Abstract
Polymorphonuclear neutrophils (PMN) infiltrate the respiratory tract early after viral infection and can contribute to both host defence and pathology. Coronaviruses are important causes of respiratory tract infections, ranging from mild to severe depending on the viral strain. This study evaluated the role of PMN during a non-fatal pulmonary coronavirus infection in the natural host. Rat coronavirus (RCoV) causes respiratory disease in adult rats, characterized by an early PMN response, viral replication and inflammatory lesions in the lungs, mild weight loss and effective resolution of infection. To determine their role during RCoV infection, PMN were depleted and the effects on disease progression, viral replication, inflammatory response and lung pathology were analysed. Compared with RCoV infection in control animals, PMN-depleted rats had worsened disease with weight loss, clinical signs, mortality and prolonged pulmonary viral replication. PMN-depleted animals had fewer macrophages and lymphocytes in the respiratory tract, corresponding to lower chemokine levels. Combined with in vitro experiments showing that PMN express cytokines and chemokines in response to RCoV-infected alveolar epithelial cells, these findings support a role for PMN in eliciting an inflammatory response to RCoV infection. Despite their critical role in the protection from severe disease, the presence of PMN was correlated with haemorrhagic lesions, epithelial barrier permeability and cellular inflammation in the lungs. This study demonstrated that while PMN are required for an effective antiviral response, they also contribute to lung pathology during RCoV infection.
Introduction
Inflammatory responses triggered by respiratory viruses are necessary for the initiation of effective antiviral immunity, but can also become dysregulated and result in acute lung injury and respiratory distress syndrome. Polymorphonuclear neutrophils (PMN) infiltrate the airways early after infection by respiratory viral pathogens including rhinoviruses, influenza viruses, respiratory syncytial virus and coronaviruses. The presence of PMN in the respiratory tract during viral infection is frequently correlated with clinical symptoms or severe disease pathology (Bradley et al., 2012; Denlinger et al., 2011; Khanolkar et al., 2009; Mckean et al., 2003; Nagata et al., 2008; Tumpey et al., 2005). In contrast, PMN have direct antiviral activities and also function in the activation of innate and adaptive immune responses, and thus can contribute to effective antiviral responses (Mantovani et al., 2011; Tate et al., 2011, 2012; Widegren et al., 2011). Because PMN can be involved in both protective and pathological immune responses, a complete understanding of their functions during viral infection may lead to the design of therapeutic strategies that exploit the beneficial functions of PMN while limiting their damaging effects in the lung.
Coronaviruses (CoV) cause respiratory diseases in humans as well as in companion and agricultural animals. Human CoV infections may result in mild common colds, more serious lower respiratory tract diseases, or the highly fatal severe acute respiratory syndrome (SARS) or Middle East Respiratory Syndrome (MERS), depending on the virus strain and the age and immune status of the host (Assiri et al., 2013; Gaunt et al., 2010; Lee et al., 2003). PMN are recruited to CoV-infected tissues, and either contribute to pathology or are necessary for an effective immune response, depending on the specific CoV and disease model. The presence of PMN corresponds to increased disease severity in humans and animals infected with SARS-CoV or human CoV-229E (Leong et al., 2006; Mckean et al., 2003; Nagata et al., 2008; Tsui et al., 2003). During neurotropic murine coronavirus infection, PMN contribute to brain pathology (Iacono et al., 2006), but are also critical for the effective resolution of infection by promoting blood–brain barrier permeability, which is needed for effective T-cell recruitment to the brain (Hosking et al., 2009; Zhou et al., 2003). Despite these findings and the fact that CoVs commonly infect the respiratory tract, the functions of PMN during respiratory CoV infections are not well understood.
Rodent models of respiratory coronavirus infection are available for SARS-CoV, but not the more common and milder CoV that circulate in human populations worldwide. We have developed a rat coronavirus (RCoV) model to determine the mechanisms that promote effective resolution of a non-fatal coronavirus infection in the lung. RCoV is a natural pathogen of rats that replicates and causes mild disease in the upper and lower respiratory tracts (Funk et al., 2009; Wojcinski & Percy, 1986). Intratracheal inoculation of adult rats with RCoV results in viral replication in the type I alveolar epithelial (AT1) cells in the lung, recruitment of PMN into the respiratory tract, expression of PMN chemotactic chemokines and transient, focal pneumonitis (Funk et al., 2009). The virus and inflammatory infiltrates within the alveoli are resolved by day 8 after infection, suggesting the rapid development of an effective antiviral response to infection. The role of PMN in this effective response to RCoV infection is not known. In this study, PMN recruitment to the lungs of RCoV-infected rats was inhibited using antibody-mediated depletion to determine the role of PMN in viral clearance, lung pathology and disease severity.
Results
PMN depletion enhances RCoV-mediated disease
There is robust recruitment of PMN to the respiratory tract during RCoV infection (Funk et al., 2009). To delineate their role during infection, rats were injected with rabbit anti-rat PMN serum (αPMN) 1 day prior to intranasal inoculation of virus. Depletion was maintained by injections of αPMN every 48 h (Fig. 1a). Control rats were injected with normal rabbit serum (NRS) on the same schedule. Several previous studies have used this polyclonal αPMN antibody to effectively deplete circulating PMN in rats without significantly altering other white blood cell populations (Janardhan et al., 2006; Li et al., 2007; Ofulue & Ko, 1999; Sir et al., 2000; Snipes et al., 1995). In agreement with these studies, αPMN effectively and specifically depleted PMN from the blood of rats for at least 4 days, followed by repopulation by day 6 post-infection (Fig. 1b). Importantly, αPMN serum did not reduce the numbers of other white blood cell types in RCoV-infected or uninfected animals (Figs 1b and S1, available in the online Supplementary Material). Thus, αPMN is an effective, specific tool for transient depletion of circulating PMN in rats.
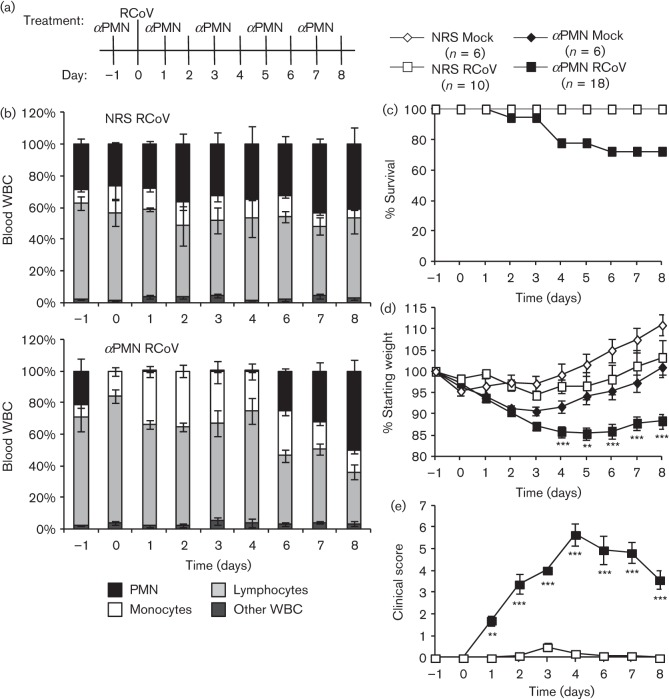
Fig. 1.
αPMN treatment depletes circulating PMN and increases disease severity during RCoV infection of rats. (a) Rats were injected with αPMN or normal rabbit serum (NRS) intraperitoneally 1 day prior to intranasal inoculation with RCoV or medium (mock), and every 48 h thereafter. (b) Blood was collected from 3–5 animals per group daily to monitor white blood cell populations. Rats were monitored for mortality (c), body weight (d) and clinical signs of disease (e). Data are means±se from 6–18 rats per treatment per day (see key). Statistically significant differences between αPMN- and NRS-treated rats were identified using one-way ANOVA followed by the Newman–Keuls post-test, **P<0.01; ***P<0.001.
PMN-depleted and NRS-treated animals were inoculated with RCoV and weighed and observed daily for clinical signs and mortality. In agreement with our previous study, RCoV infection of NRS-treated rats did not result in mortality (Funk et al., 2009). In contrast, treatment with αPMN resulted in 28 % mortality of RCoV-infected rats by day 6 (Fig. 1c). Of the 18 rats in the αPMN/RCoV group, one succumbed to infection on day 2 and four others were humanely euthanized due to excessive weight loss and severe disease. None of the mock-infected animals, either with or without αPMN treatment, died or required euthanasia during the course of the experiment. All of the treatment groups exhibited weight loss early in the study, and, except for the αPMN/RCoV group, steadily regained weight beginning on day 3 (Fig. 1d). In contrast, PMN-depleted rats that were infected with RCoV had steady weight loss through day 4, which remained low through day 8 (Fig. 1d). Clinical scores were calculated daily as described in Methods (Fig. 1e). NRS-treated rats infected with RCoV showed no or only minor clinical signs during infection. In contrast, RCoV infection of αPMN-treated rats resulted in multiple clinical signs, including hunched posture, ruffled fur, swollen face and neck, bloody ocular and nasal discharge and lethargy. Therefore, these rats had significantly increased mean clinical scores between days 1 and 8 post-infection (Fig. 1e). Surviving animals (72 %) had lower clinical scores after day 4, but did not return to complete health by day 8. The increased morbidity and mortality in rats treated with αPMN, which specifically depletes PMN from the bloodstream, suggests that PMN are needed for protection against severe disease during RCoV infection.
αPMN treatment reduces PMN recruitment and prolongs viral replication in the lungs
To confirm that treatment with αPMN inhibits recruitment of PMN to the respiratory tract, PMN were quantified in bronchoalveolar lavage fluid (BALF) on days 4, 8 and 12 post-infection. As expected from our previous study (Funk et al., 2009), PMN numbers increased in NRS/RCoV-treated rats by day 4, and declined to less than 5 % by day 8 (Fig. 2a). In rats treated with αPMN, PMN numbers in the BALF did not increase upon RCoV infection and remained low through day 12, despite their repopulation of the blood by day 6 (Fig. 1b). To determine whether PMN are needed for clearance of RCoV, viral titres from lung homogenates were compared in NRS- and αPMN-treated rats. Both groups had high levels of RCoV on day 4, which remained high in αPMN-treated rats through day 12 (Fig. 2b). In contrast, NRS-treated rats cleared the virus by day 8 post-infection. Thus, recruitment of PMN to the respiratory tract correlated with effective clearance of RCoV from the lungs.
Fig. 2.
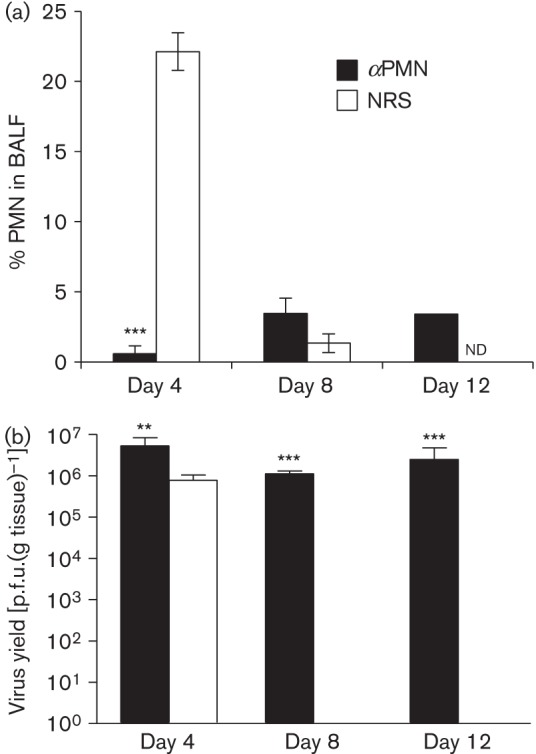
αPMN treatment reduces PMN recruitment and prolongs viral replication in the lungs. Rats were injected with αPMN or NRS intraperitoneally 1 day prior to intranasal inoculation with RCoV, and every 48 h thereafter (see Fig. 1a). (a) Cells from bronchoalveolar lavage fluid (BALF) were Giemsa–Wright stained and PMN were quantified morphologically. (b) Lung tissues were homogenized and viral titres were determined by plaque assay. Data are means±se from 3–5 rats per group. Statistically significant differences between αPMN- and NRS-treated rats were identified using an unpaired t-test, **P<0.01, ***P<0.001. nd, not determined.
PMN are needed early during RCoV infection to protect against disease
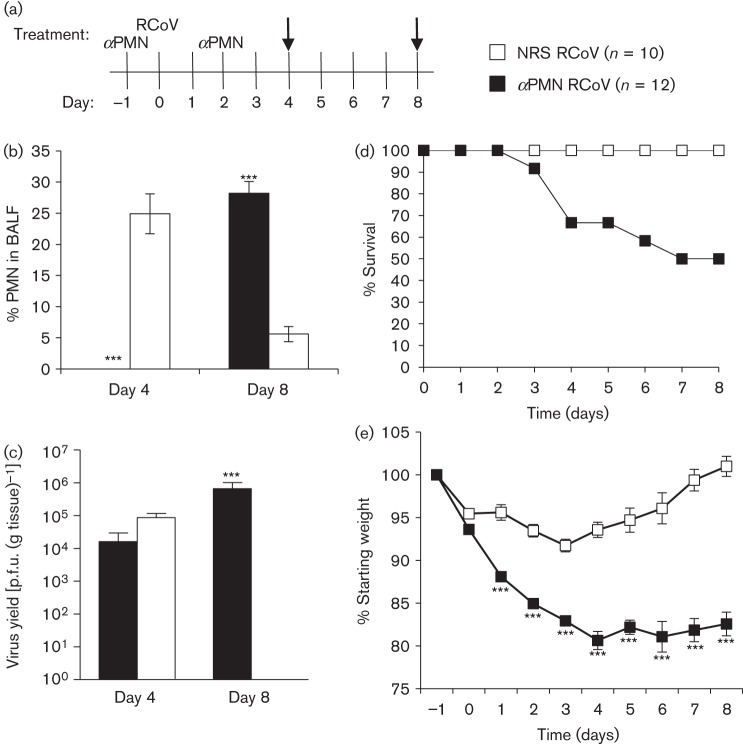
PMN are observed in the respiratory tract early after infection with RCoV (Funk et al., 2009), but it is not known whether their presence early during infection is important to later disease outcomes. To establish transient depletion of PMN early during RCoV infection, rats were treated with αPMN serum 1 day before and 2 days after RCoV inoculation (Fig. 3a). No PMN were detected in the BALF of αPMN-treated rats on day 4 post-infection, followed by recruitment of PMN to the respiratory tract by day 8 (Fig. 3b). Despite the influx of PMN into the airways, viral titres in the lungs remained high on day 8 (Fig. 3c), suggesting that the presence of PMN alone is not sufficient to clear virus late in infection. Transient PMN depletion resulted in 50 % mortality by day 8 after RCoV infection and significant weight loss compared with NRS-treated animals (Fig. 3d, e). NRS/RCoV rats initially lost weight, which they regained after day 3 (Fig. 3e). Of the six animals that died during the study, one succumbed to infection on day 3, and five were euthanized due to more than 20 % weight loss and severe disease. Clinical signs were apparent on days 2–8 post-infection and were identical to those seen in rats given αPMN throughout infection (data not shown). These findings demonstrate that delayed recruitment of PMN to the lungs cannot compensate for their absence early during RCoV infection.
Fig. 3.
PMN are needed early during RCoV infection to be protective. (a) Rats were injected with αPMN or NRS intraperitoneally 1 day prior to and 2 days after intranasal inoculation with RCoV and analysed on days 4 and 8 (arrows). (b) Cells from BALF of 3–5 rats per group were Giemsa–Wright stained and PMN were quantified morphologically. (c) Lungs from 3–6 rats per group were homogenized and viral titres were determined by plaque assay. The data are means±se. Rats (see key for numbers) were monitored for (d) survival (P = 0.046) and (e) weight loss. Statistically significant differences between αPMN- and NRS-treated rats were identified using (e) two-way ANOVA followed by a Bonferroni post-test or (b and c) unpaired t-test, ***P<0.001.
PMN promote pulmonary cellular infiltration during RCoV infection
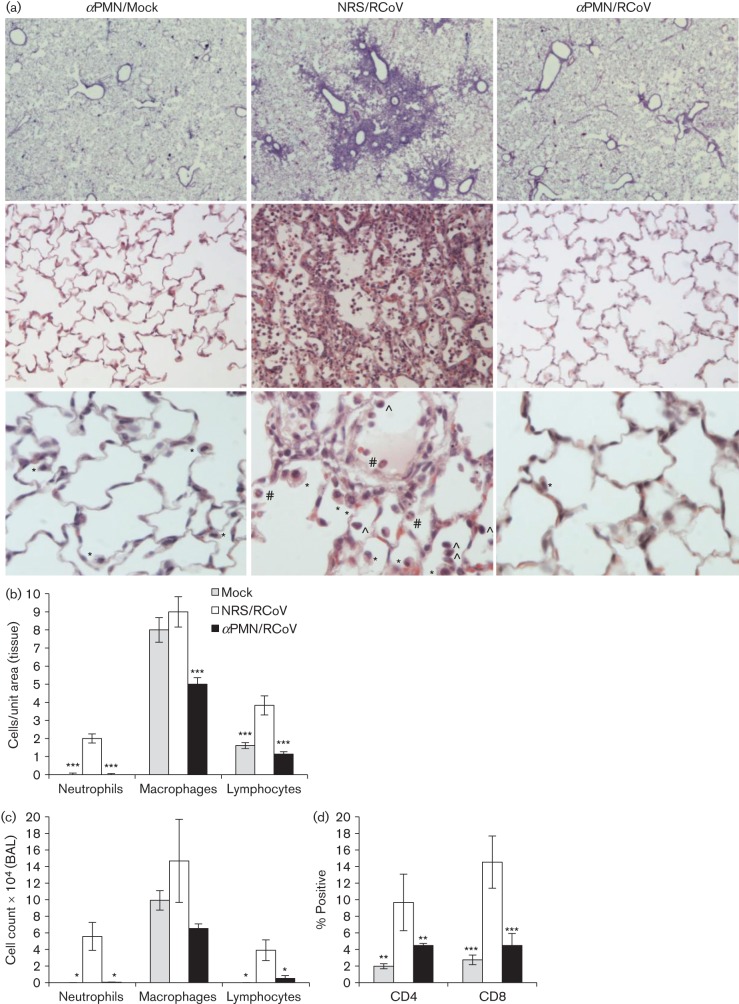
The results of transient PMN depletion suggested that PMN are needed early during RCoV infection to limit disease severity, and that later recruitment of PMN does not reduce viral titres. Therefore, we hypothesize that PMN have an indirect role in the effective response against RCoV infection. RCoV infection induces cellular infiltration into the alveolar spaces (Funk et al., 2009). To determine whether PMN are required for cellular inflammation, histological analysis of lung tissues was performed on PMN-depleted and NRS-treated rats during RCoV infection. Focal areas of pneumonitis with PMN, macrophages and lymphocytes were present in the lung sections from NRS-treated, but not αPMN-treated, animals on day 4 post-infection (Fig. 4a). Inflammatory lesions in the lungs of NRS-treated animals were mostly localized in areas surrounding the bronchioles (top panels). Quantitative analysis of density indices of PMN, macrophages and lymphocytes was performed on tissue sections from three animals per group. αPMN treatment significantly reduced the numbers of macrophages and lymphocytes in the lungs of RCoV-infected animals (Fig. 4b), corresponding to cell counts in BALF samples (Fig. 4c). To determine whether CD4- or CD8-positive lymphocytes were specifically reduced, these cells were quantified in BALF by flow cytometry. This analysis demonstrated a reduction in both CD4- and CD8-positive cells in the airways of αPMN-treated animals, compared with NRS-treated animals, upon RCoV infection (Fig. 4d). These data suggest that PMN are critical for the development of a cellular response to pulmonary RCoV infection.
Fig. 4.
PMN promote cellular inflammation in the lungs upon RCoV infection. Rats were injected with αPMN or NRS intraperitoneally 1 day prior to intranasal inoculation with RCoV, and every 48 h thereafter. On day 4 after RCoV infection, (a) lungs were formaldehyde-fixed and paraffin-embedded, and haematoxylin and eosin-stained sections were analysed for cellular inflammation. Representative tissues from three animals per group are shown at ×2 (top panels), ×20 (middle panels) and ×40 (bottom panels) magnification. Examples of cell types at ×40: neutrophil (#),macrophage (*) and lymphocyte (∧) are indicated. (b) Density indices of inflammatory cells were quantified in lung sections from three animals per group using ImageJ software. (c) White blood cells in BALF were quantified by Wright–Giemsa staining, and (d) lymphocyte subtypes by flow cytometry, using 4–6 animals per group. Statistically significant differences compared with NRS/RCoV-treated rats were identified using one-way ANOVA followed by the Newman–Keuls post-test, *P<0.05, **P<0.01, ***P<0.001.
αPMN-treated rats have reduced chemokine concentrations in BALF during RCoV infection
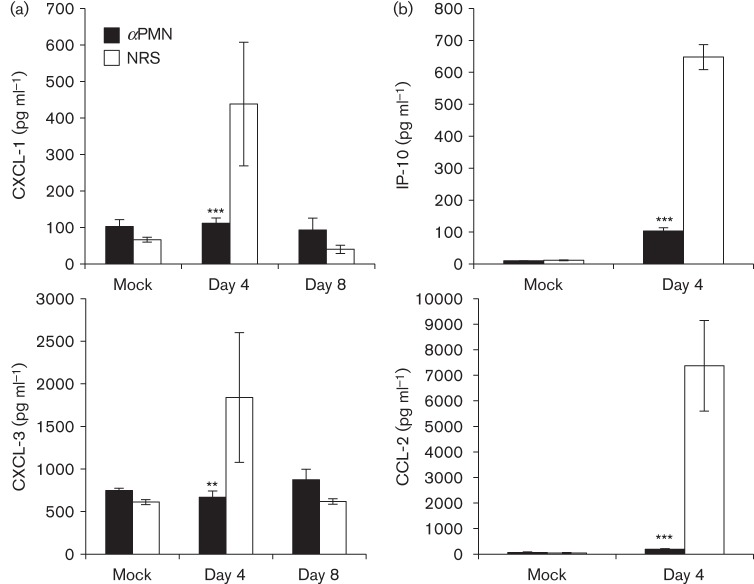
The histology data demonstrated that PMN promote pulmonary cellular infiltration during RCoV infection, and our previous studies showed RCoV-induced chemokine expression (Funk et al., 2009; Miura et al., 2007). To determine whether PMN contribute to this response, we quantified chemokines in the BALF of αPMN- and NRS-treated rats during RCoV infection. Compared with mock-inoculated animals, RCoV infection increased levels of PMN-specific chemokines (CXCL-1 and CXCL-3) in NRS-treated animals by day 4, which returned to mock levels by day 8 (Fig. 5a). In contrast, PMN-depleted rats had significantly reduced levels of CXCL-1 and CXCL-3 in the BALF, which corresponded to the lack of PMN recruitment to the lungs of depleted animals even after PMN had repopulated the blood (Figs 1b and 2a). Two additional chemokines that are induced by RCoV infection (Funk et al., 2009), interferon-inducible protein 10 (IP-10/CXCL-10) and monocyte chemoattractant protein 1 (MCP-1/CCL-2), were quantified in BALF from αPMN- and NRS-treated rats on day 4 post-infection (Fig. 5b). Both chemokines were induced by RCoV infection in NRS-treated, but not αPMN-treated rats, suggesting that PMN are needed for chemokine production.
Fig. 5.
αPMN-treated rats have reduced concentrations of chemokines in the airways during RCoV infection. ELISAs were used to quantify the concentrations of (a) PMN-specific (CXCL-1 and CXCL-3) and (b) monocyte- and lymphocyte-specific (IP-10 and CCL2) chemokines in BALF from αPMN- and NRS-treated rats after infection with RCoV or mock-inoculation. The data are means±se from 3–5 rats per treatment. Statistically significant differences between αPMN- and NRS-treated rats were identified using one-way ANOVA followed by the Newman–Keuls post-test, **P<0.01, ***P<0.001.
Proinflammatory response of PMN to RCoV-infected alveolar epithelial cells in vitro
Based on the data above, we hypothesize that PMN recruited to the airways of RCoV-infected rats produce cytokines and chemokines, including CXCL-1, CXCL-3, IP-10 and CCL-2. Type I alveolar epithelial (AT1) cells are the primary cell type infected by RCoV within the distal lung (Funk et al., 2009). Furthermore, RCoV-infected AT1-like cells direct PMN functions in vitro (Rzepka et al., 2012). To determine whether RCoV-infected AT1 cells direct expression of cytokines and chemokines by PMN, we incubated PMN isolated from rat bone marrow in conditioned medium from RCoV-infected (RCoV-AT1) or mock-infected (mock-AT1) AT1-like cells. The mRNA levels of 84 cytokines and chemokines were measured from PMN using quantitative RT-PCR arrays (Table 1). PMN that were incubated in RCoV-AT1 medium had higher mRNA levels of proinflammatory cytokines (IL-18, IL-1α, IL-1β and TNF-α), CXC chemokines (CXCL-1, CXCL-2, IP-10 and CXCL-11) and CC chemokines (CCL-2, CCL-4, CCL-7, CCL-9, CCL-12 and CCL-22) in comparison with PMN incubated in mock-AT1 medium. These findings demonstrated that PMN express proinflammatory cytokines and chemokines when exposed to RCoV-infected epithelial cells. This is in agreement with the reduced concentrations of chemokines in the BALF and cellular infiltration in the lungs of rats treated with αPMN antibody compared with NRS-treated rats, during RCoV infection.
Table 1. Cytokine and chemokine mRNAs with increased abundance in PMN exposed to medium from RCoV-infected, compared to mock-infected AT1 cells in vitro.
PMN were isolated from rat bone marrow and incubated for 4 h in conditioned medium from RCoV-infected or mock-inoculated AT1-like cells. RNA was isolated from the PMN and analysed using quantitative RT-PCR arrays specific for proinflammatory cytokines and chemokines.
| Cytokine/chemokine* | Fold-induction of mRNA† | ||
| Experiment 1 | Experiment 2 | Experiment 3 | |
| CCL-2 | 18.0 | 19.9 | 1.8 |
| CCL-4 | 5.5 | 4.0 | 2.7 |
| CCL-7 | 73.3 | 40.4 | 11.1 |
| CCL-9 | 7.9 | 8.3 | 4.5 |
| CCL-12 | 10.1 | 1.4 | 94.9 |
| CCL-22 | 5.8 | 16.5 | 3.4 |
| CXCL-1 | 39.9 | 19.6 | 10.3 |
| IP-10 | 4.9 | 17.2 | 3.1 |
| CXCL-11 | 35.1 | 159.3 | 17.4 |
| CXCL-2 | 13.8 | 23.2 | 8.7 |
| CX3CL-1 | 4.5 | 2.3 | 1.9 |
| IL-18 | 1.8 | 2.3 | 2.6 |
| IL-1α | 345.0 | 190.5 | 10.3 |
| IL-1β | 5.0 | 13.1 | 3.5 |
| TNF-α | 13.5 | 9.8 | 7.8 |
Cytokine and chemokine mRNAs that were differentially expressed in at least two of three independent experiments.
Values are fold-difference in PMN incubated in medium from RCoV-infected compared to mock-inoculated AT1-like cells.
The presence of PMN in the lungs is associated with tissue damage
Haemorrhagic lesions are observed on the surface of the lungs following the same kinetics as PMN recruitment during RCoV infection (Funk et al., 2009). Therefore, we determined whether the presence of PMN correlated with visible lesions on the surface of rat lungs. RCoV infection of NRS-treated rats resulted in gross pulmonary lesions in all animals that were analysed on day 4, and the majority of NRS-treated animals did not have lesions on day 8 (Table 2). The presence of lesions on the lungs of NRS-treated animals corresponded to increased numbers of PMN in the airways (Table 2). In contrast, none of the rats treated with αPMN throughout infection had visible lesions, which corresponded to the absence of PMN in the BALF. Likewise, in rats that received αPMN only early during infection (through day 2), no lesions were visible on the lungs on day 4, when PMN were also not detected in the BALF. Late influx of PMN to the respiratory tracts of transiently depleted rats by day 8 corresponded to the presence of pulmonary lesions in five of the six animals evaluated. In addition to surface lesions, we measured total protein in the BALF of rats as an indicator of damage to the epithelial barrier. When PMN were present in the BALF, there was a corresponding increase in protein concentration (Table 2). Furthermore, animals that had low numbers of PMN also had low total protein concentrations in the BALF. Collectively, these findings implicate PMN in causing tissue injury including gross haemorrhagic lesions and epithelial permeability.
Table 2. The presence of PMN in the lungs of RCoV-infected rats corresponds to tissue damage.
Rats were treated with αPMN or NRS throughout (Fig. 1a) or early (Fig. 3a) during infection with RCoV. Cells from BALF were Wright–Giemsa stained and quantified morphologically. The number of rats with gross lesions on the lungs was recorded. Total protein in BALF was quantified by Bradford assay.
| Treatment | % PMN in BALF* | Rats with gross lung lesions positive/total† | BALF protein* (mg ml−1) |
| NRS mock | 0.0 (0.0) | 0/6 | 0.8 (0.1) |
| NRS RCoV day 4 | 22.1 (1.3)*** | 5/5 (P = 0.0011) | 16.1 (3.3)** |
| NRS RCoV day 8 | 1.3 (0.7) | 1/5 | 1.8 (1.3) |
| αPMN mock | 2.3 (2.0) | 0/6 | 0.9 (0.3) |
| αPMN RCoV day 4 | 0.6 (0.6) | 0/5 | 0.5 (0.2) |
| αPMN RCoV day 8 | 3.5 (1.1) | 0/5 | 2.3 (0.9) |
| Early NRS mock | 6.8 (5.8) | 0/6 | 0.3 (0.2) |
| Early NRS RCoV day 4 | 24.9 (3.2)** | 4/5 (P = 0.0094) | 4.7 (0.7)*** |
| Early NRS RCoV day 8 | 5.6 (1.2) | 1/6 | 1.1 (0.2) |
| Early αPMN mock | 2.3 (0.6) | 0/4 | 2.8 (1.0) |
| Early αPMN RCoV day 4 | 0.0 (0.0) | 0/5 | 1.3 (0.3) |
| Early αPMN RCoV day 8 | 28.2 (1.9)*** | 5/6 (P = 0.0126) | 8.3 (1.6)** |
*Values are means (se) of 4–6 rats per group. Statistically significant differences compared with mock for each group as determined by one-way ANOVA with Newman–Keuls post-test; **P<0.005, ***P<0.0001.
(P values) are given for groups that differ significantly from the mock for each group as determined by a likelihood ratio test.
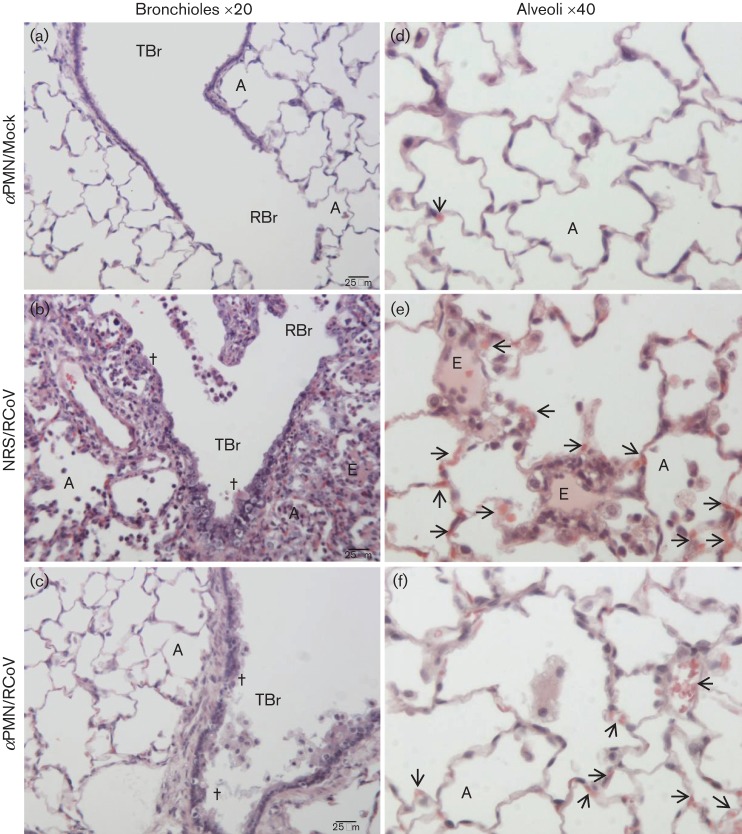
Histological analysis was performed to compare lung pathology in PMN-depleted and NRS-treated rats during RCoV infection. Corresponding with focal areas of pneumonitis (Fig. 4a), NRS/RCoV rats had severe bronchiolar and peribronchiolar inflammation, necrosis and epithelial sloughing (Fig. 6b), compared with mock-inoculated rats (Fig. 6a). Some alveoli of NRS/RCoV rats contained transudate fluid, dead cells and inflammatory cells (Fig. 6e), compared with the clear alveoli of uninfected rats (Fig. 6d). Interestingly, most of this inflammation was resolved by day 8 (Fig. S2), with mainly foamy macrophages present in the alveoli. Although inflammatory cells were absent in αPMN-treated rats on day 4 post-infection, these animals had sloughing of dead epithelial cells in the bronchioles (Fig. 6c) and engorged alveolar capillaries (Fig. 6f). In summary, pulmonary RCoV infection resulted in strikingly different histopathology in NRS vs. αPMN treated rats. PMN-dependent responses were associated with focal pneumonitis, epithelial necrosis, oedema and vascular pathology, while the lack of PMN was associated with epithelial and vascular pathology.
Fig. 6.
Differential pathology in PMN-depleted vs. NRS-treated rats during RCoV infection. Images of representative lung sections from three animals per treatment: (a, d) αPMN-treated/mock-inoculated, (b, e) NRS-treated/RCoV-infected and (c, f) αPMN-treated/RCoV-infected showing histopathology on day 4 post-infection. αPMN/mock rats had normal terminal (TBr) and respiratory (RBr) bronchioles and alveoli (A). Lungs of NRS/RCoV rats had peribronchiolar inflammation with sloughing of bronchiolar epithelium (†) and alveoli (A) filled with inflammatory cells and necrotic cell debris. Transudate-filled alveoli indicating alveolar oedema (E) were diffusely distributed in the lesions and congested capillaries (arrows) were found throughout the sections. αPMN/RCoV rats had mild pathology with sloughing of bronchiolar epithelium (†) and congested capillaries (arrows). Bars, 25 µm.
Discussion
The activities of PMN during respiratory viral infections are complex and often dichotomous: contributing to both beneficial antiviral responses and detrimental pathology. We depleted PMN from rats during infection with a non-fatal respiratory coronavirus, RCoV, to determine their contributions to an effective antiviral response. In contrast to NRS-treated rats, rats that were treated with αPMN throughout or early during RCoV infection had increased mortality and morbidity and prolonged pulmonary viral replication. Further, PMN were required for the production of chemokines in the airways and infiltration of macrophages and lymphocytes into the lungs. These findings suggest that PMN are needed early during infection to elicit an effective cellular response to control viral replication and attenuate disease severity. Despite an effective response against RCoV, NRS-treated rats had pulmonary lesions, characterized by capillary congestion, haemorrhage, oedema and epithelial permeability. Taken together, these findings highlight the dichotomous roles of PMN by contributing to effective antiviral responses, but also in mediating tissue pathology.
A rabbit anti-rat PMN antibody was used to deplete PMN in vivo without significantly affecting circulating monocytes and lymphocytes. This antibody is widely used to deplete PMN in rats but most studies do not report the effects on other cell populations (Janardhan et al., 2006; Li et al., 2007; Ofulue & Ko, 1999; Sir et al., 2000). Snipes et al. observed complete depletion of PMN and transiently reduced lymphocyte numbers in the blood of rats using this antibody (Snipes et al., 1995). However, their study and others have demonstrated that αPMN does not reduce viability of other white blood cell types in vitro at the same concentration that inactivates PMN (Ofulue & Ko, 1999; Snipes et al., 1995). We did not observe reduced lymphocyte numbers in αPMN-treated rats, but we used a lower dose of αPMN that was repeated every other day compared with a higher dose given once in the Snipes et al. study. In addition, differences in the antibody lots and genetic lines and ages of the rats may be responsible for the differences among studies. αPMN treatment alone resulted in early weight loss that was not statistically significant compared with NRS-treated rats. This was distinct from the prolonged, significant weight loss observed in the RCoV-infected αPMN-treated rats. RCoV infection of PMN-depleted rats resulted in clinical signs consistent with infection by this viral strain, which corresponded to prolonged viral replication. However, we cannot exclude a role for potential secondary infections in exacerbating these findings.
Our study indicates that PMN are needed for effective clearance of pulmonary RCoV infection. PMN may have direct antiviral functions, including phagocytosis of viruses and virus-infected cells (Fujisawa, 2008; Hartshorn et al., 1994; Hashimoto et al., 2007; Tecle et al., 2007; West et al., 1987). PMN may also contribute to protection by recruitment and activation of other immune cell types (Beauvillain et al., 2007; Radsak et al., 2000; Scapini et al., 2000). In previous studies, we showed that RCoV infects AT1 cells in the lungs and induces expression of chemokines that activate functional responses of PMN (Funk et al., 2009; Miura et al., 2007; Rzepka et al., 2012). Here, we show that PMN responded to medium from RCoV-infected AT1-like cells in vitro by increasing mRNA levels of proinflammatory cytokines and chemokines. Furthermore, depletion of PMN in vivo resulted in reduced concentrations of chemokines in the airways of RCoV-infected rats. Our in vivo findings suggest that PMN are a source of chemokines, including IP-10, CCL-2, CXCL-1 and CXCL-3, in the airways during RCoV infection. It is also possible that PMN induce other cell types to produce these chemokines. CXCL-1 and CXCL-3 recruit and activate PMN (Rzepka et al., 2012; Shibata et al., 1995). The decrease in these chemokines may explain the lack of PMN recruitment to the lungs after their numbers have returned to normal in the blood. CCL-2 mediates chemotaxis and activation of macrophages. IP-10 recruits monocytes and lymphocytes and is critical for the recruitment of both CD4+ and CD8+ T cells in response to neurotropic murine coronavirus infection (Liu et al., 2000). Histopathological and BALF analyses demonstrated robust cellular inflammation in the lungs and airways of RCoV-infected rats, which was dramatically absent in PMN-depleted rats. We specifically found reduced numbers of CD4+ and CD8+ cells in BALF from PMN-depleted animals. The numbers of circulating lymphocytes were not altered directly by αPMN treatment. Therefore, we hypothesize that the reduced numbers of lymphocytes in the respiratory tract of αPMN-treated rats was due to the absence of PMN. Others have shown that T cells are essential for effective clearance of RCoV (Weir et al., 1990). Our data support a role for PMN in recruitment of T cells to the respiratory tract, which may be responsible for viral clearance.
In addition to providing protection from RCoV-mediated disease, we found that the presence of PMN in the lungs was associated with histopathology, haemorrhagic lesions and increased epithelial permeability. While it seems contradictory that the animals with overt histopathology and lesions did not have severe clinical signs or mortality, this has been observed in other studies (Funk et al., 2009; Wojcinski & Percy, 1986). A comprehensive study using the same RCoV strain (sialodacryoadentitis virus) found significant lesions in the lower respiratory tract, including gross lesions on the lungs and interstitial pneumonitis with PMN, epithelial necrosis, occluded alveoli and oedema (Wojcinski & Percy, 1986). Despite the dramatic macroscopic and microscopic pulmonary lesions, they only observed mild clinical signs. Like our findings, the lesions observed were focal in nature and were completely resolved by day 12 post-infection. Thus, healthy rats mount an inflammatory response to RCoV infection, associated with focal, transient lesions in the lungs, which effectively limits disease severity. PMN, macrophages and lymphocytes are all present in these lesions and their individual roles have not been deciphered.
Experimental depletion of PMN in other coronavirus infection models has resulted in a range of outcomes, which may reflect their complex roles in both viral clearance and immunopathology. Infection of PMN-depleted mice with a neurotropic murine coronavirus, JHMV, causes increased viral titres in the brain and a more rapid decline to death compared with mice with PMN (Zhou et al., 2003). Furthermore, when a CXCR2-specific antibody is used to prevent PMN recruitment to the brain during JHMV infection, mortality is increased with corresponding increases in viral titres in the brain (Hosking et al., 2009). These studies suggest that PMN contribute to protection against JHMV replication and disease. In contrast, PMN depletion during infection with a recombinant JHM virus (RJHM) that induces robust recruitment of PMN to the brain, resulted in a slightly delayed time to death and reduced apoptosis in the brains of infected mice (Iacono et al., 2006), suggesting that PMN contribute to pathology during RJHM infection. Finally, depletion of PMN in interferon alpha receptor knockout (IFNAR−/−) mice, which are highly susceptible to infection by an attenuated strain of JHMV, does not affect viral replication or disease severity (Ireland et al., 2008). Like<1?show=[fo]?>wise, PMN depletion in mice infected with a hepatotropic murine coronavirus, MHV-A59, does not alter disease pathology (Cervantes-Barragán et al., 2009). The contrasting roles of PMN during murine coronavirus infections are likely dependent upon the age and strain of mice and the relative virulence of the particular virus strains being studied. These parameters, which vary among the studies, may alter the balance between the protective and pathogenic functions of PMN.
Studies to determine the role of PMN in the pathogenesis of influenza virus infections have also generated disparate conclusions depending upon the dose and strain of virus, genetic line of mice and specificity of the depletion antibody for PMN. PMN depletion in mouse models of highly virulent influenza infections results in decreased disease severity, suggesting a role for PMN in pathogenesis (Bradley et al., 2012; Crowe et al., 2009; Sakai et al., 2000). In contrast, other studies have found that depletion of PMN results in increased disease severity during influenza infection in mice, suggesting a protective role for PMN (Dienz et al., 2012; Fujisawa, 2008; Tate et al., 2008, 2009, 2011, 2012; Tumpey et al., 2005). These studies further reflect the complex, dichotomous functions of PMN during respiratory viral infections.
Many respiratory viruses cause significant morbidity without mortality in immunocompetent adults. Animal models that emulate non-fatal viral replication and pathology in the respiratory tract are critical to determine the components of immunity that protect against severe disease. RCoV infection of adult rats provides a valuable model for elucidating the mechanisms of effective immune responses to a pulmonary coronavirus infection in the natural host of the virus. Our findings demonstrate that although PMN are needed for effective resolution of RCoV infection, they contribute to lung pathology. A clear understanding of the interplay between beneficial and detrimental functions of PMN will lead to novel therapeutic strategies to reduce morbidity during respiratory viral infections.
Methods
Viruses and cell lines.
RCoV strain sialodacryoadenitis virus was propagated and titrated by plaque assay in L2P41.a cells (Gagneten et al., 1996) as described previously (Miura et al., 2007). Virus and cell stocks were obtained from Dr Kathryn Holmes (University of Colorado Denver, Aurora, CO, USA).
PMN depletion and RCoV infection.
Experiments were performed according to protocols approved by the University of Idaho Institutional Animal Care and Use Committee, following the Guide for the Care and Use of Laboratory Animals. 8-week-old male Fisher 344 rats (Harlan Laboratories) were used for infections. A pilot study was performed to determine the volume of rabbit αPMN serum (Cedarlane) that effectively depleted circulating PMN without affecting monocyte and lymphocyte numbers. To deplete PMN throughout RCoV infection, rats were injected intraperitoneally with 300 µl of αPMN or NRS 1 day prior to infection and every 48 h thereafter (Fig. 1a). Six animals for each of the mock groups (NRS/mock and αPMN/mock), 10 NRS/RCoV- and 18 αPMN/RCoV-treated rats were monitored for morbidity and mortality and surviving animals were euthanized on day 8 or 12 for tissue analyses. An additional six rats were included in the RCoV-infected groups (NRS/RCoV and αPMN/RCoV) and harvested on day 4. To obtain transient PMN depletion, rats were injected with serum on days −1 and +2 with respect to the infection (Fig. 3a). For transient depletion, 10 NRS/RCoV and 12 αPMN/RCoV rats were monitored for morbidity and mortality and the survivors were used for assays on day 8. An additional five rats per group were harvested on day 4. On day 0, rats were anaesthetized with 80 mg ketamine ml−1 and 12 mg xylazine ml−1 and inoculated intranasally with 200 µl of RCoV (4–5×105 p.f.u.) or supernatant medium from mock-inoculated L2P-41a cells. Animals were weighed daily and those that lost more than 20 % of their initial body weight were euthanized by an overdose of sodium pentobarbital, followed by exsanguination. Rats were monitored daily for clinical signs, including: 1) eye and nasal discharge, 2) lethargy, 3) sneezing or coughing, 4) ruffled fur, 5) hunched posture, 6) laboured breathing, 7) visible swelling around the face and neck, 8) porphyrin-stained eye secretions, 9) shaking and 10) death. Based on the severity of these clinical signs, rats were scored on a scale of 0–8. Healthy rats with no clinical signs received 0 points. Rats with a combination of minor clinical signs received 1–3 points, those with more severe clinical signs received 4–7 points, and dead animals received a clinical score of 8.
Blood and BALF analyses.
BALF was collected from euthanized rats by flushing the lungs with 10 ml of saline 2–3 times. Cytospin preparations of BALF cells and blood smears were differentially stained with a HEMA3 staining kit (Fisher Diagnostics). Cell-free BALF was used for quantification of protein by Bradford assay (Bio-Rad Laboratories) and chemokines by ELISAs (R&D Systems, Thermo Scientific, Lifespan Biosciences).
Analysis of cytokine gene expression by PMN in vitro.
Bone marrow PMN were isolated from uninfected rats as previously described (Rzepka et al., 2012). Type 2 alveolar epithelial cells were isolated and trans-differentiated in vitro to AT1-like cells (Miura et al., 2007; Rzepka et al., 2012). AT1-like cells were infected with RCoV for 24 h and supernatant medium was collected (RCoV-AT1) and co-cultured with freshly purified PMN for 4 h. RNA was isolated from PMN and mRNA levels were quantified using Rat Inflammatory Cytokines and Receptors RT2 Profiler Arrays (SABiosciences/Qiagen).
Lung virus titration.
Lung tissues were weighed and homogenized in Dulbecco’s Modified Eagle Medium with 50 % FBS. Plaque assay in L2P41.a cells was performed to quantify the p.f.u. g−1 of lung tissue (Miura et al., 2007).
Histopathology.
Lungs were fixed in 4 % formaldehyde, dehydrated, embedded in paraffin and sectioned. Embedding and sectioning were performed by Washington Animal Disease Diagnostic Laboratory at Washington State University (Pullman, WA). Tissue sections were deparaffinized, stained with haematoxylin and eosin (Sigma-Aldrich) and pathology was analysed by a blinded pathologist (O.B.B.). Tissue sections were imaged using a Leica microscope equipped with a Nikon DS2 digital camera. Systematic uniform random sampling was performed on representative lung sections from three animals per group to photograph (at ×20 magnification; 30–80 images per section) and count inflammatory cells per tissue area. ImageJ software (NIH) was used for cell quantification and measurement of numerical density (cells per unit area).
Flow cytometry.
Cells from BALF were incubated with FITC-conjugated CD4, CD8 or isotype control antibodies (Biolegend). Ten thousand events per sample were collected using FACS-Aria (Becton Dickinson), and data were analysed using FlowJo version 7.6.5. (Tree Star). CD4+ and CD8+ T cells were gated from macrophages by side scatter profiles.
Statistical analysis.
Statistical analyses were performed using GraphPad Prism, version 5.00 (GraphPad Software).
Acknowledgements
This work was supported by a Career Development Award from the Pacific Northwest Regional Center of Excellence (NIH/NIAID, grant U54 AI081680); by the National Center for Research Resources (NCRR), a component of the NIH, grant P20 RR015587; and by the INBRE Program of the NCRR, grant P20 RR016454. The authors would like to thank Dr Kathryn Holmes, University of Colorado Denver, for cell lines and virus stocks, Ms Ann Norton for assistance with microscopy and Dr Craig Miller for assistance with analysis.
Footnotes
Two supplementary figures are available with the online version of this paper.
References
- Assiri A., Al-Tawfiq J. A., Al-Rabeeah A. A., Al-Rabiah F. A., Al-Hajjar S., Al-Barrak A., Flemban H., Al-Nassir W. N., Balkhy H. H. & other authors (2013). Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis 13, 752–761 10.1016/S1473-3099(13)70204-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauvillain C., Delneste Y., Scotet M., Peres A., Gascan H., Guermonprez P., Barnaba V., Jeannin P. (2007). Neutrophils efficiently cross-prime naive T cells in vivo. Blood 110, 2965–2973 10.1182/blood-2006-12-063826 [DOI] [PubMed] [Google Scholar]
- Bradley L. M., Douglass M. F., Chatterjee D., Akira S., Baaten B. J. (2012). Matrix metalloprotease 9 mediates neutrophil migration into the airways in response to influenza virus-induced toll-like receptor signaling. PLoS Pathog 8, e1002641 10.1371/journal.ppat.1002641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervantes-Barragán L., Kalinke U., Züst R., König M., Reizis B., López-Macías C., Thiel V., Ludewig B. (2009). Type I IFN-mediated protection of macrophages and dendritic cells secures control of murine coronavirus infection. J Immunol 182, 1099–1106 [DOI] [PubMed] [Google Scholar]
- Crowe C. R., Chen K., Pociask D. A., Alcorn J. F., Krivich C., Enelow R. I., Ross T. M., Witztum J. L., Kolls J. K. (2009). Critical role of IL-17RA in immunopathology of influenza infection. J Immunol 183, 5301–5310 10.4049/jimmunol.0900995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denlinger L. C., Sorkness R. L., Lee W. M., Evans M. D., Wolff M. J., Mathur S. K., Crisafi G. M., Gaworski K. L., Pappas T. E. & other authors (2011). Lower airway rhinovirus burden and the seasonal risk of asthma exacerbation. Am J Respir Crit Care Med 184, 1007–1014 10.1164/rccm.201103-0585OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienz O., Rud J. G., Eaton S. M., Lanthier P. A., Burg E., Drew A., Bunn J., Suratt B. T., Haynes L., Rincon M. (2012). Essential role of IL-6 in protection against H1N1 influenza virus by promoting neutrophil survival in the lung. Mucosal Immunol 5, 258–266 10.1038/mi.2012.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa H. (2008). Neutrophils play an essential role in cooperation with antibody in both protection against and recovery from pulmonary infection with influenza virus in mice. J Virol 82, 2772–2783 10.1128/JVI.01210-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk C. J., Manzer R., Miura T. A., Groshong S. D., Ito Y., Travanty E. A., Leete J., Holmes K. V., Mason R. J. (2009). Rat respiratory coronavirus infection: replication in airway and alveolar epithelial cells and the innate immune response. J Gen Virol 90, 2956–2964 10.1099/vir.0.014282-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagneten S., Scanga C. A., Dveksler G. S., Beauchemin N., Percy D., Holmes K. V. (1996). Attachment glycoproteins and receptor specificity of rat coronaviruses. Lab Anim Sci 46, 159–166 [PubMed] [Google Scholar]
- Gaunt E. R., Hardie A., Claas E. C., Simmonds P., Templeton K. E. (2010). Epidemiology and clinical presentations of the four human coronaviruses 229E, HKU1, NL63, and OC43 detected over 3 years using a novel multiplex real-time PCR method. J Clin Microbiol 48, 2940–2947 10.1128/JCM.00636-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartshorn K. L., Crouch E. C., White M. R., Eggleton P., Tauber A. I., Chang D., Sastry K. (1994). Evidence for a protective role of pulmonary surfactant protein D (SP-D) against influenza A viruses. J Clin Invest 94, 311–319 10.1172/JCI117323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto Y., Moki T., Takizawa T., Shiratsuchi A., Nakanishi Y. (2007). Evidence for phagocytosis of influenza virus-infected, apoptotic cells by neutrophils and macrophages in mice. J Immunol 178, 2448–2457 [DOI] [PubMed] [Google Scholar]
- Hosking M. P., Liu L., Ransohoff R. M., Lane T. E. (2009). A protective role for ELR+ chemokines during acute viral encephalomyelitis. PLoS Pathog 5, e1000648 10.1371/journal.ppat.1000648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacono K. T., Kazi L., Weiss S. R. (2006). Both spike and background genes contribute to murine coronavirus neurovirulence. J Virol 80, 6834–6843 10.1128/JVI.00432-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireland D. D., Stohlman S. A., Hinton D. R., Atkinson R., Bergmann C. C. (2008). Type I interferons are essential in controlling neurotropic coronavirus infection irrespective of functional CD8 T cells. J Virol 82, 300–310 10.1128/JVI.01794-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janardhan K. S., Sandhu S. K., Singh B. (2006). Neutrophil depletion inhibits early and late monocyte/macrophage increase in lung inflammation. Front Biosci 11, 1569–1576 10.2741/1904 [DOI] [PubMed] [Google Scholar]
- Khanolkar A., Hartwig S. M., Haag B. A., Meyerholz D. K., Harty J. T., Varga S. M. (2009). Toll-like receptor 4 deficiency increases disease and mortality after mouse hepatitis virus type 1 infection of susceptible C3H mice. J Virol 83, 8946–8956 10.1128/JVI.01857-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N., Hui D., Wu A., Chan P., Cameron P., Joynt G. M., Ahuja A., Yung M. Y., Leung C. B. & other authors (2003). A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med 348, 1986–1994 10.1056/NEJMoa030685 [DOI] [PubMed] [Google Scholar]
- Leong H. N., Earnest A., Lim H. H., Chin C. F., Tan C. S., Puhaindran M. E., Tan A. C., Chen M. I., Leo Y. S. (2006). SARS in Singapore–predictors of disease severity. Ann Acad Med Singapore 35, 326–331 [PubMed] [Google Scholar]
- Li X., Kovacs E. J., Schwacha M. G., Chaudry I. H., Choudhry M. A. (2007). Acute alcohol intoxication increases interleukin-18-mediated neutrophil infiltration and lung inflammation following burn injury in rats. Am J Physiol Lung Cell Mol Physiol 292, L1193–L1201 10.1152/ajplung.00408.2006 [DOI] [PubMed] [Google Scholar]
- Liu M. T., Chen B. P., Oertel P., Buchmeier M. J., Armstrong D., Hamilton T. A., Lane T. E. (2000). The T cell chemoattractant IFN-inducible protein 10 is essential in host defense against viral-induced neurologic disease. J Immunol 165, 2327–2330 [DOI] [PubMed] [Google Scholar]
- Mantovani A., Cassatella M. A., Costantini C., Jaillon S. (2011). Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol 11, 519–531 10.1038/nri3024 [DOI] [PubMed] [Google Scholar]
- Mckean M. C., Hewitt C., Lambert P. C., Myint S., Silverman M. (2003). An adult model of exclusive viral wheeze: inflammation in the upper and lower respiratory tracts. Clin Exp Allergy 33, 912–920 10.1046/j.1365-2222.2003.01715.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura T. A., Wang J., Holmes K. V., Mason R. J. (2007). Rat coronaviruses infect rat alveolar type I epithelial cells and induce expression of CXC chemokines. Virology 369, 288–298 10.1016/j.virol.2007.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata N., Iwata N., Hasegawa H., Fukushi S., Harashima A., Sato Y., Saijo M., Taguchi F., Morikawa S., Sata T. (2008). Mouse-passaged severe acute respiratory syndrome-associated coronavirus leads to lethal pulmonary edema and diffuse alveolar damage in adult but not young mice. Am J Pathol 172, 1625–1637 10.2353/ajpath.2008.071060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofulue A. F., Ko M. (1999). Effects of depletion of neutrophils or macrophages on development of cigarette smoke-induced emphysema. Am J Physiol 277, L97–L105 [DOI] [PubMed] [Google Scholar]
- Radsak M., Iking-Konert C., Stegmaier S., Andrassy K., Hänsch G. M. (2000). Polymorphonuclear neutrophils as accessory cells for T-cell activation: major histocompatibility complex class II restricted antigen-dependent induction of T-cell proliferation. Immunology 101, 521–530 10.1046/j.1365-2567.2000.00140.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rzepka J. P., Haick A. K., Miura T. A. (2012). Virus-infected alveolar epithelial cells direct neutrophil chemotaxis and inhibit their apoptosis. Am J Respir Cell Mol Biol 46, 833–841 10.1165/rcmb.2011-0230OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai S., Kawamata H., Mantani N., Kogure T., Shimada Y., Terasawa K., Sakai T., Imanishi N., Ochiai H. (2000). Therapeutic effect of anti-macrophage inflammatory protein 2 antibody on influenza virus-induced pneumonia in mice. J Virol 74, 2472–2476 10.1128/JVI.74.5.2472-2476.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scapini P., Lapinet-Vera J. A., Gasperini S., Calzetti F., Bazzoni F., Cassatella M. A. (2000). The neutrophil as a cellular source of chemokines. Immunol Rev 177, 195–203 10.1034/j.1600-065X.2000.17706.x [DOI] [PubMed] [Google Scholar]
- Shibata F., Konishi K., Kato H., Komorita N., al-Mokdad M., Fujioka M., Nakagawa H. (1995). Recombinant production and biological properties of rat cytokine-induced neutrophil chemoattractants, GRO/CINC-2 alpha, CINC-2 beta and CINC-3. Eur J Biochem 231, 306–311 10.1111/j.1432-1033.1995.tb20701.x [DOI] [PubMed] [Google Scholar]
- Sir O., Fazal N., Choudhry M. A., Gamelli R. L., Sayeed M. M. (2000). Neutrophil depletion prevents intestinal mucosal permeability alterations in burn-injured rats. Am J Physiol Regul Integr Comp Physiol 278, R1224–R1231 [DOI] [PubMed] [Google Scholar]
- Snipes M. B., Barnett A. L., Harkema J. R., Hotchkiss J. A., Rebar A. H., Reddick L. J. (1995). Specific biological effects of an anti-rat PMN antiserum intraperitoneally infected into f344/n rats. Vet Clin Pathol 24, 11–17 10.1111/j.1939-165X.1995.tb00928.x [DOI] [PubMed] [Google Scholar]
- Tate M. D., Brooks A. G., Reading P. C. (2008). The role of neutrophils in the upper and lower respiratory tract during influenza virus infection of mice. Respir Res 9, 57 10.1186/1465-9921-9-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate M. D., Deng Y. M., Jones J. E., Anderson G. P., Brooks A. G., Reading P. C. (2009). Neutrophils ameliorate lung injury and the development of severe disease during influenza infection. J Immunol 183, 7441–7450 10.4049/jimmunol.0902497 [DOI] [PubMed] [Google Scholar]
- Tate M. D., Ioannidis L. J., Croker B., Brown L. E., Brooks A. G., Reading P. C. (2011). The role of neutrophils during mild and severe influenza virus infections of mice. PLoS ONE 6, e17618 10.1371/journal.pone.0017618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate M. D., Brooks A. G., Reading P. C., Mintern J. D. (2012). Neutrophils sustain effective CD8(+) T-cell responses in the respiratory tract following influenza infection. Immunol Cell Biol 90, 197–205 10.1038/icb.2011.26 [DOI] [PubMed] [Google Scholar]
- Tecle T., White M. R., Gantz D., Crouch E. C., Hartshorn K. L. (2007). Human neutrophil defensins increase neutrophil uptake of influenza A virus and bacteria and modify virus-induced respiratory burst responses. J Immunol 178, 8046–8052 [DOI] [PubMed] [Google Scholar]
- Tsui P. T., Kwok M. L., Yuen H., Lai S. T. (2003). Severe acute respiratory syndrome: clinical outcome and prognostic correlates. Emerg Infect Dis 9, 1064–1069 10.3201/eid0909.030362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumpey T. M., García-Sastre A., Taubenberger J. K., Palese P., Swayne D. E., Pantin-Jackwood M. J., Schultz-Cherry S., Solórzano A., Van Rooijen N. & other authors (2005). Pathogenicity of influenza viruses with genes from the 1918 pandemic virus: functional roles of alveolar macrophages and neutrophils in limiting virus replication and mortality in mice. J Virol 79, 14933–14944 10.1128/JVI.79.23.14933-14944.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir E. C., Jacoby R. O., Paturzo F. X., Johnson E. A., Ardito R. B. (1990). Persistence of sialodacryoadenitis virus in athymic rats. Lab Anim Sci 40, 138–143 [PubMed] [Google Scholar]
- West B. C., Escheté M. L., Cox M. E., King J. W. (1987). Neutrophil uptake of vaccinia virus in vitro. J Infect Dis 156, 597–606 10.1093/infdis/156.4.597 [DOI] [PubMed] [Google Scholar]
- Widegren H., Andersson M., Borgeat P., Flamand L., Johnston S., Greiff L. (2011). LTB4 increases nasal neutrophil activity and conditions neutrophils to exert antiviral effects. Respir Med 105, 997–1006 10.1016/j.rmed.2010.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcinski Z. W., Percy D. H. (1986). Sialodacryoadenitis virus-associated lesions in the lower respiratory tract of rats. Vet Pathol 23, 278–286 [DOI] [PubMed] [Google Scholar]
- Zhou J., Stohlman S. A., Hinton D. R., Marten N. W. (2003). Neutrophils promote mononuclear cell infiltration during viral-induced encephalitis. J Immunol 170, 3331–3336 [DOI] [PubMed] [Google Scholar]