Abstract
A total of 139 stool samples from wild chimpanzees, gorillas and bonobos in Cameroon and Democratic Republic of Congo (DRC) were screened for enteroviruses (EVs) by reverse transcription PCR. Enterovirus RNA was detected in 10 % of samples, comprising eight from 58 sampled chimpanzees (13.8 %), one from 40 bonobos (2.5 %) and five from 40 gorillas (12.2 %). Three viruses isolated from chimpanzees grouped with human isolate EV-A89 and four (four chimpanzees, one gorilla) represented a newly identified type, EV-A119. These species A virus types overlapped with those circulating in human populations in the same area. The remaining six strains comprised a new species D type, EV-D120, infecting one chimpanzee and four gorillas, and a single EV variant infecting a bonobo that was remarkably divergent from other EVs and potentially constitutes a new enterovirus species. The study demonstrates both the circulation of genetically divergent EV variants in apes and monkeys as well as those shared with local human populations.
Enteroviruses (EVs) are members of the genus Enterovirus within the family Picornaviridae. EVs infecting humans can be divided into four species (A to D), each containing numerous antigenically distinct (sero)types (Stanway et al., 2005). While long considered to be primarily human viruses, our recent findings of two human enteroviruses (EV-A76 and -D111) in faecal samples collected from wild chimpanzees in Cameroon (Harvala et al., 2011) provided evidence of a wider circulation of human EVs in apes, as do observations of a high seroprevalence of EV-A76 in both non-human primates (NHPs; chimpanzees and baboons) and human populations in Central Africa (Harvala et al., 2012). To further characterize EVs circulating in apes, and their relationship to human variants, we have expanded our primate faecal screening to include 139 samples obtained from wild chimpanzees and gorillas in Cameroon, and bonobos in the Democratic Republic of Congo (DRC).
A total of 58 chimpanzees (Pan troglodytes troglodytes), 40 bonobos (Pan paniscus) and 41 gorillas (western lowland gorilla, Gorilla gorilla) stool samples were studied (Peeters & Delaporte, 2012). These samples had been previously collected for simian immunodeficiency virus screening, from wild-living apes in sparsely populated forest sites with minimal human presence. Three sites were located in the southern part of Cameroon and one in the DRC (Peeters & Delaporte, 2012). Species identification was performed by amplification and sequencing of part of the mitochondrial 12S gene (Peeters & Delaporte, 2012).
RNA extraction and screening by PCR with 5′UTR primers was performed as previously described (Harvala et al., 2008). Positive samples were amplified with additional primers from the VP4 and VP1 regions and sequenced (Harvala et al., 2008, 2011). Nucleotide sequences were aligned using SSE_v1.1 (http://www.virus-evolution.org/Downloads/Software/index.html), and phylogenetic trees reconstructed using maximum-likelihood methods as implemented in the mega 5.2 software package (Tamura et al., 2011). The optimum maximum-likelihood model (lowest Bayesian information criterion score and typically greatest maximum-likelihood value) for each sequence dataset was determined first and used for phylogenetic reconstruction. Different models were selected for different datasets – VP4 and VP1, species A: Tamura Nei+gamma distribution (five rates selected) and invariant sites (TN+G+I); VP1, species D: Hasegawa-Kishino-Yano (HKY)+G+I, although running the datasets with second or third choice models created trees with identical topologies and with similar branch lengths and bootstrap values (data not shown). Phylogenetic analysis of each dataset used bootstrap resampling to determine robustness of grouping.
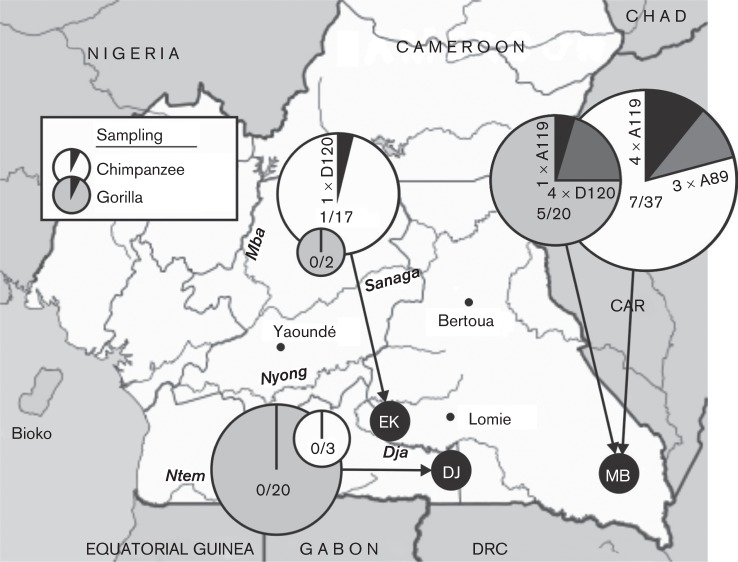
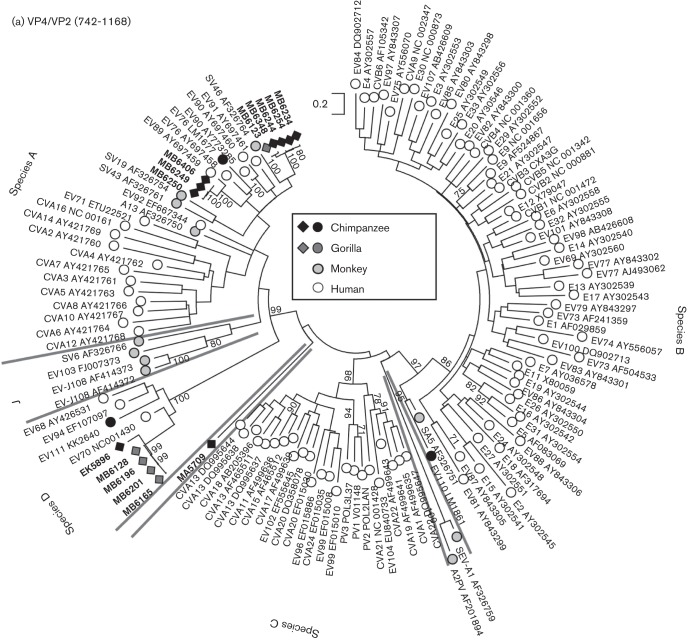
Screening of 139 faecal samples identified 14 EV-positive samples (10 %), of which eight were from 58 sampled chimpanzees (13.8 %), one from 40 bonobos (2.5 %) and five from 40 gorillas (12.2 %). Most infected NHPs were observed in samples from south-east Cameroon (five gorillas and seven chimpanzees) on the border with the Central African Republic (CAR) (Fig. 1). VP4 sequences of three chimpanzee variants grouped closely with human isolates of EV-A89. Four variants (three chimpanzees, one gorilla) represented a newly identified Cameroonian type EV-A119 based on VP4 and VP1 sequences (Fig. 2, Table 1). Five strains (one chimpanzee, four gorillas) could be classified based on VP4 sequences as a potential new species D type. Sequences from the whole VP1 region were obtained from two samples (Fig. 2b) and have been formally designated by the ICTV Picornavirus Study Group as a new enterovirus type, EV-D120. The final enterovirus identified was obtained from a bonobo sampled in Malebo in the DRC. Its VP4 sequence was remarkably divergent from other EVs and based on its basal branching (Fig. 2a) potentially represents a candidate new species. However, repeated attempts to amplify the VP1 region to verify this were unsuccessful.
Fig. 1.
Sampling locations for common chimpanzees (P. troglodytes troglodytes) and gorillas (G. gorilla gorilla) in Cameroon. Pie charts show detection frequencies of EVs in each species with EV types labelled; the size of each pie chart is proportional to the sample size in each location.
Fig. 2.
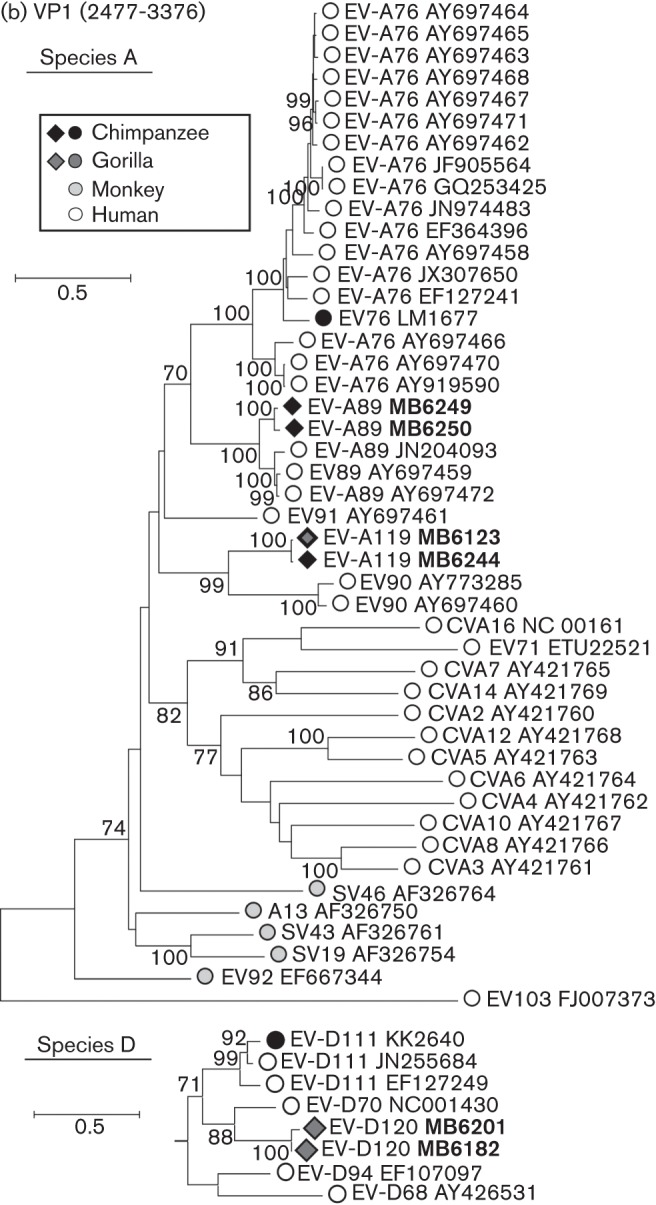
(a) Maximum-likelihood analysis (TN+G+I model) of the VP4 region (positions 742–1168 numbered using the PV3 reference sequence) of sequences amplified from infected chimpanzees and gorillas from the current study (black and medium grey filled diamonds, labelled in bold). MA5709 was obtained from a bonobo and does not group with any currently described EV species. These were compared with previously published sequences from these primates (black and medium grey filled circles) and from monkey species (light grey-filled symbols). Single, representative sequences of human enterovirus types are shown as unfilled circles. Lineages dividing EV types into species are identified by grey lines with species labelled on the periphery. Bootstrap resampling of ML trees was performed to indicate robustness of grouping (values of >70 % shown). (b) Rooted maximum-likelihood trees of the whole VP1 region (positions 2477–3376) of species A (TN+G+I model, EV103–FJ007373 as outgroup) and species D (HKY+G+I model, EV71–ETU22521 as outgroup) sequences amplified from infected chimpanzees and gorillas. Sequences are labelled as in Fig. 2(a). For each type found in both human and NHPs (A76, A89, A119 and D111), all available sequences >60 % complete in the VP1 region and >1 % divergent from each other were included in the sequence datasets.
Table 1. Enterovirus (EV) types identified in ape faecal samples.
| Type | Isolate | Primate host | Date of collection |
| EV-A89 | MB-6249 | Pan troglodytes | 15 November 2010 |
| MB-6250 | Pan troglodytes | 15 November 2010 | |
| MB-6406 | Pan troglodytes | 05 February 2011 | |
| EV-A119 | MB-6213 | Gorilla gorilla | 20 November 2010 |
| MB-6234 | Pan troglodytes | 11 December 2010 | |
| MB-6244 | Pan troglodytes | 14 November 2010 | |
| MB-6254 | Pan troglodytes | 17November 2010 | |
| MB-6384 | Pan troglodytes | 04 February 2011 | |
| EV-D120 | EK-5896 | Pan troglodytes | 05 June 2010 |
| MB-6165 | Gorilla gorilla | 13 November 2010 | |
| MB-6182 | Gorilla gorilla | 16 November 2010 | |
| MB-6196 | Gorilla gorilla | 16November 2010 | |
| MB-6201 | Gorilla gorilla | 16 November 2010 | |
| Unassigned | MA-5709 | Pan paniscus | May 2010 |
MB and EK were from Cameroon; MA was from the DRC.
The current study demonstrates an overall 10 % frequency of EV infection in chimpanzees and gorillas in different locations in Cameroon and bonobos in the DRC. Infection with a range of diverse enterovirus species corroborates our previous detection of a range of EV types in chimpanzees in other areas of Cameroon (Harvala et al., 2011). Although the numbers are relatively small (18 from the two studies combined), there was a notable preponderance of species A (A76, A89, A119) and D (D111, D120) types and an almost complete absence of species B and C variants.
While two identified types are currently unique to NHPs (EV-B110 and EV-D120), others have also been detected in humans. For example, species A variants prevalent in apes were also identified in acute flaccid paralysis (AFP) patients in Bangladesh (EV-A89; Oberste et al., 2005) and Cameroon (EV-A76; Sadeuh-Mba et al., 2013), while EV-A119 was recently described in a healthy child in Cameroon (Ayukekbong et al., 2013). This overlap in host distributions provides strong evidence for cross-species transmission of at least a subset of enteroviruses between NHP species and humans. This phenomenon extends over a wide geographical range; species A enteroviruses EV-A76 and EV-A89, detected in apes in the current study, were originally isolated from stool specimens obtained from patients presenting with AFP in Bangladesh (Oberste et al., 2005). This is a region where humans live in close proximity to NHPs and where a potential simian source was suspected following the detection of two of these viruses, EV-A76 and EV-A90, in macaques in Bangladesh (Oberste et al., 2013).
EV types recovered from apes and monkeys in previous studies, irrespective of whether co-detected in humans or not, typically segregate from human types within each species. In species B, SV5 and EV-B110 (Harvala et al., 2011) form a basally branching clade and segregate away from the 58 species B types infecting humans in the VP4 region (Fig. 2a). Furthermore, all 12 exclusively human types detected outside the identified areas of NHP overlap in Central Africa form a bootstrap-supported clade distinct from species A types found in NHPs (Fig. 2). The distinctiveness of NHP and human virus populations is further indicated by the absence of species C despite this being the commonly identified virus group in surveys of humans in both Cameroon (Ayukekbong et al., 2013) and Central Africa (Sadeuh-Mba et al., 2013).
While the stability of enteroviruses in the environment provides opportunities for NHP infection through contaminated water, the current study samples were collected from remote, forested regions of Cameroon and DRC with limited human presence. Their infection is indeed more plausibly attributable to contact with monkeys that existing surveys show to be infected with a partly overlapping subset of genetically distinct species A and B variants (Oberste et al., 2013), or with other mammalian species. Monkeys may indeed also be the conduit through which humans become infected with EV types such as A76, A89 and A119. Our previous finding of a high seroprevalence to EV-A76 in chimpanzees, baboons and other monkey species as well as human populations in Cameroon and Zimbabwe while being virtually absent in Europe (Harvala et al., 2012) is consistent with this zoonotic model for this and other NHP-associated EV types. The findings do not exclude the occurrence of human to NHP transmission, although the wide circulation and genetic diversity of EV-A76 in sub-Saharan Africa (Harvala et al., 2012; Sadeuh-Mba et al., 2013) and its rarity elsewhere (Harvala et al., 2012) nevertheless provide opportunities for human adaptation and subsequent epidemic worldwide spread into populations that lack protective immunity (Wolfe et al., 2005).
Unlike species A enteroviruses, which are widely distributed in both African and Asian monkey species, known species D infections in NHPs are confined to Central Africa. EV-D111, originally found in a chimpanzee from Cameroon (Harvala et al., 2011), has subsequently been detected in AFP cases in children in the DRC, Cameroon and CAR (Junttila et al., 2007; Bessaud et al., 2012; Sadeuh-Mba et al., 2013). The current study documents frequent infection with another species D enterovirus, now classified as EV-D120, in chimpanzees and gorillas in Cameroon. The possibility outlined above, where a NHP-derived virus may, after a period of circulation in local human populations, subsequently become pandemic can indeed be exemplified by another species D type, EV-D70. Infections were first identified in Ghana in 1969 at the start of an acute haemorrhagic conjunctivitis outbreak that rapidly spread worldwide (Kono et al., 1972). An African origin has also been suggested for EV-D94 based on its initial detection in AFP patient in the DRC (Smura et al., 2007). Although neither type has been isolated from or directly detected in NHPs, serological surveys reveal widespread exposure in a number of species and a broad in vitro tropism (Kono et al., 1981; Smura et al., 2007).
Contact points between humans and NHPs have significantly increased in Central Africa during past decades (Wolfe et al., 2005). This provides opportunities for large-scale emergence and worldwide dissemination of novel enterovirus types and species with unpredictable pathogenicities and clinical impacts.
Acknowledgements
We thank the staff and the SIV team from PRESICA for logistical support in Cameroon and the Cameroonian Ministries of Health, Environment and Forestry, and Research for permission to collect samples in Cameroon. We thank the Ministries of Health and Environment and the National Ethic Committee from the DRC for permission to perform this study and all the field staff from DRC. We thank Bila Isa Inogwabini and the staff of World Wildlife Fund for Nature in the DRC. This work was supported in part by grants from the National Institute of Health (RO1 AI 50529) and the Agence Nationale de Recherches sur le SIDA(ANRS 12125/12182/12255).
References
- Ayukekbong J., Kabayiza J. C., Lindh M., Nkuo-Akenji T., Tah F., Bergström T., Norder H. (2013). Shift of Enterovirus species among children in Cameroon–identification of a new enterovirus, EV-A119. J Clin Virol 58, 227–232 10.1016/j.jcv.2013.07.005 [DOI] [PubMed] [Google Scholar]
- Bessaud M., Pillet S., Ibrahim W., Joffret M. L., Pozzetto B., Delpeyroux F., Gouandjika-Vasilache I. (2012). Molecular characterization of human enteroviruses in the Central African Republic: uncovering wide diversity and identification of a new human enterovirus A71 genogroup. J Clin Microbiol 50, 1650–1658 10.1128/JCM.06657-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvala H., Robertson I., McWilliam Leitch E. C., Benschop K., Wolthers K. C., Templeton K., Simmonds P. (2008). Epidemiology and clinical associations of human parechovirus respiratory infections. J Clin Microbiol 46, 3446–3453 10.1128/JCM.01207-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvala H., Sharp C. P., Ngole E. M., Delaporte E., Peeters M., Simmonds P. (2011). Detection and genetic characterization of enteroviruses circulating among wild populations of chimpanzees in Cameroon: relationship with human and simian enteroviruses. J Virol 85, 4480–4486 10.1128/JVI.02285-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvala H., McIntyre C. L., Imai N., Clasper L., Djoko C. F., LeBreton M., Vermeulen M., Saville A., Mutapi F. & other authors (2012). High seroprevalence of enterovirus infections in apes and Old World monkeys. Emerg Infect Dis 18, 283–286 10.3201/eid1802.111363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junttila N., Lévêque N., Kabue J. P., Cartet G., Mushiya F., Muyembe-Tamfum J. J., Trompette A., Lina B., Magnius L. O. & other authors (2007). New enteroviruses, EV-93 and EV-94, associated with acute flaccid paralysis in the Democratic Republic of the Congo. J Med Virol 79, 393–400 10.1002/jmv.20825 [DOI] [PubMed] [Google Scholar]
- Kono R., Sasagawa A., Ishii K., Ochi M., Sugiura S., Matsumiya H., Uchida Y., Kameyama K., Kaneko M. & other authors (1972). Pandemic of new type of conjunctivitis. Lancet 299, 1191–1194 10.1016/S0140-6736(72)90921-X [DOI] [PubMed] [Google Scholar]
- Kono R., Sasagawa A., Yamazaki S., Nakazono N., Minami K., Otatsume S., Robin Y., Renaudet J., Cornet M. & other authors (1981). Seroepidemiologic studies of acute hemorrhagic conjunctivitis virus (enterovirus type 70) in West Africa. III. Studies with animal sera from Ghana and Senegal. Am J Epidemiol 114, 362–368 [DOI] [PubMed] [Google Scholar]
- Oberste M. S., Maher K., Michele S. M., Belliot G., Uddin M., Pallansch M. A. (2005). Enteroviruses 76, 89, 90 and 91 represent a novel group within the species Human enterovirus A. J Gen Virol 86, 445–451 10.1099/vir.0.80475-0 [DOI] [PubMed] [Google Scholar]
- Oberste M. S., Feeroz M. M., Maher K., Nix W. A., Engel G. A., Hasan K. M., Begum S., Oh G., Chowdhury A. H. & other authors (2013). Characterizing the picornavirus landscape among synanthropic nonhuman primates in Bangladesh, 2007 to 2008. J Virol 87, 558–571 10.1128/JVI.00837-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters M., Delaporte E. (2012). Simian retroviruses in African apes. Clin Microbiol Infect 18, 514–520 10.1111/j.1469-0691.2012.03843.x [DOI] [PubMed] [Google Scholar]
- Sadeuh-Mba S. A., Bessaud M., Massenet D., Joffret M. L., Endegue M. C., Njouom R., Reynes J. M., Rousset D., Delpeyroux F. (2013). High frequency and diversity of species C enteroviruses in Cameroon and neighboring countries. J Clin Microbiol 51, 759–770 10.1128/JCM.02119-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smura T. P., Junttila N., Blomqvist S., Norder H., Kaijalainen S., Paananen A., Magnius L. O., Hovi T., Roivainen M. (2007). Enterovirus 94, a proposed new serotype in human enterovirus species D. J Gen Virol 88, 849–858 10.1099/vir.0.82510-0 [DOI] [PubMed] [Google Scholar]
- Stanway G., Brown F., Christian P., Hovi T., Hyypia T., King A. M. Q., Knowles N. J., Lemon S. M. & other authors (2005). Family Picornaviridae. In Virus Taxonomy, 8th Report of the International Committee on Taxonomy of Viruses, pp. 757–778 Edited by Fauquet C. M., Mayo M. A., Maniloff J., Desselberger U., Ball L. A. London: Elsevier, Academic Press [Google Scholar]
- Tamura K., Peterson D., Peterson N., Stecher G., Nei M., Kumar S. (2011). MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28, 2731–2739 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe N. D., Daszak P., Marm Kilpatrick A., Burke D. (2005). Bushmeat hunting, deforestation, and prediction of zoonotic disease emergence. Emerg Infect Dis 11, 1822–1827 10.3201/eid1112.040789 [DOI] [PMC free article] [PubMed] [Google Scholar]