Abstract
For many traditional, non-industrialized populations, intensive and prolonged breastfeeding buffers infant health against poverty, poor sanitation, and limited health care. Due to novel influences on local economies, values, and beliefs, the traditional and largely beneficial breastfeeding patterns of such populations may be changing to the detriment of infant health. To assess if and why such changes are occurring in a traditional breastfeeding population, we document breastfeeding patterns in the Bolivian Tsimane, a forager-horticulturalist population in the early stages of modernization. Three predictions are developed and tested to evaluate the general hypothesis that modernizing influences encourage less intensive breastfeeding in the Tsimane: 1) Tsimane mothers in regions of higher infant mortality will practice more intensive BF; 2) Tsimane mothers who are located closer to a local market town will practice more intensive BF; and 3) Older Tsimane mothers will practice more intensive BF. Predictions were tested using a series of maternal interviews (from 2003-2011, n=215) and observations of mother-infant dyads (from 2002-2007, n=133). Tsimane breastfeeding patterns were generally intensive: 72% of mothers reported initiating BF within a few hours of birth, mean (± SD) age of CF introduction was 4.1±2.0 months, and mean (± SD) weaning age was 19.2±7.3 months. There was, however, intra-population variation in several dimensions of breastfeeding (initiation, frequency, duration, and complementary feeding). Contrary to our predictions, breastfeeding was most intensive in the most modernized Tsimane villages, and maternal age was not a significant predictor of breastfeeding patterns. Regional differences accounted for variation in most dimensions of breastfeeding (initiation, frequency, and complementary feeding). Future research should therefore identify constraints on breastfeeding in the less modernized Tsimane regions, and examine the formation of maternal beliefs regarding infant feeding.
Keywords: breastfeeding, infancy, complementary feeding, weaning, modernization, acculturation, indigenous, behavior, Tsimane
Introduction
Breastmilk contains numerous nutritional, immunological, and hormonal constituents that are tailored specifically to infant growth, metabolism, neurocognitive development, and pathogenic challenge (Hinde & Milligan, 2011). Initiation of breastfeeding (herein abbreviated BF) reduces the risk of neonatal mortality (Garcia et al., 2011), and exclusive BF for the first six months of life lowers the risk of infant morbidity across all economic scales (Kramer & Kakuma, 2004). Complementary feeding (herein abbreviated CF), i.e. the provisioning of any non-breast milk solid or liquid, should be initiated around 4-6 months of age to meet the increasing energy demands of growing infants (Dewey, 1997; McDade & Worthman, 1998; Nielsen et al., 2011). Prolonged BF in conjunction with CF provides continued immunological protection (Dettwyler, 2004; Shiva & Nasiri, 2003) and reduces infant growth faltering (Villalpando & Lopez-Alarcon, 2000), infectious morbidity, and mortality (Lamberti et al., 2011). Due to these infant health benefits, the World Health Organization recommends exclusive BF for six months, followed by mixed BF and CF for 24 months or beyond (Wagstaff et al., 2006; WHO, 2011). Promotion of increased BF initiation and duration of exclusive and mixed BF are preventative interventions included in the United Nation's Millennium Development Goals to reduce child mortality and eradicate extreme hunger by 2015 (Wagstaff et al., 2006).
A comprehensive review of ethnographic studies shows that the traditional “intensive” BF patterns of most non-industrial populations—widespread BF initiation, infant-driven “on-demand” feeding, sustained periods of exclusive BF, and continued mixed BF for up to two years or longer—are largely concordant with current recommendations (Sellen, 2001). In these populations, intensive traditional BF practices may be the most economical, nutritional and hygienic strategy for buffering infant health against the poverty, poor sanitation and limited health care they experience (Anatolitou, 2012; González de Cossio et al., 2013; Kramer & Greaves, 2007; Lutter et al., 2011; McDade, 2005). Biological evidence of infant digestive and masticatory development further suggest that recommended durations for exclusive BF and total BF may be the human ancestral norm (Sellen, 2007).
However, widespread variation in BF practices is evident even among “traditional breastfeeding populations” (Piperata & Gooden Mattern, 2011; Gray, 1995; Vitzthum, 1992) This should be expected as a suite of individual, cultural, economic, and ecological factors ultimately influence individual infants’ needs and individual mothers’ beliefs, perceptions, and constraints (Dettwyler, 1986; McDade & Worthman, 1998). Observable practices in extant traditional BF populations may also be rapidly changing due to outside contact and influences on local economies, values, and beliefs. The effects of such novel influences on the practices and patterns of traditional BF populations remain largely unexplored and, given the diversity of changing BF trends in recent decades, are particularly challenging to project.
The mid-20th century saw the widespread adoption of both bottle-feeding and increased maternal employment outside of the home in many industrialized countries, leading to drastically altered BF attitudes and behaviors, and substantial numbers of infants who were never breastfed or breastfed for only short durations (Boerma et al., 1991; Fomon, 2001; Van Esterik, 2002). Recent global campaigns to promote BF largely seek to reverse these trends. Perhaps due in part to these efforts, BF rates in the U.S. have steadily increased since the 1990s (Ryan, 1997; Wolf, 2003), though the former remain well below targeted public health goals (Forste & Hoffmann, 2008; Grummer-Strawn et al., 2008). Increased adoption of formula-feeding, shorter BF durations, and the abandonment of traditional BF practices were also observed in many developing populations worldwide during 1980s and 1990s (Adair et al., 1993; Akin et al., 1986; Ferry & Smith, 1983 ; Guthrie et al., 1983; Howrigan, 1988; Huffman, 1984; Winikoff et al., 1988). Though BF is currently on the rise in many developing countries (Lutter & Morrow, 2013), less than half of infants in these populations are exclusively breastfed for six months (Marriott et al., 2007). Durations of exclusive BF and total BF have also recently decreased among some vulnerable populations with historically high BF rates, including indigenous populations in Mexico (González de Cossio et al., 2013) and urban populations in Laos (Barennes et al., 2012).
BF patterns of developing populations are continually impacted by changes in maternal education, employment and attitudes and beliefs about infant care - instigated by changing demographics and economies (Abada et al., 2001; Quinlan et al., 2003; Raphael & Davis, 1985; Rasheed et al., 2009). Historically, the direction and magnitude of change has not been uniform. Changes in BF patterns can be positive; for example in Ghana from 1988-1998, mean BF duration increased even as large numbers of women entered the workforce for the first time (DeRose, 2007). Unfortunately, the erosion of traditional BF patterns is more commonly documented as developing populations experience rapid urbanization (Brady, 2012; Gracey, 2003; Harrison et al., 1993; Howrigan, 1988; Igun, 1982; Pérez-Escamilla, 2003; Ruel et al., 1999; Solien de González, 1963).
However, rapid urbanization does not reflect the experience of nearly half of the world's population (UNDB 2007). In Latin America and elsewhere, many populations remain relatively non-industrialized, living in rural areas and maintaining traditional subsistence practices such as hunting, gathering, fishing and horticulture (ibid). Among these populations “modernizing” influences may affect traditional BF practices differently, as modernization occurs less abruptly in rural, traditional societies than it does in rapidly urbanizing ones.
Modernization entails acculturation, or prolonged exposure to the values, institutions and technologies of a mainstream society (Sam & Berry, 2010). When non-industrialized societies begin to acculturate, subsequent transitions often occur in subsistence, diet, market integration, epidemiology, demography, and formalized education (Coimbra et al., 2002; Godoy et al., 2007; Malina et al., 2008; Nyberg, 2009; Piperata et al., 2011; Valeggia et al., 2010). These transitions often proceed in a prolonged and piecemeal fashion; for example, market foods and modern medicine may become accessible to villages still lacking in basic sanitation and public health infrastructure. While the individual and collective effects of various modernizing influences may not drive linearly predictable changes in traditional BF patterns, any change is likely to influence maternal-infant health outcomes. Ongoing and detailed studies of BF patterns in modernizing traditional populations are therefore crucial, yet research in this area is lacking.
To address this problem, we document the breastfeeding patterns of Tsimane foragerhorticulturalists of the Bolivian Amazon. Though the Tsimane remain relatively non-industrialized, access to modern medicine, village schools, and local markets has increased in recent decades (Godoy et al., 2005; Gurven et al., 2007; Reyes-García et al., 2010). Exposure to these novel influences varies across Tsimane villages, contributing to regional variation in maternal education, maternal-infant health and nutrition, and the adoption of Bolivian national values and traditions. We expect that intra-population variation in Tsimane BF patterns will reflect this regional variation in modernizing influences. Although existing Tsimane subsistence strategies and pathogen exposure (Foster et al., 2005; McDade et al., 2005; Tanner et al., 2011; Veile et al., 2012) should favor the protective effects of intensive BF, we broadly hypothesize that novel modernizing influences will contribute to decreased BF intensity in this population.
In Bolivia and elsewhere in Latin America, national BF rates have risen over the past 20 years, largely due to BF promotion campaigns (Baker et al., 2006; Lutter & Morrow, 2013). However, BF statistics derived from national-level surveys often obscure cross-cultural and regional trends, and may not reflect the BF patterns of geographically and linguistically isolated populations such as the Tsimane. For example, in Bolivia the greatest gains in BF have occurred in educated urban women (Lutter et al., 2011a; Lutter & Morrow, 2013), whereas the Tsimane reside in a Bolivian region that is overwhelmingly rural, under-educated and poor (UDAPEUNDP, 2010). Furthermore, for many indigenous Latin American populations, the benefits of health care access and health education are limited due to language barriers (Terborgh et al., 1995). Indeed BF promotion interventions in Bolivia generally are implemented in Spanish, Quechua, and Aymara, which are not spoken by the majority of Tsimane women.
Acculturation and BF Patterns
Tsimane women who reside in villages near the town of San Borja (pop~24,000) are more acculturated than Tsimane women who reside in remote villages. Acculturated Tsimane women have frequent contact with other Bolivian nationals, more exposure to formalized education, greater Spanish fluency, and participate more in agricultural labor and the local market economy. Though the effects of acculturation on Tsimane BF patterns have not been examined, we expect that they will be associated with decreased BF intensity, particularly in young Tsimane mothers. Young Tsimane women are beginning to adopt clothing styles of mainstream lowland Bolivia and other symbols of modernization (e.g. denim jeans, bras, heeled shoes, make-up and television). A handful of acculturated young women are adopting other behaviors that mimic the dominant culture, such as prolonged education and birth control.
These novel behaviors and symbols reflect changing perceptions of image and status, and represent a broader adoption of values and beliefs from the mainstream culture. We speculate that some of these novel behaviors and beliefs may ultimately serve to discourage intensive BF. For example, the Western clothing styles adopted by some young Tsimane women are restrictive and prohibit on-demand BF. Additionally, media portrayals of large-breasted women are common in stores and discotheques throughout San Borja, and the sexualization of breasts is a Western attitude that is associated with infrequent BF (Harrell, 1981). Finally, powdered baby formulas and plastic baby bottles are prevalent in San Borja pharmacies and market stands. Breastmilk substitutes were recently implicated in declines in BF rates in urban Laos (Barennes et al., 2012). Though bottle-feeding remains extremely rare in Tsimane villages, we expect that young, acculturated women will be most likely to adopt such a novel practice.
Local Ecology and BF Patterns
As suggested by the “shifting weanling's optimum” hypothesis, cross-cultural variation in BF patterns will simultaneously reflect the relative benefit of breastmilk to the infant and the relative cost of BF to the mother. (Sellen & Smay, 2001). In other words, in epidemiologically risky environments, intensive BF provides infants the best protection from disease. However, BF also poses time, energy and reproductive costs for mothers. Because of their pathogenic environment and high infant mortality rate, we expect that Tsimane women will practice intensive BF despite maternal constraints. We do not assume these behaviors reflect a conscious decision or understanding of BF benefits, but rather their historical traditions and economic conditions.
However, even long-standing traditions may change and evolve as novel epidemiologic conditions shift the costs and benefits of intensive BF, such as health care that improves infant survivorship. Among Tsimane, health care access (e.g. vaccines, antibiotics and oral rehydration therapy) is associated with higher infant survivorship (Gurven, 2012). Regional variation in health care access among the Tsimane leads to regional differences in infant mortality rates. Assuming little explicit BF education and promotion, increased access to medical care that improves infant survivorship may contribute to less intensive BF. In other words, mothers maintain intensive BF out of necessity under conditions of high infant mortality, but practice less intensive BF when survival costs are lessened by modern health care; thus, infants survive at higher rates despite less intensive BF.
To test our general hypothesis that modernization will lead to decreased BF intensity in the Tsimane, we derive predictions using three proxies of modernization. The first proxy is infant mortality rate, which varies across Tsimane regions and reflects differences in health care access. We predict that: 1) Tsimane mothers in regions of low infant mortality will practice less intensive BF. The second proxy is mother's geographic proximity to San Borja, which is associated with household income and the consumption of market foods (von Rueden & Jaeggi, 2012). We predict that 2) Tsimane mothers who are located close to San Borja will practice less intensive BF. The third proxy is maternal age. We predict that 3) Younger Tsimane mothers will practice less intensive BF.
MATERIALS AND METHODS
Ethnographic Background
Tsimane Overview
The Tsimane are indigenous forager-horticulturalists residing along the Maniqui River and nearby environs, in the Beni Department of Bolivia. The total population (~15,000) is scattered across over 90 villages that vary in size (pop. ~80-500), ecology, and proximity to the town of San Borja (Gurven, 2012). Tsimane subsistence, household labor structure, and reproductive patterns have been described elsewhere (Gurven et al., 2009; McAllister et al., 2012; Winking & Gurven, 2011).
Briefly, Tsimane practice a mixed subsistence strategy of hunting, foraging, fishing and small-scale agriculture augmented with sporadic wage labor and produce sales. Spouses extensively cooperate in household maintenance and horticultural production, but sex roles are well defined. Men acquire game and fish and engage in wage labor. Women are responsible for providing childcare, processing and preparing food, washing clothes, caring for the home and making chicha (a fermented drink made from manioc or other local cultivants). Both sexes collect forest foods, fetch firewood and water, and work in gardens and agricultural fields.
Tsimane women, on average, reach menarche at 13.9 years, marry by age 16 (Rucas et al., 2006), and have their first birth by age 18.6 (Walker et al., 2006). Tsimane women have extremely high fertility (total fertility rate = 9) (McAllister et al., 2012) and spend much of their lives in cycles of pregnancy and lactation. There are relatively few taboos concerning diet and activity of pregnant and lactating Tsimane women. Following birth, Tsimane mothers and neonates spend at least a week resting in a mosquito net. Infants are continuously held, worn close to the mother in slings, or rocked in a hammock, and breastfed more or less on-demand while mothers engage in household tasks. Affordable breastmilk substitutes (powdered milk and formulas) are available and utilized by women in San Borja, but in our observations, bottle-feeding remained rare in Tsimane villages. Toddlers and small children may be cared for by fathers, older siblings, and female kin while mothers participate in subsistence activities.
Time allocation studies indicate that Tsimane women spend roughly 2 hours/day in direct productive tasks such as agricultural work and an additional 4-6 hours per day engaged in domestic tasks including childcare (Gurven et al., 2013). Gurven and colleagues used the factorial method (FAO/WHO/UNU, 1985) in conjunction with accelerometry and heart rate monitoring (Assah et al., 2011) to construct 24-hour physical activity level estimates (PAL) for Tsimane men and women. They found that 24-hour PAL for Tsimane women (aged 20+) was 1.73. This PAL is categorized as “moderate to active” (FAO/WHO/UNU, 2004) and is only slightly higher than the mean PAL for women in industrialized societies (1.71), but slightly lower than that of women from other hunting and gathering or farming groups (Dugas et al., 2011; Gurven et al., 2013).
The Tsimane diet is nutritionally adequate; the mean BMI of a sample of nursing Tsimane mothers did not significantly differ from that of a lactationally age-matched sample of U.S. mothers (Martin et al., 2012). However, limited health care, a high-pathogen environment, poor sanitation, anemia, and micronutrient deficiencies contribute to frequent infectious-mediated morbidity and growth faltering in Tsimane infants and young children (Foster et al., 2005; Gurven, 2012; McDade et al., 2005; Tanner et al., 2009). The Tsimane infant mortality rate (herein abbreviated IMR) for 1990-2002 (defined as infant deaths in the first year of life, excluding abortions, stillbirths and miscarriages) was 126 deaths per 1000 births, based on extensive maternal reproductive histories (Gurven et al., 2007). Though the Tsimane IMR has declined over time since 1950, it remains higher than the Bolivian national average, which was 49 deaths per 1000 births in 2003, when this study began. Tsimane IMR is also unevenly distributed across villages with varying access to health care (Gurven, 2012). The extent that variation in duration of exclusive BF, age at weaning, and quality of weanling diets varies within the Tsimane population and impacts broader health and disease patterns is unknown.
Regional Variation
For the purposes of this paper, Tsimane villages are grouped into four regions that vary geographically and in many aspects of modernization. Three regions, “forest”, “riverine”, and “mission” are geographically remote, and are variable in their access to San Borja and to health care facilities. In contrast, “near-town” villages are easily accessible from San Borja year round by road.
Six villages sampled are forest villages, which are remote, composed of few families, and are the least accessible of Tsimane villages, as the road leading to them cannot be traversed during the rainy season (~ December-March). Among Tsimane regions, formal education and maternal PAL are lowest in forest villages, while IMR is highest (Gurven, 2012; Gurven et al., 2013). Lower maternal PAL in forest regions reflects their limited participation in agriculture relative to women in the more modernized regions.
Six villages sampled are riverine villages, which are located in remote regions along the Maniqui River. Riverine villages vary in their distance to San Borja, but are accessible by boat for most of the year. Village sizes (number of families) are generally intermediate compared to near-town and forest villages. IMR is slightly lower and maternal PAL and years of education are slightly higher in riverine than in forest villages. (Gurven, 2012; Gurven et al., 2013).
Four riverine villages sampled are located downstream from San Borja and are part of the Beni Biosphere Reserve. Established in 1982, the Reserve is a nationally protected area encompassing 1,350 km2 and use of its resources is restricted to Tsimane residents for subsistence (Miranda, 1995). These villages are a day's walk from Galilea, a small peasant village with a health clinic located along the main road to San Borja. The remaining two riverine villages are located upstream from San Borja and can be reached by boat in a long day's travel. They are also within a five-hour walk from a large upriver village (mission) that is attached to a Catholic mission and health clinic.
We describe and analyze mission, a large upriver village, as a separate region because it is more modernized than surrounding upriver villages. While geographically remote, it is a large village (225 families) and possesses an airstrip, health clinic, and a well-attended Catholic church. An Alsatian priest (Martin Bauer) lived there for 50 years, followed by several nuns who have since resided in the village and provide health care, health education and family planning advice to Tsimane in this and the surrounding villages, and regular schooling to residents. As a result, IMR is low, and adults receive more education compared to neighboring riverine villages (Gurven et al., 2007; von Rueden & Jaeggi, 2012).
Finally, two villages sampled are near-town villages. The near-town villages are closest to San Borja and -- with the exception of the mission village – have the largest number of families. Because health care is easily accessible, IMR is lowest of all regions (nearly half that of the remote forest and riverine villages). Adults have the most education and women are the most physically active in near-town villages. Higher PAL in near-town villages reflects their increased participation in agricultural labor (Gurven et al, 2013); agricultural plots are largest, and yields greatest, in near-town villages (von Reuden & Jaeggi, 2012).
We rank the four regions by modernization based on: 1) Infant mortality rate, low-mortality = more modernized (Gurven, 2012); 2) Adult education, greater years attained education = more modernized (von Rueden & Jaeggi, 2012); 3) Village distance from San Borja, closer to San Borja = more modernized (ibid); 4) Village size, more families = more modernized (Gurven et al., 2007) and; 5) Women's physical activity level or PAL, higher PAL = more modernized (Gurven et al., 2013). Regional characteristics are summarized with modernization rankings in Table 1.
Table 1.
Differences in indicators of modernization across four Tsimane regions. Data are from published and unpublished previous studies.
| Region | # of Villages | Number of families * | Distance to San Borja (km) | Mean years schooling (adult) * | IMR/1000** | Women's PAL *** | Modernization rank |
|---|---|---|---|---|---|---|---|
| Forest | 6 | 43 (21-66) | 31-82 | 0.6 | 171 | 1.66 | Low |
| River | 6 | 65 (37-89) | 20-59 | 2.0 | 147 | 1.69 | Medium |
| Mission | 1 | 225 | 66 | 2.9 | 111 | Not sampled | Medium-High |
| Near-town | 3 | 165 (161-169) | <30 | 3.0 | 81 | 1.81 | High |
Mean (range), von Reuden & Jaeggi, 2012 (years of schooling are age-adjusted)
IMR: 1990-2002, Gurven et al. 2007, Gurven 2012
Tsimane Health Care Access
Since 2003, pregnant Tsimane women and children under five years of age have qualified for national health insurance (Seguro Universal Materno Infantil or SUMI) enabling them to receive free preventative and outpatient services from local providers (Silva & Batista, 2010) including a public hospital in San Borja. Insured services provided included prenatal care, vaccinations, and nutritional supplements. However, health care workers do not speak Tsimane, and only in recent years has the San Borja hospital staffed a Spanish-Tsimane translator for Tsimane patients. In addition, clinics at the outskirts of San Borja, Galilea and the mission village, and traveling outreach programs are available to all Tsimane because of funding from private religious and non-governmental organizations. Clinics and outreach programs provide vaccinations, contraceptives, antibiotics, and deworming medications when available. Visitations by outreach programs occur most frequently in near-town villages (almost monthly) and at intermediate frequency or only annually in riverine and forest villages. Village outreach visits are brief and are attended by patients en masse. Thus, while expanded health care services have undoubtedly increased vaccination coverage and improved some indices of maternal and infant health among the Tsimane, services remain limited, access can be uneven and sporadic. Health care workers are limited in their abilities to provide private consultation and counseling on breastfeeding, infant care, and family planning practices. Tsimane knowledge of modern biomedical etiology, contraceptives and health care recommendations remains generally poor.
Study Sample/Data Collection
Since 2002, Tsimane health, demography, ecology, and culture have been studied under the Tsimane Health and Life History Project (herein abbreviated as THLHP, http://www.unm.edu/~tsimane). THLHP employs Bolivian physicians, laboratory technicians, and Tsimane research assistants to deliver medical care to Tsimane patients while collecting demographic and biomedical data. A multi-national team of anthropologists work in Tsimane villages collecting additional ethnographic and behavioral data. Researchers conducted initial demographic interviews from 2002-2005 (see Gurven et al. 2007); the THLHP medical team has since continually updated village censuses and patient records.
To characterize Tsimane BF practices, we have compiled four separate investigations collected under the auspices of THLHP, including interviews collected independently by three researchers and one large observational dataset. The individual researchers' interviews were collected as part of separate research projects with independent aims. We have therefore pooled data from the interview studies that overlap methodologically (i.e. same questions were asked) or thematically (including data relevant to BF behaviors that were collected across two of the studies but not all three). Specific data collection activities are summarized in Table 2.
Table 2.
Summary of data collection activities by village
| Village | Distance to San Borja (km) | Region | Number of Families | Year Interview | Sample sizes for individual study questions | Time Allocation Sample***, year | |||
|---|---|---|---|---|---|---|---|---|---|
| Breast-feeding initiation, Beliefs | Complementary feeding** | Wean Age (Practice) | Wean Age (Belief) | ||||||
| Aperecito | 82.3 | Forest | 60 | 2003 | 1 | 1 | 0 | 0 | 7, 2003 |
| Cedral | 30.7 |
Riverine (downriver) |
92 | 2006-2007 | 0 | 14 | 0 | 0 | 12, 2006- 2007 |
| Chacal | 23.3 |
Riverine (downriver) |
89 | 2006-2007 | 0 | 15 | 0 | 0 | 14, 2006- 2007 |
| Cosincho | 56.5 |
Riverine (upriver) |
107 | 2003, 2010- 2011 |
13 | 22 | 19 | 19 | 31, 2002- 2003 |
| Cuverene | 68.9 | Forest | 66 | 2003 | 10 | 4 | 5 | 5 | 9, 2002- 2003 |
| Fatima | 66.2 | Mission | 225 | 2010-2011 | 0 | 10 | 14 | 14 | 0 |
| Jamanchi 1 | 31.1 | Forest | 51 | N/A | 0 | 0 | 0 | 0 | 14, 2005- 2006 |
| Las Maras | 38.0 | Forest | 25 | 2010-2011 | 0 | 3 | 6 | 0 | 0 |
| Monte Rosa | 20.4 |
Riverine (downriver) |
44 | 2006-2007 | 0 | 10 | 0 | 0 | 0 |
| Moseruna | 44.7 | Forest | 34 | 2010-2011 | 0 | 8 | 5 | 2 | 0 |
| Munday | 59.0 |
Riverine (upriver) |
37 | 2003, 2010- 2011 |
0 | 4 | 5 | 5 | 10, 2002- 2003 |
| Nuevo Mundo |
50.6 | Forest | 21 | 2010-2011 | 0 | 10 | 3 | 1 | 0 |
| Puerto Triunfo |
26.4 |
Riverine (downriver) |
48 | 2006-2007 | 0 | 5 | 0 | 0 | 1, 2006- 2007 |
| San Miguel | 20.2 | Near-town | 169 | 2003 | 0 | 16 | 1 | 0 | 0 |
| Santa Ana* | 56.1 | Near-town | No data | 2006 | 0 | 1 | 0 | 0 | 0 |
| Tacuaral | 27.1 | Near-town | 161 | 2009, 2010- 2011 |
48 | 60 | 59 | 11 | 35, 2005- 2006 |
| Total=16 | Range= 20.2-82.3 |
Range= 21-225 |
2003-2011 | n= 72 | n= 183 | n= 117 | n= 57 | Total n=133 | |
One mother is from Santa Ana, a village that is not in the THLHP study sample. She was married to a Tsimane THLHP worker, lived in several study villages, and worked as a Spanish-English interpreter. She is analytically treated as a “near-town” woman.
While 215 CF interviews were conducted, n=183 refers only to infants who had begun CF at time of interview
Time allocation sample= number of infants sampled
Because BF patterns in traditional populations are generally understood better as a process than as discrete events (Humphrey, 2010; DW Sellen, 2009; Gray, 1996), we examine the following four dimensions of BF: (a) reported BF initiation and colostrum beliefs, (b) reported CF introduction, (c) reported age at full cessation of BF (herein termed ‘weaning age’) and weaning beliefs, and d) observed BF frequency. BF initiation, CF introduction, and weaning measures (dimensions a, b and c) are derived from maternal interviews. Between 2003 and 2011, three of the authors collectively interviewed 195 Tsimane mothers of children aged 0-36 months (herein referred to as “infants”) in 14 villages regarding past and present BF practices (see Table 2). However, retrospective interviews are subject to recall errors and biases, and mothers may not innumerate between token feeding and CF (Wilson et al., 2006); leading to discordance in reported and actual BF practices (Cosminsky et al., 1993; Vitzthum, 1994). Due to this methodological issue, interviews are augmented with a measure of BF frequency (dimension d) derived from observational studies conducted by THLHP researchers from 2003-2007 (133 infants observed). Data collection methods are described in detail below.
Prior to data collection, research protocols received Institutional Review Board approval from the University of New Mexico and the University of California, Santa Barbara. Consent was obtained from village leaders and individual participants upon initiating research activities in each village.
Collection and Analysis of Interview Data
At each phase of the study, mothers of infants (0-36 months) were identified in study villages from household censuses. In 2003 and 2006, Author 1 conducted interviews with 88 mothers in nine near-town, riverine, and forest villages (total pop ~1272). All mothers of infants were asked to participate, and no woman declined. In 2009, Author 2 conducted interviews with 51 mothers of infants in a near-town village (population ~400). All mothers of infants were asked to participate, with only one declining. From 2010-2011, Author 3 conducted interviews with 76 mothers of infants in seven near-town, riverine and forest villages (pop. ~1434). All mothers of infants were asked to participate, with only three declining.
In all interviews, mothers were first asked if their infants had begun consuming any nonbreastmilk liquids or solids. Mothers answering “yes” were asked if infants were still nursing, and how old their infants were when CF began. From this recall, infants were categorized as “exclusively breastfeeding”, “complementary feeding” or “weaned.” Multiparous mothers of nursing infants were asked the age that their previous infants were weaned. Additional questions varied among the authors: Two authors asked mothers when BF was initiated after birth and if they believed colostrum was “good” or “bad” for infants, and two authors asked what mothers believed was the “ideal” weaning age. Datasets were combined where possible.
Statistical analyses were generated using Predictive Analytics Software Statistics version 18.0 (SPSS Inc., Chicago, IL, USA). Regional comparisons of infant age, maternal age and parity (live births) were performed using one-way ANOVA. Statistical analyses assess all dimensions of BF for all mothers from all villages who were interviewed from 2003-2011: BF initiation (practice and belief), CF introduction, weaning (practice and belief), and BF frequency. Predictor variables were region (an indicator of IMR), village distance from town, and maternal age.
Though region and distance are correlated, they are examined separately in any analysis in which Fatima (mission) was sampled (CF initiation and weaning). We conduct separate analyses because the mission region is geographically remote, but also has a well-established health post and relatively low IMR. In contrast, regional and distance-related results were redundant when the mission region was not sampled (BF initiation and BF frequency). We therefore report regional results for BF initiation and BF frequency only. For all statistical models, control variables were infant age and a parity-for-age term (either the studentized residual generated from a parity*age linear regression, denoted Parityage1, or the studentized residual generated from a parity*age*age2 linear regression, denoted Parityage2).
In statistical comparisons of BF initiation and colostrum beliefs across regions, riverine and forest villages were combined due to small sample size, and are referred to collectively as remote villages. Binary logistic regression models were used to test predictors of BF initiation and colostrum beliefs. In statistical models for both CF initiation and weaning age, linear mixed models were used. Because some mothers were interviewed more than once (during different study periods) regarding CF and weaning practices of different infants, models controlled for mother ID as a random effect. Ordinary least-squares regressions were used to compare the actual and ideal weaning age reported by individual mothers.
Collection and Analysis of Observational Data
From 2002-2007, time allocation studies were conducted by Authors 1 and 3 and other THLHP researchers in nine Tsimane villages using standard scan-sampling techniques (Gross, 1984; Hames, 1992; Johnson & Behrens, 1989). Data were collected from two forest villages and two riverine villages from 2002-2003, a near-town village and a forest village from 2005-2006, and four riverine villages from 2006-2007 (observations were not collected in the mission region).
Two and three-hour household “time blocks” were conducted in household clusters based on proximity, which closely matched kinship (Winking, 2005). Clusters were sampled randomly without replacement for 2 or 3-hour time blocks, covering all hours from 7:00 a.m. to 7 p.m. During time blocks, instantaneous scans were taken every half hour, in which the activity, location, and social group of all individuals within the cluster were recorded, resulting in 4-6 time points per person per time block. The individual's activity was described as best as possible including objects and interactants. Infants were most often coded as being recipients of direct care (being held, nursed, groomed, bathed, fanned, fed, or swung) and the identity of their caretakers was noted. Other common infant behaviors included crying, sleeping, and passive or active play. Locations were standardized, allowing for houses, kitchens, yards and fields for each family, and a set of village-specific locales.
A subset of infant observations was compiled to model the transition from exclusive breastfeeding to weaning. Infants included in this subset met the following criteria: (1) aged 0-36 months with verified birthdate; (2) observed in ≥10 in-camp household scans; and (3) observed BF or CF in at least one of the observations. The resulting sample consisted of 133 infants from near-town (n=35), forest (n=30), and riverine villages (n=68) and 862 consumption observations (averaging 6.5 per infant). Observations were pooled by infant age (in months) and plotted to observe respective changes in frequency of BF versus CF over time (Figure 1). A generalized estimating equation was used to model breastfeeding frequency: “the probability that an infant was observed BF vs. CF from 0-36 months (binary outcome: 1=BF, 0=consuming nonbreastmilk food or liquids, infant ID controlled as repeated measure). Predictor variables were identical to those used in models of interview data: region (an indicator of IMR), village distance from town, and maternal age. Control variables were infant age, data coder, a parity-for-age term, the studentized residual generated from a parity*age (denoted Parityage1), or a parity*age*age2 (denoted Parityage2).
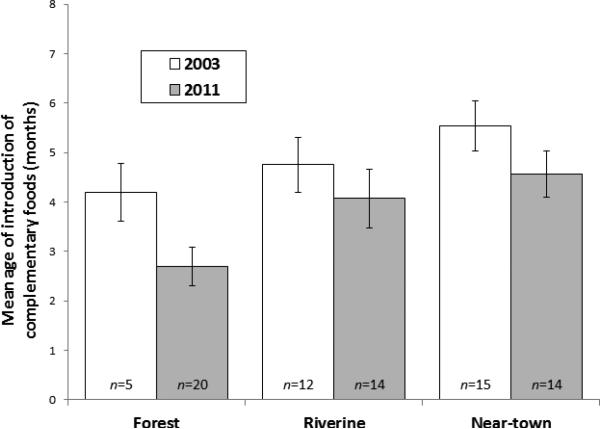
Figure 1.
Mean age of introduction of complementary foods in 2003 (white bars) and 2011 (gray bars). Means are stratified by Tsimane region, with standard errors.
RESULTS
Descriptive statistics for the 312 infants and 238 mothers sampled are provided in Table 3. Mean (± SD) infant age was 15.4 ± 10.0 months, mean (± SD) maternal age was 27.1 ± 8.1 years, and mean (± SD) parity was 4.5 ± 3.1. There were no significant differences in infant age, maternal age, and parity across regions in post-hoc tests. Mean (± SD) age of CF introduction was 4.13 ± 2.0 and varied significantly across regions (see Tables 3 and 4). Reported mean (± SD) weaning age was 19.2 ± 7.3, and did not vary significantly across regions.
Table 3.
Top: Descriptive characteristics for 312 Tsimane infants and 238 mothers combined from interview and observational datasets. Lower: Summarized interview results for CF and weaning.
| Descriptive | Forest | Riverine | Mission | Near-town | Total |
|---|---|---|---|---|---|
| Infant n | 57 | 127 | 15 | 113 | 312 |
| Mother n | 46 | 98 | 15 | 79 | 238 |
| Maternal age (years) | 26.1 ± 7.7 (15-43) | 26.9 ± 7.7 (15-47) | 30.3 ± 9.0 (17-46) | 27.4 ± 8.7 (15-48) | 27.1.0 ± 8.1 (15-48) |
| Parity | 3.9 ± 2.7 (1-13) | 4.6 ± 3.0(1-12) | 5.4 ± 3.2(1-10) | 4.6 ± 3.4(1-14) | 4.5 ± 3.1(1-14) |
| Infant age (months) | 14.2 ± 10.5 (0-36) | 15.7 ± 10.3(0-36) | 11.4± 7.4.(1.6-27) | 16.2 ± 9.6 (0-36) | 15.4 ± 10.0 (0-36) |
| Male: female infants | 1.3 | 0.9 | 0.9 | 1.3 | 1.2 |
| Interview | |||||
| CF introduction (months) | 2.9 ± 1.8 (0-7) (n= 26) | 4.4 ± 1.8 (0-9) (n= 70) | 6.0 ± 0.9 (4-8) (n= 10) | ^4.0 ± 2.1 (0-8) (n= 77) | 4.13± 2.0 (0-9) (n= 183) |
| Weaning (actual) | 17.4 ± 8.3 (10-36) (n= 19) | 17.6 ± 8.2 (5-36) (n= 24) | 22.7 ± 7.9 (12-36) (n= 14) | 19.6 ± 6.6 (9-42) (n= 60) | 19.2 ± 7.3 (5-42) (n= 117) |
| Weaning (ideal) | 21.8 ± 4.5 (12-24) (n= 8) | 22.3 ± 6.7 (12-36) (n= 24) | 25.7 ± 9.5 (12-48) (n= 14) | 19.6 ± 6.6 (18-24) (n= 11) | 23.26 ± 6.7 (12-48) (n= 57) |
* Mean, SD (range)
Table 4.
Linear mixed model for introduction of complementary feeding (Marquis et al.), forest=baseline region. Dependent variable=age of CF introduction (in months).
| Parameter | Estimate | St. Error | t-value | Sig |
|---|---|---|---|---|
| Age infant (months) | 0.171 | 0.059 | 2.899 | 0.004 |
| Age infant2 | −0.004 | 0.002 | −2.474 | 0.015 |
| Parityage2 | −0.203 | 0.140 | −1.449 | 0.149 |
| Region=Forest | 0 | n/a | n/a | n/a |
| Region=Riverine | 1.429 | 0.430 | 3.323 | 0.001 |
| Region=Mission | 2.886 | 0.698 | 4.135 | 0.000 |
| Region=Near-town | 1.086 | 0.426 | 2.834 | 0.005 |
Dimension 1: Breastfeeding initiation
Seventy-two percent of the mothers interviewed (52/72) reported that they initiated BF immediately or within a few hours following birth. The remaining 20 mothers who did not immediately breastfeed did so within 1-2 (45%) or 3-4 days (55%). Most reported delaying BF because their milk had not yet come in or their infant would not suckle. Interviews revealed no stringent taboos regarding the provisioning of colostrum, and instead suggest a degree of cultural consensus on its benefits; 62% of mothers reported that the milk produced in the first few days after birth was “jäm” (good), while 31% reported it as “jam” (“bad”), and 7% had no opinion. Eighty-three percent of mothers who reported colostrum was “good” also reported breastfeeding immediately after birth, compared to only 47% of mothers reporting colostrum was “bad” (χ2 = 8.24, p = .004).
In logistic regression models, region, but not maternal age, was a significant predictor of BF initiation and colostrum beliefs after controlling for infant age (model not shown). Mothers in low-mortality near-town villages were more likely to report immediate BF than were mothers in high-mortality, geographically remote (forest and riverine) villages (near-town 73%; remote 42%; χ2 = 11.7, p = .001), and were more likely to report that colostrum was “good” than were mothers in remote villages (near-town 85%; remote 45%; [.chi]2 = 6.36, p= .012). We cannot isolate regional from temporal differences with the available data, because interviews were conducted in remote and near-town villages during different study periods (2003 and 2009, respectively).
Dimension 2: Complementary feeding
Watery stews of plantain or rice, and meat or fish, were common introductory complementary foods. Among infants <6 months of age, 48% (28/58) had begun CF at the time of interview. Median reported age of first CF for these infants was 3.0 months. Of all non-exclusively BF infants (n= 183), the mean (± SD) reported age of CF introduction was 4.1 ± 2.0 months (median= 4.0, interquartile range= 3.0 months). Infant's age at time of interview and reported age of CF were positively correlated (Spearmans r = 0.176, p = .017), suggesting reported age of first CF may be inflated with length of recall.
In linear mixed models controlling for maternal repetition (16 mothers interviewed by Authors 1 and 2 were interviewed by Author 3 following the birth of a new child), region, but not distance from town or maternal age, was a significant predictor of timing of CF initiation (Table 4). Mothers from the forest region reported the earliest, and mothers from the mission region reported the latest age of CF introduction. Mothers from near-town and riverine regions reported intermediate ages of CF introduction. Infant age, infant age2, and parityage2 were also significant in the model.
One riverine and one near-town village were sampled twice (2003/2011 and 2009/2011, respectively). Despite having repeat measures, only 16 mothers were interviewed twice. These mothers did not vary their CF patterns from one infant to the next: paired t-test of CF initiation for previous offspring vs. next offspring was non-significant, (t = 0.149, d f= 15, p = .883).
Of the four dimensions of BF, the largest and most complete sample comes from the CF interview. We therefore assess CF trends over time by comparing data collected in forest, riverine and near-town regions in 2003 with data collected in forest, riverine and near-town regions in 2011. Temporal differences in age of mean CF introduction were not statistically significant (paired t-tests of CF initiation for 2003 vs. 2011: in near-town region (t = 1.393, df= 27, p = .175), in riverine region (t = 0.824, df = 24, p = .418), in forest region (t = 1.813, df= 23, p = .083)), suggesting little temporal change by region. The data further suggest a pattern in which regional variation persists through time (Figure 1); in 2003 and 2011, forest mothers report the earliest CF onset, followed by riverine mothers, and near-town mothers report the latest CF onset.
Dimension 3: Weaning Age
The average (± SD) age at which previous infants were reportedly fully weaned was 19.2 ± 7.3 months (n = 117). The average (± SD) ideal weaning age (a belief) reported by mothers was 23.3 ± 6.7 months (n = 57). Actual weaning age (practice) and ideal weaning age (belief) were correlated (r = 0.421, p = .001), but most mothers weaned their infants earlier than their reported ideal weaning age (paired t-test of actual vs. ideal weaning: t = -3.885, df =56, p = .001). This is likely due to a subsequent pregnancy, which most Tsimane women cite as their reason for weaning. Tsimane mothers thus have a cultural preference for prolonged BF, but rarely achieve their desired BF duration.
In linear-mixed models controlling for maternal repetition (14 mothers interviewed by Authors 1 and 2 were interviewed by Author 3 following the birth of a new child), none of the predictor variables (region, distance or maternal age) significantly predicted reported weaning age (data not shown). Only a parity-for-age term (parityage1) was a significant predictor of reported (actual) weaning age (β = -.377, p =.039), such that high-parity mothers wean sooner at all ages.
As with CF, mothers who were interviewed twice came mainly from a riverine village sampled in 2003/2011 and a near-town village sampled in 2009/2011. There was no significant variation in the timing of weaning across infants. Paired t-test of weaning previous offspring vs. next offspring was non-significant (t= -.559, df = 13, p = .585).
Dimension 4: Observations of BF Frequency
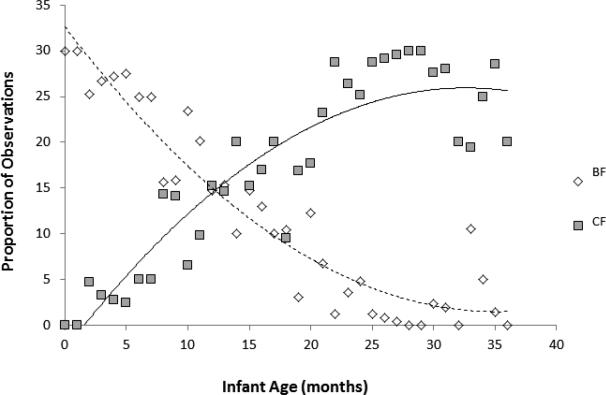
Figure 2 reveals the transition from BF to weaning using relative frequencies of each, derived from the observational dataset. The earliest CF observation was at one month and the latest BF observation at 36 months, with substantial variation observed across mother-infant dyads. Prolonged periods of mixed BF and CF were common. Observed CF consumption did not begin to exceed observed BF until 13.3 months of age.
Figure 2.
Plot of observed consumption patterns in 133 infants (0-36 months). The Y-axis shows the proportion of consumption observations in which infants were observed BF (diamonds) versus CF (squares). BF and CF observations are pooled by individual and by month. Trend lines for BF (dashed line) and CF (solid line) are shown.
In generalized estimating equations (controlling for individual infants as repeated subjects), region was a significant predictor of BF frequency, and maternal age was marginally significant after controlling for infant age and parityage1 (Table 5). BF frequency was highest in the near-town region and differed significantly between forest/near-town regions and between riverine/near-town regions, but not between forest/riverine regions. BF frequency observations were conducted during different time periods (2002-2003, 2005-2006, and 2006-2007) in different regions, and no village was sampled twice. We therefore cannot isolate regional from temporal differences with the available data.
Table 5.
Generalized estimating equation with binary logistic outcome, 1=BF (breastfeeding), 0=CF (complementary feeding: consuming non-breastmilk liquids or solids). Forest is baseline region. Model controls for repeated measures on infants.
| Parameter | Estimate | St. Error | Chi-Square | Sig |
|---|---|---|---|---|
| Age infant (months) | −0.185 | 0.014 | 167.555 | 0.002 |
| MomAge | 0.031 | 0.016 | 3.811 | 0.051 |
| Parityage1 | −0.006 | 0.127 | 0.003 | 0.960 |
| Region= Forest | 0 | n/a | n/a | n/a |
| Region= Riverine | 0.164 | 0.250 | 0.432 | 0.511 |
| Region= Acculturated | 1.000 | 0.307 | 12.617 | 0.000 |
Concordance of Observations with Interviews
Due to sampling limitations, both interview and observational data are available for only a small subset of the sample (30 mother-infant dyads). In generalized linear models, age of CF initiation (derived from interviews) was not a predictor of BF frequency before or after controlling for infant age, maternal age or parity (data not shown). We therefore see no correlation between these two separate dimensions of BF frequency (one derived from interview and one derived from observational data) in this small subset of the sample.
Discussion
In the Tsimane we find evidence of intra-population variation in BF initiation rates, BF frequency and the timing of CF. However, our general hypothesis that modernization is associated with declines in BF intensity was not supported. Our geographic proxies for modernization are instead associated with more intensive reported and observed BF, and maternal age was not generally associated with variation in BF patterns (see Table 6 for a summary of results). Region, and to a lesser extent, distance from town, was associated associated with variation in most dimensions of BF, but not in the direction expected. Mothers in remote villages have the least intensive BF practices, despites having the highest infant mortality and the least access to modernizing influences.
Table 6.
Summary of findings across four dimensions of breastfeeding (BF=breastfeeding, CF=complementary feeding). 0=Variable did not significantly predict outcome; - = Variable significantly predicted outcome, not in predicted direction; + = variable significantly predicted outcome, in predicted direction.
| Variable | Reported BF Initiation | Reported CF Introduction | Weaning Age | BFfrequency |
|---|---|---|---|---|
| 1: Distance | − | 0 | 0 | − |
| 2: Region | − | − | 0 | − |
| 3: Mother's age | 0 | 0 | 0 | +* |
Maternal age was marginally significant after controlling for parity-for-age.
Among Tsimane, distance from town generally diminishes access to markets and health care. However, regional variation encompasses more factors than just distance. For example, interior forest and riverine regions are both geographically remote. However the forest villages are much more isolated, especially in the rainy season. This geographic isolation contributes to higher infant mortality (Gurven et al., 2007), and perhaps, to less intensive BF. In contrast, the airstrip and health clinic found at the mission village seem to have a positive effect on infant health and BF patterns in the mission village and neighboring riverine villages, despite its geographically remote location. Future research should explore the respective effects of BF patterns and health care utilization on a variety of infant health outcomes, as we cannot directly link BF patterns to mortality outcomes with the data available.
Tsimane BF patterns are broadly characterized by high BF initiation, frequent BF, and prolonged BF duration—all common practices in traditional, non-industrialized societies. We have, however identified two practices that are potentially risky to infant health. First, nearly one-fourth of mothers reported delaying BF initiation; and second, most Tsimane mothers initiate CF prior to the WHO- recommended six months.
Delayed BF initiation is common in some cultures where colostrum is viewed as undesirable and dirty, and deprives newborns of vital immunological protection (Ergenekon-Ozelci et al., 2006; Rogers et al., 2011). The phenomenon of early CF is also common to many subsistence societies (Sellen & Smay, 2001) as well as in the U.S., where 40.4% of mothers introduce infants to solid foods before four months, often following the recommendation of pediatricians (Clayton et al., 2013). Exhaustive reviews indicate that exclusive BF for the first six months is optimal for infant health (Kramer & Kakuma, 2012); still others contend that initiating CF between 4-6 months is not detrimental under some circumstances (Fewtrell et al., 2007). Early CF may even be adaptive in extremely resource-scarce settings, where it reduces the energetic burden BF imposes on mothers (Gray, 1998).
It is crucial to investigate further how ecological factors and maternal constraints and beliefs might contribute to seemingly suboptimal BF practices in the less-modernized Tsimane villages. Regional comparisons of maternal physical activity level do not suggest that forest and riverine mothers have a higher workload that would be incompatible with intensive BF; women's daily PAL is highest in the near-town villages (Table 1). We also do not expect that complementary food availability is a constraint, as commonly used complementary foods such as plantain are found in all Tsimane regions. With the exception of age at weaning (which occurred earliest in high-parity women), existing children also did not seem to pose a BF constraint for Tsimane women. Parity was not associated with BF initiation, frequency, or timing of CF.
Maternal beliefs may impose constraints on BF practices in the Tsimane, based on two lines of evidence. First, maternal beliefs regarding colostrum and ideal weaning were significant predictors of BF initiation and reported age at weaning, respectively. Second, individual mothers did not vary CF or age at weaning across different infants. Future research should therefore explore in greater detail the processes by which BF and CF beliefs are established, and whether forest and riverine mothers retain “traditional beliefs” that have recently changed in the near-town and mission regions in response to modernizing influences.
This study is limited by possible reporting bias and sampling issues. We cannot demonstrate conclusively that reports of intensive BF practices reflect actual practices. For example, near-town mothers may be more likely to report recommended practice, whether or not they actually engage in it. Furthermore, we cannot directly assess recent changes in Tsimane BF patterns because of sampling limitations. Still, it is compelling that the regional variation in reported CF patterns in 2003 (and throughout the sample) resembles the variation in reported CF patterns in 2011. This suggests that regional variation in CF initiation may be robust over time. Data are currently lacking to evaluate consistency of this trend across other dimensions of BF. To better assess the nature of temporal trends, THLHP research efforts are currently focused on more consistent monitoring of Tsimane BF patterns across villages and over time.
Despite its limitations, the findings of our study have important public health implications. Prolonged BF may contribute to longer interbirth intervals, which among Tsimane women are associated with lower mortality for infants on both sides of the birth interval (Gurven 2012). Early weaning, in contrast is associated with a shorter interbirth interval and with high fertility. To work toward better maternal and infant health, this information should be communicated to Tsimane women. Public health messages must be targeted equally at all Tsimane villages, and should be ongoing. Though mothers in the forest region currently exhibit least intensive BF patterns, we suspect that changes may be looming in the near future. BF patterns of near-town mothers may change with progressive modernization; educated and acculturated women are likely to begin seeking employment outside of the home.
A possible forum for an intervention is Radio Chimane, a radio station established by New Tribes Missionaries and run by Tsimane, which broadcasts news in the Tsimane language. Radios are now present in even the most remote Tsimane regions, and most Tsimane listen to Radio Chimane regularly. Messages could emphasize the many positive aspects of Tsimane BF culture as well as the possible contraceptive effects of prolonging BF. Tsimane women report having more children than they desire, suggesting an unmet need for more effective family planning (McAllister et al., 2012). Radio messages could also advise Tsimane mothers to always feed colostrum, and encourage exclusive BF for six months. Such an intervention would be affordable and practical, and would encourage women to maintain their current practices, but adopt small changes that can help improve infant survivorship and contribute to lower fertility.
On a broader scale, ongoing, systematic documentation of breastfeeding patterns in Latin American indigenous communities needed. This is particularly important because the health needs of modernizing indigenous populations are often neglected by national and regional governments (Montenegro & Stephens, 2006). While modernization is associated with intensive BF in the Tsimane in our study, Mexico's indigenous populations have experienced a disturbing decline in BF in recent years (González de Cossio et al., 2013). Studies of BF trends in indigenous populations should therefore be conducted in a population-specific manner, and employ quantitative and qualitative data collection techniques to understand cultural and individual beliefs that inform current practice (Dettwyler, 1986). These perspectives may be especially helpful in devising educational strategies that commend and preserve beneficial breastfeeding beliefs and practices while targeting more high-risk ones.
We document breastfeeding patterns in a traditional population, the Tsimane of Bolivia
Tsimane mothers in modernized communities practiced more intensive breastfeeding patterns
Maternal beliefs predicted the timing of breastfeeding initiation and weaning
Breastfeeding promotion activities must target communities equally and in the Tsimane language
Acknowledgements
The authors thank the Tsimane women and infants who participated in the research. The authors also thank the THLHP staff, especially Stacey Rucas, who assisted with interviews in 2003, and Chris von Reuden and Adrian Jaeggi, who contributed unpublished data. Lisa McAllister was funded by a UCSB Humanities and Social Sciences Research Grant. Melanie Martin was funded by an NSF REG grant. Amanda Veile was funded by an NSF Graduate Research Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Amanda Veile, Department of Anthropology University of Massachusetts Boston 100 Morrisey Blvd. Boston, MA 02125-3393.
Melanie Martin, Michael Gurven Integrative Anthropological Sciences Program Department of Anthropology University of California-Santa Barbara Santa Barbara, CA 93106.
Lisa McAllister, Michael Gurven Integrative Anthropological Sciences Program Department of Anthropology University of California-Santa Barbara Santa Barbara, CA 93106.
References
- Abada TSJ, Trovato F, Lalu N. Determinants of breastfeeding in the Philippines : a survival analysis. Social Science and Medicine. 2001;52:71–81. doi: 10.1016/s0277-9536(00)00123-4. [DOI] [PubMed] [Google Scholar]
- Adair L, Barry P, Guilkey D. The duration of breastfeeding: how is it affected by biological, sociodemo-graphic, health sector and food industry factors? Demography. 1993;31:63–80. [PubMed] [Google Scholar]
- Akin J, Bilsborrow R, Guilkey DK, Popkin BM. Breastfeeding patterns and determinants in the near East: an analysis of four countries. Population Studies. 1986;40:247–262. [Google Scholar]
- Anatolitou F. Human milk benefits and breastfeeding. Journal of Pediatric and Neonatal Individualized Medicine. 2012;1:11–18. [Google Scholar]
- Assah FK, Ekelund U, Brage S, Wright A, Mbanya JC, Wareham NJ. Accuracy and validity of a combined heart rate and motion sensor for the measurement of free-living physical activity energy expenditure in adults in Cameroon. Int J Epidemiol. 2011;40:112–120. doi: 10.1093/ije/dyq098. [DOI] [PubMed] [Google Scholar]
- Baker E, Sanei L, Franklin N. Early initiation of and exclusive breastfeeding in large-scale community-based programmes in Bolivia and Madagascar. Journal of Health, Population and Nutrition. 2006;24:530–539. [PMC free article] [PubMed] [Google Scholar]
- Barennes H, Empis G, Quang TD, Sengkhamyong K, Phasavath P, Harimanana A, et al. Breast-Milk Substitutes: A New Old-Threat for Breastfeeding Policy in Developing Countries. A Case Study in a Traditionally High Breastfeeding Country. PLoS ONE. 2012;7:e30634. doi: 10.1371/journal.pone.0030634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerma JT, Rutstein SO, Sommerfelt AE, Bicego GT. Bottle use for infant feeding in developing countries: data from the demographic and health surveys. Has the bottle battle been lost? J Trop Pediatr. 1991;37:116–120. doi: 10.1093/tropej/37.3.116. [DOI] [PubMed] [Google Scholar]
- Brady J. Marketing breast milk substitutes: problems and perils throughout the world. Archives of Disease in Childhood. 2012;97:529–532. doi: 10.1136/archdischild-2011-301299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coimbra C, Flowers N, Salzano F, Santos R. The Xavánte in Transition. University of Michigan Press; Ann Arbor: 2002. [Google Scholar]
- Cosminsky S, Mhloyi M, Ewbank D. Child Feeding Practices in a Rural Area of Zimbabwe. Social Science and Medicine. 1993;36:937–947. doi: 10.1016/0277-9536(93)90085-i. [DOI] [PubMed] [Google Scholar]
- DeRose L. Women's Work and Breastfeeding Simultaneously Rise in Ghana. Economic Development and Cultural Change. 2007;55:583–612. [Google Scholar]
- Dettwyler K. Infant Feeding in Mali, West Africa: Variations in Belief and Practice. Social Science and Medicine. 1986;23:651–664. doi: 10.1016/0277-9536(86)90112-7. [DOI] [PubMed] [Google Scholar]
- Dettwyler K. When to Wean: Biological Versus Cultural Perspectives Clinical Obstetrics and Gynecology. 2004;47 doi: 10.1097/01.grf.0000137217.97573.01. [DOI] [PubMed] [Google Scholar]
- Dewey K. Energy and protein requirements during lactation. Annual Review of Nutrition. 1997;17:19–36. doi: 10.1146/annurev.nutr.17.1.19. [DOI] [PubMed] [Google Scholar]
- Dugas LR, Harders R, Merrill S, Ebersole K, Shoham DA, Rush EC, et al. Energy expenditure in adults living in developing compared with industrialized countries: a meta-analysis of doubly labeled water studies. Am J Clin Nutr. 2011;93:427–441. doi: 10.3945/ajcn.110.007278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ergenekon-Ozelci P, Elmaci N, Ertem M, Saka G. Breastfeeding beliefs and practices among migrant mothers in slums of Diyarbakir, Turkey, 2001. European Journal of Public Health. 2006;16:143–148. doi: 10.1093/eurpub/cki170. [DOI] [PubMed] [Google Scholar]
- FAO/WHO/UNU . Protein and energy requirements. WHO; Geneva: 1985. [Google Scholar]
- FAO/WHO/UNU . Human Energy Requirements. Report of a Joint FAO/WHO/UN Expert Consultation. WHO; Geneva: 2004. [Google Scholar]
- Ferry B, Smith DP. Breastfeeding Differentials. World Fertility Survey Comparative Studies. International Statistical Institute; London: 1983. [Google Scholar]
- Fewtrell MS, Morgan JB, Duggan C, Gunnlaugsson G, Hibberd PL, Lucas A, et al. Optimal duration of exclusive breastfeeding: what is the evidence to support current recommendations? The American Journal of Clinical Nutrition. 2007;85:635S–638S. doi: 10.1093/ajcn/85.2.635S. [DOI] [PubMed] [Google Scholar]
- Fomon SJ. Infant Feeding in the 20th Century: Formula and Beikost. The Journal of nutrition. 2001;131:409S–420S. doi: 10.1093/jn/131.2.409S. [DOI] [PubMed] [Google Scholar]
- Forste R, Hoffmann JP. Are US mothers meeting the Healthy People 2010 breastfeeding targets for initiation, duration, and exclusivity? The 2003 and 2004 National Immunization Surveys. Journal of Human Lactation. 2008;24:278–288. doi: 10.1177/0890334408317617. [DOI] [PubMed] [Google Scholar]
- Foster Z, Byron E, Reyes-García V, Huanca T, Vadez V, Apaza L, et al. Physcial growth and nutritional status of Tsimane’ Amerindian children of lowland Bolivia. American Journal of Physical Anthropology. 2005;126:343–351. doi: 10.1002/ajpa.20098. [DOI] [PubMed] [Google Scholar]
- Garcia C, Mullany L, Rahmathullah L, Katz J, Thulasiraj R, Sheeladevi S, et al. Breast-feeding initiation time and neonatal mortality risk among newborns in South India. Journal of Perinatology. 2011;31:397–403. doi: 10.1038/jp.2010.138. [DOI] [PubMed] [Google Scholar]
- Godoy R, Seyfried C, Reyes-García V, Huanca T, Leonard WR, McDade T, et al. Schooling's contribution to social capital: study from a native Amazonian society in Bolivia. Comparative Education. 2007;43:137–163. [Google Scholar]
- González de Cossio T, Escobar-Zaragoza L, Gonzalez-Castell D, Reyes-Vazquez H, Rivera-Dommarco JA. Breastfeeding in Mexico was stable, on average, but deteriorated among the poor, whereas complementary feeding improved: results from the 1999 to 2006 National Health and Nutrition Surveys. J Nutr. 2013;143:664–671. doi: 10.3945/jn.112.163097. [DOI] [PubMed] [Google Scholar]
- Gracey M. Child Health Implications of Worldwide Urbanization. Reviews on Environmental Health. 2003:51. doi: 10.1515/reveh.2003.18.1.51. [DOI] [PubMed] [Google Scholar]
- Gross DR. Time Allocation: A Tool for the Study of Cultural Behavior. Annual Review of Anthropology. 1984;13:519–558. [Google Scholar]
- Grummer-Strawn LM, Scanlon KS, Fein SB. Infant Feeding and Feeding Transitions During the First Year of Life. Pediatrics. 2008;122:S36–S42. doi: 10.1542/peds.2008-1315d. [DOI] [PubMed] [Google Scholar]
- Gurven M. Infant and fetal mortality among a high fertility and mortality population in the Bolivian Amazon. Social Science & Medicine. 2012;24:786–799. doi: 10.1016/j.socscimed.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurven M, Jaeggi A, Kaplan H, Cummings D. Physical Activity and Modernization among Bolivian Amerindians. PLoS ONE. 2013;8:e55679. doi: 10.1371/journal.pone.0055679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurven M, Kaplan H, Zelada Supa A. Mortality Experience of Tsimane Amerindians of Bolivia: Regional Variation and Temporal Trends. American Journal of Human Biology. 2007;19:376–398. doi: 10.1002/ajhb.20600. [DOI] [PubMed] [Google Scholar]
- Gurven M, Winking J, Kaplan H, von Rueden C, McAllister L. A bioeconomic approach to marriage and the sexual division of labor. Human Nature. 2009;20:151–183. doi: 10.1007/s12110-009-9062-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie GM, Guthrie HA, Fernandez TL, Estrera N. Early termination of breastfeeding among Philippine urban poor. Ecology of Food and Nutrition. 1983;12:175–202. doi: 10.1080/03670244.1983.9990716. [DOI] [PubMed] [Google Scholar]
- Hames R. Time Allocation. In: Smith EA, Winterhalder B, editors. Evolutionary Ecology and Human Behavior. Aldine De Gruyter; New York: 1992. pp. 203–235. [Google Scholar]
- Harrell BB. Lactation and Menstruation in Cultural Perspective. American Anthropologist. 1981;83:796–823. [Google Scholar]
- Harrison GG, Zaghloul ZS, Galal OM, Gabr A. Breastfeeding and weaning in a poor urban neighborhood in Cairo, Egypt: Maternal beliefs and perceptions. Social Science & Medicine. 1993;36:1063–1069. doi: 10.1016/0277-9536(93)90124-m. [DOI] [PubMed] [Google Scholar]
- Hinde K, Milligan L. Primate milk: Proximate Mechanisms and Ultimate Perspectives. Evolutionary Anthropology. 2011;20:9–23. doi: 10.1002/evan.20289. [DOI] [PubMed] [Google Scholar]
- Howrigan GA. Fertility, infant feeding, and change in Yucután. In: Levine RA, Miller PM, Maxwell West M, editors. Parental Behavior in Diverse Societies. Jossey-Bass; San Francisco: 1988. pp. 37–49. [Google Scholar]
- Huffman S. Determinants of Breastfeeding in Developing Countries: Overview and Policy Implications. Studies in Family Planning. 1984;15:170–183. [PubMed] [Google Scholar]
- Humphrey LT. Weaning behaviour in human evolution. Seminars in Cell & Developmental Biology. 2010;21:453–461. doi: 10.1016/j.semcdb.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Igun UA. Child-feeding habits in a situation of social change: The case of Maiduguri, Nigeria. Social Science & Medicine. 1982;16:769–781. doi: 10.1016/0277-9536(82)90230-1. [DOI] [PubMed] [Google Scholar]
- Johnson A, Behrens C. Time Allocation Research and Aspects of Method in Cross-Cultural Comparison. Journal of Quantitative Anthropology. 1989;1:313–334. [Google Scholar]
- Kramer K, Greaves R. Changing patterns of infant mortality and fertility among Pumé foragers and horticulturalists American Anthropologist. 2007;109:713–726. [Google Scholar]
- Kramer M, Kakuma R. The optimal duration of exclusive breast feeding: A systematic review. Advances in Experimental Medicine and Biology. 2004;554:63–77. doi: 10.1007/978-1-4757-4242-8_7. [DOI] [PubMed] [Google Scholar]
- Kramer M, Kakuma R. Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd; 2012. Optimal duration of exclusive breastfeeding. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamberti LM, Fischer Walker CL, Noiman A, Victora C, Black RE. Breastfeeding and the risk for diarrhea morbidity and mortality. BMC public health. 2011;11:S3–S15. doi: 10.1186/1471-2458-11-S3-S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutter C, Chaparro C, Grummer-Strawn L, Victora C. Backsliding on a Key Health Investment in Latin America and the Carihhean: The Case of Breastfeeding Promotion. American Joumal of Public Health. 2011;101 doi: 10.2105/AJPH.2011.300244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutter C, Morrow AL. Protection, Promotion and Support and Global Trends in Breastfeeding. Advances in Nutrition. 2013;4:213–219. doi: 10.3945/an.112.003111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malina R, Reyes M, Little B. Epidemiologic Transition in an Isolated Indigenous Community in the Valley of Oaxaca, Mexico. American Journal of Physical Anthropology. 2008;137:69–81. doi: 10.1002/ajpa.20847. [DOI] [PubMed] [Google Scholar]
- Marquis G, Habicht J, Lanata C, Black RE, Rasmussen K. Association of breastfeeding and stunting in Peruvian toddlers: an example of reverse causality. International Journal of Epidemiology. 1997;26:349–356. doi: 10.1093/ije/26.2.349. [DOI] [PubMed] [Google Scholar]
- Marriott BM, Campbell L, Hirsch E, Wilson D. Preliminary Data from Demographic and Health Surveys on Infant Feeding in 20 Developing Countries. The Journal of nutrition. 2007;137:518S–523S. doi: 10.1093/jn/137.2.518S. [DOI] [PubMed] [Google Scholar]
- Martin MA, Glassek WD, Gaulin SJC, Evans RW, Woo JG, Geraghty SR, et al. Fatty acid composition in the mature milk of Bolivian forager-horticulturalists: controlled comparisons with a U.S. sample. Maternal and Child Nutrition. 2012;8:404–418. doi: 10.1111/j.1740-8709.2012.00412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister L, Gurven M, Kaplan H, Stieglitz J. Why Do Women Have More Children Than They Want? Understanding Differences in Women's Ideal and Actual Family Size in a Natural Fertility Population. American Journal of Human Biology. 2012;24:786–799. doi: 10.1002/ajhb.22316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDade T. Life History, Maintenance, and the Early Origins of Immune Function. American Journal of Human Biology. 2005;17:81–94. doi: 10.1002/ajhb.20095. [DOI] [PubMed] [Google Scholar]
- McDade T, Leonard W, Burhop J, Vadez V, Reyes-Garcia V, Huanca T, et al. Predictors of C-reactive protein in Tsimane’ 2 to 15 year-olds in lowland Bolivia. American Journal of Physical Anthropology. 2005;128:906–913. doi: 10.1002/ajpa.20222. [DOI] [PubMed] [Google Scholar]
- McDade T, Worthman C. The weanling's dilemma reconsidered: A biocultural analysis of breastfeeding ecology. Journal of Developmental and Behavioral Pediatrics. 1998;19:286–299. doi: 10.1097/00004703-199808000-00008. [DOI] [PubMed] [Google Scholar]
- Miranda C. The Beni Biosphere Reserve. UNESCO Working Paper no. 9. 1995 [Google Scholar]
- Nielsen SB, Reilly JJ, Fewtrell MS, Eaton S, Grinham J, Wells JCK. Adequacy of Milk Intake During Exclusive Breastfeeding: A Longitudinal Study. Pediatrics. 2011;128:e907–e914. doi: 10.1542/peds.2011-0914. [DOI] [PubMed] [Google Scholar]
- Nyberg C. Market Integration, Stress, and Health: An Exploration of Hypothalamic-Pituitary-Adrenal Dynamics among the Tsimane’of the Bolivian Amazon. Anthropology: Northwestern University; 2009. [Google Scholar]
- Pérez-Escamilla R. Breastfeeding and the nutritional transition in the Latin American and Caribbean Region: a success story? Cadernos de Saúde Pública. 2003;19:S119–S127. doi: 10.1590/s0102-311x2003000700013. [DOI] [PubMed] [Google Scholar]
- Piperata B, Gooden Mattern L. Longitudinal Study of Breastfeeding Structure and Women's Work in the Brazilian Amazon. American Journal of Physical Anthropology. 2011;144:226–237. doi: 10.1002/ajpa.21391. [DOI] [PubMed] [Google Scholar]
- Piperata B, Spence J, Da-Gloria P, Hubbe M. The Nutrition Transition in Amazonia: Rapid Economic Change and its Impact on Growth and Development in Ribeirinhos. American Journal of Physical Anthropology. 2011;146:1–13. doi: 10.1002/ajpa.21459. [DOI] [PubMed] [Google Scholar]
- Quinlan RJ, Quinlan MB, Flinn MV. Parental investment and age at weaning in a Caribbean village. Evolution and Human Behavior. 2003;24:1–16. [Google Scholar]
- Raphael D, Davis F. Only Mothers Know: Patterns of Infant Feeding in Traditional Cultures. Greenwood Press; Westport CT: 1985. [Google Scholar]
- Rasheed S, Frongillo EA, Devine CM, Alam DS, Rasmussen KM. Maternal, Infant, and Household Factors Are Associated with Breast-Feeding Trajectories during Infants’ First 6 Months of Life in Matlab, Bangladesh. Journal of Nutrition. 2009;139:1582–1587. doi: 10.3945/jn.108.102392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers NL, Abdi J, Moore D, Nd'iangui S, Smith LJ, Carlson AJ, et al. Colostrum avoidance, prelacteal feeding and late breast-feeding initiation in rural Northern Ethiopia. Public Health Nutr. 2011;14:2029–2036. doi: 10.1017/S1368980011000073. [DOI] [PubMed] [Google Scholar]
- Ruel MT, Haddad L, Garrett JL. Some Urban Facts of Life: Implications for Research and Policy. World Development. 1999;27:1917–1938. [Google Scholar]
- Ryan AS. The Resurgence of Breastfeeding in the United States. Pediatrics. 1997;99:e12. doi: 10.1542/peds.99.4.e12. [DOI] [PubMed] [Google Scholar]
- S G. Butterfat feeding in early infancy in African populations: New hypotheses. American Journal of Human Biology. 1998;10:163–178. doi: 10.1002/(SICI)1520-6300(1998)10:2<163::AID-AJHB3>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Sam DL, Berry JW. Acculturation: When Individuals and Groups of Different Cultural Backgrounds Meet. Perspectives on Psychological Science. 2010;5:472–481. doi: 10.1177/1745691610373075. [DOI] [PubMed] [Google Scholar]
- Sellen D. Comparison of infant feeding patterns reported for nonindustrial populations with current recommendations. Journal of Nutrition. 2001;131:2707–2715. doi: 10.1093/jn/131.10.2707. [DOI] [PubMed] [Google Scholar]
- Sellen D. Evolution of Infant and Young Child Feeding: Implications for Contemporary Public Health Annual Review of Nutrition. 2007;27:123–148. doi: 10.1146/annurev.nutr.25.050304.092557. [DOI] [PubMed] [Google Scholar]
- Sellen D. Evolution of Human Lactation and Complementary Feeding: Implications for Contemporary Cross-Cultural Variation. In: Goldberg G, Prentice A, Prentice A, Filteau S, Simondon K, editors. Breast-feeding: Early Influences on Later Health. Springer Publishing Company; New York: 2009. [Google Scholar]
- Sellen D, Smay D. Relationship between subsistence and age at weaning in “preindustrial” societies. Human Nature: An Interdisciplinary Biosocial Perspective. 2001;12:47–87. doi: 10.1007/s12110-001-1013-y. [DOI] [PubMed] [Google Scholar]
- Shiva F, Nasiri M. A study of feeding patterns in young infants. Journal of Tropical Pediatrics. 2003;49:89–92. doi: 10.1093/tropej/49.2.89. [DOI] [PubMed] [Google Scholar]
- Silva E, Batista R. Bolivian Maternal and Child Health Policies: Successes and Failures. Canadian Foundation for the Americas (FOCAL); Ontario: 2010. [Google Scholar]
- SJ G. Correlates of breastfeeding frequency among nomadic pastoralists of Turkana, Kenya: a retrospective study. Am J Phys Anthropol. 1995;98:239–255. doi: 10.1002/ajpa.1330980302. [DOI] [PubMed] [Google Scholar]
- SJ G. The ecology of weaning among nomadic Turkana pastoralists of Kenya: Maternal thinking, maternal behavior and human adaptive strategies. Human Biology. 1996;68:437–465. [PubMed] [Google Scholar]
- Solien de González NL. Breast-feeding, weaning, and acculturation. The Journal of Pediatrics. 1963;62:577–581. doi: 10.1016/s0022-3476(63)80018-9. [DOI] [PubMed] [Google Scholar]
- Tanner S, Chuquimia-Choque M, Huanca T, McDade T, Leonard W, Reyes-García V. The effects of local medicinal knowledge and hygiene on helminth infections in an Amazonian society. Social Science and Medicine. 2011;72:701–709. doi: 10.1016/j.socscimed.2010.12.012. [DOI] [PubMed] [Google Scholar]
- Tanner S, Leonard W, McDade T, Reyes-Garcia V, Godoy R, Huanca T. Influence of Helminth Infections on Childhood Nutritional Status in Lowland Bolivia. American Journal of Human Biology. 2009;21:651–656. doi: 10.1002/ajhb.20944. [DOI] [PubMed] [Google Scholar]
- UDAPE-UNDP . Report: Human Development in the Department of Beni. In: U.N.D. Programme, editor. Social and Economic Policy Analysis Unit of the Government of Bolivia. La Paz, Bolivia: 2010. [Google Scholar]
- Valeggia C, Burke K, Fernandez-Duque E. Nutritional status and socioeconomic change among Toba and Wichí Populations of the Argentinean Chaco. Economics and Human Biology. 2010;8:100–110. doi: 10.1016/j.ehb.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Esterik P. Contemporary trends in infant feeding research. Annual Review of Anthropology. 2002;31:257–278. [Google Scholar]
- Veile A, Winking J, Gurven M, Greaves RD, Kramer KL. Infant growth and the thymus: Data from two South American native societies. American Journal of Human Biology. 2012;24:768–775. doi: 10.1002/ajhb.22314. [DOI] [PubMed] [Google Scholar]
- Villalpando S, Lopez-Alarcon M. Community and International Nutrition Growth Faltering Is Prevented by Breast-Feeding in Underprivileged Infants from Mexico City. Journal of Nutrition. 2000;130:546–552. doi: 10.1093/jn/130.3.546. [DOI] [PubMed] [Google Scholar]
- Vitzthum V. Infant nutrition and the consequences of differential market access in Nuñoa, Peru. Ecology of Food and Nutrition. 1992;28:45–63. doi: 10.1080/03670244.1992.9991259. [DOI] [PubMed] [Google Scholar]
- Vitzthum VJ. Suckling patterns: Lack of concordance between maternal recall and observational data. American Journal of Human Biology. 1994;6:551–562. doi: 10.1002/ajhb.1310060503. [DOI] [PubMed] [Google Scholar]
- von Rueden C, Jaeggi A. The origins of political inequality in a small-scale human society. American Anthropological Association Annual Meeting; San Francisco, CA.: 2012. [Google Scholar]
- Wagstaff A, Claeson M, Hecht RM. Millennium Development Goals for Health: What Will It Take to Accelerate Progress?. In: Jamison D, Breman J, AR M, editors. Disease Control Priorities in Developing Countries. World Bank; Washington DC: 2006. [PubMed] [Google Scholar]
- Walker R, Gurven M, Hill K, Migliano A, Chagnon N, De Souza R, et al. Growth rates and life histories in twenty-two small-scale societies. American Journal of Human Biology. 2006;18:295–311. doi: 10.1002/ajhb.20510. [DOI] [PubMed] [Google Scholar]
- WHO . Exclusive breastfeeding for six months best for babies everywhere. World Health Organization; WHO Media Centre: 2011. [Google Scholar]
- Wilson W, Milner J, Bulkan J, Ehlers P. Weaning practices of the Makushi of Guyana and their relationship to infant and child mortality: A preliminary assessment of international recommendations. American Journal of Human Biology. 2006;18:312–324. doi: 10.1002/ajhb.20500. [DOI] [PubMed] [Google Scholar]
- Winikoff B, Castle M, Laukaran V. Feeding Infants in Four Societies: Causes and Consequences of Mothers’ Choices. Greenwood Press, Inc.; Westport, CT: 1988. [Google Scholar]
- Winking J. Fathering among the Tsimane of Bolivia: A Test of the Proposed Goals of Paternal Care. Department of Anthropology; University of New Mexico; Albuquerque: 2005. [Google Scholar]
- Winking J, Gurven M. The total cost of father desertion. American Journal of Human Biology. 2011;23:755–763. doi: 10.1002/ajhb.21207. [DOI] [PubMed] [Google Scholar]
- Wolf JH. Low Breastfeeding Rates and Public Health in the United States. American Journal of Public Health. 2003;93:2000–2010. doi: 10.2105/ajph.93.12.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]