Abstract
Despite the continuously growing number of known avian picornaviruses (family Picornaviridae), knowledge of their genetic diversity in wild birds, especially in long-distance migrant species is very limited. In this study, we report the presence of a novel picornavirus identified from one of 18 analysed faecal samples of an Afro-Palearctic migrant bird, the European roller (Coracias garrulus L., 1758), which is distantly related to the marine-mammal-infecting seal aquamavirus A1 (genus Aquamavirus). The phylogenetic analyses and the low sequence identity (P1 26.3 %, P2 25.8 % and P3 28.4 %) suggest that this picornavirus could be the founding member of a novel picornavirus genus that we have provisionally named ‘Kunsagivirus’, with ‘Greplavirus A’ (strain roller/SZAL6-KuV/2011/HUN, GenBank accession no. KC935379) as the candidate type species.
Picornaviruses (family Picornaviridae) are small, non-enveloped viruses with single-stranded, positive-sense genomic RNA. In general, the 7.2–9.1 kb polyadenylated picornaviral genome consists predominantly of a single polyprotein coding region flanked by highly structured 5′ and 3′ untranslated regions (UTRs), although substantial divergence from the common genome organization have been observed recently (Woo et al., 2012). The viral polyprotein is co- and post-translationally processed into multiple capsid monomers: VP0 (sometimes cleaved to VP4 and VP2), VP3 and VP1, and non-structural proteins: 2A, 2B, 2C, 3A, 3BVPg, 3Cpro and 3Dpol, and the presence of a leader (L) protein upstream of the capsid proteins is also observable in some picornaviruses (Racaniello, 2007; Boros et al., 2012a).
The family Picornaviridae consists of 37 species grouped into 17 officially recognized genera (Aphthovirus, Aquamavirus, Avihepatovirus, Cardiovirus, Cosavirus, Dicipivirus, Enterovirus, Erbovirus, Hepatovirus, Kobuvirus, Megrivirus, Parechovirus, Salivirus, Sapelovirus, Senecavirus, Teschovirus and Tremovirus); and, currently 28 (but a rapidly increasing number) candidate species (Knowles et al., 2012; Adams et al., 2013; http://www.picornaviridae.com).
Free-living birds are effective hosts and dispersers of different viruses such as Newcastle disease virus (family Paramyxoviridae), Japanese encephalitis virus (family Flaviviridae) and avian influenza virus (family Orthomyxoviridae) that are potentially hazardous to livestock, poultry and even humans (Leighton & Heckert, 2007; McLean & Ubico, 2007; Stallknecht et al., 2007). Despite studies predominantly related to the human threat of avian-borne viruses, knowledge of avian picornaviruses, especially viruses in wild birds are still limited. Of the 16 so far described avian picornaviruses, only duck hepatitis A virus (genus Avihepatovirus) from mallard ducks (Anas platyrhynchos), turdivirus 1 (unassigned species), turdivirus 2 and 3 (unassigned species) from dead birds of the family Turdidae and pigeon picornavirus A and B (unassigned species) from feral pigeons (Columba livia) are thought to infect wild birds (Knowles et al., 2012; Gough & Wallis, 1986; Woo et al., 2010; Kofstad & Jonassen, 2011).
Analysis of avian picornaviruses in free-living migratory birds is particularly important because these birds are easily capable of travelling long distances, even across continents, potentially transmitting avian-borne picornaviruses to new animal populations.
The European roller (Coracias garrulus L., 1758 of the family Coraciidae) is an Afro-Palearctic migrant (long-distance migrants that breed in Europe, including Hungary, and winter in sub-Saharan Africa) bird species living mainly in loose nomadic associations and sometimes forming large flocks containing hundreds of individuals (Fry, 2001). Due to the continuous decrease in population size, this bird species is now considered to be globally ‘near threatened’ by the International Union for Conservation of Nature, and is on their Red List of Threatened Species.
This is the first report of the presence of a novel picornavirus identified in a long-distance migrant bird species and distantly related to the marine-mammal-infecting seal aquamavirus A1 (SeAV-A1, genus Aquamavirus). Here, we proposed it as the prototype species in a novel genus in the family Picornaviridae.
Faecal samples from artificial nests occupied by healthy breeding pairs and nestlings of European rollers were collected from two different Hungarian breeding territories of the Great Hungarian Plain (Dorozsma-Majsai homokhát, n = 14; Borsodi Mezőség, n = 4) in July 2011 during the regular bird ringing process. Samples were collected by qualified ornithologists with valid permission (Permit No. of the National Inspectorate For Environment, Nature and Water: 14/1368-5/2011). Two randomly selected faecal samples (one from each breeding territory) were subjected to viral metagenomics analysis using sequence independent random reverse transcriptase-PCR (RT-PCR) amplification of viral-particle associated nucleic acids and 454 GS FLX technology, as described previously (Kapoor et al., 2008a; Victoria et al., 2009). To determine the complete picornavirus genome 5′/3′ RACE, RT-PCR amplification and dye-terminator sequencing were used as described previously (Boros et al., 2011, Boros et al., 2012b).
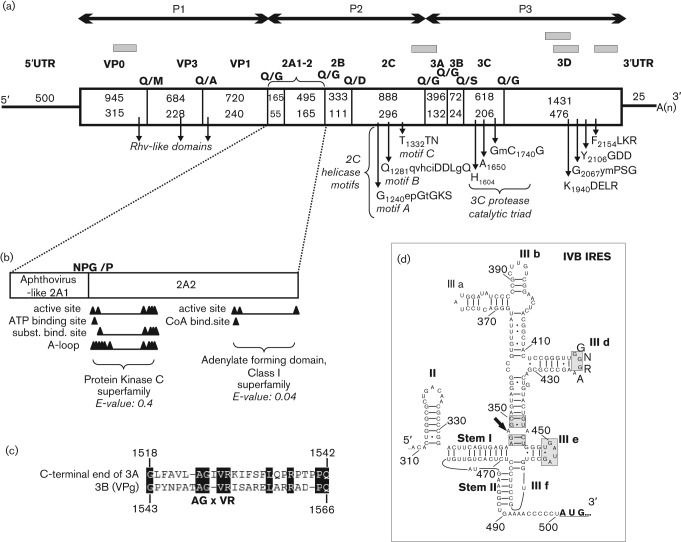
Five sequence contigs (Fig. 1a) originated from one of the sample (SZAL6) covering 26.8 % of a picornaviral genome related to SeAV-A1 (GenBank accession no. EU142040) as the closest match using blastx served as templates for virus-specific primer design (Table S1, available at JGV online). The 7272 nt RNA genome of the picornavirus strain roller/SZAL6-KuV/2011/HUN (GenBank accession no. KC935379) was predicted to possess a similar genome organization to SeAV-A1 : 5′UTR-P1(VP0-VP3-VP1)-P2(2A1-2A2-2B-2C)-P3(3A-3B-3C-3D)-3′UTR (Fig. 1). The G+C content (53.01mol %) of the entire genome is one of the highest among picornaviruses and significantly different from SeAV-A1 (Table 1).
Fig. 1.
(a) The genome organization, conserved picornaviral motifs and the predicted cleavage sites with the enlarged 2A genome region of roller/SZAL6-KuV/2011/HUN. Nucleotide (upper numbers) and amino acid (lower numbers) lengths are indicated in each gene box. The positions of the conserved picornaviral amino acid motifs are indicated with the first amino acid positions of the motif. The sequence contigs acquired from pyrosequencing are depicted as grey bars. (b) Functional sites of protein kinases and adenylate-forming domains of 2A2 of the study sequence identified by the CDD-search. (c) Alignment of the C-terminal end of the predicted 3A and the complete 3BVPg of roller/SZAL6-KuV/2011/HUN. Identical amino acids are shaded. (d) Predicted secondary RNA structure of the 5′UTR type IVB IRES of the study virus. Certain parts of the IRES structure that are conserved in type IV IRES are indicated by grey shading. The location of the specific A–A mis-pair of domain III is shown by an arrow (Hellen & de Breyne, 2007).
Table 1. Genomic features of the representative species of the 17 officially recognized and two candidate picornavirus genera and pairwise amino acid sequence identities between the P1, P2, P3, 2C and 3D proteins of roller/SZAL6-KuV/2011/HUN (KC935379) compared with those of the picornaviruses. Bold numbers indicate the highest levels of amino acid identities.
| Genus | Type species | Genome features | Roller/SZAL6-KuV/2011/HUN (KC935379) pairwise amino acid identity (%) | |||||||
| GenBank accession no. | Genome size (nt) | G+C content (mol%) | IRES type | P1 | P2 | P3 | 2C | 3D | ||
| Aphthovirus | Foot-and-mouth disease virus | AF274010 | 8115 | 54.08 | Type II | 15.5 | 16.9 | 19.2 | 21.9 | 23.8 |
| Aquamavirus | Aquamavirus A | EU142040 | 6718 | 43.85 | Type IVB | 26.3 | 25.8 | 28.4 | 34.7 | 34.9 |
| Avihepatovirus | Duck hepatitis A virus | DQ249299 | 7687 | 43.23 | Type IVB | 20.3 | 19.2 | 22.9 | 30.3 | 30.2 |
| Cardiovirus | Encephalomyocarditis virus | M81861 | 7835 | 49.47 | Type II | 15.1 | 15.5 | 19.6 | 18.9 | 22.2 |
| Cosavirus | Cosavirus A | FJ438902 | 7632 | 43.75 | Type II | 16.0 | 17.0 | 21.6 | 21.4 | 25.3 |
| Dicipivirus | Cadicivirus A | JN819202 | 8785 | 41.72 | Undefined | 13.9 | 15.9 | 19.5 | 20.8 | 24.1 |
| Enterovirus | Enterovirus C | V01149 | 7440 | 46.35 | Type I | 14.6 | 14.9 | 19.7 | 20.4 | 24.8 |
| Erbovirus | Equine rhinitis B virus | AF361253 | 8821 | 50.40 | Type II | 14.1 | 18.0 | 22.2 | 21.6 | 25.0 |
| Hepatovirus | Hepatitis A virus | M14707 | 7478 | 37.85 | Type III | 11.6 | 16.1 | 16.1 | 20.6 | 18.5 |
| Kobuvirus | Aichivirus A | AB010145 | 8251 | 58.91 | Type VB/V | 16.5 | 17.4 | 21.7 | 23.3 | 26.4 |
| Megrivirus | Melegrivirus A | HM751199 | 9075 | 46.07 | Type IV | 12.4 | 16.7 | 16.8 | 23.2 | 21.9 |
| Parechovirus | Human parechovirus | L02971 | 7339 | 39.60 | Type II | 21.7 | 18.7 | 22.8 | 27.0 | 28.8 |
| Ljungan virus | AF327920 | 7590 | 42.53 | Type II | 20.7 | 19.7 | 24.3 | 26.7 | 30.5 | |
| Salivirus | Salivirus A | GQ184145 | 7989 | 56.68 | Type V | 16.9 | 17.0 | 19.2 | 22.8 | 22.7 |
| Sapelovirus | Porcine sapelovirus | AF406813 | 7491 | 41.04 | Type IVB | 15.7 | 15.5 | 19.9 | 18.9 | 24.3 |
| Senecavirus | Seneca Valley virus | DQ641257 | 7310 | 51.62 | Type IVA | 14.5 | 16.1 | 19.4 | 21.3 | 24.3 |
| Teschovirus | Porcine teschovirus | AJ011380 | 7117 | 44.83 | Type IVB | 14.1 | 16.2 | 20.1 | 21.6 | 24.1 |
| Tremovirus | Avian encephalomyelitis virus | AJ225173 | 7055 | 44.88 | Type IVA | 10.3 | 13.7 | 17.1 | 19.0 | 21.5 |
| ‘Avisivirus’ | ‘Turkey avisivirus’ | KC465954 | 7532 | 44.97 | Type II | 20.1 | 18.4 | 23.0 | 26.1 | 27.6 |
| ‘Pasivirus’ | ‘Swine pasivirus’ | JQ316470 | 6916 | 43.20 | Undefined | 19.4 | 20.1 | 23.1 | 29.1 | 26.7 |
The complete P1 (2349 nt; 783 aa), P2 (1881 nt; 627 aa) and P3 (2517 nt; 838 aa) regions showed low amino acid sequence identity to SeAV-A1 (GenBank accession no. EU142040) (Table 1). The identity calculations were performed by BioEdit software (version 7.1.3.0) (Hall, 1999) using the pairwise alignments generated by clustal_x software (version 2.0.3). The potential proteolytic cleavage sites of roller/SZAL6-KuV/2011/HUN were mapped based on (i) the aa alignment with the two SeAV-A1 sequences: HO.02.21 (GenBank accession no. EU142040) and Holland/88 (N. J. Knowles, Pirbright Institute, personal communication, 2012) (ii) and the NetPicoRNA predictions (Blom et al., 1996). The predicted cleavage sites and the length of different genome regions are shown in Fig. 1(a).
The analysis of the P1 region did not support the presence of L protein or the maturation cleavage of VP0 similar to the members of genus Aquamavirus and other avian picornaviruses such as avihepato-, avisi-, galli-, megri- and turdiviruses (Tseng et al., 2007; Boros et al., 2013; Boros et al., 2012a; Honkavuori et al., 2011; Woo et al., 2010). No potential myristoylation motif (GxxxS/T, where x is a non-conserved amino acid) was recognizable at the N-terminal end of the viral polyprotein, which suggests that, similar to the aquamaviruses and parechoviruses, myristoylation of VP0 may not occur (Kapoor et al., 2008b).
The analysis of P2 region revealed the presence of an aphthovirus-like ‘ribosome-skipping’ motif (DxExNPG838/P) similar to SeAV-A1, leading to the release of a 55 aa 2A1 protein. The C-terminal 22 aa residues of roller/SZAL6-KuV/2011/HUN 2A1, which could be the core site of ‘ribosomal skipping’ (Ryan et al., 1991), shows 59 % amino acid identity to the 29 aa 2A1 of SeAV-A1 (EU142040). The N-terminal part (33 aa) of the 2A1 protein showed no significant sequence identity to any of the known picornaviral 2A sequences (Fig. 1b). The proteolytic cleavage site analysis revealed the presence of a second, 165 aa 2A protein that showed only 11 % amino acid identity to the 100 aa 2A2 of SeAV-A1 and did not possess any of the known picornaviral 2A characteristic motifs (e.g. catalytic sites of trypsin proteases, H-box/NC-motifs or the GxGxxGKS motifs of NTP-binding sites of 2As of avihepato- and avisiviruses) (Tseng et al., 2007; Boros et al., 2013). A Conserved Domain Database (CDD) search (Marchler-Bauer et al., 2011) identified some functional sites of protein kinases (CDD-ID: cd05094) and adenylate forming domains (CDD-ID: cl17068) in the 2A2 of roller/SZAL6-KuV/2011/HUN, although with a relatively high E-values (Fig. 1b). The 2C protein – similar to the other picornaviruses – falls into the class III helicases and all three functional motifs (A–C) were identifiable (Fig. 1a) (Hales et al., 2008).
The proteolytic cleavage site mapping strongly suggested the release of a single, 24 aa 3BVPg that was nearly half the size of the aquamavirus 46 aa 3B (encoding two VPgs in tandem) and showed only 42 % amino acid identity to the C-terminal VPg of SeAV-A1. Interestingly, the roller/SZAL6-KuV/2011/HUN VPg showed low similarity (34 %) and some conserved motifs (e.g. AGxVR) to the 25 aa peptide located at the C-terminal end of the 3A (from aa 1518 to 1542) (Fig. 1c), which suggested that the study strain originally had two VPgs, but one could have degenerated and become part of 3A.
The study sequence contains all of the conserved amino acid motifs of picornaviral 3C proteinase and 3D RNA polymerase (Fig. 1a) and showed the highest sequence identity to SeAV-A1 at the 3D region (Table 1) (Gorbalenya et al., 1989).
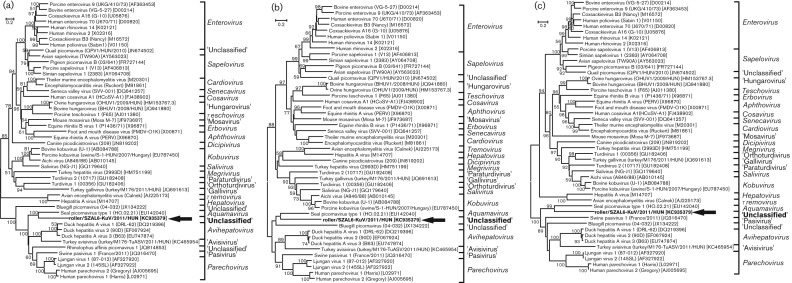
The phylogenetic analysis was performed using the amino acid sequences of the complete P1, 2C and 3CD genome regions of roller/SZAL6-KuV/2011/HUN and the representative members of the family Picornaviridae. The amino acid phylogenetic trees were constructed using the neighbour-joining method based on the Jones–Taylor–Thornton matrix-based model of mega software (version 5.0) (Tamura et al., 2011). Bootstrap values (based on 1000 replicates) for each node are shown if >50 %. All three phylogenetic trees show the consequent but distant relationship of roller/SZAL6-KuV/2011/HUN to SeAV-A1 (Aquamavirus) (Fig. 2).
Fig. 2.
Phylogenetic relationships between roller/SZAL6-KuV/2011/HUN (indicated in bold and with an arrow), representative members of the 17 picornavirus genera and unassigned picornaviruses based on amino acid sequences of the different picornavirus proteins: P1 (a), 2C (b) and 3CD (c). Bars indicate amino acid substitutions per site.
The 500 nt 5′UTR was similar in length to the 5′UTR of SeAV-A1 (506 nt) and contained three terminal uracils. The classification and analysis of the internal ribosomal entry site (IRES) (from nt 309 to 503) of the study sequence was performed by the Mfold program (Zuker, 2003). The predicted secondary structure of roller/SZAL6-KuV/2011/HUN had close similarity to the type IVB IRES structures of members of the genera Sapelovirus, Teschovirus and Aquamavirus (Table 1); thus, it contained the conservative IIIe stem–loop with highly conserved unpaired bases and the IIId G loop, but GpG instead of CpG dinucleotide pairing in the IIIf (Fig. 1d) (Hellen & de Breyne, 2007; Kapoor et al., 2008b). The 25 nt 3′UTR of the study sequence was similar in length to the 34 nt 3′UTR of SeAV-A1, the shortest among the known picornaviruses, and with a fold to a single stem–loop predicted by the Mfold program (data not shown).
Generic 3Dpol primers (Szal6-AqV-3DGen-R and Szal6-AqV-3DGen-F; Table S1) were designed based on the 3Dpol sequences of roller/SZAL6-KuV/2011/HUN and aquamaviruses for screening all of the faecal samples collected from the European rollers. No other picornaviruses were detected using this RT-PCR.
In this study, using metagenomic and RT-PCR approaches, we have reported the first complete genome sequence of a novel picornavirus (roller/SZAL6-KuV/2011/HUN) isolated from a long-distance migrant bird species, European roller, in Hungary. According to the current guidelines of the ICTV Picornaviridae Study Group (http://www.picornastudygroup.com/definitions/genus_definition.htm), novel picornavirus genera are defined by amino acid identities in the P1, P2 and P3 regions being less than <40, <40 and <50 %, respectively, compared with other genera (Table 1). Based on these guidelines, and the supporting phylogenetic analyses, roller/SZAL6-KuV/2011/HUN could be the founding member of a novel picornavirus genus. Given the lack of knowledge about the origin and pathogenic role of this picornavirus species, we propose to name it Greplavirus A (from the geographical name of the Great Hungarian Plain) in a novel genus ‘Kunsagivirus’ (from the name of the part of the Great Hungarian Plain – ‘Kunság’ – where the samples were collected), in the family Picornaviridae.
The identification of roller/SZAL6-KuV/2011/HUN from only one of the analysed faecal samples raises the possibility that the European roller is not the natural host of this virus but that it originated from another animal that was eaten. This is suspected for other enteric viruses identified using viral metagenomic approach, e.g. di-cistronic viruses from human faeces (Kapoor et al., 2010) and bat guano (Li et al., 2010). The European rollers consume primarily medium-sized (<35 mm) insects (Orthoptera, Coleoptera), although occasionally small vertebrates [e.g. pygmy shrews (Soricidae), lizards (Lacertidae)] may also serve as a food source (Molnar, 1998). Interestingly, we found co-infections (data not shown) of different rodent-origin/rodent-related picornaviruses (e.g. mosavirus and kobuvirus), mamastroviruses, picobirnavirus and Puumala virus (genus Hantavirus) with roller/SZAL6-KuV/2011/HUN in sample SZAL6 using blastx on the sequences of viral metagenomics. Five viruses related to rodent-borne viruses support the dietary origins of the identified group of viruses, although the relatively low detection rate of roller/SZAL6-KuV/2011/HUN does not necessarily imply an outside source of the virus. Further epidemiological studies and supporting experiments (e.g. follow-up and seroprevalence studies) on the possible hosts (e.g. rollers, pygmy shrews, lizards) should be conducted to answer this question.
The analysis of viruses in faecal samples of such endangered, migrant bird species may help identify viruses that are potentially capable of long-distance spread and transmission to other animal populations.
Acknowledgements
This work was supported by grants from the Hungarian Scientific Research Fund (OTKA, K83013). Á. B. was supported by TÁMOP 4.2.4. A/2-11-1-2012-0001, National Excellence Program – Elaborating and operating an inland student and researcher personal support system. The project was subsidized by the European Union and co-financed by the European Social Fund. G. R. was supported by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences and E. D. by NHLBI grant R01HL083254. We thank Attila Hunor Török, Béla Tokody (supported by the Conservation Management and Animal Health Monitoring of Natura 2000 Bird Species HU-SRB IPA CBC PROJECT) and Edit Pollák for the collection of faecal samples. We thank Nick J. Knowles (Chair, ICTV Picornaviridae Study Group) for the sequence of SeAV-A1 strain Holland/88, careful reading of the manuscript and helpful comments.
Footnotes
One supplementary table is available with the online version of this paper.
References
- Adams M. J., King A. M. Q., Carstens E. B. (2013). Ratification vote on taxonomic proposals to the International Committee on Taxonomy of Viruses (2013). Arch Virol. Apr 12. [Epub ahead of print] 10.1007/s00705-013-1688-5 [DOI] [PubMed] [Google Scholar]
- Blom N., Hansen J., Blaas D., Brunak S. (1996). Cleavage site analysis in picornaviral polyproteins: discovering cellular targets by neural networks. Protein Sci 5, 2203–2216 10.1002/pro.5560051107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boros A., Pankovics P., Simmonds P., Reuter G. (2011). Novel positive-sense, single-stranded RNA (+ssRNA) virus with di-cistronic genome from intestinal content of freshwater carp (Cyprinus carpio). PLoS ONE 6, e29145 10.1371/journal.pone.0029145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boros A., Nemes C., Pankovics P., Kapusinszky B., Delwart E., Reuter G. (2012a). Identification and complete genome characterization of a novel picornavirus in turkey (Meleagris gallopavo). J Gen Virol 93, 2171–2182 10.1099/vir.0.043224-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boros A., Pankovics P., Knowles N. J., Reuter G. (2012b). Natural interspecies recombinant bovine/porcine enterovirus in sheep. J Gen Virol 93, 1941–1951 10.1099/vir.0.041335-0 [DOI] [PubMed] [Google Scholar]
- Boros A., Nemes C., Pankovics P., Kapusinszky B., Delwart E., Reuter G. (2013). Genetic characterization of a novel picornavirus in turkeys (Meleagris gallopavo) distinct from turkey galliviruses and megriviruses and distantly related to the members of the genus Avihepatovirus. J Gen Virol 94, 1496–1509 10.1099/vir.0.051797-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry C. H. (2001). Family Coraciidae (Rollers). In Handbook of the Birds of the World (Mousebirds to Hornbills vol 6), pp. 342–377 Edited by del Hoyo J., Elliot A., Sargatal J. Barcelona: Lynx Editions [Google Scholar]
- Gorbalenya A. E., Donchenko A. P., Blinov V. M., Koonin E. V. (1989). Cysteine proteases of positive strand RNA viruses and chymotrypsin-like serine proteases: A distinct protein superfamily with a common structural fold. FEBS Lett 243, 103–114 10.1016/0014-5793(89)80109-7 [DOI] [PubMed] [Google Scholar]
- Gough R. E., Wallis A. S. (1986). Duck hepatitis type I and influenza in mallard ducks (Anas platyrhynchos). Vet Rec 119, 602. [PubMed] [Google Scholar]
- Hales L. M., Knowles N. J., Reddy P. S., Xu L., Hay C., Hallenbeck P. L. (2008). Complete genome sequence analysis of Seneca Valley virus-001, a novel oncolytic picornavirus. J Gen Virol 89, 1265–1275 10.1099/vir.0.83570-0 [DOI] [PubMed] [Google Scholar]
- Hall T. A. (1999). BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser 41, 95–98 [Google Scholar]
- Hellen C. U., de Breyne S. (2007). A distinct group of hepacivirus/pestivirus-like internal ribosomal entry sites in members of diverse picornavirus genera: evidence for modular exchange of functional noncoding RNA elements by recombination. J Virol 81, 5850–5863 10.1128/JVI.02403-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honkavuori K. S., Shivaprasad H. L., Briese T., Street C., Hirschberg D. L., Hutchison S. K., Lipkin W. I. (2011). Novel picornavirus in Turkey poults with hepatitis, California, USA. Emerg Infect Dis 17, 480–487 10.3201/eid1703.101410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor A., Victoria J., Simmonds P., Slikas E., Chieochansin T., Naeem A., Shaukat S., Sharif S., Alam M. M. & other authors (2008a). A highly prevalent and genetically diversified Picornaviridae genus in South Asian children. Proc Natl Acad Sci U S A 105, 20482–20487 10.1073/pnas.0807979105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor A., Victoria J., Simmonds P., Wang C., Shafer R. W., Nims R., Nielsen O., Delwart E. (2008b). A highly divergent picornavirus in a marine mammal. J Virol 82, 311–320 10.1128/JVI.01240-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor A., Simmonds P., Lipkin W. I., Zaidi S., Delwart E. (2010). Use of nucleotide composition analysis to infer hosts for three novel picorna-like viruses. J Virol 84, 10322–10328 10.1128/JVI.00601-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles N. J., Hovi T., Hyypiä T., King A. M. Q., Lindberg A. M., Pallansch M. A., Palmenberg A. C., Simmonds P., Skern T. & other authors (2012). Picornaviridae. In Virus Taxonomy: Classification and Nomenclature of Viruses: Ninth Report of the International Committee on Taxonomy of Viruses, pp. 855–880 Edited by King A. M. Q., Adams M. J., Carstens E. B., Lefkowitz E. J. San Diego: Elsevier [Google Scholar]
- Kofstad T., Jonassen C. M. (2011). Screening of feral and wood pigeons for viruses harbouring a conserved mobile viral element: characterization of novel astroviruses and picornaviruses. PLoS ONE 6, e25964 10.1371/journal.pone.0025964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton F. A., Heckert R. A. (2007). Newcastle disease and related avian paramyxoviruses. In Infectious diseases of wild birds, pp. 3–16 Edited by Thomas N. J., Hunter D. B., Atkinson C. T. San Diego: Wiley-Blackwell, Oxford; 10.1002/9780470344668.ch1 [DOI] [Google Scholar]
- Li L., Victoria J. G., Wang C., Jones M., Fellers G. M., Kunz T. H., Delwart E. (2010). Bat guano virome: predominance of dietary viruses from insects and plants plus novel mammalian viruses. J Virol 84, 6955–6965 10.1128/JVI.00501-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchler-Bauer A., Lu S., Anderson J. B., Chitsaz F., Derbyshire M. K., DeWeese-Scott C., Fong J. H., Geer L. Y., Geer R. C. & other authors (2011). CDD: a Conserved Domain Database for the functional annotation of proteins. Nucleic Acids Res 39 (Database issue), D225–D229 10.1093/nar/gkq1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean R. G., Ubico S. R. (2007). Arboviruses in birds. In Infectious Diseases of Wild Birds, pp. 17–62 Edited by Thomas N. J., Hunter D. B., Atkinson C. T. San Diego: Wiley-Blackwell, Oxford; 10.1002/9780470344668.ch2 [DOI] [Google Scholar]
- Molnar G. (1998). Breeding biology and foraging of Rollers (Coracias garrulus) nesting in nest-boxes. Ornis Hungarica 8 (Suppl. 1), 119–124 [Google Scholar]
- Racaniello V. (2007). Picornaviridae: The viruses and their replication. In Fields Virology, 5th edn, pp. 795–838 Edited by Knipe D. M., Howley P. M. Philadelphia: Lippincott Williams & Wilkins [Google Scholar]
- Ryan M. D., King A. M., Thomas G. P. (1991). Cleavage of foot-and-mouth disease virus polyprotein is mediated by residues located within a 19 amino acid sequence. J Gen Virol 72, 2727–2732 10.1099/0022-1317-72-11-2727 [DOI] [PubMed] [Google Scholar]
- Stallknecht D. E., Nagy E., Hunter D. B., Slemons R. D. (2007). Avian Influenza. In Infectious Diseases of Wild Birds, pp. 108–130 Edited by Thomas N. J., Hunter D. B., Atkinson C. T. San Diego: Wiley-Blackwell, Oxford; 10.1002/9780470344668.ch5 [DOI] [Google Scholar]
- Tamura K., Peterson D., Peterson N., Stecher G., Nei M., Kumar S. (2011). mega5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28, 2731–2739 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng C. H., Knowles N. J., Tsai H. J. (2007). Molecular analysis of duck hepatitis virus type 1 indicates that it should be assigned to a new genus. Virus Res 123, 190–203 10.1016/j.virusres.2006.09.007 [DOI] [PubMed] [Google Scholar]
- Victoria J. G., Kapoor A., Li L., Blinkova O., Slikas B., Wang C., Naeem A., Zaidi S., Delwart E. (2009). Metagenomic analyses of viruses in stool samples from children with acute flaccid paralysis. J Virol 83, 4642–4651 10.1128/JVI.02301-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo P. C., Lau S. K., Huang Y., Lam C. S., Poon R. W., Tsoi H. W., Lee P., Tse H., Chan A. S. & other authors (2010). Comparative analysis of six genome sequences of three novel picornaviruses, turdiviruses 1, 2 and 3, in dead wild birds, and proposal of two novel genera, Orthoturdivirus and Paraturdivirus, in the family Picornaviridae. J Gen Virol 91, 2433–2448 10.1099/vir.0.021717-0 [DOI] [PubMed] [Google Scholar]
- Woo P. C., Lau S. K., Choi G. K., Huang Y., Teng J. L., Tsoi H. W., Tse H., Yeung M. L., Chan K. H. & other authors (2012). Natural occurrence and characterization of two internal ribosome entry site elements in a novel virus, canine picodicistrovirus, in the picornavirus-like superfamily. J Virol 86, 2797–2808 10.1128/JVI.05481-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuker M. (2003). Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res 31, 3406–3415 10.1093/nar/gkg595 [DOI] [PMC free article] [PubMed] [Google Scholar]