Abstract
Potassium homeostasis plays an important role in the control of neuronal excitability, and diminished buffering of extracellular K results in neuronal Hyperexcitability and abnormal synchronization. Astrocytes are the cellular elements primarily involved in this process. Potassium uptake into astrocytes occurs, at least in part, through voltage-dependent channels, but the exact mechanisms involved are not fully understood. Although most glial recordings reveal expression of inward rectifier currents (KIR), it is not clear how spatial buffering consisting of accumulation and release of potassium may be mediated by exclusively inward potassium fluxes. We hypothesized that a combination of inward and outward rectifiers cooperate in the process of spatial buffering. Given the pharmacological properties of potassium homeostasis (sensitivity to Cs+), members of the ether-a-go-go (ERG) channel family widely expressed in the nervous system could underlie part of the process. We used electrophysiological recordings and pharmacological manipulations to demonstrate the expression of ERG-type currents in cultured and in situ hippocampal astrocytes. Specific ERG blockers (dofetilide and E 4031) inhibited hyperpolarization- and depolarization-activated glial currents, and ERG blockade impaired clearance of extracellular potassium with little direct effect on hippocampal neuron excitability. Immunocytochemical analysis revealed ERG protein mostly confined to astrocytes; ERG immunoreactivity was absent in presynaptic and postsynaptic elements, but pronounced in glia surrounding the synaptic cleft. Oligodendroglia did not reveal ERG immunoreactivity. Intense immunoreactivity was also found in perivascular astrocytic end feet at the blood–brain barrier. cDNA amplification showed that cortical astrocytes selectively expressHERG1, but not HERG2–3 genes. This study provides insight into a possible physiological role of hippocampal ERG channels and links activation of ERG to control of potassium homeostasis.
Keywords: spatial buffering, glia-neuronal interactions, epileptogenesis, long QT, synchronization, homeostasis, inherited epilepsy
Gray matter astrocytes participate in a variety of homeostatic processes, including uptake of neurotransmitters and clearance of extracellular ions (Araque et al., 1999). Perhaps the best documented role for astrocytes is the control of extracellular potassium concentration by a mechanism labeled “spatial buffering” or “siphoning” (Kuffler et al., 1966;Newman, 1995). Evidence linking astrocytic physiology to potassium homeostasis is based on simultaneous glial recordings during neuronal activity and direct measurements of [K+]out and [K+]in (Ballanyi et al., 1993). Blockade of glial potassium channels has been shown to cause abnormal [K+]outaccumulation resulting in epileptiform activity (Janigro et al., 1997a;D'Ambrosio et al., 1998). Interestingly, post-traumatic changes in hippocampal glia parallel the effects of acute blockade by external Cs+ (D'Ambrosio et al., 1999); these include changes in neuronal excitability, increased basal extracellular potassium concentrations, and abnormal potassium transients in response to sustained neuronal activity.
Potassium uptake into astrocytes occurs, at least in part, through voltage-dependent channels, but the exact mechanisms involved are not fully understood. For example, although most glial recordings reveal expression of inward rectifier currents (KIR), it is not clear how spatial buffering consisting of accumulation and release of potassium may be mediated by exclusively inward potassium fluxes. Furthermore, the spatial buffering theory is based on the assumption that glial resting membrane potential (RMP) is close to EK, an assumption frequently challenged by direct experimental evidence (McKhann et al., 1997b). Recordings from astrocytes have demonstrated that although many have an RMP consistent withKIR expression, a significant proportion of neocortical and hippocampal astrocytes have RMPs more positive than EK, and hence, this is inconsistent with an exclusive role ofKIR in determining RMP. Moreover, the biophysical properties of KIRexpressed in astrocytes cannot be fully reconciled with the known properties of cloned inward rectifiers (Kubo et al., 1993a,b). For example (1) while cloned KIRconductance is rather insensitive to [Na+]out, currents underlying inward rectification in glia are dramatically reduced in low sodium (Ransom and Sontheimer, 1995; Ransom et al., 1996); (2) in “complex” glia (D'Ambrosio et al., 1998), currents evoked by hyperpolarization are characterized by time-dependent activation/inactivation, a behavior that has not been described forKIR expressed in oocytes or in cardiac myocytes; and (3) RMP in glia does not always followEK, suggesting that currents other than KIR are involved in RMP determination. The latter issue is discussed in detail elsewhere (McKhann et al., 1997b). Briefly, resting membrane potentials of −80 mV for in situ or cultured astrocytes have been commonly reported, but these measurements were biased by an exclusion criterion. When exclusion criteria were not used, both hyperpolarized and relatively depolarized RMPs were measured (McKhann et al., 1997b). In a previous work, we demonstrated that both depolarized and hyperpolarized recordings were obtained from cells with astrocytic properties, as determined by electron microscopy of cells injected with biocytin during whole-cell recordings (D'Ambrosio et al., 1998).
Interestingly, potassium currents recorded from glia share properties with the cardiac “delayed rectifier”IKr, a current responsible for cardiac action potential repolarization. IKris encoded by the human ether-a-go-go-related gene (HERG) (Sanguinetti et al., 1995; Trudeau et al., 1995), first identified from a hippocampal cDNA library (Warmke and Ganetzky, 1994). A similar current, originally attributed to the inward rectifier family but pharmacologically related to HERG (Faravelli et al., 1996), strongly resembles the glial inward rectifier (Ransom and Sontheimer, 1995;Ransom et al., 1996). Furthermore, the characteristic time and voltage dependence of ERG produces large inward and only small outward whole-cell currents (Warmke and Ganetzky, 1994), resulting in apparent inward-going rectification such as seen in CNS astrocytes. Recently the molecular properties of two additional members of the ERG subfamily (ERG2 and ERG3), which are selectively expressed in the nervous system, have been described (Shi et al., 1997), and ERG currents have been recorded from microglia (Zhou et al., 1998). However, a role for these currents in the CNS remains elusive.
Because the data so far collected from this and other laboratories suggested that ERG-type currents may be, in part, responsible for the complex biophysical properties of CNS astrocytes, we investigated the possible involvement of the potassium channels belonging to the ERG subfamily in mechanisms of voltage-dependent potassium uptake in glia.
MATERIALS AND METHODS
Electrophysiology. Hippocampal slices were prepared from 16- to 18-d-old male Wistar rats as described elsewhere (Janigro et al., 1997a; D'Ambrosio et al., 1999). Slices were bathed at room temperature in an oxygenated saline solution containing (in mm): 120 NaCl, 3.1 KCl, 1 MgCl2, 2 CaCl2, 1.25 KH2PO4, 26 NaHCO3, and 10 dextrose (equilibrated with 95% O2 and 5% CO2 to a final pH of 7.4). Whole-cell pipettes were filled with (in mm): 140 K-Gluconate, 1 MgCl2, 2 Na2ATP, 0.3 NaGTP, 10 HEPES, 0.5 EGTA, pH of 7.2. Field recordings were performed with pipettes containing extracellular solution.
Biocytin was dissolved in the intracellular solution at 0.4%, and the dye was allowed to enter into the cells by passive diffusion after a successful recording. The detailed morphological methods used are described in D'Ambrosio et al. (1998).
Culturing and recording from cultured astrocytes was performed as described (Ransom and Sontheimer, 1995; Guatteo et al., 1996; Ransom et al., 1996; McKhann et al., 1997b). Extracellular potassium was raised (to 40 mm) to increase the contribution of HERG andKIR, and osmolarity was maintained constant by proportionally decreasing [Na+]out. Pipettes had resistance of 5–6 MΩ. Series resistance (RS) was compensated at ∼80% (lag time, 10 μsec) and monitored during the experiment. Access resistance was quite variable (5–8 MΩ) and presumably depended on the size of the electric syncytium constituted by astrocytes in hippocampal slices.
For [K+]outmeasurements, double-barreled borosilicate capillaries were prepared according to methods previously described (Janigro et al., 1997a;D'Ambrosio et al., 1998, 1999). The tip of the sylanized barrel was back-filled with a potassium selective resin (Fluka Cocktail “B”), and the rest of the barrel was filled with KCl (140 mm). We routinely performed tests for the selectivity of the electrode when the potassium channel blockers (cesium, E4031, dofetilide) were to be used during [K+]outmeasurements. A complete description of the methods used to compensate for the interfering ion can be found elsewhere (Ammann, 1986).
Immunocytochemistry and electron microscopy. Five male Sprague Dawley rats, at age of 8 weeks, were used for light microscopic (LM) and electron microscopic (EM) immunocytochemical localization of ERG channel protein (C1 and C2 segments) within the hippocampus. The details of the procedure are described elsewhere (Wenzel et al., 1997). An affinity-purified rabbit antibody specific for ERG channel protein was used to determine its ultrastructural localization. Sections were incubated for 24–48 hr at 4°C in ERG C1 or C2 antiserum and diluted 1:100 to 1:1000 in TBS containing 1% goat serum, 2% BSA, and 0.25–0.3% DMSO. C1 and C2 antisera were raised against the 1035–1049 residues (RGDVESRLDALQRQL) and residues 1145–1159 (LTSQPLHRHGSDPGS), respectively. Specificity of the immunostaining was evaluated by omitting primary antibody or after preabsorption of the primary antibody.
RT-PCR analysis of rat ERG mRNA expression. Total RNA was extracted from SH-SY5Y human neuroblastoma cells (Bianchi et al., 1998) and rat astrocytes by guanidinium isothiocyanate, after previously established procedures (Maniatis et al., 1989). To avoid contamination with genomic DNA, the extracted RNA was treated with 10U/μl of RNase-free DNase I for 1 hr at 37°C. The purity and integrity of the RNA was checked by denaturing agarose gel electrophoresis. Two micrograms of total RNA was reverse-transcribed by SuperScript II reverse transcriptase, using random hexamers following the instruction of the kit (Superscript; Life Technologies, Gaithersburg, MD). The plasmidic and retrotranscribed cDNAs were amplified in an MJ Research Minicycler by the PCR using the primers described in Table 1. The amplification protocol (44 cycles) was the following: 94°C for 1 min, 60°C for 3 min, and 72°C for 3 min. Each 50 μl reaction contained 2.5 U of AmpliTaq DNA Polymerase (Perkin-Elmer, Emeryville, CA), and 50 pmol of each primer. Only for the couple of primers 4s/4r, 10% DMSO was added to the amplification mixture. The amplification products were visualized on agarose (1.5%) gel electrophoresis, loading approximately half (25 μl) of each reaction per lane.
Table 1.
Oligonucleotide primers used for RT-PCR amplification of Erg1, Erg2 and Erg3 sequences
| Primers | Source (GenBank accession number) | nt position | Sequence (5′ to 3′) | Predicted length amplified product |
|---|---|---|---|---|
| Erg1-1s | U04270 | (1298–1323) | gaaggtcacccaggtcctgtccctg | 805 bp |
| Erg1-1r | (2078–2103) | gaagatcttctctgagttggtgttg | ||
| Erg2-2s | AF016192 | (237–260) | tcacgggcccgaacactccaagca | 673 bp |
| Erg2-2r | (888–910) | acaggtaccgcgctgtgattcgtc | ||
| Erg3-3s | AF016191 | (825–848) | atacgaaggccttgatacagccta | 316 bp |
| Erg3-3r | (1117–1141) | tctgatgtggatcccaacaggctt | ||
| Erg1-4s | Z96106 | (1994–2013) | tccagcggctgtactcgggc | 597 bp |
| Erg1-4r | (1569–2591) | tagaccaaaagtggtctgagaactc | ||
| Erg2-5s | AF016192 | (2356–2376) | aggacctggattgctggcatc | 400 bp |
| Erg2-5r | (2735–2756) | aggtgaggctagaggtgacagg | ||
| Erg3-6s | AF016191 | (3216–3236) | cagagagctgcctggggcatc | 428 bp |
| Erg3-6r | (3624–3644) | gtcaagctctgacgatgcatc |
The two pairs of primers chosen to amplify ERG2 andERG3 sequences were designed on the rat sequence available in GenBank (AF016192 and AF016191, respectively), whereas those forERG1 were designed according to the human (U04270, 1 s/1r) or the rat (Z96106, 4s/4r) sequences. Nevertheless, within the sequences recognized by the primers 1 s and 1r, the human and the rat sequences diverged by only one nucleotide; within the region recognized by the primer 4s, the human and the rat sequences were identical, whereas for 4r they diverged by only three nucleotides. Therefore, it seems reasonable to conclude that these pairs of primers were unable to discriminate species-specific differences between rErg1 and hErg1, as also suggested by their ability to amplify both rat (astrocytes) and human (SH-SY5Y and hERG1 cDNA) sequences.
RESULTS
ERG expression in hippocampal or cultured astrocytes
Hippocampal slice recordings from CA1/CA3 astrocytes reveal expression of a variety of voltage-dependent potassium currents (Janigro et al., 1997a; D'Ambrosio et al., 1998, 1999). The profile of these currents was used to classify them in three categories: “complex”, “inward rectifier”, and “linear”. The results presented herein were obtained from hippocampal astrocytes characterized by a mostly inward rectifier profile. Astrocytes cultured from different brain regions are similarly endowed with a number of potassium conductances (Guatteo et al., 1996). Because cultured spinal cord and cortical astrocytes exhibited similar biophysical and pharmacological properties in our analysis, the data from these cells were grouped together (“cultured glia”).
Voltage-dependent currents were evoked from cultured or in situ astrocytes by using the whole-cell variation of the patch-clamp technique (Janigro et al., 1997a; McKhann et al., 1997b; D'Ambrosio et al., 1998). ERG currents are enhanced in solutions containing elevated extracellular potassium (Sanguinetti et al., 1995), hence membrane currents were studied at nonphysiological potassium concentrations in cultured astrocytes (40 mm, with NaCl decreased proportionally to 106 mm). Because elevation of [K+]out in hippocampal slices is epileptogenic (McBain, 1995), slice experiments were performed in [K+]out = 4.35 mm. Inward potassium currents were induced by applying test potentials of −20 to −160 mV (at 20 sec intervals) from a holding potential of 0 mV (Fig.1A). In hippocampal slices, inward and outward currents were evoked by applying voltage steps from a holding potential of 0 mV (Fig. 1B).
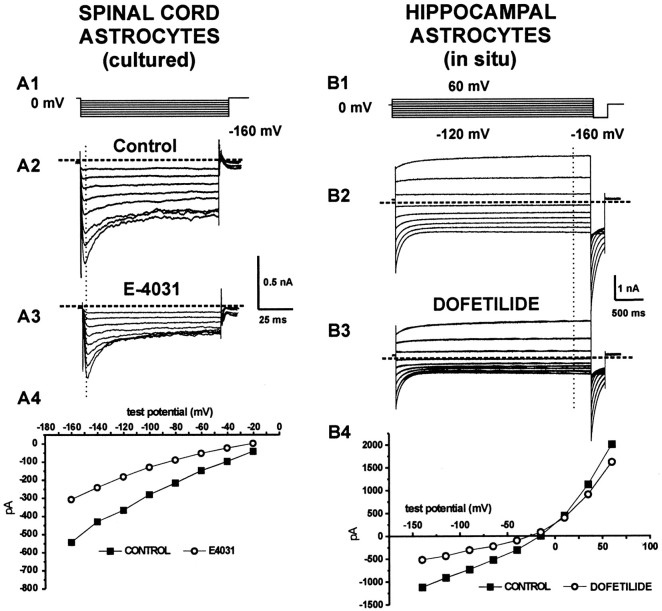
Fig. 1.
Pharmacological manipulation reveals ERG-type currents in astrocytes: effects of E4031 or dofetilide. A1, B1, Voltage-clamp protocols used to evoke whole-cell currents.A2, B2, Currents evoked in spinal cord ([K+]out = 40 mm) and in hippocampal slice ([K+]out = 4.35 mm) astrocytes. Note the characteristic time-dependent deactivation of inward currents at negative potentials (A2) and the lack of inactivation after membrane depolarization (B2). A3, B3, Partial blockade of astrocytic currents by the specific ERG channel blocker E4031 (100 nm) or dofetilide (1000 nm). Residual E4031-insensitive currents could be recorded even after prolonged exposures to the drug (>15 min). A4, B4,I–V plots determined by measuring steady-state currents before and after exposure to E4031 or dofetilide; the vertical dotted lines in A2, A3, B2, andB3 represent the time point at which current measurements were taken.
Previous studies demonstrated expression of inward rectifier-type currents in astrocytes (Guatteo et al., 1996; Ransom et al., 1996;Bordey and Sontheimer, 1997; D'Ambrosio et al., 1998, 1999). Owing to the large “tail” component of ERG, unambiguous biophysical dissociation of ERG from inward rectification is not always possible (Faravelli et al., 1996), particularly during recordings from glial syncytia where it is possible that multiple cells with different ion channels are contributing to the recorded current (McKhann et al., 1997a,b; D'Ambrosio et al., 1998, 1999). ERG and KIR currents can be differentiated, however, by applying methanesulfonanilides, a class of molecules that, at nanomolar concentrations, blocks ERG but not KIR (Kiehn et al., 1996). Potassium currents elicited in cultured (spinal cord or cortical) or hippocampal slice astrocytes were challenged with E4031 or dofetilide, ERG-specific ion channel blockers (Sanguinetti, 1992) (Fig.1). Application of E4031 (100 nm) caused a reduction of hyperpolarization-activated currents by 64 ± 10% (n = 7; p < 0.008; cultured astrocytes; Fig. 1, compare A2, A3) and by 34.52 ± 7.24% (n = 5; p < 0.001; hippocampal slice astrocytes), whereas depolarization-activated currents were reduced by 62 ± 12% (n = 7; p < 0.008) and 57.72 ± 14.49% (n = 5;p < 0.02), in hippocampal slices (Fig. 1, compareB2, B3) and in cultured astrocytes, respectively.
In addition to E4031, dofetilide has been widely used to specifically block ERG (Kiehn et al., 1996; Ficker et al., 1998) (Fig.1B). Application of dofetilide (100–1000 nm) to hippocampal slice astrocytes caused a dose-dependent current decrease within 5 min that reached a steady-state level after 10 min. Dofetilide caused a −34.6 ± 3.2%, −48.1 ± 2.9%, and −55 ± 3.9% (n= 6) decrease of peak inward currents at 100, 500, and 1000 nm, respectively. The effects on steady-state outward currents were less dramatic (−22.8 ± 2.7%, −31.3 ± 4.3%, and −36.9 ± 1.2%, at 100, 500, and 1000 nm, respectively).
Coexistence of KIR and ERG currents
These results demonstrated only partial blockade of currents recorded in astrocytes by drugs specifically targeting HERG-type currents. Furthermore, the amplitude of the currents not affected by ERG blockers varied greatly across cells, suggesting that in addition to ERG, variable levels of KIR expression are present in these glia (Guatteo et al., 1996; Bordey and Sontheimer, 1997; D'Ambrosio et al., 1998, 1999). KIRcurrents are blocked by low concentrations of Cs+ (Ransom and Sontheimer, 1995), whereas concentrations >2 mm are required to affect ERG (Faravelli et al., 1996). We compared the sensitivity of hippocampal astrocyte currents to concentrations of Cs+ specific for KIR versus treatment with dofetilide at concentrations specific for ERG (Fig. 2).
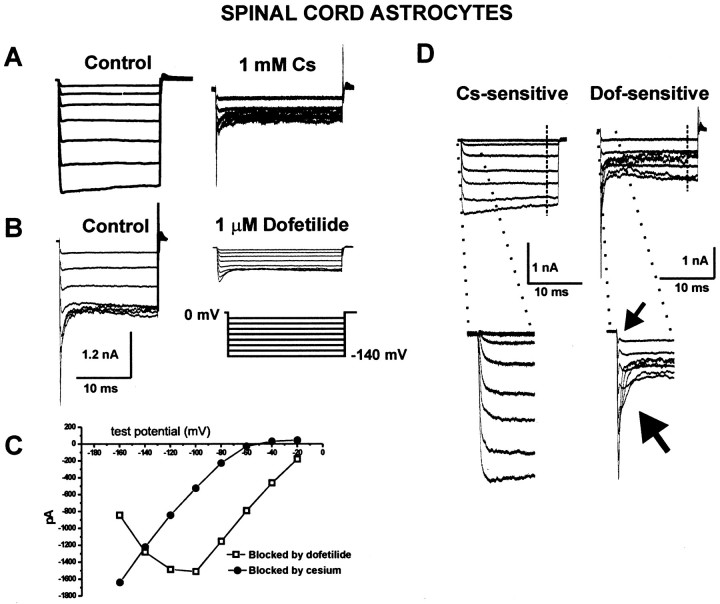
Fig. 2.
Coexistence of ERG-like and KIRcurrents: sensitivity to both dofetilide and Cs+.A, Cesium (1 mm) blockade of inward currents in cultured spinal cord astrocytes bathed in elevated [K+]out. Note that that theI–V profile of the subtraction current shown inD (IControl −Ics) is characterized by inward going rectification (C, filled symbols). In contrast, dofetilide block revealed a current (B) with complex voltage dependency and s-shaped behavior of theI–V relationship (C). The data points used to construct the I–V curve inC were obtained from the subtraction currents inD and were measured at the end of the voltage steps, as indicated by the dashed lines. D,Cs+- and dofetilide-sensitive currents have different kinetic properties. Inward rectifier currents unmasked by Cs+ blockade were characterized by little time-dependent inactivation, whereas dofetilide-sensitive currents inactivated rapidly at negative potentials (large arrow). Also note the apparent activation (or removal of inactivation) of the dofetilide-sensitive current at the beginning of the hyperpolarizing step (small arrow, bottom right panel).
After exposure to either Cs+ (1 mm) or E-4031 (100 nm), digitized currents were analyzed as previously described (Janigro et al., 1997b). Figure 2,A and B, shows the effects of Cs+ and dofetilide on hyperpolarization-activated currents. Subtraction protocols (IControl −Idrug) applied to currents evoked by hyperpolarizing steps revealed that Cs+-sensitive currents are characterized by slow voltage-independent inactivation, voltage-dependent blockade by Cs+, and inward rectification positive toEK (Fig. 2D, left panel); these properties are consistent with the biophysical properties of KIR. In contrast, dofetilide-sensitive currents displayed time and voltage-dependent relaxation (Fig. 2D, right panel) characterized by kinetic properties similar to cloned ERG (removal of inactivation followed by time-dependent deactivation) (Fig.2D1, bottom right panel, small and large arrow). Figure 2C shows the I–V properties of the subtracted currents. Whereas the results shown in Figure 2 are representative of an extreme condition (predominant expression of either KIR or ERG), in most cells the subtraction protocol revealed variable ratios of inward rectifier versus ERG currents. Such data are consistent with our previous demonstration of Cs+-sensitive and insensitive currents in astrocytes (McKhann et al., 1997b; D'Ambrosio et al., 1998,1999), further supporting the hypothesis that inward rectifier currents are not the exclusive potassium conductance in these glia.
Functional significance of ERG expression in glia
Blockade of astrocytic inward rectifier potassium channels by Cs+ exaggerates potassium transients triggered by neuronal activity and increases baseline potassium concentrations in hippocampal slices (Janigro et al., 1997a; McKhann et al., 1997a; D'Ambrosio et al., 1998, 1999). These actions were attributed to Cs+ blockade of KIR and subsequent failure of voltage-dependent potassium uptake into glia. The concentrations of cesium used in those studies (1–3 mm) were, however, also consistent with blockade of HERG-type currents. We thus hypothesized that ERG-type currents may also be involved in extracellular potassium buffering. To test this possibility, [K+]out was measured with K+-selective microelectrodes in CA1 stratum pyramidale, after stimulation of Schaffer collaterals at 0.1–10 Hz.
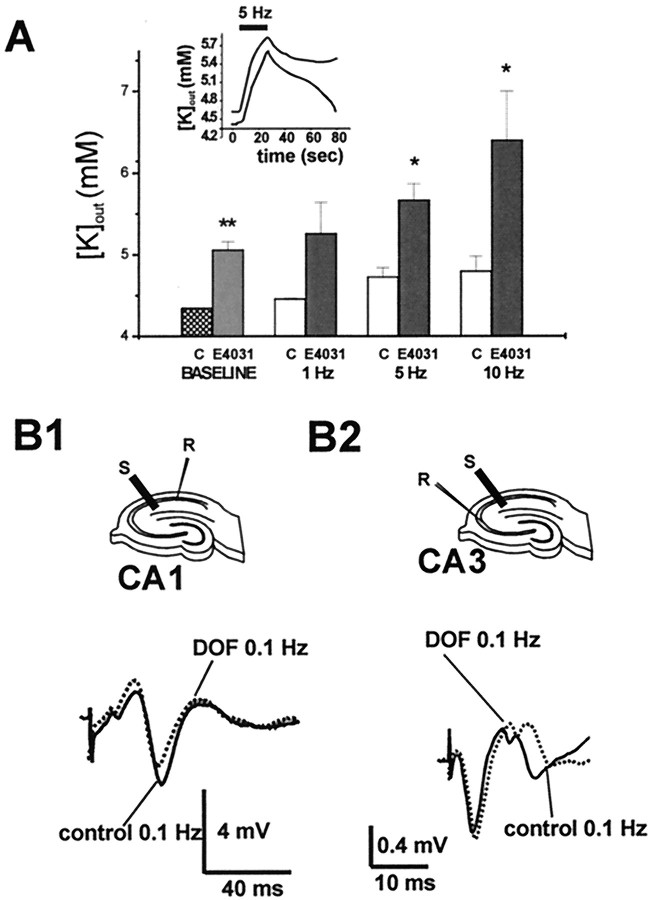
After establishing a [K+]out baseline, stimulation trains were applied for 15 sec while simultaneously acquiring field potentials with a nearby field electrode (Fig.3). Stereotyped [K+]out transients followed neuronal stimulation (Fig. 3A, inset). In the presence of E4031 (100 nm), resting potassium values increased slightly (control = 4.35 ± 0.001; E4031 = 5.06 ± 0.1; p < 0.0001;n = 10). In addition, stimulation-evoked [K+]out changes were significantly larger than in control (at 5 Hz: control = 4.73 ± 0.1; E4031 = 5.67 ± 0.2; p < 0.01; n = 4; at 10 Hz: control = 4.65 ± 0.18; E4031 = 6.4 ± 0.6; p < 0.01;n = 5). Similar results were obtained after application of 100 nm dofetilide.
Fig. 3.
Functional role for ERG currents in the hippocampus. A, Effect of ERG blockade by E4031 on CA1 resting potassium level and potassium accumulation induced by neuronal activity (inset). The resting (baseline) [K+]out increased slightly after the drug treatment, whereas larger increases were measured after afferent stimulation at 5 and 10 Hz (*p < 0.01; **p < 0.0001); the [K+]out changes induced by stimulation at low frequency (1 Hz) were not significantly affected by treatment with E-4031. B, Field recordings in CA1 and CA3 (cell body layer), in the presence of 100 nm E4031, fail to reveal any significant increase in neuronal excitability as reflected in population spike amplitude; the result suggests that the K changes produced under these conditions were too small to induce gross changes in neuronal excitability. The traces shown were taken after stimulation at 0.1 Hz, but no significant differences (control vs treatment) were observed after stimulation at higher (1, 5, and 10 Hz) frequencies.
Increases in accumulation of extracellular potassium may be caused by increased neuronal activation, as well as by decreased buffering by glia. In fact, if the effects of ERG channel blockers were mediated by blockade of repolarizing neuronal currents, one would expect abnormal potassium accumulation associated with neuronal hyperexcitability. To rule out this possibility, we recorded and analyzed the postsynaptic field responses to Schaffer collateral stimulation in stratum pyramidale, obtained before and after treatment with dofetilide (Fig.3B). Instead of neuronal hyperexcitability (predicted if the drug were causing blockade of neuronal K currents), dofetilide treatment resulted in no significant change of the population spike, either in CA1 (3.8 ± 1.1 vs 3.6 ± 1.2 mV in control vs dofetilide; n = 5) or in CA3 (0.3 ± 0.09 mV vs 0.3 ± 0.08 in control vs dofetilide, n = 3).
Hippocampal expression of ERG is confined to astrocytes
Immunocytochemical investigation of the ERG channel protein within the hippocampus indicated that ERG channels are preferentially distributed in hippocampal astrocytes (Figs.4–6). Using two different antibodies raised against two epitopes located in carboxyl terminus of the cloned ERG channel protein, we found a relatively specific pattern of ERG immunoreactivity in the astrocytes of both hippocampus and dentate gyrus; hippocampal oligodendrocytes and endothelial cells did not show any ERG immunoreactivity (Figs.4C, 5B–D, F–H).
Fig. 4.
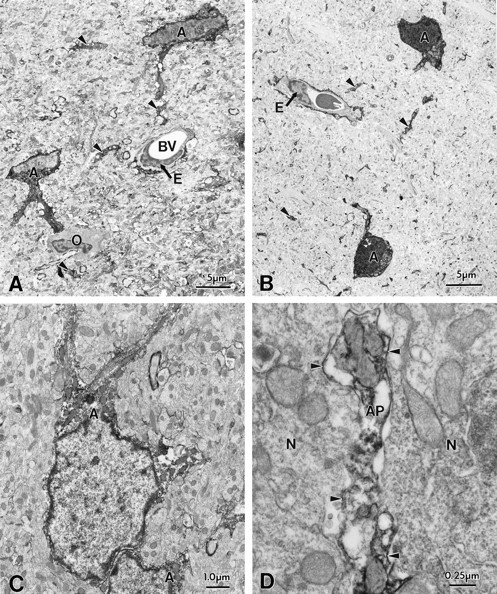
Immunocytochemical localization of ERG (C1 antibody) channel protein in hippocampal astrocytes. Electron microscopy of biocytin-filled astrocytes (A, C) and immunocytochemistry of ERG (C1) channel protein in astrocytes (B, D) within the hippocampal CA1 subregion. Note the similar morphological features of hippocampal astrocytes of biocytin-filled and ERG-immunopositive cell bodies (A, B,) and their processes (arrows, C, D). ERG immunoreactivity was confined within the cell body cytoplasm of astrocytes and within large primary astrocytic processes and on small branched processes within the neuropil. Note that an oligodendrocyte (O) was immunonegative (A). Pyramidal cells did not show ERG immunoreactivity.
Fig. 5.
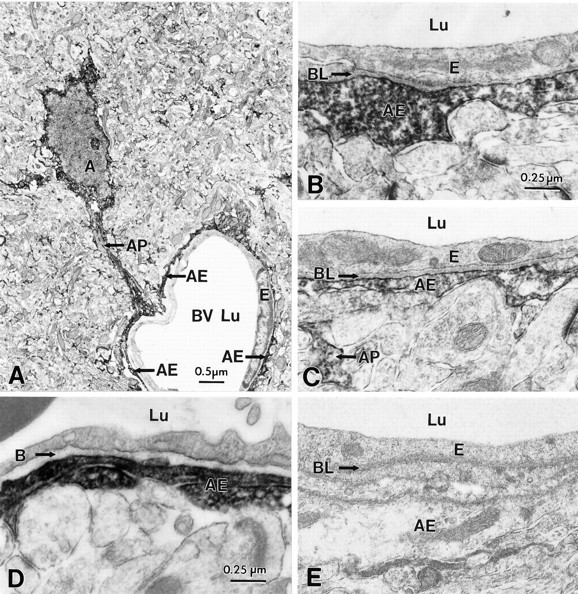
Immunocytochemical localization of ERG channel protein in astrocytes surrounding blood vessels in hippocampal CA1 (A,D); comparison with biocytin-filled cells (B,C). A, Low-power magnification of an ERG-immunoreactive astrocyte process (AP) forming an astrocytic endfoot (AE) around a capillary (BV). The endothelial cell (E) of the capillary wall does not show ERG immunoreactivity. As comparison, the capillary wall from a biocytin-filled astrocytic end foot is shown in B. D, Immunocytochemical localization of ERG channel protein in astrocytes surrounding blood vessels in hippocampal CA3 subregion; note the immunonegative basal lamina (BL). C, Comparison with the capillary wall of biocytin-filled astrocytes. E, Specificity of the ERG antibody is demonstrated in a control section after preabsorption of the primary antibody.
Direct comparison of immunocytochemically characterized ERG-positive cells with the profiles of electrophysiologically characterized astrocytes filled with biocytin revealed almost identical morphological features (Fig. 4A,B, immunocytochemistry and biocytin, respectively). Cells of comparable morphology were characterized morphologically and electrophysiologically in a previous study (D'Ambrosio et al., 1998).
Astrocytes in the neuropil of hippocampal CA1 and CA3 subregions and the dentate molecular layer showed ERG immunoreactivity in the cytoplasm of the cell body and its tapering primary processes, which branched into many fine immunopositive processes. Immunocytochemical localization of ERG (C1) channel protein in astrocytes within the hippocampal CA1 and CA3 subregions was very similar (Fig.4C,D represent CA1 and CA3, respectively). ERG immunoreactivity was evident within the cytoplasm of astrocytic cell bodies, their large processes (large arrowheads) and small branched processes (small arrowheads) within the neuropil. In agreement with our electrophysiological data, oligodendrocytes (Fig. 4A), were not immunopositive for ERG.
At the electron microscopic level, these immunopositive astrocytic processes were often seen in association with blood vessels, forming astrocytic end feet around the capillaries (Fig. 5). Similar to the overall immunocytochemical profile described above for astrocytic cell bodies, we found no significant difference in the pattern of perivascular immunoreactivity between CA1 (Fig. 5A–D) and CA3 subregions (Fig. 5F–H). In both regions, the cellular specificity for ERG staining was remarkable, and immunopositive layers of perivascular glia were clearly distinguishable from the immunonegative blood–brain barrier endothelial cells (Figs.5B–D, F–H).
In contrast to the complete absence of ERG immunoreactivity in oligodendrocytes and endothelial cells, cell bodies and proximal dendrites of hippocampal pyramidal neurons and dentate granule cells showed lightly stained cell bodies and nuclei when antibody concentration was raised. Smaller dendrites, axons, and synaptic profiles did not show ERG immunoreactivity at either LM or EM levels. However, occasional axonal elements (seen in all hippocampal subfields, and particularly in fiber tracts such as the fimbria hippocampi and alveus) were immunopositive. ERG-positive astrocytic processes were also observed in apposition to dendrites, small axons, and surrounding synaptic profiles (Figs. 6C,7).
Fig. 7.
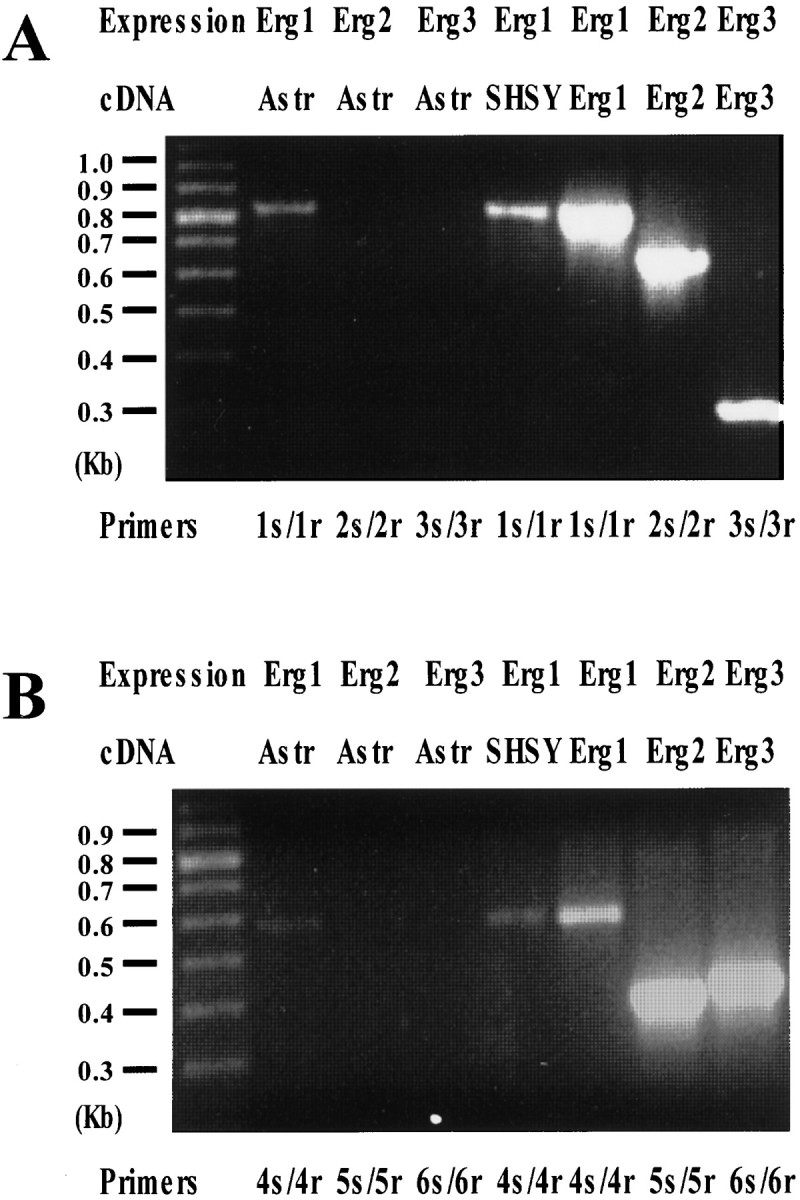
Expression of Erg1, Erg2, and Erg3 in rat astrocytes. A, RNA extracted from cultured rat cortical astrocytes (lanes 2, 3, and 4) or from SH-SY5Y cells (lane 5) were retrotranscribed as described in Materials and Methods. cDNAs from these RT reactions, as well as cloned cDNAs encoding for hErg1 (lane 6), rErg2 (lane 7), and rErg3 (lane 8), were amplified using the following primers: 1s/1r (specific for Erg1) for lanes 2, 5, and 6; 2s/2r (specific for Erg2) for lanes 3 and 7; and 3s/3r (specific for Erg3) for lanes 4 and 8. Molecular weights of the expected bands were: 805, 673, and 316 bp for 1s/1r, 2s/2r, and 3s/3r, respectively. Lane 1 shows the position of the molecular weight DNA markers (100 bp ladder from Pharmacia, Piscataway, NJ). Half (25 μl) of each amplification reaction per lane was loaded on the gel. B, RNA extracted from cultured rat cortical astrocytes (lanes 2, 3, and 4) or from SH-SY5Y cells (lane 5) were retrotranscribed as described in Materials and Methods. cDNAs from these RT reaction, as well as cloned cDNAs encoding for hErg1 (lane 6), rErg2 (lane 7), and rErg3 (lane 8), were amplified using the following primers: 4s/4r (specific for Erg1) for lanes 2, 5, and 6; 5s/5r (specific for Erg2) for lanes 3 and 7; and 6s/6r (specific for Erg3) for lanes 4 and 8. Molecular weights of the expected bands were: 597, 400, and 428 bp for 4s/4r, 5s/5r, and 6s/6r, respectively.Lane 1 shows the position of the molecular weight DNA markers (100 bp ladder from Pharmacia). Half (25 μl) of each amplification reaction per lane was loaded on the gel.
Fig. 6.
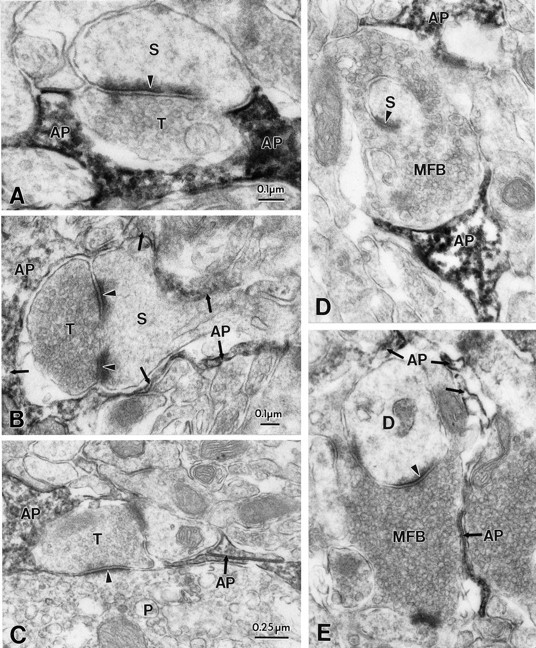
Perisynaptic glia, but not neuronal synaptic elements, are immunopositive for ERG protein. A, B, Electron micrographs of synapses in stratum radiatum of the CA1 subregion which are in close apposition to astrocytic processes that are immunopositive for ERG (C1) channel protein (AP). Note the absence of immunoreactivity in presynaptic (T) and postsynaptic (S) components. C, Immunonegative axon terminal (T) forming an asymmetric synapse with a neruonal cell body (P) and a small dendrite, both also immunonegative; D, Electron micrographs of synapses in stratum lucidum of the CA3 subregion showing ERG-immunopositive astrocytic processes (AP) in close apposition to immunonegative presynaptic and postsynaptic elements (MFB, mossy fiber bouton). E, Mossy fiber boutons (MFB) in synaptic contact with dendritic spines (D). Note that large portions of the mossy fiber bouton surface is apposed to astrocytic processes that are immunopositive for ERG channel protein (AP).
Cell type-specific immunostaining was most notable at the synaptic level (Fig. 6) In both hippocampal subregions (Fig.6A–D, Ca1, E–H, CA3) both presynaptic and postsynaptic elements were immunonegative, whereas the glial ensheathment surrounding the synaptic cleft was markedly stained by the antibody.
Molecular nature of hippocampal astrocyte ERG
At least three genes encode ERG currents in the mammalian nervous system (Shi et al., 1997). AlthoughERG2–3 appear to be exclusively expressed in nervous tissues, ERG1 is found in both nervous and non-nervous cells. To elucidate the molecular nature of astrocytic ERG, we performed RT-PCR experiments to study the molecular correlates of the dofetilide- and E-4031-sensitive currents recorded from in situ and cultured astrocytes (Fig. 5). As shown in Figure 5, PCR amplification of the cDNA in vitro retrotranscribed from cultured rat astrocyte RNA with ERG1-specific primers (pair 1 s/1r) allowed the identification of a band of the molecular weight expected from ERG1 nucleotide sequence (805 bp). This band was identical to that identified also in SH-SY5Y human neuroblastoma cells, which are known to express ERG1 (Bianchi et al., 1998), as well as on PCR amplification of the cloned HERG cDNA. Furthermore, the expression of ERG1 in rat astrocytes was also confirmed by showing that another pair of Erg1-specific primers (pair 4s/4r), was able to amplify a 597 bp region of theERG1 cDNA, as expected from the primer position with respect to the primary sequence. It should be underlined that these primers were highly specific for the ERG1 sequence, because they failed to amplify either ERG2 or ERG3 cloned cDNAs (data not shown). In contrast to ERG1 expression, no band was detected when the two pairs of primers specifically designed to amplify ERG2 (primers 2s/2r and 5s/5r) or ERG3(primers 3s/3r and 6s/6r) were used on the same RT product from rat astrocytes. These primers were effectively able to specifically amplifyERG2 and ERG3 sequences, respectively, as suggested by the appearance of bands of the expected molecular weights (673 bp with 2s/2r; 400 bp with 5s/5r; 316 bp with 3s/3r; 428 bp with 6s/6r) when the Erg2 and Erg3 cloned cDNAs were used as templates.
DISCUSSION
There is increasing evidence suggesting that astrocytes play an important role in the regulation of neuronal excitability by participating in the control of ion and neurotransmitter homeostasis (Linden, 1997; Araque et al., 1999). Pharmacological studies have demonstrated that preventing astrocytic voltage-dependent potassium buffering results in abnormal epileptiform hippocampal activity, and that “epileptic”, as well as post-traumatic hippocampi are partially devoid of astrocytic ion currents underlying potassium uptake (O'Connor et al., 1998; D'Ambrosio et al., 1999). The biophysical and molecular properties of these potassium channels has not been fully elucidated, but several reports suggested a significant contribution of inward rectifier-type channels (Ransom and Sontheimer, 1995; Ransom et al., 1996; Janigro et al., 1997a; D'Ambrosio et al., 1998). Our results indicate that in addition to KIR,in situ and cultured astrocytes also express a current similar to HERG. This conclusion is based on the current's pharmacological sensitivity to agents known to block selectively HERG (and not KIR), its resistance to submillimolar Cs+ (a KIR channel blocker), and on the ERG immunocytochemical results demonstrating an almost exclusive presence of immunoreactivity in hippocampal astrocytes. Finally, molecular analysis by RT-PCR revealed that cultured astrocytes express ERG1, but not ERG2–3genes, suggesting that neuron-specific genes are not constitutively expressed in these glia.
The biophysical properties of cloned and native ERG currents are rather complex, and the apparent inward going rectification of these currents closely resembles KIR (Faravelli et al., 1996). Under our experimental conditions, direct investigations of the biophysical properties of astrocytic ERG were impossible, because the syncytial nature of hippocampal (and cultured) astrocytic networks prevents optimal space clamp, and because this current was rarely found in isolation from contaminating currents. However, pharmacological subtraction of whole-cell currents revealed that the properties of astrocytic ERG are similar to those reported for clonedHERG1 (Fig. 1B2) (Shi et al., 1997). The presence of rat ERG1 was further confirmed by RT-PCR experiments in cultured glia.
Previous studies have shown that cultured as well as hippocampal astrocytes display a wide range of RMPs, inconsistent with an exclusive role for KIR that predicts RMPs close toEK; the biophysical properties of ERG, in contrast, predict a more positive resting potential (Arcangeli et al., 1995; Bianchi et al., 1998), as found in a large subpopulation of astrocytes (Janigro et al., 1997a; D'Ambrosio et al., 1998). In the present study, we found variable levels of ERG-KIR coexpression, consistent with the variable resting potentials found across astrocytes. Detailed investigations of RMP values in cultured astrocytes have shown a bimodal distribution with peak values atEK (approximately ∼70 mV) and approximately −40 mV (McKhann et al., 1997b). Although the first RMP values are consistent with a predominant role for KIR in determining RMP, the more positive potentials are identical to those reported for cancer cells expressing HERG (−40 to −44 mV) (Bianchi et al., 1998). It is thus possible that glial syncytia are comprised of two different subpopulations of cells, one expressing predominantly KIR (and responsible for K uptake) and one endowed with high levels of HERG (to facilitate potassium release). During syncytial recordings such as those described herein, an electrotonically weighted average is recorded, resulting in RMP values distributed continuously betweenEK and the RMP predicted by predominant expression of ERG.
Blockade of glial ERG in hippocampal slices caused a small but significant increase in basal [K+]out, together with a pronounced increase of potassium transients induced by neuronal activity. Both effects are qualitatively identical to what was observed after exposure of the tissue to millimolar concentrations of Cs+ (Janigro et al., 1997a; D'Ambrosio et al., 1998). The effect of ERG blockade was not attributable to increased neuronal excitability, as also shown for Cs+-mediated effects on [K+]out dynamics (Janigro et al., 1997a; D'Ambrosio et al., 1998, 1999) or to effects on GABAB inhibition (Maccaferri et al., 1994). Although our results do not address the issue of the relative contribution of ERG versus KIR in astrocytic potassium homeostasis, they nevertheless suggest that ERG currents are involved in this process. Because blockade of “potassium buffering” by glia has been shown to be pro-epileptogenic (Janigro et al., 1997a;McKhann et al., 1997a; D'Ambrosio et al., 1998, 1999), it is tempting to speculate that an inherited defect in glial ERG function may be associated with some forms of human epilepsy. Clinically, it is well established that HERG mutations are responsible for one form of the “long QT” syndrome, an inherited cardiac disorder causing syncope, seizures, and sudden death (Curran et al., 1995). In addition, there are several recent reports linking seizure susceptibility to HERG dysfunction or long QT (Drake et al., 1993; Gordon, 1994; Pacia et al., 1994; Yang et al., 1998). Thus, in addition to altered cardiac rhythm, mutated HERG may also affect CNS function by causing hyperexcitability by a mechanism involving astrocytes. Further studies will elucidate the possible phenotypic changes deriving from CNS HERG1mutations.
Current day orthodoxy predicts that seizure disorders are largely attributable to changes in phenotypic expression of neuronal ion channels regulating excitability. In support of this postulate is the fact that pharmacological manipulations of neuronal excitability (e.g., blockade of IPSCs or neuronal after-spike repolarization) readily result in epileptic-like discharge. Similarly, “knock-out” mutants lacking neuronal voltage-dependent channels exhibit an “epileptic” phenotype (Smart et al., 1998). However, the possibility that non-neuronal ion channel/transporter mutations (i.e., in glia) are linked to epilepsy is supported by an increasing body of experimental evidence (Bennett et al., 1995; Lee et al., 1995; Schwartzkroin et al., 1998; Janigro, 1999). The results presented here suggest that electrophysiologically relevant mutations that affect the coordination of cardiac rhythm, may also influence neuronal activity by an indirect mechanism, involving changes in glial homeostatic control of brain function.
Footnotes
This work was supported by the Epilepsy Foundation (A.E.) and by National Institute of Health Grants ES07033, NS38195, HL51614 (D.J.), NS35548 (P.A.S.), HL55973, and a National Science Foundation CAREER award (G.A.R.). M.T. is supported by Telethon 1058, National Research Council (Consiglio Nazionale delle Ricerche) n. 97.04512.CT04, 97.01230.PF49, and 98.03149.CT04. We are indebted to Dr. M. T. Keating (Salt Lake City, UT) for hERG1 cDNA and Drs. R. Wymore and J. Exton (New York, NY) for rERG2 and rERG3 cDNA clones.
Correspondence should be addressed to Dr. Damir Janigro, Director, Cerebrovascular Research, Cleveland Clinic Foundation NB-20, 9500 Euclid Avenue, Cleveland, OH 44195. E-mail: janigrd@ccf.org.
REFERENCES
- 1.Ammann D. Ion sensitive electrodes. Springer; Berlin: 1986. [Google Scholar]
- 2.Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999;22:208–215. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- 3.Arcangeli A, Bianchi L, Becchetti A, Faravelli L, Coronnello M, Mini E, Olivotto M, Wanke E. A novel inward-rectifying K+ current with a cell-cycle dependence governs the resting potential of mammalian neuroblastoma cells. J Physiol (Lond) 1995;489:455–471. doi: 10.1113/jphysiol.1995.sp021065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ballanyi K, Branchereau P, Champagnat J, Fortin G, Velluti J. Extracellular potassium, glial and neuronal potentials in the solitary complex of rat brainstem slices. Brain Res. 1993;607:99–107. doi: 10.1016/0006-8993(93)91493-c. [DOI] [PubMed] [Google Scholar]
- 5.Bennett SA, Stevenson B, Staines WA, Roberts DC. Periodic acid-Schiff (PAS)-positive deposits in brain following kainic acid-induced seizures: relationships to fos induction, neuronal necrosis, reactive gliosis, and blood–brain barrier breakdown. Acta Neuropathol Berl. 1995;89:126–138. doi: 10.1007/BF00296356. [DOI] [PubMed] [Google Scholar]
- 6.Bianchi L, Wible B, Arcangeli A, Taglialatela M, Morra F, Castaldo P, Crociani O, Rosati B, Faravelli L, Olivotto M, Wanke E. HERG encodes a K current highly conserved in tumors of different histogenesis: a selective advantage for cancer cells? Cancer Res. 1998;58:815–822. [PubMed] [Google Scholar]
- 7.Bordey A, Sontheimer H. Postnatal development of ionic currents in rat hippocampal astrocytes in situ. J Neurophysiol. 1997;78:461–477. doi: 10.1152/jn.1997.78.1.461. [DOI] [PubMed] [Google Scholar]
- 8.Curran ME, Splawski I, Timothy KW, Vincent GM, Green ED, Keating MT. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell. 1995;80:795–803. doi: 10.1016/0092-8674(95)90358-5. [DOI] [PubMed] [Google Scholar]
- 9.D'Ambrosio R, Wenzel J, Schwartzkroin PA, Janigro D. Functional specialization and topographic segregation of hippocampal astrocytes. J Neurosci. 1998;18:1–14. doi: 10.1523/JNEUROSCI.18-12-04425.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D'Ambrosio R, Maris DO, Grady MS, Winn HR, Janigro D. Impaired K homeostasis and altered electrophysiological properties of post-traumatic hippocampal glia. J Neurosci. 1999;19:8152–8162. doi: 10.1523/JNEUROSCI.19-18-08152.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drake ME, Reider CR, Kay A. Electrocardiography in epilepsy patients without cardiac symptoms. Seizure. 1993;2:63–65. doi: 10.1016/s1059-1311(05)80104-9. [DOI] [PubMed] [Google Scholar]
- 12.Faravelli L, Arcangeli A, Olivotto M, Wanke E. A HERG-like K channel in rat F-11 DRG cell line: pharmacological and biophysical identification. J Physiol (Lond) 1996;496:13–23. doi: 10.1113/jphysiol.1996.sp021661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ficker E, Jarolimek W, Kiehn J, Baumann A, Brown AM. Molecular determinants of dofetilide block of HERG K channels. Circ Res. 1998;82:395. doi: 10.1161/01.res.82.3.386. [DOI] [PubMed] [Google Scholar]
- 14.Gordon N. The long Q-T syndromes. Brain Dev. 1994;16:153–155. doi: 10.1016/0387-7604(94)90054-x. [DOI] [PubMed] [Google Scholar]
- 15.Guatteo E, Stanness KA, Janigro D. Hyperpolarization-activated currents in cultured rat cortical and spinal cord astrocytes. Glia. 1996;16:196–209. doi: 10.1002/(SICI)1098-1136(199603)16:3<196::AID-GLIA2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 16.Janigro D. Blood–brain barrier, ion homeostasis, and epilepsy: possible implication toward the understanding of ketogenic diet mechanisms. Epilepsy Res. 1999;37:223–232. doi: 10.1016/s0920-1211(99)00074-1. [DOI] [PubMed] [Google Scholar]
- 17.Janigro D, Gasparini S, D'Ambrosio R, McKhann GM, DiFrancesco D. Reduction of K+ uptake in glia prevents LTD maintenance and causes epileptiform activity. J Neurosci. 1997a;17:2813–2824. doi: 10.1523/JNEUROSCI.17-08-02813.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janigro D, Martenson ME, Baumann TK. Preferential blockade of Ih in rat trigeminal ganglion neurons by DK-AH 268. J Membr Biol. 1997b;160:101–109. doi: 10.1007/s002329900299. [DOI] [PubMed] [Google Scholar]
- 19.Kiehn J, Lacerda AE, Brown AM. Molecular physiology and pharmacology of HERG. Circulation. 1996;94:2572–2579. doi: 10.1161/01.cir.94.10.2572. [DOI] [PubMed] [Google Scholar]
- 20.Kubo Y, Baldwin TJ, Jan YN, Jan LY. Primary structure and functional expression of a mouse inward rectifier potassium channel. Nature. 1993a;362:127–133. doi: 10.1038/362127a0. [DOI] [PubMed] [Google Scholar]
- 21.Kubo Y, Reuveny E, Slesinger PA, Jan YN, Jan YL. Primary structure and functional expression of a rat G-protein-coupled muscarinic potassium channel. Nature. 1993b;364:802–806. doi: 10.1038/364802a0. [DOI] [PubMed] [Google Scholar]
- 22.Kuffler SW, Nichols JG, Orkand RK. Physiological properties of glial cells in the central nervous system of amphibia. J Neurophysiol. 1966;29:768–787. doi: 10.1152/jn.1966.29.4.768. [DOI] [PubMed] [Google Scholar]
- 23.Lee SH, Magge S, Spencer DD, Sontheimer H, Cornell-Bell AH. Human epileptic astrocytes exhibit increased gap junction coupling. Glia. 1995;15:195–202. doi: 10.1002/glia.440150212. [DOI] [PubMed] [Google Scholar]
- 24.Linden DJ. Long-term potentiation of glial synaptic currents in cerebellar culture. Neuron. 1997;18:983–994. doi: 10.1016/s0896-6273(00)80337-2. [DOI] [PubMed] [Google Scholar]
- 25.Maccaferri G, Janigro D, Lazzari A, DiFrancesco D. Cesium prevents maintenance of long-term depression in rat hippocampal CA1 neurons. NeuroReport. 1994;5:1813–1816. doi: 10.1097/00001756-199409080-00032. [DOI] [PubMed] [Google Scholar]
- 26.Maniatis T, Fritsh EF, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor; New York, NY: 1989. [Google Scholar]
- 27.McBain CJ. Hippocampal inhibitory neuron activity in the elevated potassium model of epilepsy. J Neurophysiol. 1995;72:2853–2863. doi: 10.1152/jn.1994.72.6.2853. [DOI] [PubMed] [Google Scholar]
- 28.McKhann GM, D'Ambrosio R, Newell DW, Janigro D. Synchronous interictal-like burst discharges induced by cesium are neurotoxic in organotypic hippocampal cultures. Soc Neurosci Abstr. 1997a;23:55. [Google Scholar]
- 29.McKhann GM, D'Ambrosio R, Janigro D. Heterogeneity of astrocyte resting membrane potentials revealed by whole cell and gramicidin-perforated patch recordings from cultured neocortical and hippocampal slice astrocytes. J Neurosci. 1997b;17:6850–6863. doi: 10.1523/JNEUROSCI.17-18-06850.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Newman EA. Glial cell regulation of extracellular potassium. In: Kettenmann H, Ransom BR, editors. Neuroglia. Oxford UP; New York: 1995. pp. 717–731. [Google Scholar]
- 31.O'Connor ER, Sontheimer H, Spencer DD, de-Lanerolle NC. Astrocytes from human hippocampal epileptogenic foci exhibit action potential-like responses. Epilepsia. 1998;39:347–354. doi: 10.1111/j.1528-1157.1998.tb01386.x. [DOI] [PubMed] [Google Scholar]
- 32.Pacia SV, Devinsky O, Luciano DJ, Vazquez B. The prolonged QT syndrome presenting as epilepsy: a report of two cases and literature review. Neurology. 1994;44:1408–1410. doi: 10.1212/wnl.44.8.1408. [DOI] [PubMed] [Google Scholar]
- 33.Ransom CB, Sontheimer H. Biophysical and pharmacological characterization of inwardly rectifying potassium currents in rat spinal cord astrocytes. J Neurophysiol. 1995;73:333–346. doi: 10.1152/jn.1995.73.1.333. [DOI] [PubMed] [Google Scholar]
- 34.Ransom CB, Sontheimer H, Janigro D. Astrocytic inwardly-rectifying potassium currents are dependent on extracellular sodium ions. J Neurophysiol. 1996;76:626–630. doi: 10.1152/jn.1996.76.1.626. [DOI] [PubMed] [Google Scholar]
- 35.Sanguinetti MC. Modulation of potassium channels by antiarrhythmic and antihypertensive drugs. Hypertension. 1992;19:228–236. doi: 10.1161/01.hyp.19.3.228. [DOI] [PubMed] [Google Scholar]
- 36.Sanguinetti MC, Jiang C, Curran ME, Keating MT. A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell. 1995;81:299–307. doi: 10.1016/0092-8674(95)90340-2. [DOI] [PubMed] [Google Scholar]
- 37.Schwartzkroin PA, Baraban SC, Hochman DW. Osmolarity, ionic flux, and changes in brain excitability. Epilepsy Res. 1998;32:275–285. doi: 10.1016/s0920-1211(98)00058-8. [DOI] [PubMed] [Google Scholar]
- 38.Shi W, Wymore RS, Wang HS, Pan Z, Cohen IS, McKinnon D, Dixon JE. Identification of two nervous system-specific members of the erg potassium channel gene family. J Neurosci. 1997;17:9423–9432. doi: 10.1523/JNEUROSCI.17-24-09423.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smart SL, Lopantsev V, Zhang CL, Robbins CA, Wang H, Chiu SY, Schwartzkroin PA, Messing A, Tempel BL. Deletion of the K(V)1.1 potassium channel causes epilepsy in mice. Neuron. 1998;20:809–819. doi: 10.1016/s0896-6273(00)81018-1. [DOI] [PubMed] [Google Scholar]
- 40.Trudeau MC, Warmke JW, Ganetzky B, Robertson GA. HERG, a human inward rectifier in the voltage-gated potassium channel family. Science. 1995;269:92–95. doi: 10.1126/science.7604285. [DOI] [PubMed] [Google Scholar]
- 41.Warmke JW, Ganetzky B. A family of potassium channel genes related to eag in Drosophila and mammals. Proc Natl Acad Sci USA. 1994;91:3438–3442. doi: 10.1073/pnas.91.8.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wenzel HJ, Buckmaster PS, Anderson NL, Wenzel ME, Schwartzkroin PA. Ultrastructural localization of neurotransmitter immunoreactivity in mossy cell axons and their synaptic targets in the rat dentate gyrus. Hippocampus. 1997;7:559–570. doi: 10.1002/(SICI)1098-1063(1997)7:5<559::AID-HIPO11>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 43.Yang WP, Levesque PC, Little WA, Conder ML, Ramakrishnan P, Neubauer MG, Blanar MA. Functional expression of two KvLQT1-related potassium channels responsible for an inherited idiopathic epilepsy. J Biol Chem. 1998;273:19419–19423. doi: 10.1074/jbc.273.31.19419. [DOI] [PubMed] [Google Scholar]
- 44.Zhou W, Cayabyab FS, Pennefather PS, Schlichter LC, DeCoursey TE. HERG-like channels in microglia. J Gen Physiol. 1998;111:781–794. doi: 10.1085/jgp.111.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]