Abstract
Arenaviruses can cause fatal human haemorrhagic fever (HF) diseases for which vaccines and therapies are extremely limited. Both the New World (NW) and Old World (OW) groups of arenaviruses contain HF-causing pathogens. Although these two groups share many similarities, important differences with regard to pathogenicity and molecular mechanisms of virus infection exist. These closely related pathogens share many characteristics, including genome structure, viral assembly, natural host selection and the ability to interfere with innate immune signalling. However, members of the NW and OW viruses appear to use different receptors for cellular entry, as well as different mechanisms of virus internalization. General differences in disease signs and symptoms and pathological lesions in patients infected with either NW or OW arenaviruses are also noted and discussed herein. Whilst both the OW Lassa virus (LASV) and the NW Junin virus (JUNV) can cause disruption of the vascular endothelium, which is an important pathological feature of HF, the immune responses to these related pathogens seem to be quite distinct. Whereas LASV infection results in an overall generalized immune suppression, patients infected with JUNV seem to develop a cytokine storm. Additionally, the type of immune response required for recovery and clearance of the virus is different between NW and OW infections. These differences may be important to allow the viruses to evade host immune detection. Understanding these differences will aid the development of new vaccines and treatment strategies against deadly HF viral infections.
Introduction
Arenaviruses cause deadly haemorrhagic fever (HF) infections that are often neglected tropical diseases. Some reports indicate that Lassa virus (LASV) infects up to 300 000 people leading to 5000 deaths each year (Günther & Lenz, 2004; McCormick et al., 1987a), whilst other reports indicate as many 2 million infected individuals resulting in 5000–10 000 deaths annually (McCormick, 1999). Although most individuals exposed to LASV are able to mount an appropriate immune response that is capable of clearing the virus, those individuals who are unable to do so experience severe disease that often culminates in death. At present, no vaccine is available for the prevention of Lassa HF, and treatment options are extremely limited. The only antiviral used to combat arenavirus infection is ribavirin, which is effective only when given early during the course of infection and has significant toxic side effects (Günther & Lenz, 2004). Junin virus (JUNV), the causative agent of Argentine haemorrhagic fever (AHF), is also a pathogen of significant concern for humans. The Candid #1 vaccine is effective in protecting against AHF and may also provide cross-protection against Machupo virus (MACV) infection, although it is currently only licensed for use in Argentina (Jahrling et al., 1988). Although this vaccine is available for the prevention of AHF, safety concerns exist. Therefore, development of better treatment methods for arenavirus-induced HFs is still needed. Several animal models exist for characterizing arenavirus-induced disease pathogenesis. Some of these include non-human primates, inbred and outbred guinea pigs and Syrian golden hamsters, as well as various immunodeficient strains of mice. For recent review articles detailing these model systems, see Lukashevich (2013) and Vela (2012).
Arenaviruses are bi-segmented ambisense RNA viruses (Fig. 1). The large (L) RNA segment encodes the L RNA-dependent RNA polymerase (RdRp) in a negative-sense orientation. The L polymerase is responsible for transcribing viral mRNAs as well as replicating the genome. This is an extremely large protein (~250 kDa) and contains four conserved domains (Brunotte et al., 2011). Domain I contains endonuclease activity, which is thought to be involved in cleaving 5′ caps from cellular mRNAs in the process known as cap snatching for the purpose of priming viral mRNA transcription (Morin et al., 2010; Raju et al., 1990). Domain III of the L polymerase contains the RdRp domain (Vieth et al., 2004). The potential functional roles of the conserved domains II and IV are currently unknown. The Z matrix protein is also encoded on the L genomic segment but in a positive-sense orientation. The L and Z genes are separated by an intergenic region with stable secondary structure that is thought to be involved in transcriptional termination and stability of arenavirus mRNAs, which are not polyadenylated (Pinschewer et al., 2005). The small (S) genomic segment encodes the nucleoprotein (NP) gene in the negative-sense orientation and the glycoprotein (GP) precursor (GPC) in the positive-sense orientation, separated by an intergenic region. The GPC protein must be proteolytically processed into three fragments: the stable signal peptide (SSP), globular head (GP1), and transmembrane domain (GP2). The SSP is cleaved in the endoplasmic reticulum by signal peptidase, whilst GP1 and GP2 are cleaved by the cellular protease subtilisin kexin isozyme-1(SKI-1)/site 1 protease (S1P) (Beyer et al., 2003; Eichler et al., 2003a, b; York & Nunberg, 2007). The SSP remains associated with GP2, and these structures trimerize to form a functional glycoprotein (York et al., 2004). The 5′ and 3′ untranslated regions of each of the viral genomic segments are complementary to each other, and their base pairing allows the genome segments to circularize, thus forming panhandle structures (Perez & de la Torre, 2003).
Fig. 1.
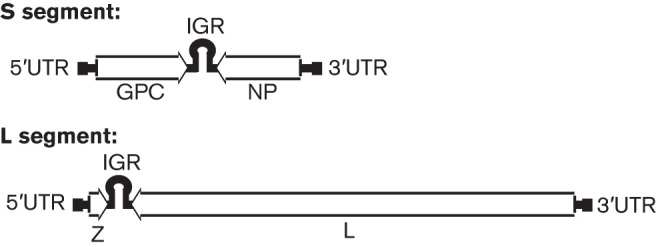
Arenavirus genome structure. Arenaviruses are bi-segmented, ambisense RNA viruses. The genomic large (L) segment encodes the Z matrix protein in the positive-sense orientation and the L polymerase in the negative-sense orientation. The two genes are separated by an intergenic region (IGR) with secondary structure. The genomic small (S) segment contains the glycoprotein (GPC) gene in the positive-sense orientation and the nucleoprotein (NP) gene in the negative-sense orientation. Both segments have 5′ and 3′ untranslated regions (UTRs) with complementarity, allowing the genome segments to circularize into a panhandle structure.
Despite the fact that arenavirus infections in humans share many characteristics in terms of disease manifestation, pathology and viral biology, differences in other aspects of virus infection also exist. In the following sections, we aim to provide a comprehensive analysis of current knowledge about New World (NW) and Old World (OW) arenavirus-induced HFs and to outline some of the significant differences between the major viruses (e.g. LASV and JUNV) that are responsible for severe HF diseases in humans. Understanding the similarities and differences between different arenavirus infections is important for the development of new methods of treatment and for vaccines against these deadly viral pathogens.
Phylogenetic and epidemiological differences between NW and OW arenaviruses
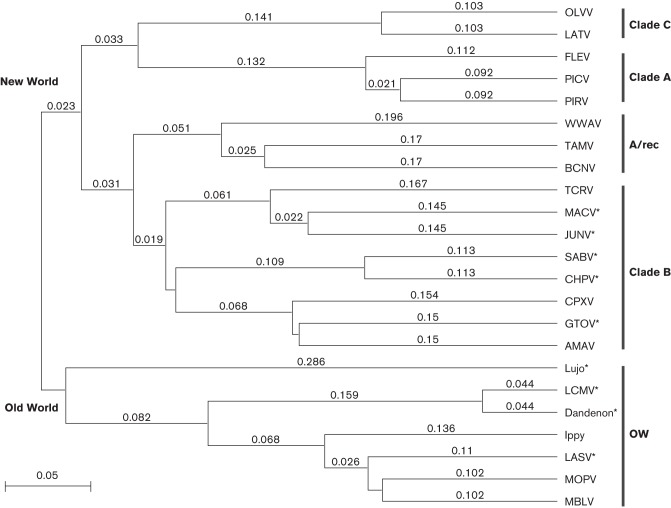
The family Arenaviridae is classified into two groups: the NW and the OW arenaviruses (Table 1). This distinction is based on geographical distribution and serological relatedness, as well as phylogeny (Fig. 2). Here, we will focus on the arenaviruses that have pathogenic potential for humans. The OW arenaviruses that have potential to cause human diseases include LASV, lymphocytic choriomeningitis virus (LCMV) and Lujo virus (LUJV). The reservoir species for nearly all arenaviruses capable of causing diseases in humans are various members of the rodent order (Table 1). These natural hosts maintain high viral loads, despite the lack of any inherent disease (Buchmeier et al., 2001). The viruses can be maintained in the rodent hosts via either vertical or horizontal transmission, or by both mechanisms. Female rodents infected with JUNV or MACV demonstrate reduced fertility, thereby necessitating horizontal transmission in the host population. In contrast, LCMV does not cause infertility in its host species, and can be maintained solely through vertical transmission (Childs & Peters, 1993; Webb et al., 1975; Zapata & Salvato, 2013). LASV is carried by Mastomys natalensis, a multimammate rat common in West Africa where LASV infection is endemic and enzootic. The host range for LCMV is much broader, as its host, Mus musculus (the common mouse), has a worldwide distribution and thus LCMV does as well.
Table 1. Arenavirus distribution, host species and disease incidence in humans.
References: Aguilar et al. (2009); Ambrosio et al. (2011); Charrel & de Lamballerie (2003); Delgado et al. (2008); Enria et al. (2008); Fulhorst et al. (2008); Harrison et al. (1999); Coimbra et al. (1994); McCormick (1999); Paweska et al. (2009).
| Virus | Geographical location | Natural host species | Incidence of disease |
| Old World | |||
| LCMV | Worldwide | Mus musculus (common mouse) | Over 5 % of people show evidence of previous exposure, <1 % mortality |
| Lassa virus | West Africa | Mastomys natalensis (multimammate rat) | Approximately 2 million infections annually and between 5000–10 000 deaths |
| Lujo virus | South Africa | Unknown | Five identified cases, four fatal |
| New World | |||
| Junín virus | Argentina | Calomys musculinus (drylands vesper mouse) | 300–1000 cases year−1 before vaccine, 30–50 cases year−1 after introduction of vaccine, 15–30 % mortality |
| Machupo virus | Bolivia | Calomys callosus (large vesper mouse) | 1962–1964 : 1000 cases; 1990s: 19 cases; 2007–2008: >200 cases; ~20 % mortality |
| Sabiá virus | Brazil | Unknown | One naturally occurring case, fatal |
| Guanarito virus | Venezuela | Zygodontomys brevicauda (common cane mouse) | 618 cases, 23 % fatal |
| Chapare virus | Bolivia | Unknown | One confirmed case, fatal |
Fig. 2.
Arenavirus phylogenetic tree. Multiple sequence alignment of the complete GPC protein sequences from different arenaviruses by clustal w analysis. A phylogenetic tree was generated using the unweighted pair group method with arithmetic mean, using MacVector 12.6.0. Horizontal distances represent protein differences: bar, 0.05 amino acid changes per site. Arenavirus GPCs included are: Oliveros virus (OLVV) (GenBank accession no. YP_001649210), Latino virus (LATV) (Q8B121), Flexal virus (FLEV) virus (YP_001936019), Pichinde virus (PICV) (ABU39904), Pirital virus (PIRV) (YP025080), White Water Arroyo virus (WWAV) (Q911P0), Tamiami virus (TAMV) (Q8AYY5), Bear Canyon virus (BCNV) (A0PJ25), Tacaribe virus (TCRV) (P18141), Machupo virus (MACV) (NP899212), Junín virus (JUNV) (P26313), Sabia virus (SABV) (Q90037), Chapare virus (CHPV) (B2C4J0), Cupixi virus (CPXV) (Q8B115), Guanarito virus (GTOV) (Q8AYW1), Amapari virus (AMAV) (YP_001649208), Lujo virus (YP 002929490), lymphocytic choriomeningitis virus (LCMV) (AAX49341), Dandenon virus (ABY20729), Ippy virus (Q27YE4), Lassa virus (LASV) (P08669), Mopeia virus (MOPV) (P19240) and Mobala virus (MBLV) (Q2A069). OW, Old World arenaviruses. New World arenaviruses are divided into clades A, B and C, as well as the A/rec recombinant lineage. Pathogenic arenaviruses are indicated by an asterisk.
The South American arenaviruses that cause diseases in humans are JUNV, Guanarito virus (GTOV), MACV, Sabia virus (SABV) and Chapare virus (CHPV). These NW viruses are responsible for causing human HF in Argentina, Venezuela, Bolivia, Brazil and Bolivia, respectively. Only one naturally occurring case each has been reported for SABV and CHPV infections. All of the NW arenaviruses that cause human diseases belong to NW clade B. The fact that non-pathogenic viruses are also found in this clade suggests that phylogenetic relatedness is not a good indicator of pathogenicity for arenaviruses. The reservoir species for the NW arenaviruses that cause human diseases are also rodents: JUNV is found in Calomys musculinus (drylands vesper mouse) (Mills et al., 1991), GTOV has been shown to be carried by Zygodontomys brevicauda (common cane mouse) (Fulhorst et al., 1997) and the natural reservoir for MACV is Calomys callosus (large vesper mouse) (Johnson et al., 1966). The natural range of the host species for each respective virus is the determining factor for the endemicity of human disease. Consumption of food contaminated with urine or faeces from infected rodents is a common route of infection. Infection may also occur via inhalation of aerosolized particles or through contaminated medical equipment.
The incidence of disease varies for each of these arenaviruses. Although LCMV has the largest worldwide distribution, and therefore the potential to infect larger numbers of people, the incidence of disease caused by this virus is low. Acquired LCMV infection is not a significant cause for concern in adult populations. Whereas more than 5 % of humans show evidence of previous exposure to LCMV, the disease has a mortality rate of less than 1 % (Peters, 2006). In contrast, congenital LCMV infection can be quite serious, and may even result in spontaneous abortion or fetal death. However, the number of congenital infections is unknown, as only severe cases are reported. Therefore, we cannot objectively estimate the disease incidence for congenital LCMV infection (Bonthius, 2012). Recently, LCMV has proven to be an important pathogen in immunocompromised individuals. Fourteen cases have been reported of LCMV infection resulting from organ transplant, and 11 of these cases proved fatal (CDC, 2008; Fischer et al., 2006; MacNeil et al., 2012). Whilst LCMV infection is serious for immunocompromised individuals, this virus does not pose a significant threat to healthy adults. In contrast, LASV infection can be quite serious and annually infects a significant portion of the human population. In some areas, as much as 55 % of the population shows evidence of previous exposure to LASV infection, underscoring the importance of this pathogen as a risk to endemic populations (Lukashevich et al., 1993). LUJV infection has been identified in only five individuals to date. However, four of these cases proved fatal, highlighting the pathogenic potential for this newly discovered OW arenavirus (Paweska et al., 2009).
Although the numbers of NW arenavirus infections are not as impressive as the number of LASV infections, these viruses still cause significant human disease. Of these viruses, JUNV is responsible for the highest levels of morbidity and mortality, resulting in approximately 300–1000 cases diagnosed per year before the development of the Candid #1 vaccine. Since the implementation of Candid #1 vaccination, the infection rate has decreased to 30–50 cases annually (Ambrosio et al., 2011; Enria et al., 2008; Harrison et al., 1999). The mortality rate for untreated cases of JUNV infection is high, at 15–30 % (Harrison et al., 1999). MACV infection is also a significant cause for concern. However, unlike JUNV infection, which maintains a constant presence in endemic regions, MACV infections have surfaced sporadically in the form of outbreaks. From 1962 to 1964, there were 1000 reported cases. In the 1990s, 19 cases were reported. Over 200 cases of MACV infection were reported in 2007–2008. Even though this virus only seems to emerge sporadically, the mortality rate is high at 20 %, and warrants the development of vaccines and therapies for those afflicted with the disease (Aguilar et al., 2009; Charrel & de Lamballerie, 2003). To date, 618 cases of GTOV infection have been reported, with 23 % of these cases resulting in death (Fulhorst et al., 2008). Whilst GTOV infection is not common, the mortality rate is high, giving cause for concern. Only one naturally occurring case has been reported each for CHPV and SABV infections, but in both cases the disease resulted in death, underscoring the seriousness of the disease caused by arenavirus-induced HFs. Additionally, there was a case of laboratory-acquired SABV infection that was not fatal (Coimbra et al., 1994; Delgado et al., 2008).
Similarities and differences in disease manifestations caused by NW and OW arenaviruses
Arenavirus HFs vary widely in their disease manifestations (Table 2). Some of the signs and symptoms that seem to be shared among patients with severe arenavirus infections are fever, leukopenia, oedema, shock, petechiae, elevated liver transaminases, myalgia and vomiting. Interestingly, even individuals infected with the same virus can show a wide variety of signs and symptoms, which can make diagnosis difficult. This is very common with patients infected with LASV, and individuals infected with LASV are frequently misdiagnosed. Currently, the only treatment of Lassa HF is the nucleoside analogue ribavirin. However, this treatment is only effective if given early on during the course of infection. Therefore, misdiagnosis can have serious consequences. In addition to the general signs and symptoms of severe arenavirus infections described above, Lassa fever infections can also result in pharyngitis, retrosternal pain, proteinuria, sore throat, mucosal bleeding, deafness, pleural effusion, pericardial effusion, malaise, headache, nausea, diarrhoea and thrombocytopenia (McCormick et al., 1987b; Moraz & Kunz, 2011). Lassa cases which typically result in death may also exhibit mucosal bleeding, pulmonary oedema, respiratory distress, shock, encephalopathy, seizures, and coma (Moraz & Kunz, 2011). Approximately 15 % of Lassa patients suffer from sensorineural deafness (Cummins et al., 1990a). With such a wide range of signs and symptoms, which may range from asymptomatic to multi-systemic failure and death, Lassa fever has proven difficult to diagnose. The wide variety of symptoms in combination with the fact that early signs and symptoms are common to many virus infections result in the infection sometimes being mistaken for malaria, typhoid fever or influenza, among other febrile illnesses (Monath & Casals, 1975).
Table 2. Disease signs and symptoms of arenavirus HFs.
References: Aguilar et al. (2009); Delgado et al. (2008); Enria et al. (2008); Harrison et al. (1999); Kilgore et al. (1997); Coimbra et al. (1994); Maiztegui et al. (1979); de Manzione et al. (1998); Moraz & Kunz (2011); Paweska et al. (2009); Salas et al. (1991); Vainrub & Salas (1994).
| Sign/symptom | Old World | New World | |||||
| LASV | LUJV | JUNV | MACV | GTOV | SABV | CHPV | |
| Haemorrhage | Mild | Yes | Infrequent | Infrequent | Yes | Yes | Yes |
| Fever | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Leukopenia | Yes | Yes | Yes | Yes | Yes | Yes | – |
| Thrombocytopenia | infrequent | Yes | Yes | Yes | Yes | – | – |
| Oedema | Yes | Yes | Yes | Yes | Yes | Yes | – |
| Shock | Yes | – | Yes | Yes | Yes | Yes | – |
| Petechiae | Yes | Yes | Yes | Yes | Yes | Yes | – |
| Elevated AST/ALT* | Yes | Yes | Yes | Yes | Yes | Yes | – |
| Late neurological syndrome | No | – | Yes | – | – | – | – |
| Seizure | Yes | – | Yes | Yes | Yes | Yes | – |
| Respiratory distress | Yes | Yes | – | – | – | – | – |
| Myalgia | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Vomiting | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Arthralgia | Yes | – | Yes | Yes | Yes | – | Yes |
| Sensorineural deafness | Yes | – | No | No | No | – | – |
| Hypotension | Yes | – | Yes | Yes | – | – | – |
| Vascular lesions | No | – | No | No | – | – | – |
| Elevated cytokines | No | – | Yes | Yes | – | – | – |
AST, aspartate aminotransferase; ALT, alanine aminotransferase.
Whilst LUJV has only recently been discovered and only five cases identified, this virus elicits many shared symptoms as those displayed during LASV infection. Patients suffering from LUJV infection are reported to experience diarrhoea, vomiting, fever, chest pain, sore throat, rash, myalgia, facial swelling, respiratory distress, cerebral oedema, thrombocytopenia, elevated liver transaminases, fever and leukopenia. Mild bleeding was observed in three of the five patients (Paweska et al., 2009). Although only five cases have been identified, four of these resulted in death, underscoring the seriousness of the disease.
Among the NW arenaviruses, CHPV and SABV infections have only been identified as single incidents, whilst JUNV, MACV and GTOV have infected many people. The disease manifestations caused by infection with these viruses display many of the same symptoms observed with OW arenavirus-induced HFs. Additional symptoms produced by JUNV infection, the most serious of the NW arenaviruses in terms of human infections, include mild hypotension and conjunctivitis. Neurological symptoms such as irritability, lethargy and hyporeflexia have also been observed. Severe cases may exhibit haemorrhagic manifestations, leukopenia, thrombocytopenia, shock and seizures (Harrison et al., 1999). Similarly, patients infected with MACV may exhibit gingival haemorrhage, nausea, gastrointestinal haemorrhage, thrombocytopenia, haematuria, tremor, anorexia and respiratory distress (Aguilar et al., 2009), as well as some of the symptoms listed above as common to arenavirus HFs. Additional signs and symptoms reported for individuals infected with GTOV include malaise, headache, sore throat, abdominal pain, diarrhoea, convulsions, thrombocytopenia and various haemorrhagic manifestations (de Manzione et al., 1998). Whereas there is variation among the disease manifestations, many of these arenavirus infections display shared symptoms. It is important to note that, whilst disease manifestation can vary based on the infecting virus, a wide range of symptoms may be exhibited by individuals infected even with the same arenavirus, in which some patients may experience very mild disease, whereas others present with severe HF.
Pathological analyses of patients infected with JUNV (NW) or LASV (OW)
Although diagnosis of arenaviral HF presents challenges because of the wide spectrum of symptoms displayed by infected patients, determination of the cause of death has proven even more challenging. The cause of death from arenaviral HF is not well understood. Recent studies are making progress in understanding the pathology of the disease. Although similarities are seen between the pathologies of HF induced by OW versus NW viruses, many differences also exist.
One consistent predictor of disease outcome in cases of LASV HF is the level of viraemia. Those individuals with high viral loads display more exacerbated disease and are given a poor prognosis (Johnson et al., 1987). Patients with viral loads higher than 8.5 log10 p.f.u. ml−1 typically succumb to the disease (Oldstone & Campbell, 2011). In contrast, individuals with lower initial viral loads typically are capable of clearing the infection and survive (Johnson et al., 1987). Whilst LASV infects many vital organs and cell types and replicates to high titres, the resulting histological damage in any of these organs and tissues alone is not severe enough to account for the cause of death in these patients. High viral titres can be found in the blood, liver, spleen, lung and adrenal gland. The most consistent histopathological lesions are found in the liver, lung and adrenal gland, with the most severe pathology being found in the liver. Hepatocellular necrosis, mononuclear phagocytic reaction and focal hepatocellular cytoplasmic degeneration are the most common liver pathologies but with little recruitment of inflammatory cells into this organ. However, none of the pathological lesions found are severe enough to account for the cause of death (Walker et al., 1982a).
Whereas the level of viraemia is a good indicator of disease prognosis for individuals infected with LASV, endogenous levels of IFN-α are indicative of disease outcome in JUNV-infected patients. During the second week of disease, the IFN-α levels are extremely high in cases that result in fatality (Levis et al., 1985). Haemorrhage, neurological changes, leukopenia and thrombocytopenia are much more common in AHF patients than in LASV-infected patients (Pfau, 1996). Although viral hepatitis is common in LASV-infected patients, it is uncommon or mild in individuals infected with JUNV (Pfau, 1996). Renal papillary necrosis is also noted in JUNV infection, and these necrotic sites coincide with the presence of viral antigen production (Elsner et al., 1973; Maiztegui et al., 1975). Myocarditis and secondary bacterial infection in the lungs are also observed in fatal cases (Elsner et al., 1973). Cases of GTOV and MACV infection resemble JUNV-mediated pathology, which is not unexpected given the phylogenetic relatedness of these viral pathogens (de Manzione et al., 1998; Johnson et al., 1967; Stinebaugh et al., 1966; Tesh et al., 1994).
Differences in coagulopathies caused by JUNV (NW) and LASV (OW) infections
Although arenaviruses can cause HF in humans, the capacity for different arenaviruses to result in haemorrhage or coagulopathy differs among viruses. LASV is atypical, in the sense that haemorrhage is not common in infected individuals. Only a small percentage of patients develop haemorrhage, and this haemorrhage is limited primarily to mucosal surfaces (McCormick & Fisher-Hoch, 2002). Additionally, the amount of blood loss and pathological lesions are not sufficient to account for the terminal shock and death that follows in lethal cases (Walker et al., 1982b). In the relatively few cases where bleeding does occur, it is typically associated with coagulopathy including thrombocytopenia and platelet dysfunction (Cummins et al., 1989; Fisher-Hoch et al., 1988). The platelet malfunction has been attributed to a plasma inhibitor of platelet aggregation, which has yet to be identified (Cummins et al., 1989).
LASV has a non-lytic cell cycle and does not cause any evident cellular damage in infected monocytes, macrophages or endothelial cells (Lukashevich et al., 1999). The virus is able to efficiently infect the vascular endothelium, and infection of these cells yields high viral titres without causing cell death (Lukashevich et al., 1999). Infection of these cells is crucial to the pathology of the virus, as in both experimentally infected non-human primates as well as human patients disruption of the function of the vascular endothelium is closely followed by shock and death (Walker et al., 1982b). Oedema is closely associated with death in infected patients, and this is most likely due to increased vascular permeability. Whilst autopsies on LASV-infected patients and experimentally infected non-human primates fail to reveal vascular lesions, which correlates with the absence of cytopathic effect observed for this virus, vascular permeability is affected in the course of this disease. The mechanism for increased permeability of the vascular endothelium has yet to be determined but is most likely due to virus infection of this tissue causing cellular changes that allow increased fluid flow, which results in the oedema observed in severe disease. This, in combination with thrombocytopenia and platelet dysfunction, may be the cause of shock, leading ultimately to death. In other viral HFs, such as dengue and Ebola, a cytokine storm interferes with the integrity of the vascular endothelium (Paessler & Walker, 2013). However, this does not appear to be the case for LASV infection (Mahanty et al., 2001). Infected macrophages are not activated, and pro-inflammatory cytokines are not released (Baize et al., 2004; Mahanty et al., 2003). Increased levels of pro-inflammatory cytokines are also not detected in LASV-infected patients (Mahanty et al., 2001). Additionally, LASV-infected human umbilical vein endothelial (HUVEC) cells have been shown to produce relatively low levels of IL-8, as opposed to those that are infected with the apathogenic Mopeia arenavirus (MOPV) (Lukashevich et al., 1999). These relatively low levels of IL-8 are also observed in LASV-infected patients (Mahanty et al., 2001). Although the release of inflammatory mediators does not appear to be the cause of increased vascular permeability, LASV infection may be influencing cellular integrity by some other mechanism(s).
Despite the fact that haemorrhage is not a common feature of LASV-infected patients, for those suffering from AHF due to JUNV infection, haemorrhage is much more common. Even though an increase in the occurrence of haemorrhage is observed in JUNV infection, vascular damage is limited, as seen with LASV infection (Weissenbacher et al., 1987). The receptor for JUNV, transferrin receptor 1 (TfR1), is highly expressed on vascular endothelial cells, and these cells support the high levels of virus replication as observed in vitro (Andrews et al., 1978; Radoshitzky et al., 2007). As this virus is not cytopathic, vascular lesions are not observed in vivo (Gomez et al., 2003; Weissenbacher et al., 1987). Experimentally infected endothelial cells show an increase in nitric oxide and prostaglandin PGI2 production (Gomez et al., 2003). The release of these vasoactive mediators may be the cause of the increased vascular permeability upon JUNV infection of endothelial cells, and may contribute to the subsequent shock seen in patients. Experimentally infected cells also show an increase in the cellular adhesion molecules ICAM-1 and VCAM-1 (Gomez et al., 2003). Interestingly, whereas JUNV-infected HUVEC cells show reduced levels of the coagulation von Willebrand factor (VWF), patients infected with JUNV display increased levels of VWF in serum samples (Gomez et al., 2003; Molinas et al., 1989). This discrepancy suggests that VWF is originating from some other source rather than endothelial cells. Additionally, an as-yet-unknown inhibitor of platelet aggregation exists in plasma, as evidenced by the ability of AHF patient plasma to inhibit aggregation of normal platelets in vitro, a characteristic also found in LASV-infected patients (Cummins et al., 1990b). Infected individuals also exhibit thrombocytopenia and reduced complement activity (de Bracco et al., 1978). The coagulation activity of blood in infected patients is also low (Heller et al., 1995). All of these factors may contribute to the coagulopathy and oedema observed in AHF.
NW and OW arenaviruses trigger different immune responses
The outcome of arenavirus HF is heavily dependent on an effective immune response. However, differences in immune responses are noted between NW and OW arenavirus infections that play a critical role in clearance of the virus. In the case of LASV infection, an effective T-cell-mediated response appears to be critical for recovery from infection. In experimentally infected macaques, animals that survive infection have activated T-cells in circulation, but fatally infected animals display low and delayed T-cell activation. The surviving animals are also able to control virus replication, whereas those that succumb to the disease exhibit high viral loads (Baize et al., 2009). In hospitalized patients, high IgG and IgM titres are not associated with the outcome of the disease; however, high viral titres are associated with a poor outcome, indicating that the antibody response is not effective in controlling virus replication and the resulting pathology (Johnson et al., 1987).
In order to generate an adaptive response, a virus must first be recognized by antigen-presenting cells (APCs), which initiate the production of an appropriate immune response. APCs, such as dendritic cells (DCs) and macrophages, are early targets of arenavirus infection. Although both macrophages and DCs are targeted by LASV infection, DCs seem to be a more important target, as they produce much more virus than macrophages upon LASV infection (Baize et al., 2004). Whilst macrophages and DCs are readily infected with LASV, they fail to become activated upon infection (Baize et al., 2004). No increase is observed in the levels of activating markers, such as CD80, CD86, CD40, CD54 and HLAs, or of cytokines, such as TNF-α, IL-1β, IL-6 and IL-12. In addition, infected DCs fail to mature, as evidenced by the absence of increased levels of phagocytic activity. The failure of APCs to become activated upon virus infection is consistent with the generalized immune suppression that is one of the hallmarks of LASV infection.
A closely related virus, MOPV, is apathogenic in humans and can actually provide protection against LASV infection in non-human primates (Fisher-Hoch et al., 2000). Interestingly, MOPV infection also fails to activate DCs (Pannetier et al., 2004). Similar to LASV infection, MOPV primarily targets DCs and macrophages. Upon infection with MOPV, DCs fail to upregulate expression of pro-inflammatory cytokines and of CD80, CD86, CD54, CD40 and HLA-abc. However, MOPV infection of macrophages increases the transcription of messages encoding IFN-α, IFN-β, TNF and IL-6 (Pannetier et al., 2004). Therefore, whilst MOPV fails to activate DCs, macrophages are capable of becoming activated upon virus infection. However, in a DC and T-cell co-culture model, DCs were shown to be capable of activation in response to MOPV infection. This has been speculated to be the effect of cross-talk with T-cells (Fig. 3). These activated DCs were capable of inducing strong T-cell responses, whereas DCs infected with LASV using the same co-culture model remained inactive (Pannetier et al., 2011). The activation of the APCs is critical for generating an effective T-cell response, which is required for clearance of the virus and patient recovery (Fig. 3).
Fig. 3.
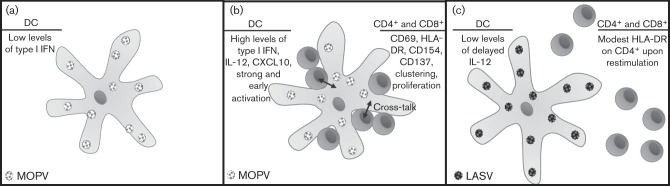
Activation of DCs and T-cells in the presence of MOPV infection versus LASV infection. (a) Response of DCs to infection by MOPV in the absence of T-cells. (b) Response of DCs to infection by MOPV in the presence of a T-cell co-culture results in early and strong activation of DCs and activation of T-cells, suggesting cross-talk between the cell types, possibly involving bystander activation of T-cells. (c) LASV infection of DCs in a T-cell co-culture model does not result in highly activated DCs and T-cells (Pannetier et al., 2011).
Whilst the hallmark of LASV infection is a generalized immune suppression, JUNV-infected patients display elevated cytokine levels. Infected patients show increases in levels of TNF-α, IFN-α, IL-6 and IL-10 (Heller et al., 1992; Levis et al., 1985; Marta et al., 1999). Patients with exacerbated disease and fatal cases consistently show elevated levels of TNF-α and IFN-α (Heller et al., 1992; Levis et al., 1985). Although these increased levels of cytokines are observed in JUNV-infected patients, in vitro-infected macrophages show no increase in cytokine production, such as IFN-α, IFN-β, TNF-α, IL-10, IL-6 and IL-12 (Groseth et al., 2011). Therefore, the increased cytokine levels observed in patients must originate from another source, possibly DCs whose role in cytokine production upon JUNV infection has yet to be established. The exact role of cytokines in the pathogenesis of AHF has yet to be determined. A proposed theory is that cytokines may be important in controlling virus replication in the early stages of infection, yet a delayed response could contribute to pathogenesis, as seen in patients with severe disease and high levels of cytokines (Groseth et al., 2011).
Although the antibody response seems to be ineffective in controlling LASV infection, this is not true in the case of JUNV infection. The reason for the disparity is unknown but underscores the differences in haemorrhagic diseases caused by OW and NW arenaviruses. When immune plasma from previously exposed individuals is administered to other JUNV-infected patients early during the course of infection, the mortality rate can be reduced from 16 to 1 % (Maiztegui et al., 1979). However, this treatment is only effective when given within the first week of illness, emphasizing the importance of early diagnosis (Enria & Maiztegui, 1994). Plasma banks have been set up in endemic areas in order to collect serum from individuals who have survived AHF. The efficacy of immune plasma treatment appears to be due the ability of the antibodies to neutralize the virus, as viraemia levels of patients are reduced after transfusion with immune plasma (Montardit et al., 1979). Although immune plasma therapy is highly effective, alternative therapies are needed. Potential complications associated with plasma transfusions, such as inadvertent pathogen transmission, must also be taken into consideration. Additionally, a late neurological syndrome has been observed in 10 % of patients treated with immune plasma, which typically resolves but underscores the need for alternative therapies (Maiztegui et al., 1979). Ribavirin has been tested in a small study, given on day 8 after the onset of illness. Treatment in this later stage of illness did lower viral titres and endogenous IFN levels whilst prolonging the time to death but failed to reduce mortality (Enria et al., 1987). Ribavirin treatment in LASV-infected patients is only beneficial when given during the early stages of infection, and perhaps would be more effective for JUNV-infected patients if administered earlier.
Effects of molecular differences between OW and NW arenaviruses on infection, innate detection and immune activation
Even though NW and OW arenaviruses share many similarities with regard to structural composition and viral biology, differences in their molecular strategies of virus infection do exist. At the molecular level, these viruses demonstrate differences in receptor usage and endosomal trafficking, and slight variations in the functions of some of the viral proteins. Here, we discuss some of the major differences between the arenavirus groups and explore the effects these molecular strategies may have on virus infection and interaction with the immune system.
Receptor usage
Receptor usage is key in determining cellular tropism. Arenaviruses are known to target DCs and macrophages early on during the course of infection. OW arenaviruses LASV and LCMV, in addition to the pathogenic NW arenaviruses Oliveros virus and Latino virus (LATV), use cellular α-dystroglycan (αDG) to gain entry into host cells (Kunz et al., 2001; Spiropoulou et al., 2002). This is a cell-surface molecule that is used for attachment to the extracellular matrix (ECM), and as such is expressed fairly ubiquitously on cells, accounting for the ability of LASV to infect many cell types. Normally, αDG binds laminin, a component of the extracellular matrix, whilst the transmembrane portion of the receptor, βDG, anchors the receptor by binding dystrophin in the cytoplasm. When LASV infection occurs, laminin is displaced by the effect of binding of the virus to αDG, which causes the membrane to destabilize. The disturbance of the membrane could result in an interference with cellular signalling, which may also contribute to HF disease pathogenesis (Rojek et al., 2012). Additionally, OW arenavirus infection results in the downregulation of αDG, which may contribute further to destabilization of the membrane (Rojek et al., 2007a). αDG has been shown to be highly expressed on macrophages and DCs in mice, which may explain why these cells are early targets of arenavirus infection (Sevilla et al., 2000). This may have important implications for the immune evasion employed by arenaviruses, as early infection of APCs without activation would prevent initiation of an appropriate immune response.
Recent studies have provided evidence for LASV exposure resulting in positive selection on genes required for the proper functioning of αDG in certain human populations. Before αDG can be expressed on the cell surface, it must first be modified post-translationally by the cellular like-acetylglucosaminyltransferase (LARGE). Without glycosylation by LARGE, αDG is incapable of interacting with either the extracellular matrix or the arenavirus GP (Kunz et al., 2005; Rojek et al., 2007b). The International HapMap project, which has analysed over 3 million human polymorphisms, has identified positive selection of two genes (LARGE and dystrophin) in a Nigerian population (Andersen et al., 2012; Sabeti et al., 2007). Dystrophin is a cytosolic adaptor protein that is necessary for αDG to function properly. In the identified population, over 21 % of individuals showed evidence of exposure to LASV infection, which may be responsible for the increase in the allelic variants of LARGE and dystrophin. These variants may alter the ability of LASV GP to bind αDG, therefore providing the immune system with an advantage, and conferring protection against LASV infection (Andersen et al., 2012; Sabeti et al., 2007).
LASV may use alternative receptors besides αDG for entry into cells. Evidence for this comes from the fact that addition of laminin has been found to be incapable of blocking LASV GP-mediated infection of Vero cells. This suggests that the virus can infect cells through the use of alternative receptors (Kunz et al., 2005). Recently, four cellular receptors have been found to be capable of mediating LASV infection: Axl, Tyro3, LSECtin and DC-SIGN. Although entry through these receptors was less efficient, these molecules were found to be capable of mediating LASV infection independently of αDG (Shimojima et al., 2012). These receptors may be important in infection of cell types such as hepatocytes, which display high viral titres despite the seeming lack of αDG expression on the cell membrane (Walker et al., 1982b; Yamamoto et al., 2004). Interestingly, Ebola virus has also been shown to be capable of using these same receptors for entry into cells (Alvarez et al., 2002; Brindley et al., 2011; Gramberg et al., 2005). Ebola and LASV share the same cellular tropism, infecting macrophages, DCs, endothelial cells and the liver.
The NW arenaviruses that are responsible for human disease gain cellular entry by binding human TfR1 (Radoshitzky et al., 2007). The normal function of this receptor is to mediate endocytosis of iron-bound transferrin, thereby transporting iron across the cell membrane and allowing its subsequent release into the cytoplasm (Andrews et al., 1999). The specificity for the human homologue of TfR1 appears to be an important determinant of pathogenesis, as non-pathogenic NW arenaviruses Amapari virus (AMAV) and Tacaribe virus (TCRV) are capable of using TfR1 orthologues but are incapable of binding the human receptor. In addition, a single mutation in TCRV GP allows binding of human TfR1, whilst a combination of four mutations in AMAV GP allows this interaction to occur. This suggests that modest mutations in the GP of these apathogenic arenaviruses may allow infection of human cells (Abraham et al., 2009). Like αDG, TfR1 is expressed on a wide variety of cell types, which would therefore allow pantropic infection, as is also observed during NW arenavirus infections.
Similar to the positive selection on genes affecting αDG expression and modification as a result of LASV exposure, recent studies have revealed evolutionary pressure exerted on TfR1 expression as a result of NW arenavirus infection. An analysis of rodent TfR1 sequences has identified residues that appear to be positively selected, and these residues are located in the domain of TfR1 that interacts with MACV GP. These naturally occurring mutations are able to prevent binding by MACV whilst retaining TfR1 functionality (Demogines et al., 2013). This is notable, as most mutations that confer protection against infection do so at some cost to the host, due to a loss in functionality of the protein. These variations are able to confer protection in rodents, and similar variants could potentially also protect humans from MACV infection (Demogines et al., 2013).
Endosomal trafficking
As many of the OW and NW arenaviruses use different cellular receptors for entry into host cells, they also use different routes of intracellular trafficking as part of this entry process. OW arenaviruses that bind αDG enter the cell via internalization through smooth vesicles in a process that is cholesterol dependent but independent of both clathrin and caveolin (Borrow & Oldstone, 1994; Quirin et al., 2008; Rojek et al., 2008a, b; Vela et al., 2007). Recent studies have found that binding of LASV to αDG causes phosphorylation of βDG by receptor tyrosine kinases. This phosphorylation is associated with the dissociation of βDG from the cytoskeletal adaptor protein utrophin, which may facilitate endocytosis (Moraz et al., 2013). The virus–receptor complex is then delivered via the multivesicular body to the late endosome, and this process is dependent on microtubular transport (Quirin et al., 2008; Rojek et al., 2008b). The virus–receptor complexes appear to be sorted by the ESCRT (endosomal sorting complexes required for transport) complex into intraluminal vesicles in the multivesicular body prior to being transported to the late endosome (Pasqual et al., 2011). Through this route, the virus subverts classical routes of endosomal trafficking and bypasses the early endosome. This compartment contains the Toll-like receptors responsible for detection of RNA viruses, and by avoiding transport to the early endosome, OW arenaviruses can evade detection by these innate immune receptors. This may partly explain the observed failure of the innate immune system to detect LASV infection, resulting in uncontrolled virus infection (McCormick & Fisher-Hoch, 2002).
Although OW arenavirus internalization is independent of clathrin, the NW arenavirus JUNV infection involves internalization via clathrin-coated pits at the plasma membrane, which is not unexpected, as TfR1 normally associates with clathrin-coated pits (Martinez et al., 2007). Additionally, cholesterol sequestration has only a minor effect on JUNV infection, unlike LASV entry which is cholesterol dependent (Martinez et al., 2007). Upon binding, TfR1 is internalized and transported to the early endosome (Martinez et al., 2009). The early endosome has a pH of ~6.0, but JUNV GP requires a pH of <5.5 for optimal fusion activity. Thus, the virus must be transported to the late endosome, which is a more acidic compartment, which would allow for fusion to occur (Martinez et al., 2009). This is not a route normally taken in TfR1 trafficking, and may indicate that the virus can somehow reroute the normal pattern of TfR1 recycling. The trafficking of TfR1 seems to reply on the multimeric state of the receptor, as monomers are quickly recycled through the early endosome, whilst larger oligomers are retained in this compartment for an extended period of time (Marsh et al., 1995). Arenavirus binding may exert some influence on the oligomeric state of TfR1, and may potentially influence the trafficking of the receptor by this method (Rojek & Kunz, 2008). Exactly what effects the altered route of endosomal trafficking of JUNV has on infection and immune detection remain to be determined.
IFN inhibition by viral proteins
One of the hallmarks of severe LASV infection is generalized immune suppression. The innate response to virus infection is essential in the development of an effective adaptive immune response, which is needed to clear the infection. One mechanism employed by arenaviruses to interfere with the development of effective immune responses is the inhibition of type I IFN production. Pathological arenavirus infections by both NW and OW arenaviruses are characterized by the ability of viral proteins to inhibit type I IFN. A potent inhibitor of type I IFN is the viral NP. The NP proteins of OW LASV and LCMV as well as NW JUNV, MACV, White Water Arroyo virus, TCRV and LATV have all been shown to display this ability to inhibit type I IFN through the inhibition of IFN regulatory factor 3 translocation. Despite the fact that it has been suggested that the NP protein of TCRV does not have the ability to suppress type I IFN (Martínez-Sobrido et al., 2007), recent studies have shown that it is able to do so (Harmon et al., 2013; Jiang et al., 2013). The same residues that are responsible for the IFN inhibitory function of NP have also been shown to be involved in the 3′→5′ exoribonuclease activity of this protein, tying the two functions together. The proposed model is that the exoribonuclease function of NP degrades viral pathogen-associated molecular pattern RNAs (i.e. dsRNA), thereby preventing pathogen recognition by the innate pathogen recognition receptors [i.e. retinoic acid-inducible gene 1 (RIG-I) and melanoma differentiation-associated protein 5 (MDA5)], leading to the suppression of IFN production (Hastie et al., 2011; Qi et al., 2010). Interestingly, these same residues have also been implicated in binding of the NP protein to IκB kinase ϵ, thereby inhibiting the nuclear translocation and transcriptional activity of NF-κB, which may indicate an overlap in functional domains of the protein or may be the result of an allosteric effect (Pythoud et al., 2012; Rodrigo et al., 2012).
The viral Z protein appears to play a similar role in inhibiting IFN production. The Z proteins of NW arenaviruses JUNV, GTOV, MACV and SABV bind RIG-I, resulting in downregulation of the IFN-β response (Fan et al., 2010). Based on published data to date, the NW arenavirus Z protein can bind to RIG-I, but those of the OW viruses (LASV and LCMV) do not have this ability. NW arenavirus Z protein seems to inhibit the binding of mitochondrial antiviral signalling protein (MAVS) to RIG-I, thus preventing the downstream signalling that would result in production of type I IFN (Fan et al., 2010).
Vaccine development for the NW (JUNV) and OW (LASV) viruses
Multiple attempts have been made to generate an effective LASV vaccine. However, at present, no licensed vaccines are available for the prevention of Lassa fever. An attempt to generate a whole-virus vaccine via inactivation of LASV by gamma irradiation generated good humoral responses in non-human primates, but this response failed to protect animals against lethal challenge with LASV (McCormick et al., 1992), suggesting that T-cell responses rather than humoral responses are important for protection against LASV infection. Studies in humanized mice confirm the need for a cell-mediated immune defence against LASV. Control of LASV infection in humanized mice has been shown to be T-cell dependent. Interestingly, these mice could be protected from disease through T-cell depletion, indicating a role for T-cells in LASV pathogenesis as well as defence. This contradiction could be reconciled by the hypothesis that T-cells are necessary for rapid clearance of the virus, but if the host response proves incapable of doing so, the disease becomes mediated by the same cells that would otherwise protect against it (Flatz et al., 2010).
MOPV infection has been shown to be capable of providing protective heterologous immunity to LASV infection in non-human primates (Fisher-Hoch et al., 2000). Additionally, the recombinant ML29 vaccine expressing the L segment of MOPV and the S segment of LASV has been shown to protect marmosets against LASV infection by inducing sterilizing cell-mediated responses (Lukashevich et al., 2008). However, there are obvious safety concerns for these as vaccine candidates, as the genome of this recombinant virus contains the S segment from LASV, a Biosafety Level 4 (BSL-4) virus, and the L segment from MOPV, which is currently classified as a BSL-3 virus. Also, in regions endemic for LASV, a large portion of the population also suffers from human immunodeficiency virus infection. Therefore, the efficacy and safety of such a vaccine when applied to immunocompromised individuals must also be addressed. In addition to the cross-protection against LASV provided by MOPV infection, studies have shown that T-cell epitopes can be cross-protective for LASV and LCMV infections, providing proof of concept for a multivalent vaccine that would protect against different strains and potentially species of OW arenaviruses (Botten et al., 2010). A recombinant vaccinia virus vaccine has also been developed expressing combinations of LASV NP, GP1 and GP2 proteins. Recombinants expressing all three proteins, or the combination of GP1 and GP2, were able to protect macaques from fatal infection but not from viraemia. Again, this vaccine would not be applicable for use in immunocompromised individuals. In addition, pre-existing immunity from the smallpox vaccine would potentially be problematic (Fisher-Hoch et al., 2000). A recombinant vesicular stomatitis virus vaccine expressing LASV GPC has also been found to be protective in non-human primates. Although the animals were protected, LASV viraemia was detected in the animals at day 7 post-challenge (Geisbert et al., 2005). A similar approach was taken using the yellow fever virus vaccine YF17D as a vector for LASV GP. One construct contained both GPs from yellow fever virus and LASV, and was capable of protecting strain 13 guinea pigs from LASV challenge. However, it replicated poorly and was not stable upon sequential passages in tissue culture (Bredenbeek et al., 2006; Jiang et al., 2011). Another construct expressing only LASV GP also protected strain 13 animals from death but did not prevent disease or viraemia, and was poorly immunogenic (Jiang et al., 2011).
Although development of a protective vaccine for LASV infection has proven challenging, development of a vaccine against JUNV infection has been more fruitful. The Candid #1 strain of JUNV has been shown to be a highly successful vaccine against AHF. This strain was generated by passaging the virus twice in guinea pigs, followed by sequential passaging in suckling mice and tissue culture (Goñi et al., 2006). Unfortunately, it is not an appealing vaccine candidate for commercial production because of its small target population. However, the Argentine government has taken up production, and Candid #1 is now used in Argentina as an effective vaccine against JUNV infection. This vaccine has not been approved by the US Food and Drug Administration (FDA) due to a lack of proper FDA-compliant documentation, the lack of a detailed genetic composition of the vaccine strain, and the association with foot-and-mouth disease in several regions of Argentina. More importantly, the molecular basis for the attenuated phenotype of Candid #1 remains unresolved. Recently, a reverse genetics system has been developed for Candid #1, which has provided much-needed insight as to the molecular determinants of attenuation for the vaccine strain. A single residue change, F427I, in the G2 transmembrane domain of the GP appears to be responsible for the attenuated phenotype. The mechanism for this remains to be addressed, but, as this substitution does not affect the ability of the virus to use TfR1 as a receptor, it is possible that the F427I change may affect viral fusion or maturation (Albariño et al., 2011). The reverse genetics system has the potential to address most, if not all, of the concerns regarding Candid #1, and will hopefully provide a safe and effective vaccine that will meet FDA approval criteria (Emonet et al., 2011). In addition, although humoral responses seem to be unimportant in LASV infection, serum treatment has been shown to be efficacious in the treatment of Argentine HF (Maiztegui et al., 1979). Encouragingly, the Candid #1 vaccine has also been shown to be efficacious in providing cross-protection against MACV infection in experimentally infected guinea pigs and rhesus monkeys (Barrera Oro & Eddy, 1982; Barrera Oro & Lupton, 1988; Jahrling et al., 1988). Despite the fact that infection with JUNV can be prevented with Candid #1 or treated with patient serum, measures against LASV infection remain lacking.
Concluding remarks
Arenaviruses are pathogens of significant human morbidity and mortality, and their status as neglected tropical pathogens warrants further investigation into the mechanisms of pathogenesis, the immune response and the viral biology of these important viral pathogens. Although arenaviruses share many characteristics, such as genome structure, viral assembly, natural host reservoirs and interference with immune responses, many differences do exist between the viruses. NW and OW viruses appear to use different receptors for and mechanisms of viral entry into cells. Although individuals infected with different arenaviruses may share many features of HF, differences in signs and symptoms do exist. Compounding these differences is the fact that even individuals infected with the same arenavirus species show varied disease manifestations, and some individuals may recover from the illness, whilst others display severe and fatal diseases. The same can be said for the differences in pathological lesions observed in patients. Although haemorrhage is much more common in the case of JUNV infection than for LASV infection, disruption of the vascular endothelium is an important hallmark for both virus infections in terms of exacerbated disease. Likewise, the type of immune response generated for arenavirus HFs is critical for clearance of the virus. Whilst an effective T-cell-mediated response is critical for clearance of LASV, the antibody response seems to be important for recovery from JUNV infection. The interaction with the immune system also demonstrates critical differences between OW and NW arenavirus infections. Whereas severe LASV infection is characterized by a generalized immune suppression, JUNV infection seems to result in a cytokine storm. Interestingly, although both NW and OW NP proteins are capable of inhibiting the production of type I IFN, NW arenaviruses appear to take an extra step to ensure evasion of host immune detection through the use of the viral Z protein. Recent studies have also provided intriguing evidence for evolution of certain host genes that can potentially provide natural protection from pathogenic NW and OW arenavirus infections. Taken together, whilst these viruses share many similarities in molecular mechanisms as well as characteristics of disease manifestation, understanding the differences between these related viruses will be important for the development of new vaccines and treatment modalities against these deadly viral pathogens.
Acknowledgements
We apologize to colleagues whose work cannot be cited in this review due to space constraints. Work in the authors’ laboratories was supported in part by NIH grants R01AI093580 and R56AI091805 to H. L. and R01AI083409 to Y. L.
References
- Abraham J., Kwong J. A., Albariño C. G., Lu J. G., Radoshitzky S. R., Salazar-Bravo J., Farzan M., Spiropoulou C. F., Choe H. (2009). Host-species transferrin receptor 1 orthologs are cellular receptors for nonpathogenic new world clade B arenaviruses. PLoS Pathog 5, e1000358 10.1371/journal.ppat.1000358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar P. V., Camargo W., Vargas J., Guevara C., Roca Y., Felices V., Laguna-Torres V. A., Tesh R., Ksiazek T. G., Kochel T. J. (2009). Reemergence of Bolivian hemorrhagic fever, 2007–2008. Emerg Infect Dis 15, 1526–1528 10.3201/eid1509.090017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albariño C. G., Bird B. H., Chakrabarti A. K., Dodd K. A., Flint M., Bergeron É., White D. M., Nichol S. T. (2011). The major determinant of attenuation in mice of the Candid1 vaccine for Argentine hemorrhagic fever is located in the G2 glycoprotein transmembrane domain. J Virol 85, 10404–10408 10.1128/JVI.00856-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez C. P., Lasala F., Carrillo J., Muñiz O., Corbí A. L., Delgado R. (2002). C-type lectins DC-SIGN and L-SIGN mediate cellular entry by Ebola virus in cis and in trans. J Virol 76, 6841–6844 10.1128/JVI.76.13.6841-6844.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosio A., Saavedra M., Mariani M., Gamboa G., Maiza A. (2011). Argentine hemorrhagic fever vaccines. Hum Vaccin 7, 694–700 10.4161/hv.7.6.15198 [DOI] [PubMed] [Google Scholar]
- Andersen K. G., Shylakhter I., Tabrizi S., Grossman S. R., Happi C. T., Sabeti P. C. (2012). Genome-wide scans provide evidence for positive selection of genes implicated in Lassa fever. Philos Trans R Soc Lond B Biol Sci 367, 868–877 10.1098/rstb.2011.0299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews B. S., Theofilopoulos A. N., Peters C. J., Loskutoff D. J., Brandt W. E., Dixon F. J. (1978). Replication of dengue and Junin viruses in cultured rabbit and human endothelial cells. Infect Immun 20, 776–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews N. C., Fleming M. D., Levy J. E. (1999). Molecular insights into mechanisms of iron transport. Curr Opin Hematol 6, 61–64 10.1097/00062752-199903000-00001 [DOI] [PubMed] [Google Scholar]
- Baize S., Kaplon J., Faure C., Pannetier D., Georges-Courbot M.-C., Deubel V. (2004). Lassa virus infection of human dendritic cells and macrophages is productive but fails to activate cells. J Immunol 172, 2861–2869 [DOI] [PubMed] [Google Scholar]
- Baize S., Marianneau P., Loth P., Reynard S., Journeaux A., Chevallier M., Tordo N., Deubel V., Contamin H. (2009). Early and strong immune responses are associated with control of viral replication and recovery in Lassa virus-infected cynomolgus monkeys. J Virol 83, 5890–5903 10.1128/JVI.01948-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrera Oro J. G., Eddy G. A. (1982). Characteristics of candidate live attenuated Junin virus vaccine. In IV International Conference on Comparative Virology, Banff, Alberta, Canada: Abstract S4–10. [Google Scholar]
- Barrera Oro J. G., Lupton H. W. (1988). Cross-protection against Machupo virus with Candid #1 live-attenuated Junin virus vaccine. I. The postvaccination prechallenge immune response. In Second International Conference on the Impact of Viral Diseases on the Development of Latin American Countries and the Caribbean Region, Buenos Aires, Argentina [Google Scholar]
- Beyer W. R., Pöpplau D., Garten W., von Laer D., Lenz O. (2003). Endoproteolytic processing of the lymphocytic choriomeningitis virus glycoprotein by the subtilase SKI-1/S1P. J Virol 77, 2866–2872 10.1128/JVI.77.5.2866-2872.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonthius D. J. (2012). Lymphocytic choriomeningitis virus: an underrecognized cause of neurologic disease in the fetus, child, and adult. Semin Pediatr Neurol 19, 89–95 10.1016/j.spen.2012.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrow P., Oldstone M. B. A. (1994). Mechanism of lymphocytic choriomeningitis virus entry into cells. Virology 198, 1–9 10.1006/viro.1994.1001 [DOI] [PubMed] [Google Scholar]
- Botten J., Whitton J. L., Barrowman P., Sidney J., Whitmire J. K., Alexander J., Kotturi M. F., Sette A., Buchmeier M. J. (2010). A multivalent vaccination strategy for the prevention of Old World arenavirus infection in humans. J Virol 84, 9947–9956 10.1128/JVI.00672-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredenbeek P. J., Molenkamp R., Spaan W. J. M., Deubel V., Marianneau P., Salvato M. S., Moshkoff D., Zapata J., Tikhonov I. & other authors (2006). A recombinant Yellow Fever 17D vaccine expressing Lassa virus glycoproteins. Virology 345, 299–304 10.1016/j.virol.2005.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brindley M. A., Hunt C. L., Kondratowicz A. S., Bowman J., Sinn P. L., McCray P. B., Jr, Quinn K., Weller M. L., Chiorini J. A., Maury W. (2011). Tyrosine kinase receptor Axl enhances entry of Zaire ebolavirus without direct interactions with the viral glycoprotein. Virology 415, 83–94 10.1016/j.virol.2011.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunotte L., Lelke M., Hass M., Kleinsteuber K., Becker-Ziaja B., Günther S. (2011). Domain structure of Lassa virus L protein. J Virol 85, 324–333 10.1128/JVI.00721-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmeier M., Bowen M., Peters C. (2001). Arenaviridae: The Viruses and Their Replication. In Fields Virology, 4th edn, pp. 1635–1668 Edited by Knipe D., Howley P. Philadelphia, PA: Lippincott-Raven Publisher [Google Scholar]
- CDC (Centers for Disease Control and Prevention) (2008). Brief report: Lymphocytic choriomeningitis virus transmitted through solid organ transplantation – Massachusetts, 2008. MMWR Morb Mortal Wkly Rep 57, 799–801 [PubMed] [Google Scholar]
- Charrel R. N., de Lamballerie X. (2003). Arenaviruses other than Lassa virus. Antiviral Res 57, 89–100 10.1016/S0166-3542(02)00202-4 [DOI] [PubMed] [Google Scholar]
- Childs J., Peters C. (1993). Ecology and epidemiology of arenaviruses and their hosts. In The Arenaviridae, pp. 331–384 Edited by Salvato M. New York: Plenum Press; 10.1007/978-1-4615-3028-2_19 [DOI] [Google Scholar]
- Coimbra T. L. M., Nassar E. S., de Souza L. T. M., Ferreira I. B., Rocco I. M., Burattini M. N., Travassos da Rosa A. P. A., Vasconcelos P. F. C. & other authors (1994). New arenavirus isolated in Brazil. Lancet 343, 391–392 10.1016/S0140-6736(94)91226-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins D., Fisher-Hoch S. P., Walshe K. J., Mackie I. J., McCormick J. B., Bennett D., Perez G., Farrar B., Machin S. J. (1989). A plasma inhibitor of platelet aggregation in patients with Lassa fever. Br J Haematol 72, 543–548 10.1111/j.1365-2141.1989.tb04321.x [DOI] [PubMed] [Google Scholar]
- Cummins D., McCormick J. B., Bennett D., Samba J. A., Farrar B., Machin S. J., Fisher-Hoch S. P. (1990a). Acute sensorineural deafness in Lassa fever. JAMA 264, 2093–2096 10.1001/jama.1990.03450160063030 [DOI] [PubMed] [Google Scholar]
- Cummins D., Molinas F. C., Lerer G., Maiztegui J. I., Faint R., Machin S. J. (1990b). A plasma inhibitor of platelet aggregation in patients with Argentine hemorrhagic fever. Am J Trop Med Hyg 42, 470–475 [DOI] [PubMed] [Google Scholar]
- de Bracco M. M. E., Rimoldi M. T., Cossio P. M., Rabinovich A., Maiztegui J. I., Carballal G., Arana R. M. (1978). Argentine hemorrhagic fever. Alterations of the complement system and anti-Junin-virus humoral response. N Engl J Med 299, 216–221 10.1056/NEJM197808032990502 [DOI] [PubMed] [Google Scholar]
- de Manzione N., Salas R. A., Paredes H., Godoy O., Rojas L., Araoz F., Fulhorst C. F., Ksiazek T. G., Mills J. N. & other authors (1998). Venezuelan hemorrhagic fever: clinical and epidemiological studies of 165 cases. Clin Infect Dis 26, 308–313 10.1086/516299 [DOI] [PubMed] [Google Scholar]
- Delgado S., Erickson B. R., Agudo R., Blair P. J., Vallejo E., Albariño C. G., Vargas J., Comer J. A., Rollin P. E. & other authors (2008). Chapare virus, a newly discovered arenavirus isolated from a fatal hemorrhagic fever case in Bolivia. PLoS Pathog 4, e1000047 10.1371/journal.ppat.1000047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demogines A., Abraham J., Choe H., Farzan M., Sawyer S. L. (2013). Dual host–virus arms races shape an essential housekeeping protein. PLoS Biol 11, e1001571 10.1371/journal.pbio.1001571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichler R., Lenz O., Strecker T., Eickmann M., Klenk H.-D., Garten W. (2003a). Identification of Lassa virus glycoprotein signal peptide as a trans-acting maturation factor. EMBO Rep 4, 1084–1088 10.1038/sj.embor.7400002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichler R., Lenz O., Strecker T., Garten W. (2003b). Signal peptide of Lassa virus glycoprotein GP-C exhibits an unusual length. FEBS Lett 538, 203–206 10.1016/S0014-5793(03)00160-1 [DOI] [PubMed] [Google Scholar]
- Elsner B., Schwarz E., Mando O. G., Maiztegui J., Vilches A. (1973). Pathology of 12 fatal cases of Argentine hemorrhagic fever. Am J Trop Med Hyg 22, 229–236 [PubMed] [Google Scholar]
- Emonet S. F., Seregin A. V., Yun N. E., Poussard A. L., Walker A. G., de la Torre J. C., Paessler S. (2011). Rescue from cloned cDNAs and in vivo characterization of recombinant pathogenic Romero and live-attenuated Candid #1 strains of Junin virus, the causative agent of Argentine hemorrhagic fever disease. J Virol 85, 1473–1483 10.1128/JVI.02102-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enria D. A., Maiztegui J. I. (1994). Antiviral treatment of Argentine hemorrhagic fever. Antiviral Res 23, 23–31 10.1016/0166-3542(94)90030-2 [DOI] [PubMed] [Google Scholar]
- Enria D. A., Briggiler A. M., Levis S., Vallejos D., Maiztegui J. I., Canonico P. G. (1987). Tolerance and antiviral effect of ribavirin in patients with Argentine hemorrhagic fever. Antiviral Res 7, 353–359 10.1016/0166-3542(87)90017-9 [DOI] [PubMed] [Google Scholar]
- Enria D. A., Briggiler A. M., Sánchez Z. (2008). Treatment of Argentine hemorrhagic fever. Antiviral Res 78, 132–139 10.1016/j.antiviral.2007.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L., Briese T., Lipkin W. I. (2010). Z proteins of New World arenaviruses bind RIG-I and interfere with type I interferon induction. J Virol 84, 1785–1791 10.1128/JVI.01362-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer S. A., Graham M. B., Kuehnert M. J., Kotton C. N., Srinivasan A., Marty F. M., Comer J. A., Guarner J., Paddock C. D. & other authors (2006). Transmission of lymphocytic choriomeningitis virus by organ transplantation. N Engl J Med 354, 2235–2249 10.1056/NEJMoa053240 [DOI] [PubMed] [Google Scholar]
- Fisher-Hoch S., McCormick J. B., Sasso D., Craven R. B. (1988). Hematologic dysfunction in Lassa fever. J Med Virol 26, 127–135 10.1002/jmv.1890260204 [DOI] [PubMed] [Google Scholar]
- Fisher-Hoch S. P., Hutwagner L., Brown B., McCormick J. B. (2000). Effective vaccine for lassa fever. J Virol 74, 6777–6783 10.1128/JVI.74.15.6777-6783.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatz L., Rieger T., Merkler D., Bergthaler A., Regen T., Schedensack M., Bestmann L., Verschoor A., Kreutzfeldt M. & other authors (2010). T cell-dependence of Lassa fever pathogenesis. PLoS Pathog 6, e1000836 10.1371/journal.ppat.1000836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulhorst C. E., Bowen M. D., Salas R. A., de Manzione N. M. C., Duno G., Utrera A., Ksiazek T. G., Peters C. J., Nichol S. T. & other authors (1997). Isolation and characterization of pirital virus, a newly discovered South American arenavirus. Am J Trop Med Hyg 56, 548–553 [DOI] [PubMed] [Google Scholar]
- Fulhorst C. F., Cajimat M. N. B., Milazzo M. L., Paredes H., de Manzione N. M. C., Salas R. A., Rollin P. E., Ksiazek T. G. (2008). Genetic diversity between and within the arenavirus species indigenous to western Venezuela. Virology 378, 205–213 10.1016/j.virol.2008.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisbert T. W., Jones S., Fritz E. A., Shurtleff A. C., Geisbert J. B., Liebscher R., Grolla A., Ströher U., Fernando L. & other authors (2005). Development of a new vaccine for the prevention of Lassa fever. PLoS Med 2, e183 10.1371/journal.pmed.0020183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez R. M., Pozner R. G., Lazzari M. A., D’Atri L. P., Negrotto S., Chudzinski-Tavassi A. M., Berría M. I., Schattner M. (2003). Endothelial cell function alteration after Junin virus infection. Thromb Haemost 90, 326–333 [DOI] [PubMed] [Google Scholar]
- Goñi S. E., Iserte J. A., Ambrosio A. M., Romanowski V., Ghiringhelli P. D., Lozano M. E. (2006). Genomic features of attenuated Junín virus vaccine strain candidate. Virus Genes 32, 37–41 10.1007/s11262-005-5843-2 [DOI] [PubMed] [Google Scholar]
- Gramberg T., Hofmann H., Möller P., Lalor P. F., Marzi A., Geier M., Krumbiegel M., Winkler T., Kirchhoff F. & other authors (2005). LSECtin interacts with filovirus glycoproteins and the spike protein of SARS coronavirus. Virology 340, 224–236 10.1016/j.virol.2005.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groseth A., Hoenen T., Weber M., Wolff S., Herwig A., Kaufmann A., Becker S. (2011). Tacaribe virus but not Junin virus infection induces cytokine release from primary human monocytes and macrophages. PLoS Negl Trop Dis 5, e1137 10.1371/journal.pntd.0001137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Günther S., Lenz O. (2004). Lassa virus. Crit Rev Clin Lab Sci 41, 339–390 10.1080/10408360490497456 [DOI] [PubMed] [Google Scholar]
- Harmon B., Kozina C., Maar D., Carpenter T. S., Branda C. S., Negrete O. A., Carson B. D. (2013). Identification of critical amino acids within the nucleoprotein of Tacaribe virus important for anti-interferon activity. J Biol Chem 288, 8702–8711 10.1074/jbc.M112.444760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison L. H., Halsey N. A., McKee K. T., Jr, Peters C. J., Barrera Oro J. G., Briggiler A. M., Feuillade M. R., Maiztegui J. I. (1999). Clinical case definitions for Argentine hemorrhagic fever. Clin Infect Dis 28, 1091–1094 10.1086/514749 [DOI] [PubMed] [Google Scholar]
- Hastie K. M., Kimberlin C. R., Zandonatti M. A., MacRae I. J., Saphire E. O. (2011). Structure of the Lassa virus nucleoprotein reveals a dsRNA-specific 3′ to 5′ exonuclease activity essential for immune suppression. Proc Natl Acad Sci U S A 108, 2396–2401 10.1073/pnas.1016404108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller M. V., Saavedra M. C., Falcoff R., Maiztegui J. I., Molinas F. C. (1992). Increased tumor necrosis factor-α levels in Argentine hemorrhagic fever. J Infect Dis 166, 1203–1204 10.1093/infdis/166.5.1203 [DOI] [PubMed] [Google Scholar]
- Heller M. V., Marta R. F., Sturk A., Maiztegui J. I., Hack C. E., Cate J. W., Molinas F. C. (1995). Early markers of blood coagulation and fibrinolysis activation in Argentine hemorrhagic fever. Thromb Haemost 73, 368–373 [PubMed] [Google Scholar]
- Jahrling P. B., Trotter R. W., Barrera Oro J. G., Lupton H. W., Cosgriff T. M., Lewis R. W., Parrish D. B., Smith S. B., Peters C. J. (1988). Cross-protection against Machupo virus with Candid #1 Junin virus vaccine: III, post-challenge clinical findings. In Second International Conference on the Impact of Viral Diseases on the Development of Latin American Countries and the Caribbean Region, p. E3 Mar del Plata, Argentina [Google Scholar]
- Jiang X., Dalebout T. J., Bredenbeek P. J., Carrion R., Jr, Brasky K., Patterson J., Goicochea M., Bryant J., Salvato M. S., Lukashevich I. S. (2011). Yellow fever 17D-vectored vaccines expressing Lassa virus GP1 and GP2 glycoproteins provide protection against fatal disease in guinea pigs. Vaccine 29, 1248–1257 10.1016/j.vaccine.2010.11.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X., Huang Q., Wang W., Dong H., Ly H., Liang Y., Dong C. (2013). Structures of arenaviral nucleoproteins with triphosphate dsRNA reveal a unique mechanism of immune suppression. J Biol Chem 288, 16949–16959 10.1074/jbc.M112.420521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. M., Kuns M. L., Mackenzie R. B., Webb P. A., Yunker C. E. (1966). Isolation of Machupo virus from wild rodent Calomys callosus. Am J Trop Med Hyg 15, 103–106 [DOI] [PubMed] [Google Scholar]
- Johnson K. M., Halstead S. B., Cohen S. N. (1967). Hemorrhagic fevers of Southeast Asia and South America: a comparative appraisal. Prog Med Virol 9, 105–158 [PubMed] [Google Scholar]
- Johnson K. M., McCormick J. B., Webb P. A., Smith E. S., Elliott L. H., King I. J. (1987). Clinical virology of Lassa fever in hospitalized patients. J Infect Dis 155, 456–464 10.1093/infdis/155.3.456 [DOI] [PubMed] [Google Scholar]
- Kunz S., Sevilla N., McGavern D. B., Campbell K. P., Oldstone M. B. A. (2001). Molecular analysis of the interaction of LCMV with its cellular receptor α-dystroglycan. J Cell Biol 155, 301–310 10.1083/jcb.200104103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz S., Rojek J. M., Kanagawa M., Spiropoulou C. F., Barresi R., Campbell K. P., Oldstone M. B. A. (2005). Posttranslational modification of α-dystroglycan, the cellular receptor for arenaviruses, by the glycosyltransferase LARGE is critical for virus binding. J Virol 79, 14282–14296 10.1128/JVI.79.22.14282-14296.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levis S. C., Saavedra M. C., Ceccoli C., Feuillade M. R., Enria D. A., Maiztegui J. I., Falcoff R. (1985). Correlation between endogenous interferon and the clinical evolution of patients with Argentine hemorrhagic fever. J Interferon Res 5, 383–389 10.1089/jir.1985.5.383 [DOI] [PubMed] [Google Scholar]
- Lukashevich I. S. (2013). The search for animal models for Lassa fever vaccine development. Expert Rev Vaccines 12, 71–86 10.1586/erv.12.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukashevich I. S., Clegg J. C. S., Sidibe K. (1993). Lassa virus activity in Guinea: distribution of human antiviral antibody defined using enzyme-linked immunosorbent assay with recombinant antigen. J Med Virol 40, 210–217 10.1002/jmv.1890400308 [DOI] [PubMed] [Google Scholar]
- Lukashevich I. S., Maryankova R., Vladyko A. S., Nashkevich N., Koleda S., Djavani M., Horejsh D., Voitenok N. N., Salvato M. S. (1999). Lassa and Mopeia virus replication in human monocytes/macrophages and in endothelial cells: different effects on IL-8 and TNF-α gene expression. J Med Virol 59, 552–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukashevich I. S., Carrion R., Jr, Salvato M. S., Mansfield K., Brasky K., Zapata J., Cairo C., Goicochea M., Hoosien G. E. & other authors (2008). Safety, immunogenicity, and efficacy of the ML29 reassortant vaccine for Lassa fever in small non-human primates. Vaccine 26, 5246–5254 10.1016/j.vaccine.2008.07.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNeil A., Ströher U., Farnon E., Campbell S., Cannon D., Paddock C. D., Drew C. P., Kuehnert M., Knust B. & other authors (2012). Solid organ transplant-associated lymphocytic choriomeningitis, United States, 2011. Emerg Infect Dis 18, 1256–1262 10.3201/eid1808.120212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahanty S., Bausch D. G., Thomas R. L., Goba A., Bah A., Peters C. J., Rollin P. E. (2001). Low levels of interleukin-8 and interferon-inducible protein-10 in serum are associated with fatal infections in acute Lassa fever. J Infect Dis 183, 1713–1721 10.1086/320722 [DOI] [PubMed] [Google Scholar]
- Mahanty S., Hutchinson K., Agarwal S., McRae M., Rollin P. E., Pulendran B. (2003). Cutting edge: impairment of dendritic cells and adaptive immunity by Ebola and Lassa viruses. J Immunol 170, 2797–2801 [DOI] [PubMed] [Google Scholar]
- Maiztegui J. I., Laguens R. P., Cossio P. M., Casanova M. B., de la Vega M. T., Ritacco V., Segal A., Fernández N. J., Arana R. M. (1975). Ultrastructural and immunohistochemical studies in five cases of Argentine hemorrhagic fever. J Infect Dis 132, 35–43 10.1093/infdis/132.1.35 [DOI] [PubMed] [Google Scholar]
- Maiztegui J. I., Fernandez N. J., de Damilano A. J. (1979). Efficacy of immune plasma in treatment of Argentine haemorrhagic fever and association between treatment and a late neurological syndrome. Lancet 314, 1216–1217 10.1016/S0140-6736(79)92335-3 [DOI] [PubMed] [Google Scholar]
- Marsh E. W., Leopold P. L., Jones N. L., Maxfield F. R. (1995). Oligomerized transferrin receptors are selectively retained by a lumenal sorting signal in a long-lived endocytic recycling compartment. J Cell Biol 129, 1509–1522 10.1083/jcb.129.6.1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marta R. F., Montero V. S., Hack C. E., Sturk A., Maiztegui J. I., Molinas F. C. (1999). Proinflammatory cytokines and elastase-α-1-antitrypsin in Argentine hemorrhagic fever. Am J Trop Med Hyg 60, 85–89 [DOI] [PubMed] [Google Scholar]
- Martinez M. G., Cordo S. M., Candurra N. A. (2007). Characterization of Junin arenavirus cell entry. J Gen Virol 88, 1776–1784 10.1099/vir.0.82808-0 [DOI] [PubMed] [Google Scholar]
- Martinez M. G., Forlenza M. B., Candurra N. A. (2009). Involvement of cellular proteins in Junin arenavirus entry. Biotechnol J 4, 866–870 10.1002/biot.200800357 [DOI] [PubMed] [Google Scholar]
- Martínez-Sobrido L., Giannakas P., Cubitt B., García-Sastre A., de la Torre J. C. (2007). Differential inhibition of type I interferon induction by arenavirus nucleoproteins. J Virol 81, 12696–12703 10.1128/JVI.00882-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick J. B. (1999). Lassa fever. In Emergence and Control of Rodent-borne Viral Diseases, pp. 177–195 Edited by Saluzzo J. F., Dodet B. Amsterdam: Elsevier [Google Scholar]
- McCormick J. B., Fisher-Hoch S. P. (2002). Lassa fever. Curr Top Microbiol Immunol 262, 75–109 10.1007/978-3-642-56029-3_4 [DOI] [PubMed] [Google Scholar]
- McCormick J. B., Webb P. A., Krebs J. W., Johnson K. M., Smith E. S. (1987a). A prospective study of the epidemiology and ecology of Lassa fever. J Infect Dis 155, 437–444 10.1093/infdis/155.3.437 [DOI] [PubMed] [Google Scholar]
- McCormick J. B., King I. J., Webb P. A., Johnson K. M., O’Sullivan R., Smith E. S., Trippel S., Tong T. C. (1987b). A case–control study of the clinical diagnosis and course of Lassa fever. J Infect Dis 155, 445–455 10.1093/infdis/155.3.445 [DOI] [PubMed] [Google Scholar]
- McCormick J. B., Mitchell S. W., Kiley M. P., Ruo S., Fisher-Hoch S. P. (1992). Inactivated Lassa virus elicits a non protective immune response in rhesus monkeys. J Med Virol 37, 1–7 10.1002/jmv.1890370102 [DOI] [PubMed] [Google Scholar]
- Mills J. N., Ellis B. A., McKee K. T., Jr, Ksiazek T. G., Oro J. G., Maiztegui J. I., Calderon G. E., Peters C. J., Childs J. E. (1991). Junin virus activity in rodents from endemic and nonendemic loci in central Argentina. Am J Trop Med Hyg 44, 589–597 [DOI] [PubMed] [Google Scholar]
- Molinas F. C., de Bracco M. M. E., Maiztegui J. I. (1989). Hemostasis and the complement system in Argentine hemorrhagic fever. Rev Infect Dis 11 (Suppl. 4), S762–S770 10.1093/clinids/11.Supplement_4.S762 [DOI] [PubMed] [Google Scholar]
- Monath T. P., Casals J. (1975). Diagnosis of Lassa fever and the isolation and management of patients. Bull World Health Organ 52, 707–715 [PMC free article] [PubMed] [Google Scholar]
- Montardit A. I., Fernandez M. J., DeSensi M. R. F., Damilano A. J., Maiztegui J. I. (1979). Neutralizacion de la viremia en enfermos de fiebre hemorragica Argentina tratados con plasma inmune. Medicina (B Aires) 39, 799. [PubMed] [Google Scholar]
- Moraz M.-L., Kunz S. (2011). Pathogenesis of arenavirus hemorrhagic fevers. Expert Rev Anti Infect Ther 9, 49–59 10.1586/eri.10.142 [DOI] [PubMed] [Google Scholar]
- Moraz M.-L., Pythoud C., Turk R., Rothenberger S., Pasquato A., Campbell K. P., Kunz S. (2013). Cell entry of Lassa virus induces tyrosine phosphorylation of dystroglycan. Cell Microbiol 15, 689–700 10.1111/cmi.12078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin B., Coutard B., Lelke M., Ferron F., Kerber R., Jamal S., Frangeul A., Baronti C., Charrel R. & other authors (2010). The N-terminal domain of the arenavirus L protein is an RNA endonuclease essential in mRNA transcription. PLoS Pathog 6, e1001038 10.1371/journal.ppat.1001038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone M. B. A., Campbell K. P. (2011). Decoding arenavirus pathogenesis: essential roles for alpha-dystroglycan-virus interactions and the immune response. Virology 411, 170–179 10.1016/j.virol.2010.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paessler S., Walker D. H. (2013). Pathogenesis of the viral hemorrhagic fevers. Annu Rev Pathol 8, 411–440 10.1146/annurev-pathol-020712-164041 [DOI] [PubMed] [Google Scholar]
- Pannetier D., Faure C., Georges-Courbot M.-C., Deubel V., Baize S. (2004). Human macrophages, but not dendritic cells, are activated and produce alpha/beta interferons in response to Mopeia virus infection. J Virol 78, 10516–10524 10.1128/JVI.78.19.10516-10524.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannetier D., Reynard S., Russier M., Journeaux A., Tordo N., Deubel V., Baize S. (2011). Human dendritic cells infected with the nonpathogenic Mopeia virus induce stronger T-cell responses than those infected with Lassa virus. J Virol 85, 8293–8306 10.1128/JVI.02120-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasqual G., Rojek J. M., Masin M., Chatton J.-Y., Kunz S. (2011). Old world arenaviruses enter the host cell via the multivesicular body and depend on the endosomal sorting complex required for transport. PLoS Pathog 7, e1002232 10.1371/journal.ppat.1002232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paweska J. T., Sewlall N. H., Ksiazek T. G., Blumberg L. H., Hale M. J., Lipkin W. I., Weyer J., Nichol S. T., Rollin P. E. & other authors (2009). Nosocomial outbreak of novel arenavirus infection, southern Africa. Emerg Infect Dis 15, 1598–1602 10.3201/eid1510.090211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez M., de la Torre J. C. (2003). Characterization of the genomic promoter of the prototypic arenavirus lymphocytic choriomeningitis virus. J Virol 77, 1184–1194 10.1128/JVI.77.2.1184-1194.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters C. J. (2006). Lymphocytic choriomeningitis virus – an old enemy up to new tricks. N Engl J Med 354, 2208–2211 10.1056/NEJMp068021 [DOI] [PubMed] [Google Scholar]
- Pfau C. J. (1996). Arenaviruses. In Medical Microbiology, 4th edn Edited by Baron S. Galveston, TX: University of Texas Medical Branch at Galveston; [PubMed] [Google Scholar]
- Pinschewer D. D., Perez M., de la Torre J. C. (2005). Dual role of the lymphocytic choriomeningitis virus intergenic region in transcription termination and virus propagation. J Virol 79, 4519–4526 10.1128/JVI.79.7.4519-4526.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pythoud C., Rodrigo W. W. S. I., Pasqual G., Rothenberger S., Martínez-Sobrido L., de la Torre J. C., Kunz S. (2012). Arenavirus nucleoprotein targets interferon regulatory factor-activating kinase IKKϵ. J Virol 86, 7728–7738 10.1128/JVI.00187-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi X., Lan S., Wang W., Schelde L. M., Dong H., Wallat G. D., Ly H., Liang Y., Dong C. (2010). Cap binding and immune evasion revealed by Lassa nucleoprotein structure. Nature 468, 779–783 10.1038/nature09605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirin K., Eschli B., Scheu I., Poort L., Kartenbeck J., Helenius A. (2008). Lymphocytic choriomeningitis virus uses a novel endocytic pathway for infectious entry via late endosomes. Virology 378, 21–33 10.1016/j.virol.2008.04.046 [DOI] [PubMed] [Google Scholar]
- Radoshitzky S. R., Abraham J., Spiropoulou C. F., Kuhn J. H., Nguyen D., Li W., Nagel J., Schmidt P. J., Nunberg J. H. & other authors (2007). Transferrin receptor 1 is a cellular receptor for New World haemorrhagic fever arenaviruses. Nature 446, 92–96 10.1038/nature05539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju R., Raju L., Hacker D., Garcin D., Compans R., Kolakofsky D. (1990). Nontemplated bases at the 5′ ends of Tacaribe virus mRNAs. Virology 174, 53–59 10.1016/0042-6822(90)90053-T [DOI] [PubMed] [Google Scholar]
- Rodrigo W. W. S. I., Ortiz-Riaño E., Pythoud C., Kunz S., de la Torre J. C., Martínez-Sobrido L. (2012). Arenavirus nucleoproteins prevent activation of nuclear factor kappa B. J Virol 86, 8185–8197 10.1128/JVI.07240-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojek J. M., Kunz S. (2008). Cell entry by human pathogenic arenaviruses. Cell Microbiol 10, 828–835 10.1111/j.1462-5822.2007.01113.x [DOI] [PubMed] [Google Scholar]
- Rojek J. M., Campbell K. P., Oldstone M. B. A., Kunz S. (2007a). Old World arenavirus infection interferes with the expression of functional α-dystroglycan in the host cell. Mol Biol Cell 18, 4493–4507 10.1091/mbc.E07-04-0374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojek J. M., Spiropoulou C. F., Campbell K. P., Kunz S. (2007b). Old World and clade C New World arenaviruses mimic the molecular mechanism of receptor recognition used by α-dystroglycan’s host-derived ligands. J Virol 81, 5685–5695 10.1128/JVI.02574-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojek J. M., Perez M., Kunz S. (2008a). Cellular entry of lymphocytic choriomeningitis virus. J Virol 82, 1505–1517 10.1128/JVI.01331-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojek J. M., Sanchez A. B., Nguyen N. T., de la Torre J.-C., Kunz S. (2008b). Different mechanisms of cell entry by human-pathogenic Old World and New World arenaviruses. J Virol 82, 7677–7687 10.1128/JVI.00560-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojek J. M., Moraz M.-L., Pythoud C., Rothenberger S., Van der Goot F. G., Campbell K. P., Kunz S. (2012). Binding of Lassa virus perturbs extracellular matrix-induced signal transduction via dystroglycan. Cell Microbiol 14, 1122–1134 10.1111/j.1462-5822.2012.01784.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabeti P. C., Varilly P., Fry B., Lohmueller J., Hostetter E., Cotsapas C., Xie X., Byrne E. H., McCarroll S. A. & other authors (2007). Genome-wide detection and characterization of positive selection in human populations. Nature 449, 913–918 10.1038/nature06250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevilla N., Kunz S., Holz A., Lewicki H., Homann D., Yamada H., Campbell K. P., de La Torre J. C., Oldstone M. B. A. (2000). Immunosuppression and resultant viral persistence by specific viral targeting of dendritic cells. J Exp Med 192, 1249–1260 10.1084/jem.192.9.1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimojima M., Ströher U., Ebihara H., Feldmann H., Kawaoka Y. (2012). Identification of cell surface molecules involved in dystroglycan-independent Lassa virus cell entry. J Virol 86, 2067–2078 10.1128/JVI.06451-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiropoulou C. F., Kunz S., Rollin P. E., Campbell K. P., Oldstone M. B. A. (2002). New World arenavirus clade C, but not clade A and B viruses, utilizes α-dystroglycan as its major receptor. J Virol 76, 5140–5146 10.1128/JVI.76.10.5140-5146.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinebaugh B. J., Schloeder F. X., Johnson K. M., Mackenzie R. B., Entwisle G., De Alba E. (1966). Bolivian hemorrhagic fever. A report of four cases. Am J Med 40, 217–230 10.1016/0002-9343(66)90103-3 [DOI] [PubMed] [Google Scholar]
- Tesh R. B., Jahrling P. B., Salas R., Shope R. E. (1994). Description of Guanarito virus (Arenaviridae: Arenavirus), the etiologic agent of Venezuelan hemorrhagic fever. Am J Trop Med Hyg 50, 452–459 [DOI] [PubMed] [Google Scholar]
- Vela E. (2012). Animal models, prophylaxis, and therapeutics for arenavirus infections. Viruses 4, 1802–1829 10.3390/v4091802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vela E. M., Zhang L., Colpitts T. M., Davey R. A., Aronson J. F. (2007). Arenavirus entry occurs through a cholesterol-dependent, non-caveolar, clathrin-mediated endocytic mechanism. Virology 369, 1–11 10.1016/j.virol.2007.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieth S., Torda A. E., Asper M., Schmitz H., Günther S. (2004). Sequence analysis of L RNA of Lassa virus. Virology 318, 153–168 10.1016/j.virol.2003.09.009 [DOI] [PubMed] [Google Scholar]
- Walker D. H., McCormick J. B., Johnson K. M., Webb P. A., Komba-Kono G., Elliott L. H., Gardner J. J. (1982a). Pathologic and virologic study of fatal Lassa fever in man. Am J Pathol 107, 349–356 [PMC free article] [PubMed] [Google Scholar]
- Walker D. H., McCormick J. B., Johnson K. M., Webb P. A., Komba-Kono G., Elliott L. H., Gardner J. J. (1982b). Pathologic and virologic study of fatal Lassa fever in man. Am J Pathol 107, 349–356 [PMC free article] [PubMed] [Google Scholar]
- Webb P. A., Justines G., Johnson K. M. (1975). Infection of wild and laboratory animals with Machupo and Latino viruses. Bull World Health Organ 52, 493–499 [PMC free article] [PubMed] [Google Scholar]
- Weissenbacher M. C., Laguens R. P., Coto C. E. (1987). Argentine hemorrhagic fever. Curr Top Microbiol Immunol 134, 79–116 10.1007/978-3-642-71726-0_4 [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Kato Y., Karita M., Kawaguchi M., Shibata N., Kobayashi M. (2004). Expression of genes related to muscular dystrophy with lissencephaly. Pediatr Neurol 31, 183–190 10.1016/j.pediatrneurol.2004.03.020 [DOI] [PubMed] [Google Scholar]
- York J., Nunberg J. H. (2007). Distinct requirements for signal peptidase processing and function in the stable signal peptide subunit of the Junín virus envelope glycoprotein. Virology 359, 72–81 10.1016/j.virol.2006.08.048 [DOI] [PubMed] [Google Scholar]
- York J., Romanowski V., Lu M., Nunberg J. H. (2004). The signal peptide of the Junín arenavirus envelope glycoprotein is myristoylated and forms an essential subunit of the mature G1-G2 complex. J Virol 78, 10783–10792 10.1128/JVI.78.19.10783-10792.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapata J. C., Salvato M. S. (2013). Arenavirus variations due to host-specific adaptation. Viruses 5, 241–278 10.3390/v5010241 [DOI] [PMC free article] [PubMed] [Google Scholar]