Abstract
A few patients with obsessive-compulsive disorder (OCD) remain severely impaired despite exhausting best-practice treatments. For them, neurosurgery (stereotactic ablation or deep brain stimulation) might be considered. We investigated the proportion of treatment-seeking OCD patients, in a naturalistic clinical sample, who met contemporary neurosurgery selection criteria. Using comprehensive baseline data on diagnosis, severity, and treatment history for adult patients from the NIMH-supported Brown Longitudinal OCD Study, only two of 325 patients met screening criteria for neurosurgery. This finding prompts consideration of new models for clinical trials with limited samples as well as methods of refining entry criteria for such invasive treatments.
Keywords: Obsessive-compulsive disorder, Deep brain stimulation, Neurosurgery, Humanitarian device exemption
Introduction
Obsessive-compulsive disorder (OCD) is marked by recurring intrusive thoughts (i.e., obsessions) and ritualistic behaviors aimed at reducing distress (i.e., compulsions). Its 12-month prevalence is approximately 1.2% in the U.S.,[1] with annual incidence of 0.55 per 1000 person-years.[2] OCD can be quite debilitating, with significant impairment in functioning and quality of life.[3, 4] The disorder generally improves after evidence-based psychological and/or pharmacological interventions, including exposure and response prevention (ERP), serotonin reuptake inhibitors (SRIs) and ERP combined with SRIs.[5] Several medications may be combined with SRIs in efforts to augment benefit.[6]
Patients with “intractable” OCD remain very severely ill and impaired despite first- and second-line treatments. For them, neurosurgery (stereotactic ablation or deep brain stimulation; DBS) may be an option. Both kinds of procedures alter activity in neural networks implicated in the illness. DBS, in contrast to ablation, is generally reversible.[7] Given significant risks and burdens imposed by either approach, prospective patients must meet high eligibility thresholds. Estimates of how many OCD sufferers are surgical candidates vary widely, as indicated by recent controversy over FDA humanitarian approval (a Humanitarian Device Exemption or HDE) of DBS for OCD[8, 9]. The HDE is intended for conditions where adequately-powered randomized controlled trials are not feasible given the small number of patients affected. Fins and colleagues[9] suggested that the number of candidates for DBS for OCD may be as high as 20–30% of total cases and that the HDE was therefore misused. The FDA’s HDE approval suggests drastically lower rates, given its statutory limitation to conditions affecting less than 4,000 people in the U.S. annually. A high estimate of the affected population is also discordant with the number of annual procedures reported in Belgium (0.6 per million inhabitants).[10] To derive a realistic estimate of the relevant population, we systematically applied DBS selection criteria to a well-characterized naturalistic clinical sample.
Materials and methods
Participants
We analyzed baseline measures for all 325 adults in the NIMH-supported Brown Longitudinal Obsessive-Compulsive Study (BLOCS). The BLOCS sample was restricted to treatment-seeking patients who identified OCD as their “primary” diagnosis (the disorder they considered to be most problematic overall; see [11] for details) and were willing to participate in annual interviews for a minimum of five years. Participants must have sought OCD treatment within five years prior to study entry. Demographic and clinical characteristics of this sample have been published elsewhere[11] and are consistent with other studies of OCD clinical samples. Briefly, the sample was predominantly white (98%), 54% female, and had an average age at baseline of 40 (± 12.8) years.
Measures
Lifetime Axis I diagnoses were assessed using the Structured Clinical Interview for DSM-IV,[12] and functional impairment was measured with Global Assessment of Functioning.[13] OCD severity was evaluated by the Yale-Brown Obsessive Compulsive Scale (YBOCS).[14] Treatment histories, including current and past medications for OCD symptoms, were gathered via a semi-structured, rater-administered questionnaire (the Butler Hospital OCD Database). The Treatment Adherence Survey – Patient Version [15] and a modified version of the Psychosocial Treatment Interview[16] assessed amount and quality of previous exposure-based cognitive-behavioral therapy.
DBS inclusion/exclusion criteria
Inclusion and exclusion criteria applied in this study were based on our ongoing controlled trial of DBS for OCD.[17] Among other requirements, criteria for participation in the controlled trial include presence of severe and significantly impairing OCD for a minimum of five years, and unsuccessful trials of a number of medications and behavior therapy. While several DBS entry criteria could not be fully evaluated using the BLOCS dataset, we replicated them as closely as possible. Criteria used in the current study are presented in Table 1, along with the controlled trial criteria. Whenever available data did not allow for perfect replication of the controlled trial criteria, we biased the results toward subject inclusion so as not to underestimate the number of potential surgical candidates. For example, in assessing past SRI trials, the OCD database inquires whether subjects have tried each medication for at least 1 month; however controlled trial inclusion criteria require 3-month trials (at minimum) for these medications. Thus, for purposes of the present study, any reported use of these medications for OCD was counted as a trial. However, stricter criteria were used for benzodiazepine and neuroleptic trial length (minimum of 1 month), due to available information in the BLOCS data.
Table 1.
DBS for OCD Inclusion and Exclusion Criteria*
| Inclusion and exclusion criteria applied in present study | DBS controlled trial inclusion & exclusion criteria |
|---|---|
Inclusion Criteria
|
Inclusion Criteria
|
Exclusion Criteria
|
Exclusion Criteria
|
Only criteria comparable to those used in this study are listed here. Full inclusion and exclusion criteria are available elsewhere[18]. Controlled trial DBS criteria were replicated as closely as possible with available data in the present study. Whenever available data did not allow for perfect replication of the controlled trial criteria, we attempted to bias results toward subject inclusion so as not to underestimate the number of potential surgical candidates.
Including olanzapine, risperidone, haloperidol, clorpromazine, thiothixene, clozapine, quetiapine, ziprasidone, aripiprazole, paliperidone.
Including clonazepam, alprazolam, and lorazepam
Results
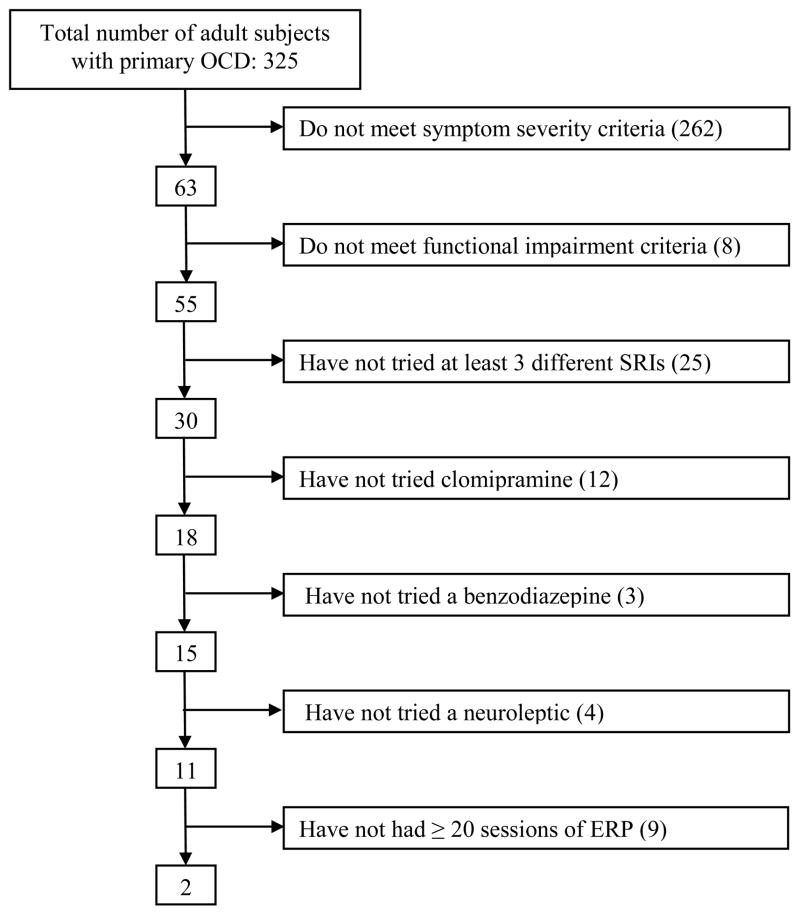
Figure 1 shows the number of the 325 subjects remaining after application of each criterion. Inclusion criteria were applied one at a time to the sample, followed by exclusion criteria. Approximately 19% of the sample met the severity criterion; this number dropped to 17% (55 of 325) when the functional impairment criterion was also applied. When the remaining inclusion criteria were applied to ensure that potential candidates had sufficient trials of medications and ERP, the pool shrank to 0.6% (2 of 325) of treatment seeking patients. Exclusion criteria were then applied to the remaining subject pool, and neither of the remaining two subjects met any of the exclusion criteria. Using the YBOCS severity criterion of 30 from our pilot OCD studies, rather than the 28 in the current multicenter trial, made no difference in the final number.
Figure 1.
Breakdown of BLOCS sample by inclusion criterion
Discussion
Meeting the stringent criteria to qualify for DBS is rare among the general OCD population. Considering incidence rates for OCD (.55 per 1000 person-years),[2] 12-month prevalence rates (1.2% of the US population),[1] and that only approximately 25% of people with OCD seek treatment for this disorder[4, 18] our findings suggest that the pool of potential surgical candidates is extremely small. Using these values, we estimate the number of DBS candidates to be in the range of 184–4020 patients per year, depending on whether yearly incidence or prevalence is considered, respectively (based on the 2010 US population of individuals aged 18–79).[19] However, this estimate should be interpreted with reasonable caution as DBS candidacy is clearly a low base-rate event. Based on our findings, previous estimates[9] suggesting 20–30% of all OCD patients could be surgery candidates appear more consistent with the percentage of OCD patients with severe symptoms; however, as is apparent in the present study, the presence of severe symptoms is a necessary, but not sufficient condition for candidacy for neurosurgical intervention.
Although quite low, the final number of eligible candidates from the present analysis still may be an overestimate. When exact surgery criteria could not be replicated from available data, criteria used erred on the side of inclusion whenever possible so as not to underestimate the number of potential candidates. Also, while we examined some entry criteria here, others could not be included due to insufficient information: comorbid neurological or other relevant disorders, whether psychiatric medications were prescribed at high-therapeutic doses, general health, acute suicidality, etc. Applying these criteria may have further reduced the number of potential candidates. Additionally, the sample used is in all likelihood biased, since half or more of the patients had been treated in a well-known OCD specialty clinic, which they may have sought out because of severity of their symptoms, or failures of previous treatments. While a limitation of the current study, it probably increases the likelihood of having participants who meet criteria for DBS by virtue of severity, impairment, and their likelihood of receiving adequate conventional treatments. Another potential limitation is that participation in the BLOCS sample required willingness to participate in annual interviews which may have excluded some more impaired participants.
In our research group’s experience with independent reviews of patients’ qualification for inclusion in our DBS controlled trial questions from reviewers most commonly focus on adequacy of past ERP attempts. ERP is highly efficacious and generally considered a first-line treatment for OCD.[5] Prior to labeling a case “intractable” it is essential to ensure that adequate trials of ERP with a well-qualified therapist were conducted without substantial benefit. In our experience, patients qualifying for DBS typically exceed the minimum requirements for previous ERP trials.
However, our analysis highlights an underutilization of efficacious treatments. Most severely affected individuals had not tried a sufficient variety of medications to have pharmacotherapy ruled out as a potentially effective option. Similarly, as has been previously noted in this sample and others,[20–22] behavioral psychotherapies are often underutilized in the treatment of OCD and other anxiety disorders. This is especially concerning as ERP is highly efficacious first line treatment for OCD, with or without concurrent SRIs.[6] Previous work examining utilization of cognitive-behavioral therapy within the BLOCS sample has suggested a variety of reasons for this underutilization, including financial cost of treatment, fear of treatment, difficulty attending sessions, and lack of clinician recommendation[20]. Barriers to receipt of efficacious treatments need to be addressed, as certainly some of the patients in the present study would be expected to benefit from additional treatments.
In this study, 53 of the 55 participants met severity and impairment cutoffs, but had not exhausted all treatment options. We are, of course, unable to predict outcomes for these patients if additional treatment options were aggressively pursued. Similarly, the reasons why additional treatments had not been pursued in these cases (e.g., client or clinician decision, accessibility of treatment, etc.) remain unknown. Given that many of these treatment approaches are highly efficacious, particularly behavior therapy, at least some of these patients would be expected to improve, though the number remaining severe under these hypothetical circumstances cannot be reasonably anticipated. Prospective longitudinal studies are required to answer questions about outcomes for such patients.
Given the very small population of OCD patients who receive neurosurgical interventions, it is too premature to put forth specific recommendations for improving identification of optimal DBS candidates. It has been difficult to characterize this small group and even more difficult to begin to identify characteristics of those candidates most likely to benefit from these treatments. While it is too early to say which characteristics may typify optimal DBS candidates, we can recommend several lines of research which will serve to better characterize intractable OCD populations as well as those who may respond well to DBS versus alternative interventions. First, investigations into the course of treatment refractory OCD are needed to establish the stability of severe OCD over time; longitudinal studies may also play an important role in delineating the burden of illness despite aggressive treatments. Second, when sample sizes permit, investigations of neurosurgical and other interventions for intractable OCD should seek to identify subgroups of patients who may respond differentially to treatment. For example, some reports suggest that those with primary incompleteness type OCD may be less likely to respond to these treatments[23]. Third, the formation of a national/international registry of psychiatric neurosurgery cases would allow systematic collection of data which may serve to further inform candidate selection and optimize patient outcomes for this small and unique group of patients.[24] Finally, more information is needed on viable alternatives to neurosurgical intervention, including intensive residential treatment programs. While surgical candidates are typically encouraged to try residential treatment, and many have already tried and failed to benefit from these programs by the time they seek out neurosurgery, previous participation in a specialized residential treatment program is not currently a requirement for DBS. Additional information is needed to determine whether such programs may be a practical and effective alternative to neurosurgery for some candidates.
To maximize benefit and minimize risk for patients with intractable OCD, it is important to identify which neurosurgery inclusion/exclusion criteria should be retained and whether others should be added or discarded. Overly inclusive criteria may be problematic by allowing non-optimal cases to assume the risks associated with neurosurgery; however, other risks exist with overly stringent criteria, such as risk of suicide while a subject is waiting for evaluation or told to try another intervention before qualifying for surgery [e.g., 10]. Entry criteria for neurosurgery must continue to evolve, influenced by ongoing interdisciplinary research.
In part, refining selection criteria will depend on understanding more about the clinical features of this population, following the recommended lines of research above, as well as proposed biomarkers that may predict outcomes. Empirically evaluating existing criteria and attempts to refine these will require thorough systematic collection of longitudinal data in patients with disabling, highly refractory OCD. Such studies should focus on both those who are and are not treated surgically in order to compare clinical characteristics, as well as outcomes. Regarding the narrower population of patients who do undergo surgical intervention, collecting systematic baseline and follow-up data on patients undergoing DBS and ablative surgeries targeting the same circuitry would be highly informative. The quality and type of data collected will also be paramount and should include not only information on symptom presentation and severity, but also patterns of comorbidity, core features of the illness, neuroimaging, neuropsychiatric functioning, and measures of observable behavior.
Acknowledgments
Work on this project was funded by the following grants from the National Institute of Mental Health: R01MH060218, T32MH067553, P50MH086400, and U01MH076179. The content of this manuscript does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health. Additional author disclosures include: BG has received meeting travel expenses from Medtronic, Inc. and Hoffman-La Roche Pharmaceuticals. WG has served as a consultant for Avanir Pharmaceuticals, Inc., Alexza Pharmaceuticals, Inc., F. Hoffman-La Roche Ltd., and Otsuka Pharmaceutical.
References
- 1.Kessler RC, Chiu WT, Demler O, et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nestadt G, Bienvenu OJ, Cai G, et al. Incidence of obsessive-compulsive disorder in adults. J Nerv Ment Dis. 1998;186:401–406. doi: 10.1097/00005053-199807000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Eisen JL, Mancebo MA, Pinto A, et al. Impact of obsessive-compulsive disorder on quality of life. Compr Psychiatry. 2006;47:270–275. doi: 10.1016/j.comppsych.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruscio AM, Stein DJ, Chiu WT, et al. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol Psychiatry. 2010;15:53–63. doi: 10.1038/mp.2008.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eddy KT, Dutra L, Bradley R, et al. A multidimensional meta-analysis of psychotherapy and pharmacotherapy for obsessive-compulsive disorder. Clin Psychol Rev. 2004;24:1011–1030. doi: 10.1016/j.cpr.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 6.American Psychiatric Association. Practice guideline for the treatment of patients with obsessive-compulsive disorder. Arlington, VA: American Psychiatric Association (APA); 2007. [PubMed] [Google Scholar]
- 7.Greenberg BD, Rauch SL, Haber SN. Invasive circuitry-based neurotherapeutics: stereotactic ablation and deep brain stimulation for OCD. Neuropsychopharmacology. 2010;35:317–336. doi: 10.1038/npp.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dickson V. FDA calls study criticizing HDE biased, riddled with omissions. Devices & Diagnostics Letter 2011. 2011:38. [Google Scholar]
- 9.Fins JJ, Mayberg HS, Nuttin B, et al. Misuse of the FDA’s humanitarian device exemption in deep brain stimulation for obsessive-compulsive disorder. Health Aff (Millwood) 2011;30:302–311. doi: 10.1377/hlthaff.2010.0157. [DOI] [PubMed] [Google Scholar]
- 10.Gabriels L, Nuttin B, Cosyns P. Applicants for stereotactic neurosurgery for psychiatric disorders: role of the Flemish advisory board. Acta Psychiatr Scand. 2008;117:381–389. doi: 10.1111/j.1600-0447.2008.01166.x. [DOI] [PubMed] [Google Scholar]
- 11.Pinto A, Mancebo MC, Eisen JL, et al. The Brown Longitudinal Obsessive Compulsive Study: clinical features and symptoms of the sample at intake. J Clin Psychiatry. 2006;67:703–711. doi: 10.4088/jcp.v67n0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) NY: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 13.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 2002. Text Revision. [Google Scholar]
- 14.Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry. 1989;46:1006–1011. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- 15.Mancebo MC, Pinto A, Rasmussen SA, et al. Development of the Treatment Adherence Survey-patient version (TAS-P) for OCD. J Anxiety Disord. 2008;22:32–43. doi: 10.1016/j.janxdis.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steketee G, Perry JC, Goisman RM, et al. The psychosocial treatments interview for anxiety disorders: a method for assessing psychotherapeutic procedures in anxiety disorders. J Psychother Pract Res. 1997;6:194–210. [PMC free article] [PubMed] [Google Scholar]
- 17.National Institute of Mental Health; Butler Hospital. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000. Effectiveness of Deep Brain Stimulation for Treating People with Treatment Resistant Obsessive-Compulsive Disorder. [cited 2011 Dec 12]. Available from: http://clinicaltrials.gov/show/NCT00640133 NLM Identifier: NCT00640133. [Google Scholar]
- 18.Regier DA, Narrow WE, Rae DS, et al. The de facto US mental and addictive disorders service system. Epidemiologic catchment area prospective 1-year prevalence rates of disorders and services. Arch Gen Psychiatry. 1993;50:85–94. doi: 10.1001/archpsyc.1993.01820140007001. [DOI] [PubMed] [Google Scholar]
- 19.U.S. Census Bureau. Age and Sex Composition: 2010. Retrieved December, 2011 from http://www.census.gov/prod/cen2010/briefs/c2010br-03.pdf.
- 20.Mancebo MC, Eisen JL, Sibrava N, et al. Patient utilization of cognitive-behavioral therapy for OCD. Behav Ther. 2011;42:399–412. doi: 10.1016/j.beth.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goisman RM, Rogers MP, Steketee G, et al. Utilization of behavioral methods in a multicenter anxiety disorders study. J Clin Psychiat. 1993;54:213–218. [PubMed] [Google Scholar]
- 22.Zayfert C, DeViva JC, Becker, et al. Exposure utilization and completion of cognitive behavioral therapy in PTSD in a “real world” clinical practice. J Trauma Stress. 2005;18:637–645. doi: 10.1002/jts.20072. [DOI] [PubMed] [Google Scholar]
- 23.Greenberg BD, Malone DA, Friehs GM, et al. Three-year outcomes in deep brain stimulation for highly resistant obsessive-compulsive disorder. Neuropsychopharmacology. 2006;31:2384–93. doi: 10.1038/sj.npp.1301165. [DOI] [PubMed] [Google Scholar]
- 24.U. S. Department of Health, Education, and Welfare. Protection of human subjects. Use of psychosurgery in practice and research: Report and recommendations of National Commission for the Protection of Human Subjects. Fed Regist. 1977;42:26318–26332. [PubMed] [Google Scholar]