of the EMR group (P = 0.0300). The 3 cases of intraprocedural perforation included 2 cases of NET located in the duodenal bulb, and 1 case of early cancer of the depressed type located in the descending part of the duodenum. Delayed perforation was not observed in any of the cases in either group. Emergency surgery was necessitated in 2 cases of the ESD group, but not in any of the cases of the EMR group. The observed postprocedural bleeding was acute bleeding that occurred within 24 h of the procedure in 1 case of the ESD group. On the other hand, no postprocedural bleeding was observed in any of the cases of the EMR group. In the ESD group, closure of the wound with clips after the procedure was not performed in any of the 9 cases of lesions located in the duodenal bulb, but the wound was closed with clips in 4 of the 5 cases of lesions located in the descending part of the duodenum. In the remaining case where closure by clipping was not performed, postprocedural bleeding occurred on the day after the procedure. In the EMR group, the wound was closed with clips in 6 of the 7 cases of lesions located in the duodenal bulb and 18 of the 24 cases of lesions located in the descending part of the duodenum. The mean postoperative length of hospitalization was 8.2 ± 2.9 d (range: 5-16 d) in the ESD group and 6.1 ± 2.0 d (range: 2-12 d) in the EMR group (P = 0.0067) (Table 2).
Table 2.
Clinical outcomes of 15 endoscopic submucosal dissection and 31 endoscopic mucosal resection performed in 44 patients
| Total | ESD (n = 15) | EMR (n = 31) | P value | |
| En bloc resection rate | 84.8% | 86.7% | 83.9% | > 0.9999 |
| Complete resection rate | 78.3% | 86.7% | 74.2% | 0.4599 |
| Procedure time, min, mean ± SD (range) | 37.1 ± 51.4 (2-217) | 86.5 ± 63.1 (15-217) | 13.2 ± 17.0 (2-89) | < 0.0001 |
| Complication | 6 (13.0%) | 5 (35.7%) | 1 (3.4%) | 0.0105 |
| Perioperative perforation | 3 | 3 | 0 | 0.0300 |
| Perioperative bleeding | 3 | 2 | 1 | 0.2444 |
| Postoperative bleeding | 1 | 1 | 0 | 0.3261 |
| Emergency surgery | 2 | 2 | 0 | 0.1014 |
| Postoperative hospital stay, day, mean ± SD (range) | 6.8 ± 2.5 (2-16) | 8.2 ± 2.9 (5-16) | 6.1 ± 2.0 (2-12) | 0.0067 |
As of December 2013, follow-up by endoscopy had been completed in 26 cases (56.5%). The mean follow-up period was 9.7 ± 12.6 mo (range: 1.4-60.0 mo). Recurrence was observed at 7 mo after the procedure in only 1 case of the EMR group.
DISCUSSION
Endoscopic treatment of duodenal lesions is associated with a high incidence of complications, such as bleeding and perforation, because of poor operability using a scope and the thin duodenal wall. In particular, duodenal ESD is technically difficult, requires a longer procedure time, and is associated with a high risk of bleeding and perforation[6,9]. Therefore, the operator should be sufficiently skilled, with experience of safe and steady implementation of ESD at least for lesions of the stomach, esophagus, and large bowel.
Indications for ESD in patients with duodenal tumors should be determined taking into consideration the histopathology, macroscopic morphology, and size of the lesions. Duodenal adenomas are well known to have the potential for malignant transformation[10,11]. In particular, adenomas measuring 2 cm or greater in diameter or those that histopathologically show high-grade dysplasia are more likely to become malignant[12-14]; therefore, resection of such lesions is recommended. In a study that investigated 128 lesions of early duodenal cancer treated by surgery or endoscopic polypectomy, no lymph node metastasis was detected in any of the cases of intramucosal carcinoma[15]. Based on these findings, endoscopic treatment can be considered for differentiated non-invasive carcinomas not showing invasion of the submucosal layer. Although EMR has been reported to be safe and useful for the treatment of duodenal tumors[16-22], lesions greater than 2 cm in diameter are likely to require piecemeal resection[16,22].
We have previously reported the use of ESD for carcinoids, which are currently called NETs. EMR is adequate for lesions measuring less than 1 cm in diameter that are superficial, reaching only up to the submucosal layer, especially those with a polypoid morphology. However, ESD may be useful in cases where en bloc resection by EMR is difficult. Surgical treatment should be considered for tumors whose lower edges are found to be widely adjoining the muscular layer, because treatment of such tumors by ESD is associated with a high risk of perforation, and because accurate pathologic diagnosis of the deep margin may be difficult[7].
Although the present study did not reveal any significant differences between the EMR and ESD groups, both the en bloc and complete resection rates were lower in the EMR group. During EMR of lesions located in the duodenal bulb, the pyloric ring may become an obstacle to snaring. In the descending part of the duodenum, the distance between the mucosal folds is short, and there are relatively numerous lesions extending over the folds. Based on these observations, it may be difficult to ensure snaring of lesions located in the descending part of the duodenum by EMR. The rates of persistence and recurrence are higher in cases of piecemeal resection than in the case of en bloc resection[22]. Moreover, because en bloc resection allows accurate pathological evaluation of the deep and lateral resection margins[23], ESD may be preferable for endoscopic treatment of cancer and NET, which require en bloc resection, if en bloc resection by EMR is difficult. On the other hand, EMR may be sufficient for endoscopic treatment of adenomas, because piecemeal resection seems to be acceptable for these lesions. However, because 3 of the 4 cancers in the EMR group in this study had been diagnosed as adenomas preoperatively, caution is necessary, especially in cases of preoperative diagnosis of adenoma.
Among the complications associated with endoscopic treatment for duodenal lesions, bleeding is the most frequent and usually occurs within 24 h after the procedure. The reported incidence of acute bleeding after EMR for adenomas is 4%-33%[16-22]. As for perforation, caution is required not only against intraprocedural perforation, but also against delayed perforation due to exposure to bile and pancreatic juice[6]. The wound should be closed by clipping after resection in order to prevent complications such as postprocedural bleeding and delayed perforation[9]. In this study, the frequency of perforation was significantly higher and the postoperative length of hospitalization was significantly longer in the ESD group than in the EMR group. Although delayed perforation was not observed in any of the cases, either in the ESD or in the EMR group, postprocedural bleeding was observed in 1 case of the ESD group, where closure of the wound with clips was not performed. In an effort to ensure safer treatment, we have been performing ESD for duodenal tumors under general anesthesia in the presence of a surgeon in an operating room since 2010.
The limitations of this study were that it was a single-institution study and the sample size was small. Because of the retrospective design, ESD was often selected as the treatment procedure for lesions for which en bloc resection by EMR was expected to be difficult, such as depressed-type lesions and NET; inevitably therefore, there would have been a selection bias.
In conclusion, we consider that duodenal ESD may be indicated for well-differentiated intramucosal carcinoma and NETs measuring less than 1 cm in diameter and not invading deeper than the submucosal layer lesions, which are unlikely to be amenable to en bloc resection by EMR. However, because duodenal ESD is associated with a relatively high incidence of complications, its use should be considered carefully. Accumulation of further clinical data is required for a clearer elucidation of the short-term and long-term prognoses.
COMMENTS
Background
In recent years, the indications for endoscopic submucosal dissection (ESD) have been expanded to include esophageal and colorectal cancer. There have also been some reports of the application of ESD for duodenal tumors. However, there have been no reports of comparison between ESD and endoscopic mucosal resection (EMR) for the treatment of duodenal tumors.
Research frontiers
The technical difficulty of ESD for lesions located in the duodenum is extremely high, because of the poor operability of endoscopes in this location, the thin duodenal wall, high degree of fibrillization of the submucosal layer. The risk of accidental complications such as delayed bleeding and perforation due to exposure to bile and duodenal juice is also high. Therefore, the indications for ESD remain controversial.
Innovations and breakthroughs
ESD was associated with a longer procedure time and a higher incidence of intraprocedural perforation. EMR was associated with a lower rate of complete resection. For early duodenal cancer and neuroendocrine tumors (NET), which require en bloc resection, ESD is preferable if en bloc resection by EMR is difficult, while EMR is sufficient for endoscopic treatment of adenomas.
Applications
Duodenal ESD may be indicated for well-differentiated intramucosal carcinoma and NETs measuring less than 1 cm in diameter and not invading deeper than the submucosal layer lesions, which are unlikely to be amenable to en bloc resection by EMR.
Terminology
Gastrointestinal tract neuroendocrine cells sometimes go through certain changes that cause them to grow too much and form tumors. These tumors are known as NET; in the past, they were called carcinoids. But in 2000, the World Health Organization reclassified carcinoids as neuroendocrine tumors and neuroendocrine cancers.
Peer review
In this study authors aimed to determine an appropriate compartmentalization of ESD or EMR for duodenal tumors. Although the study group is relatively small, it is observed that ESD was associated with a longer procedure time and a higher incidence of perforation. On the other hand EMR was associated with a lower rate of complete resection. It is concluded that while ESD is preferable for early duodenal cancer, EMR is sufficient for endoscopic treatment of adenomas. This is basically well written paper of an interesting topic.
Footnotes
P- Reviewers: Arroyo A, Elpek GO, Hara K, Rolle U, Tang WF S- Editor: Qi Y L- Editor: A E- Editor: Liu XM
References
- 1.Kim KO, Kim SJ, Kim TH, Park JJ. Do you have what it takes for challenging endoscopic submucosal dissection cases? World J Gastroenterol. 2011;17:3580–3584. doi: 10.3748/wjg.v17.i31.3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gotoda T, Yamamoto H, Soetikno RM. Endoscopic submucosal dissection of early gastric cancer. J Gastroenterol. 2006;41:929–942. doi: 10.1007/s00535-006-1954-3. [DOI] [PubMed] [Google Scholar]
- 3.Fujishiro M, Yahagi N, Kakushima N, Kodashima S, Muraki Y, Ono S, Yamamichi N, Tateishi A, Shimizu Y, Oka M, et al. Endoscopic submucosal dissection of esophageal squamous cell neoplasms. Clin Gastroenterol Hepatol. 2006;4:688–694. doi: 10.1016/j.cgh.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 4.Kakushima N, Yahagi N, Fujishiro M, Kodashima S, Nakamura M, Omata M. Efficacy and safety of endoscopic submucosal dissection for tumors of the esophagogastric junction. Endoscopy. 2006;38:170–174. doi: 10.1055/s-2005-921039. [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto H, Yahagi N, Oyama T. Mucosectomy in the colon with endoscopic submucosal dissection. Endoscopy. 2005;37:764–768. doi: 10.1055/s-2005-870166. [DOI] [PubMed] [Google Scholar]
- 6.Honda T, Yamamoto H, Osawa H, Yoshizawa M, Nakano H, Sunada K, Hanatsuka K, Sugano K. Endoscopic submucosal dissection for superficial duodenal neoplasms. Dig Endosc. 2009;21:270–274. doi: 10.1111/j.1443-1661.2009.00908.x. [DOI] [PubMed] [Google Scholar]
- 7.Matsumoto S, Miyatani H, Yoshida Y, Nokubi M. Duodenal carcinoid tumors: 5 cases treated by endoscopic submucosal dissection. Gastrointest Endosc. 2011;74:1152–1156. doi: 10.1016/j.gie.2011.07.029. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki S, Ishii N, Uemura M, Deshpande GA, Matsuda M, Iizuka Y, Fukuda K, Suzuki K, Fujita Y. Endoscopic submucosal dissection (ESD) for gastrointestinal carcinoid tumors. Surg Endosc. 2012;26:759–763. doi: 10.1007/s00464-011-1948-y. [DOI] [PubMed] [Google Scholar]
- 9.Matsumoto S, Miyatani H, Yoshida Y. Endoscopic submucosal dissection for duodenal tumors: a single-center experience. Endoscopy. 2013;45:136–137. doi: 10.1055/s-0032-1310123. [DOI] [PubMed] [Google Scholar]
- 10.Galandiuk S, Hermann RE, Jagelman DG, Fazio VW, Sivak MV. Villous tumors of the duodenum. Ann Surg. 1988;207:234–239. doi: 10.1097/00000658-198803000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller JH, Gisvold JJ, Weiland LH, McIlrath DC. Upper gastrointestinal tract: villous tumors. AJR Am J Roentgenol. 1980;134:933–936. doi: 10.2214/ajr.134.5.933. [DOI] [PubMed] [Google Scholar]
- 12.Rosen M, Zuccaro G, Brody F. Laparoscopic resection of a periampullary villous adenoma. Surg Endosc. 2003;17:1322–1323. doi: 10.1007/s00464-002-4527-4. [DOI] [PubMed] [Google Scholar]
- 13.Lépilliez V, Napoléon B, Ponchon T, Saurin JC. [Duodenal adenomas: diagnostic and treatment] Gastroenterol Clin Biol. 2009;33:240–246. doi: 10.1016/j.gcb.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Okada K, Fujisaki J, Kasuga A, Omae M, Kubota M, Hirasawa T, Ishiyama A, Inamori M, Chino A, Yamamoto Y, et al. Sporadic nonampullary duodenal adenoma in the natural history of duodenal cancer: a study of follow-up surveillance. Am J Gastroenterol. 2011;106:357–364. doi: 10.1038/ajg.2010.422. [DOI] [PubMed] [Google Scholar]
- 15.Nagatani K, Takekoshi T, Baba Y, Kaku Y, Koizumi K, Fujii A. Indications for endoscopic treatment of early duodenal cancer: based on cases reported in the literature (in Japanese with English abstract) Endosc Dig. 1993;7:969–976. [Google Scholar]
- 16.Kim HK, Chung WC, Lee BI, Cho YS. Efficacy and long-term outcome of endoscopic treatment of sporadic nonampullary duodenal adenoma. Gut Liver. 2010;4:373–377. doi: 10.5009/gnl.2010.4.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Apel D, Jakobs R, Spiethoff A, Riemann JF. Follow-up after endoscopic snare resection of duodenal adenomas. Endoscopy. 2005;37:444–448. doi: 10.1055/s-2005-861287. [DOI] [PubMed] [Google Scholar]
- 18.Hirasawa R, Iishi H, Tatsuta M, Ishiguro S. Clinicopathologic features and endoscopic resection of duodenal adenocarcinomas and adenomas with the submucosal saline injection technique. Gastrointest Endosc. 1997;46:507–513. doi: 10.1016/s0016-5107(97)70005-1. [DOI] [PubMed] [Google Scholar]
- 19.Ahmad NA, Kochman ML, Long WB, Furth EE, Ginsberg GG. Efficacy, safety, and clinical outcomes of endoscopic mucosal resection: a study of 101 cases. Gastrointest Endosc. 2002;55:390–396. doi: 10.1067/mge.2002.121881. [DOI] [PubMed] [Google Scholar]
- 20.Oka S, Tanaka S, Nagata S, Hiyama T, Ito M, Kitadai Y, Yoshihara M, Haruma K, Chayama K. Clinicopathologic features and endoscopic resection of early primary nonampullary duodenal carcinoma. J Clin Gastroenterol. 2003;37:381–386. doi: 10.1097/00004836-200311000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Lépilliez V, Chemaly M, Ponchon T, Napoleon B, Saurin JC. Endoscopic resection of sporadic duodenal adenomas: an efficient technique with a substantial risk of delayed bleeding. Endoscopy. 2008;40:806–810. doi: 10.1055/s-2008-1077619. [DOI] [PubMed] [Google Scholar]
- 22.Alexander S, Bourke MJ, Williams SJ, Bailey A, Co J. EMR of large, sessile, sporadic nonampullary duodenal adenomas: technical aspects and long-term outcome (with videos) Gastrointest Endosc. 2009;69:66–73. doi: 10.1016/j.gie.2008.04.061. [DOI] [PubMed] [Google Scholar]
- 23.Sohn JW, Jeon SW, Cho CM, Jung MK, Kim SK, Lee DS, Son HS, Chung IK. Endoscopic resection of duodenal neoplasms: a single-center study. Surg Endosc. 2010;24:3195–3200. doi: 10.1007/s00464-010-1114-y. [DOI] [PubMed] [Google Scholar]


