Abstract
The initiation of drug therapy results in a reduction in the human immunodeficiency virus type 1 (HIV-1) population, which represents a potential genetic bottleneck. The effect of this drug-induced genetic bottleneck on the population dynamics of the envelope (Env) regions has been addressed in several in vivo studies. However, it is difficult to investigate the effect on the env gene of the genetic bottleneck induced not only by entry inhibitors but also by non-entry inhibitors, particularly in vivo. Therefore, this study used an in vitro selection system using unique bulk primary isolates established in the laboratory to observe the effects of the antiretroviral drug-induced bottleneck on the integrase and env genes. Env diversity was decreased significantly in one primary isolate [KP-1, harbouring both CXCR4 (X4)- and CCR5 (R5)-tropic variants] when passaged in the presence or absence of raltegravir (RAL) during in vitro selection. Furthermore, the RAL-selected KP-1 variant had a completely different Env sequence from that in the passage control (particularly evident in the gp120, V1/V2 and V4-loop regions), and a different number of potential N-glycosylation sites. A similar pattern was also observed in other primary isolates when using different classes of drugs. This is the first study to explore the influence of anti-HIV drugs on bottlenecks in bulk primary HIV isolates with highly diverse Env sequences using in vitro selection.
Introduction
Human immunodeficiency virus type 1 (HIV-1) shows a high degree of genetic diversity owing to its high rates of replication and recombination and the high mutation rate of the HIV-1 reverse transcriptase (Nájera et al., 2002). Even in a single infected individual, the virus can best be described as a population of distinct, but closely related, genetic variants or ‘quasi-species’ (Eigen, 1993; Nijhuis et al., 1998). The quasi-species behaviour of viruses is recognized as a key element in our understanding and modelling of viral evolution and disease control (Vignuzzi et al., 2006).
Combination antiretroviral (ARV) therapy results in a contraction of the viral population, which represents a potential genetic bottleneck (Charpentier et al., 2006; Delwart et al., 1998; Ibáñez et al., 2000; Kitrinos et al., 2005; Nijhuis et al., 1998; Nora et al., 2007; Sheehy et al., 1996; Zhang et al., 1994). Whilst this bottleneck has a direct effect on the region that is being targeted by the drugs (e.g. protease or reverse transcriptase), it also affects other regions of the viral genome. Indeed, the effect of the drug-induced genetic bottleneck on the population dynamics of the envelope (Env) regions has been addressed in several in vivo studies (Charpentier et al., 2006; Delwart et al., 1998; Ibáñez et al., 2000; Kitrinos et al., 2005; Nijhuis et al., 1998; Nora et al., 2007; Sheehy et al., 1996; Zhang et al., 1994).
Virus bottleneck evolution of the HIV-1 env gene might be important when choosing the optimal drugs to treat a particular patient. Indeed, a CCR5 antagonist (maraviroc, MVC) and a fusion inhibitor (enfuvirtide, T-20) have now been approved for use as HIV-1 entry inhibitors. Analysing the dynamics of drug-induced genetic bottlenecks and studying drug-resistant mutation profiles in response to HIV-1-specific ARV drugs are both important if we are to understand fully HIV-1 drug resistance and pathogenesis.
The aim of the present study was to understand better the effect of in vivo drug-induced genetic bottlenecks. In vitro selection of different primary HIV-1 isolates was performed using the recently approved HIV integrase inhibitor raltegravir (RAL) (Steigbigel et al., 2008). Two R5-, one X4-, one dual- and one mixed R5/X4-tropic isolates were passaged through a RAL-induced genetic bottleneck. We also performed in vitro selection of the R5/X4 isolate using lamivudine (3TC), saquinavir (SQV) and MVC, and compared the results with those from the RAL-selected isolate.
Results
Genotypic profiles of the HIV-1 primary isolates
Four genetically heterogeneous HIV-1 primary isolates (KP-1–4) from Japanese drug-naïve patients were used to assess the extent to which RAL affected the selection of bulk primary viruses in vitro. A laboratory isolate, strain 89.6, was also used in the study (rather than a molecular clone) to allow escape mutants to be selected from each quasi-species pool and to be generated de novo. First, the sequences of the integrase (IN) regions of the four primary isolates were determined. Table 1 shows the detailed evaluation of the R5/X4 mixture subtype B (KP-1), R5-CRF08_BC (KP-2), R5 subtype B (KP-3) and X4-CRF01_AE (KP-4) primary isolates, and the dual-tropic subtype B laboratory virus (89.6). Although some naturally occurring polymorphisms were observed within the IN regions of these isolates compared with the subtype B consensus sequence available from the Los Alamos National Laboratory HIV sequence database, we did not identify any primary resistant mutations to RAL. Three baseline viruses (KP-1, KP-4 and 89.6) were sensitive to RAL, with IC50 values ranging from 1.2 to 4 nM, which are comparable with those reported previously (Kobayashi et al., 2008). However, KP-2 and KP-3 showed minor resistance to RAL, with IC50 values of 16 and 32 nM, respectively. These two isolates contained amino acid mutations at positions 72, 125 and 201 within the IN region [previously reported as L-870,810 and S-1360 resistance mutations (Hombrouck et al., 2008; Rhee et al., 2008), but not as RAL-resistance mutations]. KP-2 also contained a unique insertion at position 288 (NQDME) at the C-terminal end of the IN region.
Table 1. Susceptibility of HIV-1 isolates to RAL and distinct differences in IN region sequences between RAL-selected and control-passaged viruses.
| Isolate | Subtype | Tropism | Passage no. | Concn (nM) | RAL-selected variant* | Passage control | ||
| IN sequence | RAL IC50 (nM) | IN sequence | RAL IC50 (nM) | |||||
| KP-1 | B | Mix | 0 | 0 | K/R7, K/R111, Q/H216, D/N278 | 4 | K/R7, K/R111, Q/H216, D/N278 | 4 |
| 8 | 20 | K111, H216, D278 | 31 (7.8) | R7, R111, Q216, N278 | 4.5 (1.2) | |||
| 17† | 20 | K7, K111, H216, D278 | 26 (6.5) | R7, R111, Q216, N278 | 0.4 (0.1) | |||
| KP-2 | CRF08_BC | R5 | 0 | 0 | I201, ins289NQDME | 16 | I201, ins289NQDME | 16 |
| 18 | 40 | G189G/R, I201, ins289NQDME | 32 (2) | I201, ins289NQDME | 16 (1) | |||
| 30 | 85 | G189R, I201, ins289NQDME | 55 (3.4) | I201, ins289NQDME | 25 (1.6) | |||
| KP-3 | B | R5 | 0 | 0 | V72, A125 | 32 | V72, A125 | 32 |
| 11 | 25 | V72, A125 | 25 (0.78) | V72, A125 | 33 (1) | |||
| 22 | 27.5 | V72, A125 | 37 (1.2) | V72, A125T | 13 (0.41) | |||
| KP-4 | CRF01_AE | X4 | 0 | 0 | – | 2.1 | – | 2.1 |
| 8 | 40 | – | 33 (16) | R166R/K, D279N | 4.4 (2.1) | |||
| 29 | 40 | T210I | 22 (10) | G163E, R166R/K, D279N/S | 4.1 (2) | |||
| 89.6 | B | R5X4 | 0 | 0 | – | 1.2 | – | 1.2 |
| 8 | 15 | – | 34 (28) | – | 4.4 (3.7) | |||
| 34 | 20 | – | 11 (9.2) | V180I | 1.2 (1) | |||
Amino acid changes in each passage variant are shown. Italicized letters represent mutations relative to the consensus subtype BC or B present in the baseline isolates. Bold letters represent amino acids selected out of the quasi-species cloud. The fold increase in RAL IC50 values is shown in parentheses for in vitro-selected variants compared with those in the baseline isolates.
The RAL variant selected after 17 passages was compared with the control selected after 20 passages.
In vitro selection of variants of the primary isolates and 89.6 using RAL
To induce RAL-selected HIV-1 variants in vitro, PM1/CCR5 cells, a T-cell line expressing high levels of CCR5, were exposed to the four primary isolates and strain 89.6. The viruses were then serially passaged in the presence of RAL. As a control, each isolate was passaged under the same conditions, but without RAL, to allow monitoring of spontaneous changes occurring in the viruses during prolonged PM1/CCR5 cell passage (the passage control). The selected viruses were initially propagated at a RAL concentration equal to each IC50 value. The RAL concentrations were then increased from 20 to 85 nM during the course of the selection procedure (Table 1).
Only small shifts in the IC50 to RAL were observed in four of the five isolates (KP-1, KP-2, KP-4 and 89.6), with fold changes in IC50 values of 3.4, 6.5, 16 and 9.2, respectively. KP-3 did not show resistance to RAL. IC50 values in all the passage controls were comparable with those of the baseline viruses (Table 1).
IN region sequences in RAL-selected variants
The full-length IN genes were amplified and cloned to determine the genetic basis of selection in the presence or absence of RAL. Ten to 12 clones from each sample were sequenced.
Substitutions within IN were observed at passages 30 (G189R) and 29 (T210I) in two RAL-selected isolates (KP-2 and KP-4, respectively). Neither of these has been reported as IN inhibitor-resistant mutations. No substitutions in the IN regions of KP-3 and 89.6 were found. However, A125T and V180I substitutions were observed in the KP-3 and 89.6 control variants at the last passage. No previously reported mutations were identified in the IN region of KP-1 (an R5/X4 mixture isolate) after 17 passages. However, four amino acids (K7/K111/H216/D278) were selected by RAL from the baseline quasi-species, whereas different amino acids (R7/R111/Q216/N278) were selected in the control-passage variants (Table 1).
Taken together, these findings showed that RAL-induced selection pressure causes adaptation within the IN regions of bulk primary viruses during in vitro passage in the target cells, and confirmed that this system can be used to analyse drug-selected variants in vitro.
Comparison of env gene sequences in RAL-selected and passage-control isolates
A highly diverse gp120 region was observed in the baseline R5/X4 mixture isolate, KP-1; however, the viral diversity of variants passaged in the presence or absence of RAL decreased significantly during in vitro selection (overall mean distance after RAL selection of 0.056 at baseline to 0.007 after passage 17; mean overall distance in the passage control of 0.01 after 20 passages, Table 2). Moreover, the RAL-selected and control variants utilized CCR5 to enter the target cell; neither variant used CXCR4 (Table 3).
Table 2. Comparison of amino acid length and number of PNGs between RAL-selected and control-passage KP-1 variants.
| Passage no. | Genetic diversity* | Mean ENV 1–474 length (range)† | Mean V1/V2 length (range) | Mean V3 length (range) | Mean V4 length (range) | Mean PNGs (range) | |
| Baseline | 0 | 0.056 | 472 (461–480) | 69 (60–74) | 34 (33–34) | 30 (29–31) | 24 (22–28) |
| RAL-selected virus | 2 | 0.038 | 479 (472–480)‡ | 74 (71–74)‡ | 34 (33–34)‡ | 31 (29–31)‡ | 27 (25–28) ‡ |
| 8 | 0.0070 | 480 | 74 | 34 | 31 | 28 (26–29) | |
| 17 | 0.0070 | 480 | 74 | 34 | 31 | 27 (26–27)§ | |
| Passage control | 2 | 0.045 | 464 (461–466)‡ | 64 (60–74)‡ | 34 (33–34)‡ | 29 (29–31)‡ | 24 (22–27)‡ |
| 8 | 0.0070 | 463 (462–463) | 62 | 34 | 29 | 23 (22–23) | |
| 10 | 0.0080 | 462 (459–463) | 62 | 34 | 29 | 23 (22–23) | |
| 20 | 0.010 | 463 | 62 | 34 | 29 | 23 (22–23)§ | |
| P value | <0.0001‡ | <0.0001‡ | 0.91‡ | 0.0048‡ | 0.0019‡ | ||
| <0.0001§ |
Overall mean distance.
Sequence from gp120 SP to the V5 region (aa 1–474).
, § P values were calculated using the homoscedastic t-test between the RAL-selected and the passage-control variants indicated by the same symbols above.
Table 3. Comparison of amino acid length, number of potential N-linked glycosylation sites, V3 sequences and co-receptor usage between anti-retroviral drug-selected and control-passaged KP-1 variants.
| Passage no. | Genetic diversity* | Mean ENV1–474 length (range)† | Mean V1/V2 length (range) | Mean V3 length (range) | Mean V4 length (range) | Mean PNGs (range) | V3 region | Geno2 pheno (%)§ | ||
| Prevalence (%) | Sequence‡ | |||||||||
| Baseline | 0 | 0.056 | 472 (461–480) | 69 (60–74) | 34 (33–34) | 30 (29–31) | 24 (22–28) | 41.9 | CTRPNNNTRKGIHIGPGKFYATGAIIGDIRQAHC | 41.2 |
| 22.6 | .........................V........ | 41.2 | ||||||||
| 16.1 | ....-..I.......T.R..T.RD...N..K... | 1.7 | ||||||||
| 13.0 | ....-..I.......T.R..T.KT...N.KK... | 2.9 | ||||||||
| 3.2 | ....-..I.......................... | 7.4 | ||||||||
| 3.2 | .......................D.......... | 55.3 | ||||||||
| Passage control | 8 | 0.0070 | 463 (462–463) | 62 | 34 | 29 | 23 (22–23) | 100.0 | .........................V........ | 41.2 |
| RAL-selected virus | 8 | 0.0070 | 480 | 74 | 34 | 31 | 28 (26–29) | 100.0 | .................................. | 41.2 |
| 3TC-selected virus | 6 | 0.020 | 478 (475–480) | 74 | 34 | 31 (29–31) | 27 (25–28) | 83.3 | .................................. | 41.2 |
| SQV-selected virus | 11 | 0.0040 | 474 | 71 | 34 | 31 | 26 | 100.0 | .................................. | 41.2 |
| MVC-selected virus | 7 | 0.0080 | 469 (468–469) | 69 | 33 | 29 | 24 (23–24) | 100.0 | ....-..I....R..T.R..T.KT...N.KK... | 1.7 |
Overall mean distance.
Sequence from gp120 SP to the V5 region (aa 1–474).
V3 sequences of each variant are shown. Dots denote sequence identity and dashes indicate a deletion mutation.
Prediction of viral co-receptor tropism using Geno2pheno based on a selectable ‘false positive rate’.
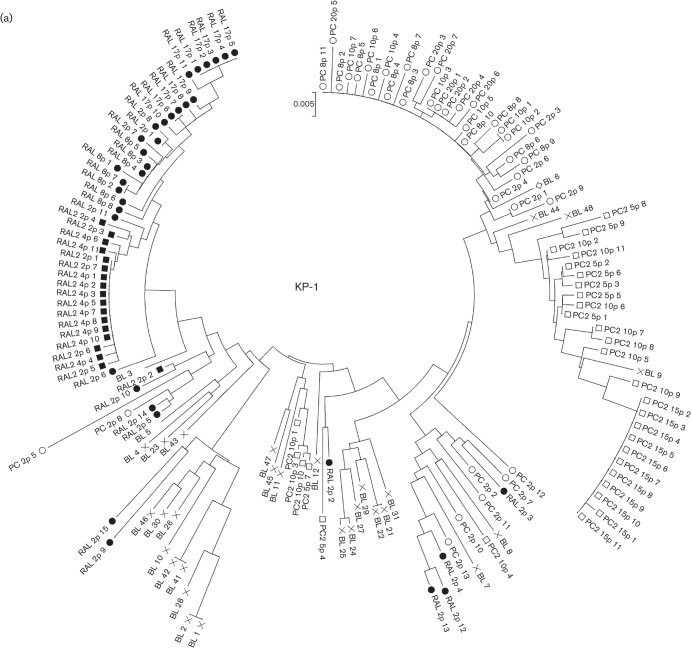
Interestingly, the low-diversity RAL-selected variant contained a completely different Env sequence from that of the passage-control variant (Fig. 1a). Different regions spanning the whole envelope sequence [from the signal peptide (SP) to V5] were compared in the RAL-selected and passage-control viruses. The results showed that, after only two passages, the gp120, V1/V2 and V4-loop regions within RAL-selected variants were longer than those in the control variants, and the number of putative N-linked glycosylation sites (PNGs) was significantly higher than that in the control-passage viruses (Table 2). This phenomenon was seen consistently in two independent experiments.
Fig. 1.
Comparison of the gp120 sequences between RAL-selected and control-passaged viruses. The gp120 sequences of baseline, RAL-selected and the passage-control viruses were aligned for KP-1 (a), KP-2 (b), KP-3 (c), KP-4 (d) and strain 89.6 (e). Each amino acid in (a)–(e) is numbered relative to the HIV-1 HXB2 reference sequence. The V3 sequences from the JR-FL-V3Lib baseline library, RAL-selected and passage-control viruses were aligned (f). Filled cells denote the most dominant amino acids observed in RAL-selected variants at the latest passage, open cells denote the most dominant amino acids observed in the passage-control variants at the latest passage and shaded cells show amino acids deleted by the end of both passages, whilst ‘–’ indicates a deletion mutation. The number of passages is indicated, e.g. 17p for passage 17.
We also analysed the gp120 sequences in the other four isolates. Although the number of positional differences between the RAL-selected and passage-control variants for these four isolates was lower than that in KP-1 (between three and nine, compared with >40), there was a similar pattern of separation between the Env sequences (Fig. 1). In three of the four isolates (KP-2, KP-3 and KP-4), positional differences were observed in SP, C1 and all the variable regions of gp120 (Fig. 1b–d). In strain 89.6, differences were observed in the C2, C3 and V4 regions (Fig. 1e).
These results suggested that RAL treatment of target cells causes a decrease in viral diversification within quasi-species Env regions via a route different from that in untreated target cells.
In vitro induction of RAL-selected V3-loop library virus variants
To investigate further the effects of RAL on viral Env sequences, we used the V3-loop library virus (JR-FL-V3Lib) developed by Yusa et al. (2005), which carries a set of random combinations from zero to ten substitutions (27 648 possibilities) in the V3 loop (residues 305, 306, 307, 308, 309, 317, 319, 322, 323 and 326; V3 loop from Cys296 to Cys331). The variants contained in the library were polymorphic mutations derived from 31 R5 clinical isolates (Yusa et al., 2005). PM1/CCR5 cells were exposed to the JR-FL-V3Lib and serially passaged in the presence of RAL. After two passages, the V3 sequence within the RAL-selected variant was completely different from that in the passage control (Fig. 1f). This suggested that, under pressure from RAL, the infectious clone harbouring different V3 region sequence from the passage control had adapted to the target cells, despite containing the same IN sequences.
Phylogenetic analysis of the Env regions after passage with or without RAL
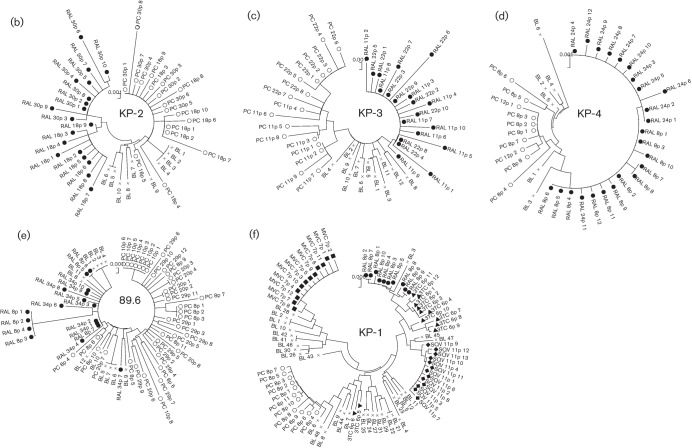
To confirm the temporal and spatial differences observed in each of the RAL-selected and passage-control viruses, phylogenetic analyses were conducted using complete SP–V5 sequences. The neighbour-joining phylogenetic tree showed a clear and distinct branching between RAL-selected and passage-control KP-1 viruses (Fig. 2a). We also identified a similar pattern in all the other isolates tested (Fig. 2b–e).
Fig. 2.
Phylogenetic analyses of the Env regions from in vitro-passaged viruses selected with or without ARV drugs. (a–e) Phylogenetic trees were constructed using gp120 SP–V5 sequences from RAL-selected and passage-control variants of KP-1 (a), KP-2 (b), KP-3 (c), KP-4 (d) and strain 89.6 (e). An ‘×’ represents baseline (BL) variants, and closed and open symbols represent RAL-selected (RAL) and passage-control (PC) variants, respectively. In (a), the results of the second experiment are indicated as RAL2 and PC2, respectively. (f) A phylogenetic tree was constructed using gp120 SP–V5 sequences from RAL-, 3TC-, SQV-, MVC-selected and control-passaged variants of KP-1. ○, Control variants after eight passages; •, RAL-selected variants after eight passages; ▴, 3TC-selected variants after six passages; ⧫, SQV-selected variants after 11 passages; ▪, MVC-selected variants after seven passages. The trees were constructed using the neighbour-joining algorithm embedded within the mega software.
In vitro selection of KP-1 variants by 3TC, SQV and MVC
To determine whether other HIV drugs also changed the route of adaptation to the target cells, we attempted to select KP-1 variants using a reverse transcriptase inhibitor (3TC), a protease inhibitor (SQV) and a CCR5 inhibitor (MVC). As shown in Fig. 2(f), the pattern of clustering at distinct positions between the selected isolates and the passage-control variants was similar to that observed for the RAL-selected variants. The selected variants showed decreased diversity in the gp120 sequences; however, the length of the gp120, V1/V2 and V4 sequences increased (apart from in the MVC-selected variants). In addition, the number of PNGs within gp120 was higher than that in the control (Table 3). We also compared the V3 sequences between the passage-control and each of the drug-selected variants. The V3 sequences in all the SQV-selected variants and 83.3 % of those in the 3TC-selected variants, were comparable with those in the RAL-selected variants. This was not the case for the passage controls. Comparison of variants passaged with RAL and 3TC showed that the length of the V1/V2 and V4 regions and the number of PNGs was similar; however, these parameters were different in the SQV-selected variants (Table 3). This indicated that the time at which a drug acts (e.g. during the early or late phase of the HIV life cycle) influences the selection of Env sequences. During selection with MVC, CXCR4-tropic variants were selected from the baseline mixture after seven passages.
Taken together, these results suggested that, in treated cells, different classes of anti-HIV drugs may suppress the variability of quasi-species during in vitro selection via a route different from that in untreated cells.
Discussion
This study evaluated the impact of anti-HIV drugs on the Env bottleneck in bulk HIV-1 primary isolates during selection in vitro. RAL-, 3TC- and SQV-selected variants of the unique viral isolate, KP-1, harbouring both X4 and R5 variants and with a very high level of baseline viral diversity, were used to study the final destination (genetic bottleneck) of a large variety of Env sequences. Interestingly, the phylogenetic clustering of RAL-selected KP-1 variants was completely different from that of non-drug-treated controls (Fig. 2). Our results also confirmed differences in the length of the gp120, V1/V2 and V4-loop regions and in the number of PNGs (Tables 2 and 3).
It is not clear why viruses cultured under pressure from the non-Env-directed drug RAL result in different env genotypes compared with those without the drug. Thus, we cloned the IN–env region of the proviral genome from passaged viruses and sequenced the env and IN regions on the same cloned plasmid, and compared them among the baseline and passages 1, 2, 8 and 17 of the KP-1 virus. Under low concentrations of the IN inhibitor RAL, K7 was selected for at a late passage after accumulation of the other three amino acids, K111, D278 and H216, in IN. During the sequential accumulation of these four amino acids (K111, D278, H216 and K7), the RAL-selected Env sequences at passage 17 (the Env sequences shown as filled boxes in Fig. 1) sequentially accumulated mutations in the same proviral genome (Fig. S1, available in JGV Online). However, we did not find a clone including both the RAL-selected Env at passage 17 and RAL-selected IN at passage 17 in the baseline or each passaged virus, except for in the last passage. We also examined the gp120 and IN sequences of the 3TC- and SQV-selected KP-1 variants. Compared with the RAL-selected region, the variable regions of gp120 in these selected variants were very similar to each other, except for the V1/V2 region (Fig. S2). However, the passage-control variant was very different from the drug-selected variants (Fig. 1a). Furthermore, the IN sequences were different in each passaged virus: K111/D278/H216/K7 in RAL-selected, R111/D278/Q216/R7 in 3TC-selected, K111/D278/H216/R7 in SQV-selected and R111/N278/Q216/R7 in virus without drug treatment (underlined residues indicate amino acids different from those in viruses without drug treatment). To explain these results, we believe that, under pressure from anti-HIV drugs (non-entry ARVs), the virus might show a primitive reaction to select for the Env sequence and recombine from quasi-species to gain advantage for entry and/or enhance replication in target cells. Meanwhile, IN was selected from quasi-species by a direct and/or indirect effect of RAL-induced pressure. The combination of both selective pressures may affect the selection for Env and IN during adaptation in drug-treated conditions (Figs 1a and S2). These results suggest that non-entry inhibitors, such as RAL, 3TC and SQV, might also affect cell adaptation to PM1/CCR5 cells.
Many in vivo studies have reported the effects of the anti-HIV drug-induced bottleneck on the env gene (Charpentier et al., 2006; Delwart et al., 1998; Ibáñez et al., 2000; Kitrinos et al., 2005; Nijhuis et al., 1998; Nora et al., 2007; Sheehy et al., 1996; Zhang et al., 1994). However, these studies had several limitations. Because viruses were placed under in vivo selective pressure using at least two anti-HIV drugs and by the host immune response, it is difficult to separate the different effects and to draw clear conclusions, particularly in vivo. Delwart et al. (1998) and Kitrinos et al. (2005) avoided some of these limitations by employing a heteroduplex tracking assay, although in vivo peculiarities still remained. Therefore, we used an in vitro selection system using unique bulk primary isolates established in our laboratory (Hatada et al., 2010; Shibata et al., 2007; Yoshimura et al., 2006, 2010b) to observe the effects of the anti-retroviral drug-induced bottleneck on the IN and env genes.
This selection provides a sensitive approach for analysing virus population dynamics. The effectiveness of ARV drugs can be examined during the in vitro passage of a single variant or mixture of variants without being affected by many of the factors encountered in vivo. In addition, differences in the Env sequences between the baseline and selected variants can be compared after any number of passages. The results of the present study provide important information that will enhance our understanding of the drug-induced genetic bottleneck. This phenomenon can be examined in vitro using bulk primary isolates treated with or without drugs.
Recently, several new ARV drugs have been licensed for use in HIV-1-infected patients. MVC, approved in 2006, is the first CCR5 inhibitor (Gulick et al., 2008). One important advantage associated with this drug is the absence of cross-resistance with previously available ARV compounds (Gulick et al., 2008; Steigbigel et al., 2008). However, as is usual with anti-HIV drugs, resistant variants with mutations in the Env, gp120 and gp41 sequences are induced both in vivo and in vitro (Anastassopoulou et al., 2009; Berro et al., 2009; Tilton et al., 2010; Yoshimura et al., 2009, 2010a). As shown in the present study, distinct Env sequences from each quasi-species might be selected by the different anti-HIV drugs (e.g. length of the V1/2 and/or V4 regions, V3 region depletion and the number of PNGs). Moreover, many of the novel anti-retroviral drugs in pre-clinical trials are viral entry inhibitors (e.g. PRO140, ibalizumab, BMS-663068 and PF-232798; Jacobson et al., 2010; McNicholas et al., 2010; Nettles et al., 2011; Stupple et al., 2011; Toma et al., 2011). Therefore, it is necessary to examine whether such entry inhibitors are effective when used alongside conventional drugs.
In conclusion, we studied the genetic bottleneck in bulk primary HIV-1 isolates from untreated patients and drugs targeting the Env (and other) regions. The results showed, for the first time, the presence of drug-selected Env sequences in these isolates. Although our observations were based on a limited number of HIV-1 isolates and need to be confirmed by independent studies, we believe that they provide a new paradigm for HIV-1 evolution in the new combination ARV therapy era.
Methods
Patients and isolates.
Primary HIV-1 isolates were isolated from four drug-naïve patients in our laboratory (KP-1–4) and passaged in phytohaemagglutinin-activated PBMCs. Infected PBMCs were then co-cultured for 5 days with PM1/CCR5 cells (a kind gift from Dr Y. Maeda; Maeda et al., 2008; Yusa et al., 2005) and the culture supernatants were stored at −150 °C (Hatada et al., 2010; Shibata et al., 2007; Yoshimura et al., 2006, 2010b).
After isolation of the primary viruses, we checked the sensitivity of each primary isolate to MVC. The KP-1 isolate was relatively MVC-resistant compared with KP-2 and KP-3 (54 vs 5.9 and 8.7 nM, respectively). KP-1 became MVC sensitive after eight passages in PM1/CCR5 cells [IC50, 3.4 nM; Geno2pheno value (see below), 41.2 %], whilst under the pressure of MVC, KP-1 became highly resistant to MVC after eight passages (IC50, >1000 nM; Geno2pheno value, 1.7 %). These results indicated that the bulk KP-1 isolate used in this study harboured primarily R5 viruses with X4- or dual-tropic viruses as a minor population.
Cells, culture conditions and reagents.
PM1/CCR5 cells were maintained in RPMI 1640 (Sigma) supplemented with 10 % heat-inactivated FCS (HyClone Laboratories), 50 U penicillin ml−1, 50 µg streptomycin ml−1 and 0.1 mg G418 (Nacalai Tesque) ml−1. MVC, RAL and SQV were kindly provided by Pfizer, Merck & Co. and Roche Products, respectively. 3TC was purchased from Wako Pure Chemical Industries.
The laboratory-adapted HIV-1 strain 89.6, which was obtained through the NIH AIDS Research and Reference Reagent Program, was propagated in phytohaemagglutinin-activated PBMCs. The viral-competent library pJR-FL-V3Lib, which contains 176 bp V3–loop DNA fragments with 0–10 random combinations of amino acid substitutions, was introduced into pJR-FL, as described previously (Yusa et al., 2005).
In vitro selection of HIV-1 variants using anti-HIV drugs.
The four primary HIV isolates (KP-1–4), strain 89.6 and JR-FL-V3Lib were treated with various concentrations of RAL and used to infect PM1/CCR5 cells to induce the production of RAL-selected HIV-1 variants, as described previously, with minor modifications (Hatada et al., 2010; Shibata et al., 2007; Yoshimura et al., 2006, 2010b). Briefly, PM1/CCR5 cells (4×104 cells) were exposed to 500 TCID50 HIV-1 isolates and cultured in the presence of RAL. Virus replication in PM1/CCR5 cells was monitored by observing the cytopathic effects. The culture supernatant was harvested on day 7 and used to infect fresh PM1/CCR5 cells for the next round of culture in the presence of increasing concentrations of RAL. When the virus began to propagate in the presence of the drug, the compound concentration was increased further. Proviral DNA was extracted from lysates of infected cells at different passages using a QIAamp DNA Blood Mini kit (Qiagen). The proviral DNAs obtained were then subjected to nucleotide sequencing. In vitro selection of the KP-1 isolate using SQV, 3TC and MVC was also performed using the procedure described above.
Amplification of proviral DNA and nucleotide sequencing.
Proviral DNA was subjected to PCR amplification using PrimeSTAR GXL DNA polymerase and Ex-Taq polymerase (Takara), as described previously (Hatada et al., 2010; Shibata et al., 2007; Yoshimura et al., 2006, 2010b). The primers used were 1B and H for the gp120 region (Hatada et al., 2010; Shibata et al., 2007; Yoshimura et al., 2006, 2010b), IN 1F (5′-CAGACTCACAATATGCATTAGG-3′) and IN 1R (5′-CCTGTATGCAGACCCCAATATG-3′) for the IN region, and IN 1F and H for the IN–gp120 region. The first-round PCR products were used directly in a second round of PCR using primers 2B and F (Hatada et al., 2010; Shibata et al., 2007; Yoshimura et al., 2006, 2010b) for gp120, IN 2F (5′-CTGGCATGGGTACCAGCACACAA-3′) and IN 2R (3′-CCTAGTGGGATGTGTACTTCTGAACTTA-3′) for IN, and IN 2F and F for IN–gp120. The PCR conditions used were as described above. The second-round PCR products were purified and cloned into a pGEM-T Easy Vector (Promega) or pCR-XL-TOPO Vector (Invitrogen), and the env and IN regions in both the passaged and selected viruses were sequenced using an Applied Biosystems 3500xL Genetic Analyzer and a BigDye Terminator v3.1 Cycle Sequencing kit (Applied Biosystems). Phylogenetic reconstructions were generated using the neighbour-joining method embedded in the mega software (http://www.megasoftware.net) (Tamura et al., 2007). Overall, mean distances for viral diversity were also calculated using mega software. The number and location of putative PNGs were estimated using N-GlycoSite (http://www.hiv.lanl.gov/content/sequence/GLYCOSITE/glycosite.html) from the Los Alamos National Laboratory database.
Susceptibility assay.
The sensitivity of the passaged viruses to various drugs was determined as described previously with minor modifications (Hatada et al., 2010; Shibata et al., 2007; Yoshimura et al., 2006, 2010b). Briefly, PM1/CCR5 cells (2×103 cells per well) in 96-well round-bottomed plates were exposed to 100 TCID50 of the viruses in the presence of various concentrations of drugs and incubated at 37 °C for 7 days. The IC50 values were then determined using a Cell Counting Kit-8 assay (Dojindo Laboratories). All assays were performed in duplicate or triplicate.
Predicting co-receptor usage by the V3 sequence.
HIV-1 tropism was inferred using Geno2pheno [coreceptor] program, with a false rate positive (FPR) value of 5.0 %, which is freely available (http://coreceptor.bioinf.mpi-inf.mpg.de/index.php). This genotyping tool more accurately predicts virological responses to the CCR5 antagonist MVC in ARV-naïve patients than a reference phenotypic tropism test (Sing et al., 2007).
Statistical analyses.
Pairwise comparisons of the different parameters between variants in the two groups was calculated using the homoscedastic t-test. A P value of <0.05 was considered statistically significant.
Acknowledgements
We are grateful to Dr Yosuke Maeda for providing the PM1/CCR5 cells. We also thank Syoko Yamashita, Yoko Kawanami, Noriko Shirai and Akiko Shibata for technical assistance. This study was supported in part by the Ministry of Education, Culture, Sports, Science and Technology, Japan, by a Grant-in-Aid for Young Scientists (B-22790163); grants from the Ministry of Health, Labour and Welfare; the Program of Founding Research Centers for Emerging and Re-emerging Infectious Diseases; and the Global COE program Global Education and Research Center Aiming at the Control of AIDS.
Footnotes
Two supplementary figures are available with the online version of this paper.
References
- Anastassopoulou C. G., Ketas T. J., Klasse P. J., Moore J. P. (2009). Resistance to CCR5 inhibitors caused by sequence changes in the fusion peptide of HIV-1 gp41. Proc Natl Acad Sci U S A 106, 5318–5323 10.1073/pnas.0811713106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berro R., Sanders R. W., Lu M., Klasse P. J., Moore J. P. (2009). Two HIV-1 variants resistant to small molecule CCR5 inhibitors differ in how they use CCR5 for entry. PLoS Pathog 5, e1000548 10.1371/journal.ppat.1000548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpentier C., Nora T., Tenaillon O., Clavel F., Hance A. J. (2006). Extensive recombination among human immunodeficiency virus type 1 quasispecies makes an important contribution to viral diversity in individual patients. J Virol 80, 2472–2482 10.1128/JVI.80.5.2472-2482.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delwart E. L., Pan H., Neumann A., Markowitz M. (1998). Rapid, transient changes at the env locus of plasma human immunodeficiency virus type 1 populations during the emergence of protease inhibitor resistance. J Virol 72, 2416–2421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eigen M. (1993). The origin of genetic information: viruses as models. Gene 135, 37–47 10.1016/0378-1119(93)90047-7 [DOI] [PubMed] [Google Scholar]
- Gulick R. M., Lalezari J., Goodrich J., Clumeck N., DeJesus E., Horban A., Nadler J., Clotet B., Karlsson A. & other authors (2008). Maraviroc for previously treated patients with R5 HIV-1 infection. N Engl J Med 359, 1429–1441 10.1056/NEJMoa0803152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatada M., Yoshimura K., Harada S., Kawanami Y., Shibata J., Matsushita S. (2010). Human immunodeficiency virus type 1 evasion of a neutralizing anti-V3 antibody involves acquisition of a potential glycosylation site in V2. J Gen Virol 91, 1335–1345 10.1099/vir.0.017426-0 [DOI] [PubMed] [Google Scholar]
- Hombrouck A., Voet A., Van Remoortel B., Desadeleer C., De Maeyer M., Debyser Z., Witvrouw M. (2008). Mutations in human immunodeficiency virus type 1 integrase confer resistance to the naphthyridine L-870,810 and cross-resistance to the clinical trial drug GS-9137. Antimicrob Agents Chemother 52, 2069–2078 10.1128/AAC.00911-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibáñez A., Clotet B., Martínez M. A. (2000). Human immunodeficiency virus type 1 population bottleneck during indinavir therapy causes a genetic drift in the env quasispecies. J Gen Virol 81, 85–95 [DOI] [PubMed] [Google Scholar]
- Jacobson J. M., Thompson M. A., Lalezari J. P., Saag M. S., Zingman B. S., D’Ambrosio P., Stambler N., Rotshteyn Y., Marozsan A. J. & other authors (2010). Anti-HIV-1 activity of weekly or biweekly treatment with subcutaneous PRO 140, a CCR5 monoclonal antibody. J Infect Dis 201, 1481–1487 10.1086/652190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitrinos K. M., Nelson J. A., Resch W., Swanstrom R. (2005). Effect of a protease inhibitor-induced genetic bottleneck on human immunodeficiency virus type 1 env gene populations. J Virol 79, 10627–10637 10.1128/JVI.79.16.10627-10637.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M., Nakahara K., Seki T., Miki S., Kawauchi S., Suyama A., Wakasa-Morimoto C., Kodama M., Endoh T., Oosugi E. (2008). Selection of diverse and clinically relevant integrase inhibitor-resistant human immunodeficiency virus type 1 mutants. Antiviral Res 80, 213–222 10.1016/j.antiviral.2008.06.012 [DOI] [PubMed] [Google Scholar]
- Maeda Y., Yusa K., Harada S. (2008). Altered sensitivity of an R5X4 HIV-1 strain 89.6 to coreceptor inhibitors by a single amino acid substitution in the V3 region of gp120. Antiviral Res 77, 128–135 10.1016/j.antiviral.2007.11.001 [DOI] [PubMed] [Google Scholar]
- McNicholas P., Wei Y., Whitcomb J., Greaves W., Black T. A., Tremblay C. L., Strizki J. M. (2010). Characterization of emergent HIV resistance in treatment-naive subjects enrolled in a vicriviroc phase 2 trial. J Infect Dis 201, 1470–1480 10.1086/652189 [DOI] [PubMed] [Google Scholar]
- Nájera R., Delgado E., Pérez-Alvarez L., Thomson M. M. (2002). Genetic recombination and its role in the development of the HIV-1 pandemic. AIDS 16 (Suppl. 4), S3–S16 [DOI] [PubMed] [Google Scholar]
- Nettles R., Schurmann D., Zhu L., Stonier M., Huang S. P., Chien C., Krystal M., Wind-Rotolo M., Bertz R., Grasela D. (2011). Pharmacodynamics, safety, and pharmacokinetics of BMS-663068: a potentially first-in-class oral HIV attachment inhibitor. In 18th Conference on Retroviruses and Opportunistic Infections, abstract 49. Boston, MA.
- Nijhuis M., Boucher C. A., Schipper P., Leitner T., Schuurman R., Albert J. (1998). Stochastic processes strongly influence HIV-1 evolution during suboptimal protease-inhibitor therapy. Proc Natl Acad Sci U S A 95, 14441–14446 10.1073/pnas.95.24.14441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nora T., Charpentier C., Tenaillon O., Hoede C., Clavel F., Hance A. J. (2007). Contribution of recombination to the evolution of human immunodeficiency viruses expressing resistance to antiretroviral treatment. J Virol 81, 7620–7628 10.1128/JVI.00083-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee S.-Y., Liu T. F., Kiuchi M., Zioni R., Gifford R. J., Holmes S. P., Shafer R. W. (2008). Natural variation of HIV-1 group M integrase: implications for a new class of antiretroviral inhibitors. Retrovirology 5, 74 10.1186/1742-4690-5-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehy N., Desselberger U., Whitwell H., Ball J. K. (1996). Concurrent evolution of regions of the envelope and polymerase genes of human immunodeficiency virus type 1 observed during zidovudine (AZT) therapy. J Gen Virol 77, 1071–1081 10.1099/0022-1317-77-5-1071 [DOI] [PubMed] [Google Scholar]
- Shibata J., Yoshimura K., Honda A., Koito A., Murakami T., Matsushita S. (2007). Impact of V2 mutations on escape from a potent neutralizing anti-V3 monoclonal antibody during in vitro selection of a primary human immunodeficiency virus type 1 isolate. J Virol 81, 3757–3768 10.1128/JVI.01544-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sing T., Low A. J., Beerenwinkel N., Sander O., Cheung P. K., Domingues F. S., Büch J., Däumer M., Kaiser R. & other authors (2007). Predicting HIV coreceptor usage on the basis of genetic and clinical covariates. Antivir Ther 12, 1097–1106 [PubMed] [Google Scholar]
- Steigbigel R. T., Cooper D. A., Kumar P. N., Eron J. E., Schechter M., Markowitz M., Loutfy M. R., Lennox J. L., Gatell J. M. & other authors (2008). Raltegravir with optimized background therapy for resistant HIV-1 infection. N Engl J Med 359, 339–354 10.1056/NEJMoa0708975 [DOI] [PubMed] [Google Scholar]
- Stupple P. A., Batchelor D. V., Corless M., Dorr P. K., Ellis D., Fenwick D. R., Galan S. R., Jones R. M., Mason H. J. & other authors (2011). An imidazopiperidine series of CCR5 antagonists for the treatment of HIV: the discovery of N-(1S)-1-(3-fluorophenyl)-3-[(3-endo)-3-(5-isobutyryl-2-methyl-4,5,6,7-tetrahydro-1H-imidazo[4,5-c]pyridin-1-yl)-8-azabicyclo[3.2.1]oct-8-yl]propylacetamide (PF-232798). J Med Chem 54, 67–77 10.1021/jm100978n [DOI] [PubMed] [Google Scholar]
- Tamura K., Dudley J., Nei M., Kumar S. (2007). mega4: Molecular Evolutionary Genetics Analysis (mega) software version 4.0. Mol Biol Evol 24, 1596–1599 10.1093/molbev/msm092 [DOI] [PubMed] [Google Scholar]
- Tilton J. C., Wilen C. B., Didigu C. A., Sinha R., Harrison J. E., Agrawal-Gamse C., Henning E. A., Bushman F. D., Martin J. N. & other authors (2010). A maraviroc-resistant HIV-1 with narrow cross-resistance to other CCR5 antagonists depends on both N-terminal and extracellular loop domains of drug-bound CCR5. J Virol 84, 10863–10876 10.1128/JVI.01109-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toma J., Weinheimer S. P., Stawiski E., Whitcomb J. M., Lewis S. T., Petropoulos C. J., Huang W. (2011). Loss of asparagine-linked glycosylation sites in variable region 5 of human immunodeficiency virus type 1 envelope is associated with resistance to CD4 antibody ibalizumab. J Virol 85, 3872–3880 10.1128/JVI.02237-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignuzzi M., Stone J. K., Arnold J. J., Cameron C. E., Andino R. (2006). Quasispecies diversity determines pathogenesis through cooperative interactions in a viral population. Nature 439, 344–348 10.1038/nature04388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura K., Shibata J., Kimura T., Honda A., Maeda Y., Koito A., Murakami T., Mitsuya H., Matsushita S. (2006). Resistance profile of a neutralizing anti-HIV monoclonal antibody, KD-247, that shows favourable synergism with anti-CCR5 inhibitors. AIDS 20, 2065–2073 10.1097/01.aids.0000247587.31320.fe [DOI] [PubMed] [Google Scholar]
- Yoshimura K., Harada S., Hatada M., Matsushita S. (2009). Mutations in V4 and C4 regions of the HIV-1 CRF08-BC envelope induced by the in vitro selection of Maraviroc Confer cross-resistance to other CCR5 inhibitors. In 16th Conference on Retroviruses and Opportunistic Infections, p. 640. Montreal, Canada [Google Scholar]
- Yoshimura K., Harada S., Matsushita S. (2010a). Two step escape pathway of the HIV-1 subtype C primary isolate induced by the in vitro selection of Maraviroc. In 17th Conference on Retroviruses and Opportunistic Infections, abstract 535. San Francisco, CA [Google Scholar]
- Yoshimura K., Harada S., Shibata J., Hatada M., Yamada Y., Ochiai C., Tamamura H., Matsushita S. (2010b). Enhanced exposure of human immunodeficiency virus type 1 primary isolate neutralization epitopes through binding of CD4 mimetic compounds. J Virol 84, 7558–7568 10.1128/JVI.00227-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusa K., Maeda Y., Fujioka A., Monde K., Harada S. (2005). Isolation of TAK-779-resistant HIV-1 from an R5 HIV-1 GP120 V3 loop library. J Biol Chem 280, 30083–30090 10.1074/jbc.M414360200 [DOI] [PubMed] [Google Scholar]
- Zhang Y. M., Dawson S. C., Landsman D., Lane H. C., Salzman N. P. (1994). Persistence of four related human immunodeficiency virus subtypes during the course of zidovudine therapy: relationship between virion RNA and proviral DNA. J Virol 68, 425–432 [DOI] [PMC free article] [PubMed] [Google Scholar]