Abstract
Aim
To determine whether fluid flow-induced shear stress affects the differentiation of bone marrow-derived human mesenchymal stem cells (hMSCs) into osteogenic cells.
Materials & methods
hMSCs cultured with or without osteogenic differentiation medium were exposed to fluid flow-induced shear stress and analyzed for alkaline phosphatase activity and expression of osteogenic genes.
Results
Immediately following shear stress, alkaline phosphatase activity in osteogenic medium was significantly increased. At days 4 and 8 of culture the mRNA expression of bone morphogenetic protein-2 and osteopontin was significantly higher in hMSCs subjected to shear stress than those cultured in static conditions. However, hMSCs cultured in osteogenic differentiation medium were less responsive in gene expression of alkaline phosphatase and bone morphogenetic protein-2.
Conclusion
These data demonstrate that shear stress stimulates hMSCs towards an osteoblastic phenotype in the absence of chemical induction, suggesting that certain mechanical stresses may serve as an alternative to chemical stimulation of stem cell differentiation.
Keywords: alkaline phosphatase, bone repair, mesenchymal, osteogenesis, shear stress, stem cells
Mesenchymal stem cells (MSCs) are defined as self-renewing multipotent cells of the mesenchymal lineage with the capacity to differentiate into cell types that form bone, cartilage, adipose, tendon, muscle, neural and other connective tissues [1-6]. There is much interest in the potential of using human MSCs (hMSCs) in bone and cartilage regenerative medicine for the treatment of musculoskeletal trauma and disease [6,7], since they can be obtained from the patient to be treated [8].
Mesenchymal stem cells have been successfully isolated from bone marrow [9] and adipose tissue [10] and are directed towards osteogenic differentiation in vitro when cultured in the presence of dexamethasone, β-glycerophosphate and l-ascorbic acid-2-phosphate [11]. Dexamethasone, a synthetic hormone, is a member of the glucocorticoid family of steroids and regulates bone morphogenetic proteins (BMPs), which are major inducers of osteogenesis [12]. β-glycerophosphate provides phosphate for calcium phosphate deposition [13] and ascorbic acid is essential for the production of collagen [14]. Rat MSCs differentiate into osteogenic cells upon stimulation with BMPs. However, the use of BMPs for osteogenic differentiation may not be as effective in hMSCs as in nonhuman cells [15]. Dexamethasone is a main component of osteogenic, chondrogenic and adipogenic media for MSCs, and does not specifically induce osteogenesis. In long-term in vitro osteogenic differentiation conditions, it can promote adipogenesis [16]. Systematic administration of glucocorticoids in human patients has deleterious effects such as bone loss [17] and can induce cell apoptosis [18].
Besides chemical signaling, mechanical stress regulates bone mass and strength across multiple species [19,20]. Among the multiple theories regarding the primary mechanical signal to which bone cells respond, strain-induced fluid shear stress has received abundant experimental support [21,22]. Fluid shear stress occurs in the interstitial spaces around bone cells during repetitive loading and unloading of bone [23] and probably in the bone marrow cavity. Fluid shear stress regulates cell function by stimulating multiple intracellular signaling pathways [24], including those involved in differentiation. Accordingly, there is growing interest in the regulation of differentiation of bone progenitor cells by mechanical stress. With mechanical stimulation, the time in culture required to predifferentiate cells may be greatly reduced. Recent data indicate that mechanical stimulation may be used to stimulate the osteogenic differentiation of bone marrow MSCs on both 2D planar substrates and 3D scaffolds [25,26]. In 2D culture, rat MSCs exposed to shear stress showed increases in bone sialoprotein (BSP) and osteopontin (OP) gene expression, as well as alkaline phosphatase (ALP) activity [27]. Increased cell proliferation and production of osteogenic markers, including ALP and calcium, were found when MSCs derived from rat bone were mechanically stimulated in a 3D scaffold [28]. However, it is difficult to calculate shear stress magnitudes applied to a 3D scaffold, given the variety of materials and structures in use [29].
We hypothesized that shear stress alone might be a potential osteogenic stimulator for preculture of hMSCs prior to regenerative medicine therapies owing to the demonstrated disadvantages of chemical supplementation discussed above. We applied shear stress in a simplified and well characterized parallel plate flow chamber system, which has potential for use in scaled-up and routine preculture protocols. We show that a fluid shear stress increases the expression of genes that are regulated during the differentiation of hMSCs to bone cells.
Materials & methods
Cell isolation & culture
Isolated bone marrow (AllCells, Berkeley, CA, USA) from multiple healthy human donors aged between 20 and 26 years (seven male and one female) was enriched for hMSCs using RosetteSep® (StemCell Technologies, Vancouver, BC, Canada). Bone marrow was incubated with a cocktail of antibodies (50 μl per 1 ml of bone marrow) that bind to marrow cells other than MSCs, for 20 min at room temperature. The cells were then diluted twofold in phosphate-buffered saline (PBS; Cambrex, East Rutherford, NJ, USA), containing 2% fetal bovine serum (FBS) and 1 mM EDTA solution. The cells were layered on Ficoll-Paque® (StemCell Technologies) and centrifuged at 300 × g for 25 min. The cell layer enriched for MSCs at the Ficoll-Paque plasma interface was removed and seeded at a density of 6.7–13.3 × 103 cells/cm2. The cells were cultured in basal culture media, which consisted of Dulbecco’s modified Eagle’s medium-low glucose (Sigma, St Louis, MO, USA), 1% antibiotic (containing 10 units/l penicillin G sodium, 10 mg/ml streptomycin sulfate and 0.25 mg/ml amphotericine B; Gibco, Invitrogen Corporation, Carlsbad, CA, USA) and 10% FBS (Atlanta Biologicals, Norcross, GA, USA). The cells were incubated at 37°C in the presence of 5% CO2 and 95% humidity with fresh media changes every 3–4 days. Nonpooled cells from passages 3–5 were plated at a density of 7.6–8.6 × 103 cells/cm2 on polycarbonate slides for ALP experiments and glass slides for gene expression experiments. The hMSCs were cultured in basal media and basal media containing the osteogenic supplements 100 nM dexamethasone, 50 μg/ml l-ascorbic acid-2-phosphate and 10 mM β-glycerophosphate. Cells grown in osteogenic media are assumed to be preosteoblastic (hMSC-Ost).
Fluid shear stress
Cells were exposed to fluid shear stress using a parallel plate flow chamber as shown in Figure 1 [30]. The flow chamber consists of a machine-milled polycarbonate base, a rectangular silastic (0.20 in size, Dow Corning, Midland, MI, USA) gasket and the slide with the attached cells. The gasket provides a seal between the base and the slide and also creates the channel by providing separation between the slide and the base. Media enters and exits the flow chamber through slits in the polycarbonate plate. Wall shear stress on the cell monolayer can be calculated assuming Newtonian fluid and parallel-plate geometry:
where Q is the flow rate (cm2/s); μ is the viscosity of the media; h is the channel height (0.022 cm); b is the slit width (2.5 cm); and τ is the wall shear stress (dynes/cm2).
Figure 1. Hydrostatic pressure device attached to the flow chamber with cells on the slide used for the fluid shear stress experiments.
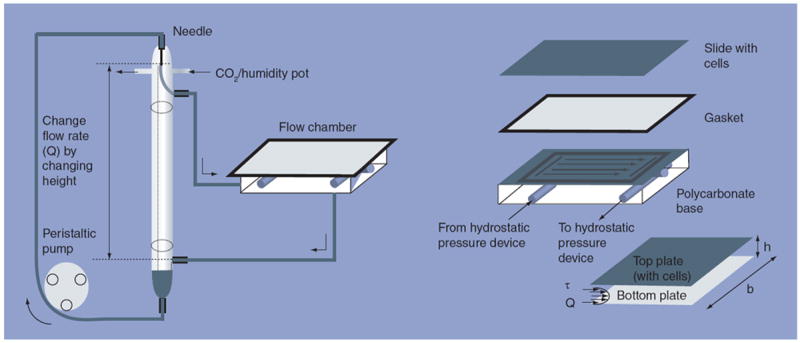
Cells are exposed to steady laminar fluid shear stress in the direction of the arrows. Media flow follows arrows from the assembled flow chamber to the lower reservoir of the hydrostatic pressure device and is then pumped to the upper reservoir via a peristaltic pump. The media then flows back into the flow chamber via the upper reservoir of the hydrostatic pressure system. The shear stress, τ, is defined by the flow rate, Q, the height of the channel, h, the width of the channel, b, and the viscosity of the liquid, μ (not shown). The flow rate, Q, and thus shear stress, τ, in the flow chamber, can be varied by changing the height between the opening of the upper reservoir and inlet of the hydrostatic pressure system. The entire system is in a 37°C environment and has humidified 5% CO2 flowing into the system where noted.
The recirculating hydrostatic pressure drop system consists of two glass reservoirs, one above the other, with a parallel-plate flow chamber connected to them. Media exits the flow chamber and is then pumped though the system from the lower to the upper reservoir. Media from the upper reservoir then re-enters the flow chamber. The hydrostatic pressure head created by the distance between the opening of the upper reservoir and the inlet of the lower reservoir can be varied by adding or removing glass tubes between the reservoirs. By varying this distance, and thus Q, and keeping all other variables constant in the previous equation, the shear stress (τ) within the chamber can be varied.
Cells to be analyzed for gene expression were switched to serum-free respective media for cell cycle synchronization 12–16 h before experimentation. The assembled flow chamber was sealed and attached to the hydrostatic pressure system, which was filled with 20 ml basal (hMSC) or osteogenic supplemented (hMSC-Ost) media, with the substitution of 2% FBS for 10% FBS [31]. All shear stress experiments were conducted in standard culture conditions of 37°C, 5% CO2 and 95% humidity. Cells not exposed to shear stress (designated as ‘control’ for the remainder of this paper) were given 20 ml of respective experimental media and placed in an incubator.
ALP activity stain
After experimentation, cells were washed twice with ice-cold PBS. The cells were incubated in a staining solution that consisted of 0.5 mg/ml napthol AS-BI phosphate in N,N′-dimethyl formamide and 1 mg/ml ‘fast red’ in 50 mM Tris and filtered through Whatman paper #1 for 30–60 min at 37°C. The cells were then washed with PBS, fixed for 20 min in 3.7% formaldehyde at room temperature in PBS and imaged.
ALP activity quantification
Cells were lysed using 0.1% Triton X-100 in PBS. At the same time as cell collection the conditioned media (12–15 ml) was concentrated using Amicon Ultra-15 centrifugal filter units (Millipore, Billerica, MA, USA) with 50 kDa cutoff, centrifuged at 4000 × g at 4°C for 20 min. ALP activity was assayed using a colorimetric assay (Raichem, San Diego, CA, USA) per the manufacturer’s instructions and normalized to DNA content. The assay measures the rate at which ALP converts p-nitrophenol phosphate (colorless) to p-nitrophenyl (yellow). For analysis of ALP activity, 25 μl of cellular lysate or concentrated media was added to 250 μl of p-nitrophenol phosphate in a 96-well plate. Samples were read in a microplate reader (BioRad 680 microplate reader; Hercules, CA, USA) at 405 nm. The change in color intensity was measured to give a quantification of ALP as nmol of substrate converted/minutes using a standard curve. ALP activity per 1 ml of concentrated media was normalized to the total amount of experimental media (20 ml). For DNA quantification a kit was used (BioRad) per the manufacturer’s instructions. A total of 10 μl of cell lysate was added to 2 ml of 0.1 mg/ml Hoechst dye in TEN buffer. Absorbance at 360 nm excitation and 460 nm emission was read using a VersaFluor Fluorometer System (BioRad) and DNA concentration (ng/ml) was determined from a DNA standard curve.
Real-time PCR
For gene expression analysis, cells were washed twice with ice-cold PBS and total RNA was isolated using Trizol Reagent (Invitrogen) or RNeasy (Qiagen, Valencia, CA, USA) according to the manufacturers’ instructions. RNA concentration was quantified using a spectrophotometer at 260 nm and quality was assessed using gel electrophoresis. A total of 2 μg of RNA was reverse transcribed in 5 mM MgCl2, 1X PCR Buffer II, 1 mM each of dGTP, dATP, dTTP and dCTP, 1 U/ml RNase inhibitor, 2.5 U/ml MuLV reverse transcriptase and 2.5 mM random hexamers solution (GeneAMP RNA PCR Kit, Applied Biosystems, Foster City, CA, USA). The reverse transcription reaction was carried out in an iCycler Thermal Cycler (BioRad) with the following parameters: 25°C for 10 min, 42°C for 15 min and 99°C for 5 min. Samples were kept on ice until analyzed by real-time PCR.
For real-time PCR reaction, 2 × TaqMan Master Mix (Applied Biosystem) was mixed with 100 ng of transcribed sample mRNA. Samples, nontemplate controls and primers (Applied Biosystems assay-on-Demand) were added to a 96-well plate. Endogeneous primers were 18S (assay ID: Hs99999901_s1) and glyceralde-hyde-3-phosphatase dehydrogenase (GAPDH) (assay ID: Hs00266705_g1). Target primers were BMP-2 (assay ID: Hs00154192_m1), BSP (assay ID: Hs00173720_m1) and OP (assay ID: Hs00167093_m1). Each gene expression profile for each sample was completed in duplicate. The cDNA was amplified in Applied Biosystems ABI PRISM 7000 Sequence Detection System using the following thermal cycling parameters: 50°C, 2 min (1 cycle); 95°C, 10 min (1 cycle); PCR (40 cycles): step 1, denaturation: 95°C, 15 s; step 2, annealing/extension/detection: 60°C, 1 min.
Data were analyzed using Applied Biosystems ABI PRISM® 7000 System Software, v1.2.3 with relative quantification study. Baseline and cycle threshold values were determined automatically via the software and verified in each case. The ratio of treated mRNA levels to control mRNA levels was calculated using the ΔΔ cycle threshold method.
Statistics
Control and experimental cells were compared using a paired student’s t-test. For real-time PCR gene expression experiments, a Mann–Whitney Rank Sum Test was performed for cells exposed to osteogenic supplements (hMSC-Osts) compared with cells in basal media (hMSCs) at each time point and a Kruskal–Wallis one-way analysis of variance (ANOVA) on ranks was performed with all shear stress groups relative to the control group for each cell type at each time point.
Results
Effect of shear stress on cell morphology
Human MSCs and hMSC-Osts were exposed to 9 dynes/cm2 shear stress for 24 h 3 days after plating on slides and the addition of the basal and osteogenic media, respectively. Control (no exposure to shear stress) hMSCs were spindle-shaped with a fibroblast-like morphology (Figure 2A). After exposure to osteogenic supplements, the hMSC-Osts (Figure 2C) had a cuboidal shape similar to that of osteoblasts. Shear stress had little effect on the alignment and shape of hMSCs and hMSC-Osts (Figure 2A VSB & C VS D).
Figure 2. Morphology of human mesenchymal stem cells and human mesenchymal cells exposed to osteogenic supplements exposed to shear stress (left to right) for 24 h.
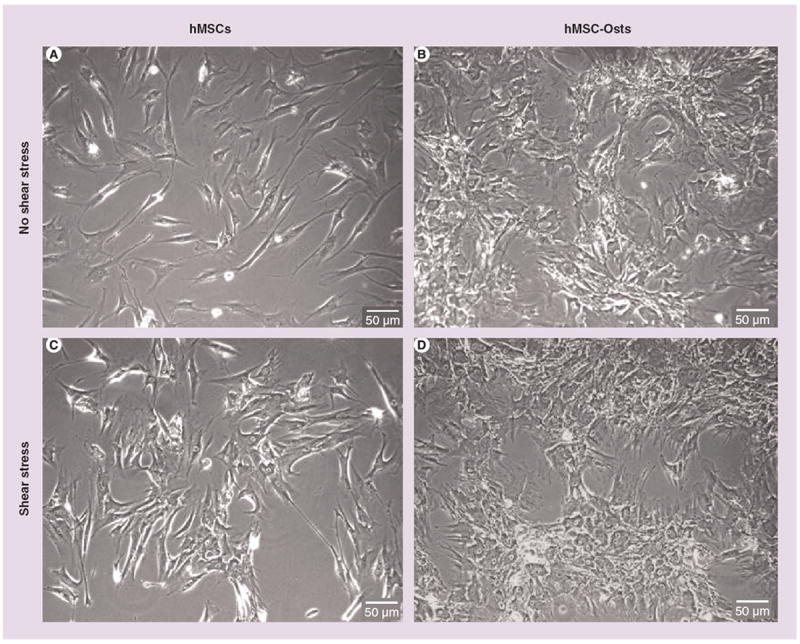
Phase contrast microscopy images of (A) hMSCs and (B) hMSC-Osts not exposed to shear stress, and (C) hMSCs and (D) hMSC-Osts exposed to shear stress. hMSCs changed their fibroblast-like, spindle-shaped morphology to a more cuboidal morphology after exposure to osteogenic supplements (A vs B). There were no obvious changes in morphology of hMSCs (A vs C) and hMSC-Osts (B vs D) after 24 h exposure to 9 dynes/cm2 steady shear stress. hMSC: Human mesenchymal cell; hMSC-Osts: Human mesenchymal cells exposed to osteogenic supplement.
Effect of shear stress on cell membrane ALP activity
Cells were treated as above. Control hMSC-Osts displayed a marked increase in ALP staining intensity compared with control hMSCs (Figure 3A VS C). Staining intensity increased from days 7 to 11 for hMSC-Osts (Figure 3B & D VS F & H). Shear stress had no noticeable effect on ALP staining intensity of the hMSCs and hMSC-Osts (Figure 3A VS B, C VS D, E VS F & G VS H).
Figure 3. Alkaline phosphatase stain of human mesenchymal stem cells and human mesenchymal cells exposed to osteogenic supplements exposed to shear stress.

(A) hMSCs and (B) hMSC-Osts, in a 7 day experiment, not exposed to shear stress; (C) hMSCs and (D) hMSCs-Osts in a 7 day experiment, exposed to shear stress; (E) hMSCs and (F) hMSCs-Osts, in an 11 day experiment, not exposed to shear stress; and (G) hMSCs and (H) hMSCs-Osts in an 11 day experiment, exposed to shear stress. An increase in alkaline phosphatase expression evidenced by red staining was displayed after 7 days exposure to osteogenic supplements (A vs B), (E vs F). There were minimal differences in both cell groups upon exposure to fluid shear stress for 24 h (A vs C), (B vs D), (E vs G), (F vs H).
hMSC: Human mesenchymal cell; hMSC-Osts: Human mesenchymal cells exposed to osteogenic supplement.
Effect of shear stress on cellular & media ALP activity
For all time points, ALP activity was greater in hMSC-Osts than in hMSCs (Figure 4A). Cells were treated as above and assayed for ALP activity immediately and 3 days after 24 h of shear stress exposure, and ALP activity of hMSC-Osts exposed to shear stress was significantly lower than that of control hMSC-Osts at day 7 and 11 (38 and 80% less, respectively) (Figure 4A). There was no change in hMSC and hMSC-Ost ALP activity immediately after exposure to shear stress.
Figure 4. Alkaline phosphatase activity regulation of human mesenchymal stem cells and human mesenchymal cells exposed to osteogenic supplements by fluid shear stress.

Fluid SS significantly reduced sustained cellular ALP activity in hMSC-Osts exposed to 9 dynes/cm2 steady SS for 24 h compared with hMSC-Osts not exposed to SS. Cellular ALP was analyzed 3 days after experimentation at both 7 and 11 days, and immediately after experimentation at 8 days (A). Fluid SS significantly increased released ALP activity in hMSC-Osts exposed to 9 dynes/cm2 steady SS for 24 h compared with hMSC-Osts not exposed to SS. Media ALP was analyzed 3 days after experimentation at 8 days (B). Values are mean ± standard error of the mean of independent experiments (7 days, n = 4; 8 days, hMSC n = 6, hMSC-Ost n = 7; 11 days, n = 5; cellular ALP, n = 6; released ALP, n = 4; cellular plus released ALP, n = 4).
*p < 0.05; **p < 0.01.
ALP: Alkaline phosphatase; hMSC: Human mesenchymal cell; hMSC-Osts: Human mesenchymal cells exposed to osteogenic supplement; SS: Shear stress.
Since cellular ALP significantly decreased after exposure to shear stress, we wanted to determine if this was due to release of ALP into the media during shear stress exposure. On day 8, immediately after exposure to 24 h shear stress on day 7, media ALP activity was significantly higher for hMSC-Osts (6.98 vs 13.71 mU/ng/ml) (Figure 4B) than for control hMSC-Osts at 8 days.
Effect of osteogenic supplements on BMP-2, BSP & OP gene expression
After 4 days, BMP-2 and BSP gene expression levels were not significantly different between hMSC-Osts and hMSCs. However, OP expression was 0.8-fold less than in hMSCs (Figure 5). After 8 days, there was a significantly higher (0.9-fold) BSP expression in hMSC-Osts relative to hMSCs (Figure 5).
Figure 5. Real-time PCR gene expression of human mesenchymal cells exposed to osteogenic media.
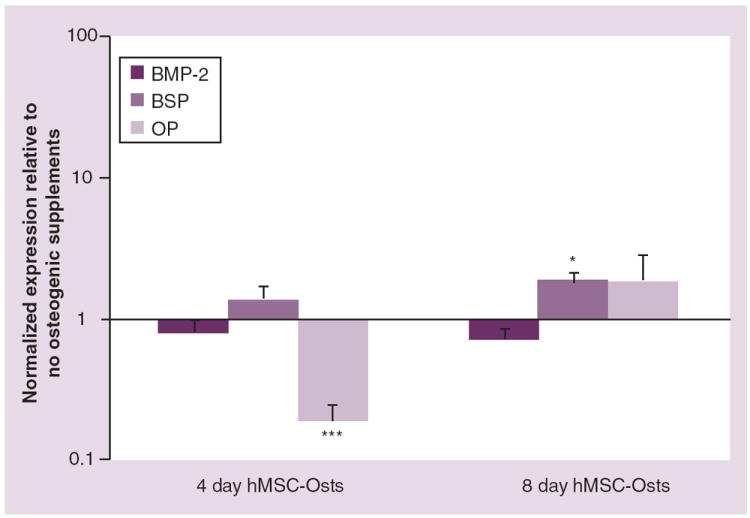
Human MSCs were cultured in osteogenic supplemented media for 4 and 8 days (hMSC-Osts). The gene expressions analyzed were BMP-2, BSP and OP; all were calculated using the ΔΔ cycle threshold method normalized to GAPDH. Those values were then normalized to undifferentiated hMSCs and mean values ± standard error of the mean plotted as relative values on a log scale.
*p < 0.05; ***p < 0.001 compared with no osteogenic supplements, Mann–Whitney Rank Sum Test.
BMP: Bone morphogenetic protein; BSP: Bone sialoprotein; hMSC-Osts: Human mesenchymal cells exposed to osteogenic supplement; OP: Osteopontin.
Effect of shear stress on BMP-2, BSP & OP gene expression
To analyze the effect of shear stress on gene expression, hMSCs and hMSC-Osts cultured for 3 and 7 days were exposed to 4, 15 and 22 dynes/cm2 shear stress for 24 h. The expressions of genes affiliated with an osteogenic phenotype were increased in hMSCs in response to 24 h of shear stress after 3 days of static culture. BMP-2 expression was significantly upregulated in a shear stress magnitude-dependent manner compared with control hMSCs (Figure 6A). BSP expression appeared to increase with increasing shear stress magnitudes; however, it was not significantly higher than in controls for any magnitude of shear stress (Figure 6B). OP mRNA was significantly (2.1-fold) higher after exposure to 22 dynes/cm2 shear stress (Figure 6C).
Figure 6. Real-time PCR gene expression of human mesenchymal cells exposed to shear stress.

Cells were exposed to 4, 15 and 22 dynes/cm2 for 24 h after 3 and 7 days in culture in basal (hMSC) and osteogenic supplemented (hMSC-Osts) media. The gene expression of BMP-2 (A), BSP (B) and OP (C) was analyzed. They were calculated using the ΔΔ cycle threshold method and all were normalized to GAPDH. Those values were then normalized to hMSCs and hMSC-Osts not exposed to shear stress and mean values ± standard error of the mean plotted as relative values on a log scale. *p < 0.05 relative to no shear stress in the same group, Kruskal–Wallis one-way ANOVA on ranks. BMP: Bone morphogenetic protein; BSP: Bone sialoprotein; hMSC: Human mesenchymal cell; hMSC-Osts: Human mesenchymal cells exposed to osteogenic supplement; OP: Osteopontin.
Human MSCs subjected to shear stress on day 7 showed greater effects on mRNA expression compared with those subjected to the stress on day 3. BMP-2 expression was highest after 22 dynes/cm2 of shear stress compared with control cells (Figure 6A). BSP mRNA was significantly increased 9.4- and 6.3-fold at 15 and 22 dynes/cm2, respectively (Figure 6B). There was a significant 7.7-fold increase in OP expression in hMSCs exposed to 22 dynes/cm2 shear stress (Figure 6C). In hMSC-Osts cultured for 3 days and then exposed to 24 h shear stress, only OP mRNA levels were increased 1.8- and 3.8-fold at 4 and 22 dynes/cm2, respectively, relative to their controls (Figure 6C).
Discussion
Cells for tissue engineering bone repair/replacement should be readily available and have osteogenic potential. MSCs meet this requirement as they can be harvested from the patient, expanded and differentiated into bone cells [6]. Here we show that mechanical stimulation alone affects the differentiation of hMSCs by upregulating the expression of osteogenic factors, BMP-2, a growth factor, and BSP and OP, matrix proteins whose production increases in response to chemical osteogenic treatments [32,33].
Alkaline phosphatase activity is an early marker of osteogenic differentiation [11] and plays a role in early mineralization via its enzymatic hydrolysis activity [34]. This was demonstrated in the current study, where cellular ALP activity was higher in MSCs grown in osteogenic supplements. There was no effect of shear stress on cellular ALP levels found in non-osteogenic conditions. However, in osteogenic conditions, ALP levels were significantly lower 3 days after shear stress exposure. Interestingly, Grellier et al. subjected hMSCs to shorter bouts of fluid shear stress in osteogenic conditions and showed that 30 min exposure upregulated ALP mRNA but 90 min reduced it to almost basal levels [35]. Furthermore, MSCs exposed to an oscillatory fluid flow profile showed reduced ALP activity despite upregulating OP and osteocalcin mRNA under the same conditions [36]. In other studies, tensile loading of hMSCs upregulated ALP activity in nonosteogenic medium [26] and compression loading of MSCs in a porous scaffold upregulated ALP activity in nonosteogenic conditions but not when osteogenic supplements were added [25]. Clearly, the effect of mechanical stress on ALP activity and the downstream outcomes of ALP are complex.
Despite no change in cellular ALP activity levels, we found that 24 h exposure to shear stress following 7 days culture in osteogenic supplemented media induced a greater amount of ALP activity in the media than control cells, indicating the possibility that ALP is being produced and secreted into the media. ALP is a glycosylphosphatidylinisotol protein that is anchored to the cell membrane via a phosphoethanolamine bound to an oligosaccharide [37]. It is possible that fluid shear stresses of sufficient magnitude, such as those used in our experiments, may be able to break these bonds. A release of ALP to cell media during MC3T3 differentiation has been noted with the onset of mineralization [38]. If more ALP was released in cells subjected to shear stress this would have created a deficit of ALP within the cells (as measured 3 days after shear stress exposure) and an increase of ALP in the surrounding media. This could explain why in the studies discussed above, there was lower cellular ALP activity in experiments in which fluid flow was the mechanical stimulus [26] but higher ALP when substrate strain was the stimulus [25]. One of the pathways through which shear stress stimulates osteogenic differentiation of MSCs may include the regulation of autocrine factors. Here we show that BMP-2, an important inducer of osteogenic differentiation, is regulated in hMSCs by shear stress in a magnitude-dependent manner in vitro. This is particularly interesting given that osteogenic supplements did not upregulate BMP-2 mRNA in our experiments and hMSCs do not respond well to exogenous BMP-2 in standard osteogenic media conditions [39], although many genes involved in the BMP-2 pathways have been shown to be upregulated in hMSCs in osteogenic medium [33]. In vivo, there is a rise in BMP-2 levels in bone tissue and an induction of remodeling with an increase in loads [40]. This upregulation in BMP-2 expression may be due not only to an increase in its production by fully differentiated bone cells but also by bone marrow MSCs, which are also likely to be exposed to increased levels of shear stress as a result of loading on the whole bone. By contrast, there was a less consistent effect of shear stress on BMP-2 mRNA when cells were stimulated with osteogenic supplements. This is in agreement with Sharp et al., who showed no effect of 24 h continuous shear stress in osteogenic conditions on BMP-2 mRNA levels immediately post flow [41].
It has been previously shown that OP mRNA is upregulated by hMSC exposure to osteogenic supplements [32,33]. However, OP expression by stem cells exposed to osteogenic supplements has been found to be variably regulated; for instance, Diefenderfer et al. also showed that dexamethasone slightly reduced OP mRNA expression in MSCs from some human donors [42]. In differentiated bone cells, OP expression is increased upon exposure to shear stress in vitro [43], cyclic strain [44,45] and hydrostatic pressure [46]. Likewise, in vivo, OP is upregulated after mechanical loading [47]. In the current experiments, OP gene expression was higher in hMSCs exposed to shear stress and was the only gene significantly upregulated by shear stress in hMSC-Osts. Similarly, after rat MSCs were exposed to continuous shear stress in osteogenic media, there was no effect on BMP-2, as noted above, while there was an effect on OP [41]. Less is known about the response of BSP to mechanical loading in mature bone, but its role as a mechanosensitive extracellular matrix protein in stimulation of hMSCs is becoming better understood. Here we show that BSP is significantly upregulated by shear stress in the 8-day group, reflecting its later appearance in osteogenic differentiation, where it is associated with the onset of mineralization [48].
Disruption of the cytoskeleton can alter the response of cells to shear stress [49,50]. Previously, we have shown that when differentiating to osteoblasts, the cytoskeleton of hMSCs changes from thick, defined actin stress fibers to a more dispersed actin fiber arrangement. Likewise, the cell becomes less stiff upon differentiation [51,52]. In the present study, we found that hMSCs are more sensitive to shear stress than hMSCs differentiated into bone-like cells. We suggest that hMSCs have greater sensitivity to shear stress than early osteoblast-like cells differentiating from hMSCs because of the higher cell stiffness caused by the more defined cytoskeleton in the undifferentiated cells. The cell signaling pathways involved in transmitting the mechanical signal into an osteogenic response by the cells are unknown; however, they are highly likely to involve the MAPK pathways, as shear stress was shown to stimulate upregulation of mRNA for a MAPK kinase in hMSCs [53] and the ERK and P38 pathways in osteoblasts [43]. MAPK have been well demonstrated to be key regulators in the cytoskeleton-mediated osteogenesis of hMSCs [54,55].
It is interesting that without soluble factors to stimulate differentiation along any specific lineage, cells exposed to shear stress exhibited bone matrix-specific differentiation markers such as OP and BSP. While we did not test for markers of other lineages, this indicates that they preferentially differentiate to osteoblasts rather than alternative mesenchymal lineage cells, such as myoblasts, adipocytes or fibroblasts. This may be because specific mechanical forces allow differentiation to specific lineages. For example, human MSCs seeded on collagen gels and subjected to dynamic tensile and torsional forces in the range of those experienced by tendons and ligaments differentiated to ligament-like fibroblasts without the aid of exogenous growth differentiation stimulants [56]. Another explanation is that these cells were grown on a hard substrate (polycarbonate or glass) and MSCs are more likely to differentiate towards the osteogenic lineage on hard compared with soft substrates in the absence of other stimulators of cell differentiation [57]. It would be interesting to examine whether the osteogenic response to shear stress is as robust on softer substrates, which tend to favor myogenic differentiation [58] or cell quiescence [59]. A substrate-specific effect on the expression of chondrogenic versus osteogenic markers in hMSCs grown in 3D has also been observed [60].
Conclusion
Human bone marrow MSCs from multiple donors and passage numbers respond to fluid shear stress by increasing the expression of the bone markers BMP-2, BSP and OP. Human MSCs are more sensitive to fluid shear stress than chemically differentiated osteoblast-like cells from hMSCs and sensitivity of hMSCs to shear stress is greater after they have a longer attachment time prior to shear stress exposure. It is important to apply the appropriate pretreatment to hMSC prior to clinical application and that the pretreatment does not induce unwanted side effects. Exposure of proliferating MSCs to shear stress may be a cost-effective and straightforward method of predifferentiating cells without chemical treatment for orthopedic and other applications.
Future perspective
There is interest in increasing the economic efficiency of the processing of human MSCs in sterile bioreactor conditions for therapeutic use of these cells. Much recent work in the field indicates that mechanical preconditioning of these cells may be an important step in this culture process. Culturing cells in the presence of fluid flow-induced shear stress without chemical differentiation has the potential to become a routine method for preconditioning.
Executive summary.
-
▪
Human mesenchymal stem cells respond to laminar fluid flow-induced shear stress applied in a parallel plate flow chamber with upregulation of osteogenic markers. This response is increased after several days of preculture.
-
▪
Human mesenchymal stem cells cultured in osteogenic media do not show a consistent additive affect of shear stress exposure.
-
▪
Mechanical conditioning could be a useful way to avoid chemical differentiation in the preculture of human mesenchymal stem cells for therapeutic use.
Acknowledgments
This work was supported by the NIH, grant number: NIH5RC2DE020767.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Bibliography
- 1.Bennett JH, Joyner CJ, Triffitt JT, Owen ME. Adipocytic cells cultured from marrow have osteogenic potential. J Cell Sci. 1991;99(Pt 1):131–139. doi: 10.1242/jcs.99.1.131. [DOI] [PubMed] [Google Scholar]
- 2.Young RG, Butler DL, Weber W. Use of mesenchymal stem cells in a collagen matrix for achilles tendon repair. J Orthop Res. 1998;16:406–413. doi: 10.1002/jor.1100160403. [DOI] [PubMed] [Google Scholar]
- 3.Azizi SA, Stokes D, Augelli BJ. Engraftment and migration of human bone marrow stromal cells implanted in the brains of albino rats – similarities to astrocyte grafts. Proc Natl Acad Sci USA. 1998;95:3908–3913. doi: 10.1073/pnas.95.7.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrari G, Cusella-De Angelis G, Coletta M. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998;279:1528–1530. doi: 10.1126/science.279.5356.1528. [DOI] [PubMed] [Google Scholar]
- 5.Alhadlaq A, Elisseeff JH, Hong L, et al. Adult stem cell driven genesis of human-shaped articular condyle. Ann Biomed Eng. 2004;32(7):911–923. doi: 10.1023/b:abme.0000032454.53116.ee. [DOI] [PubMed] [Google Scholar]
- 6.Marion NW, Mao JJ. Mesenchymal stem cells and tissue engineering. Methods Enzymol. 2006;420:339–361. doi: 10.1016/S0076-6879(06)20016-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee CH, Marion NW, Hollister S, Mao JJ. Tissue formation and vascularization in anatomically shaped human joint condyle ectopically in vivo. Tissue Eng Part A. 2009;15(12):3923–3930. doi: 10.1089/ten.tea.2008.0653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caplan AI, Bruder SP. Mesenchymal stem cells: building blocks for molecular medicine in the 21st century. Trends Mol Med. 2001;7:259–264. doi: 10.1016/s1471-4914(01)02016-0. [DOI] [PubMed] [Google Scholar]
- 9.Alhadlaq A, Mao JJ. Mesenchymal stem cells: isolation and therapeutics. Stem Cells Dev. 2004;13(4):436–448. doi: 10.1089/scd.2004.13.436. [DOI] [PubMed] [Google Scholar]
- 10.Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7(2):211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 11.Pittenger MF, Mackay AM, Beck SC, et al. Multilieneage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 12.Cooper JA, Hewison M, Stewart PM. Glucocorticoid activity, inactivity and the osteoblast. J Endocrinol. 1999;163:159–164. doi: 10.1677/joe.0.1630159. [DOI] [PubMed] [Google Scholar]
- 13.Maniatopoulos C, Sodek J, Melcher AH. Bone formation in vitro by stromal cells obtained from bone marrow of young adult rats. Cell Tissue Res. 1988;254:317–330. doi: 10.1007/BF00225804. [DOI] [PubMed] [Google Scholar]
- 14.Choong PF, Martin TJ, Ng KW. Effects of ascorbic acid, calcitriol, and retinoic acid on the differentiation of preosteoblasts. J Orthop Res. 1993;11(5):638–647. doi: 10.1002/jor.1100110505. [DOI] [PubMed] [Google Scholar]
- 15.Osyczka AM, Diefenderfer DL, Bhargave G, Leboy PS. Different effects of BMP-2 on marrow stromal cells from human and rat bone. Cells Tissues Organs. 2004;176(1–3):109–119. doi: 10.1159/000075032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nuttall ME, Patton AJ, Olivera DL, Nadeau DP, Gowen M. Human trabecular bone cells are able to express both osteoblastic and adipocytic phenotype: implications for osteopenic disorders. J Bone Miner Res. 1998;13(3):371–382. doi: 10.1359/jbmr.1998.13.3.371. [DOI] [PubMed] [Google Scholar]
- 17.Reid IR. Glucocorticoid-induced osteoporosis. Baillieres Best Pract Res Clin Endocrinol Metab. 2000;14(2):279–298. doi: 10.1053/beem.2000.0074. [DOI] [PubMed] [Google Scholar]
- 18.Weinstein RS, Jilka RL, Parfitt AM, Manolagas SC. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. Potential mechanisms of their deleterious effects on bone. J Clin Invest. 1998;102(2):274–282. doi: 10.1172/JCI2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Batson EL, Reilly GC, Currey JD, Balderson DS. Postexercise and positional variation in mechanical properties of the radius in young horses. Equine Vet J. 2000;32:95–100. doi: 10.2746/042516400777591570. [DOI] [PubMed] [Google Scholar]
- 20.Kopher RA, Mao JJ. Suture growth modulated by the oscillatory component of micromechanical strain. J Bone Miner Res. 2003;18:521–528. doi: 10.1359/jbmr.2003.18.3.521. [DOI] [PubMed] [Google Scholar]
- 21.You J, Yellowley CE, Donahue HJ, Zhang Y, Chen Q, Jacobs CR. Substrate deformation levels associated with routine physical activity are less stimulatory to bone cells relative to loading-induced oscillatory fluid flow. J Biomech Eng. 2000;122(4):387–393. doi: 10.1115/1.1287161. [DOI] [PubMed] [Google Scholar]
- 22.McGarry JG, Klein-Nulend J, Mullender MG, Prendergast PJ. A comparison of strain and fluid shear stress in stimulating bone cell responses – a computational and experimental study. FASEB J. 2005;19(3):482–484. doi: 10.1096/fj.04-2210fje. [DOI] [PubMed] [Google Scholar]
- 23.Tami AE, Schaffler MB, Tate MLK. Probing the tissue to subcellular level structure underlying bone’s molecular sieving function. Biorheology. 2003;40(6):577–590. [PubMed] [Google Scholar]
- 24.Reich KM, Gay CV, Frangos JA. Fluid shear stress as a mediator of osteoblast cyclic adenosine monophosphate production. J Cell Physiol. 1990;143:100–104. doi: 10.1002/jcp.1041430113. [DOI] [PubMed] [Google Scholar]
- 25.Sittichockechaiwut A, Scutt AS, Reilly GC. Short bouts of mechanical loading are as effective as dexamethasone at inducing matrix production by human bone marrow mesenchymal stem cell. Eur Cell Mater. 2010;20:45–57. doi: 10.22203/ecm.v020a05. [DOI] [PubMed] [Google Scholar]
- 26.Friedl G, Schmidt H, Rehak I, Kostner G, Schauenstein K, Windhager R. Undifferentiated human mesenchymal stem cells (hMSCs) are highly sensitive to mechanical strain: transcriptionally controlled early osteo–chondrogenic response in vitro. Osteoarthritis Cartilage. 2007;15(11):1293–1300. doi: 10.1016/j.joca.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 27.Kreke MR, Huckle WR, Goldstein AS. Fluid flow stimulates expression of osteopontin and bone sialoprotein by bone marrow stromal cells in a temporally dependent manner. Bone. 2005;36(6):1047–1055. doi: 10.1016/j.bone.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 28.Datta N, Pham QP, Sharma U, Sikavitsas VI, Jansen JA, Mikos AG. In vitro generated extracellular matrix and fluid shear stress synergistically enhance 3D osteoblastic differentiation. Proc Natl Acad Sci USA. 2006;103(8):2488–2493. doi: 10.1073/pnas.0505661103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jungreuthmayer C, Donahue SW, Jaasma MJ, et al. A comparative study of shear stresses in collagen–glycosaminoglycan and calcium phosphate scaffolds in bone tissue-engineering bioreactors. Tissue Eng Part A. 2009;15(5):1141–1149. doi: 10.1089/ten.tea.2008.0204. [DOI] [PubMed] [Google Scholar]
- 30.Frangos JA, Eskin SG, McIntire LV, Ives CL. Flow effects on prostacyclin production by cultured human endothelial cells. Science. 1985;227:1477–1479. doi: 10.1126/science.3883488. [DOI] [PubMed] [Google Scholar]
- 31.Allen FD, Hung CT, Pollack SR, Brighton CT. Serum modulates the intracellular calcium response of primary cultured bone cells to shear flow. J Biomech. 2000;33(12):1585–1591. doi: 10.1016/s0021-9290(00)00144-5. [DOI] [PubMed] [Google Scholar]
- 32.Frank O, Heim M, Jakob M, et al. Real-time quantitative RT-PCR analysis of human bone marrow stromal cells during osteogenic differentiation in vitro. J Cell Biochem. 2002;85(4):737–746. doi: 10.1002/jcb.10174. [DOI] [PubMed] [Google Scholar]
- 33.Kulterer B, Friedl G, Jandrositz A, et al. Gene expression profiling of human mesenchymal stem cells derived from bone marrow during expansion and osteoblast differentiation. BMC Genomics. 2007;8:70. doi: 10.1186/1471-2164-8-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sugawara Y, Suzuki K, Koshikawa M, Ando M, Iida J. Necessity of enzymatic activity of alkaline phosphatase for mineralization of osteoblastic cells. Jpn J Pharmacol. 2002;88(3):262–269. doi: 10.1254/jjp.88.262. [DOI] [PubMed] [Google Scholar]
- 35.Grellier M, Bareille R, Bourget C, Amedee J. Responsiveness of human bone marrow stromal cells to shear stress. J Tissue Eng Regen Med. 2009;3(4):302–309. doi: 10.1002/term.166. [DOI] [PubMed] [Google Scholar]
- 36.Li YJ, Batra NN, You L, et al. Oscillatory fluid flow affects human marrow stromal cell proliferation and differentiation. J Orthop Res. 2004;22(6):1283–1289. doi: 10.1016/j.orthres.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 37.Low MG, Saltiel AR. Structural and functional roles of glycosyl-phosphatidylinositol in membranes. Science. 1988;239(4837):268–275. doi: 10.1126/science.3276003. [DOI] [PubMed] [Google Scholar]
- 38.Yoshikawa M, Suzuki K, Takashi K, Koshikawa M, Imai T, Matsumoto A. Quantitative analysis of alkaline phosphtase activity and mineralization of a clonal osteoblast-like cell mc3t3-e1. J Hard Tis Bio. 1999;8(2):37–42. [Google Scholar]
- 39.Diefenderfer DL, Osyczka AM, Reilly GC, Leboy PS. BMP responsiveness in human mesenchymal stem cells. Connect Tissue Res. 2003;44(Suppl. 1):305–311. [PubMed] [Google Scholar]
- 40.Radomisli TE, Moore DC, Barrach HJ, Keeping HS, Ehrlich MG. Weight-bearing alters the expression of collagen types I and II, BMP 2/4 and osteocalcin in the early stages of distraction osteogenesis. J Orthop Res. 2001;19(6):1049–1056. doi: 10.1016/S0736-0266(01)00044-4. [DOI] [PubMed] [Google Scholar]
- 41.Sharp LA, Lee YW, Goldstein AS. Effect of low-frequency pulsatile flow on expression of osteoblastic genes by bone marrow stromal cells. Ann Biomed Eng. 2009;37(3):445–453. doi: 10.1007/s10439-008-9632-7. [DOI] [PubMed] [Google Scholar]
- 42.Diefenderfer DL, Osyczka AM, Garino JP, Leboy PS. Regulation of BMP-induced transcription in cultured human bone marrow stromal cells. J Bone Joint Surg Am. 2003;85–A(Suppl. 3):19–28. doi: 10.2106/00004623-200300003-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.You J, Reilly GC, Zhen X, et al. Osteopontin gene regulation by oscillatory fluid flow via intracellular calcium mobilization and activation of mitogen-activated protein kinase in mc3t3-e1 osteoblasts. J Biol Chem. 2001;276:13365–13371. doi: 10.1074/jbc.M009846200. [DOI] [PubMed] [Google Scholar]
- 44.Harter LV, Hruska KA, Duncan RL. Human osteoblast-like cells respond to mechanical strain with increased bone matrix protein production independent of hormonal regulation. Endocrinology. 1995;136(2):528–535. doi: 10.1210/endo.136.2.7530647. [DOI] [PubMed] [Google Scholar]
- 45.Sittichockechaiwut A, Scutt AM, Ryan AJ, Bonewald LF, Reilly GC. Use of rapidly mineralising osteoblasts and short periods of mechanical loading to accelerate matrix maturation in 3D scaffolds. Bone. 2009;44(5):822–829. doi: 10.1016/j.bone.2008.12.027. [DOI] [PubMed] [Google Scholar]
- 46.Klein-Nulend J, Roelofsen J, Semeins CM, Bronckers AL, Burger EH. Mechanical stimulation of osteopontin mrna expression and synthesis in bone cell cultures. J Cell Physiol. 1997;170(2):174–181. doi: 10.1002/(SICI)1097-4652(199702)170:2<174::AID-JCP9>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 47.Miles RR, Turner CH, Santerre R, et al. Analysis of differential gene expression in rat tibia after an osteogenic stimulus in vivo: mechanical loading regulates osteopontin and myeloperoxidase. J Cell Biochem. 1998;68(3):355–365. doi: 10.1002/(sici)1097-4644(19980301)68:3<355::aid-jcb6>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 48.Ganss B, Kim RH, Sodek J. Bone sialoprotein. Crit Rev Oral Biol Med. 1999;10(1):79–98. doi: 10.1177/10454411990100010401. [DOI] [PubMed] [Google Scholar]
- 49.Ajubi NE, Klein-Nulend J, Nijweide PJ, Vrijheid-Lammers T, Alblas MJ, Burger EH. Pulsating fluid flow increases prostaglandin production by cultured chicken osteocytes – a cytoskeleton-dependent process. Biochem Biophys Res Commun. 1996;225(1):62–68. doi: 10.1006/bbrc.1996.1131. [DOI] [PubMed] [Google Scholar]
- 50.McGarry JG, Klein-Nulend J, Prendergast PJ. The effect of cytoskeletal disruption on pulsatile fluid flow-induced nitric oxide and prostaglandin E2 release in osteocytes and osteoblasts. Biochem Biophys Res Commun. 2005;330(1):341–348. doi: 10.1016/j.bbrc.2005.02.175. [DOI] [PubMed] [Google Scholar]
- 51.Yourek G, Hussain MA, Mao JJ. Cytoskeletal changes of mesenchymal stem cells during differentiation. ASAIO J. 2007;53(2):219–228. doi: 10.1097/MAT.0b013e31802deb2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yourek G, Alhadlaq A, Patel A, et al. Biological Nanostructures And Applications Of Nanostructures In Biology Electrical Mechanical And Optical Properties. Kluwer Academic Publishing; NY, USA: 2004. Nanophysical properties of living cells: the cytoskeleton; pp. 69–97. [Google Scholar]
- 53.Glossop JR, Cartmell SH. Effect of fluid flow-induced shear stress on human mesenchymal stem cells: differential gene expression of IL1B and MAP3K8 in MAPK signaling. Gene Expr Patterns. 2009;9(5):381–388. doi: 10.1016/j.gep.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 54.Osyczka AM, Leboy PS. Bone morphogenetic protein regulation of early osteoblast genes in human marrow stromal cells is mediated by extracellular signal-regulated kinase and phosphatidylinositol 3-kinase signaling. Endocrinology. 2005;146(8):3428–3437. doi: 10.1210/en.2005-0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marie PJ, Fromigue O. Osteogenic differentiation of human marrow-derived mesenchymal stem cells. Regen Med. 2006;1(4):539–548. doi: 10.2217/17460751.1.4.539. [DOI] [PubMed] [Google Scholar]
- 56.Altman GH, Horan RL, Martin I, et al. Cell differentiation by mechanical stress. FASEB J. 2002;16(2):270–272. doi: 10.1096/fj.01-0656fje. [DOI] [PubMed] [Google Scholar]
- 57.Reilly GC, Engler AJ. Intrinsic extracellular matrix properties regulate stem cell differentiation. J Biomech. 2009;43:55–62. doi: 10.1016/j.jbiomech.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 58.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 59.Winer JP, Janmey PA, McCormick ME, Funaki M. Bone marrow-derived human mesenchymal stem cells become quiescent on soft substrates but remain responsive to chemical or mechanical stimuli. Tissue Eng Part A. 2009;15(1):147–154. doi: 10.1089/ten.tea.2007.0388. [DOI] [PubMed] [Google Scholar]
- 60.Kopher RA. PhD thesis. University of Illinois at Chicago College of Engineering; IL, USA: 2006. Engineered osteogenesis of adult stem cells in polymeric scaffolds exposed to various mechanical stimuli. [Google Scholar]


