Abstract
Postnatal orofacial tissues contain rare cells that exhibit stem/progenitor cell properties. Despite a tremendous unmet clinical need for regeneration of tissues lost in congenital anomalies, infections, trauma or tumor resection, how orofacial stem/progenitor cells contribute to tissue development, pathogenesis and regeneration is largely a mystery. This perspective article critically analyzes the current status of orofacial stem/progenitor cells, identifies gaps in our understanding and highlights pathways for the development of regenerative therapies.
Keywords: dental stem cells, dental pulp stem cells, periodontal ligament stem cells, alveolar bone stem cells, bone regeneration, tooth regeneration, periodontal regeneration, scaffolds, growth factors, animal models
Introduction
The face consists of vastly diverse tissues, which not only are vital for esthetics, but also exert several indispensable functions including breathing, chewing, speech, sight, and smell. Orofacial tissues are lost in congenital anomalies, infections, trauma or tumor resection. There is a tremendous and unmet clinical need for regeneration of lost orofacial tissues and restoration of both function and esthetics. Postnatally, some orofacial stem/progenitor cells can be readily isolated, for example, from surgically removed gingiva or teeth, without undue trauma to the patient. However, enthusiasm for harnessing the presumed therapeutic power of orofacial stem/progenitor cells must be matched with sufficient scientific rigor to study their potency and limitations and in randomized clinical trials that determine whether/how orofacial stem/progenitor cells might be used in patients.
Facial development, including that of the tooth and oral cavity, is a classic act of interactions by stem cells of the epithelium, craniofacial mesoderm and neural crest-derived mesenchyme (Thesleff, 2006; Cordero et al., 2011). For example, tooth enamel derives from oral epithelium, whereas the remaining dental structures, including the pulp, dentin and cementum, originate from neural crest derived mesenchyme (Thesleff and Tummers, 2008). Endoderm makes little contribution to orofacial development with the exception of taste buds and small glands of the tongue (Rothova et al., 2012). Salivary glands are generated by epithelial stem cells growing into the underlying mesoderm that gives rise to glandular stromal cells through a process that is similar to invagination of oral epithelial cells into the underlying mesenchyme during tooth development (Tucker, 2007). Even some of the seemingly simple flat bones of the skull are formed by a patchwork of mesodermal cells and neural crest-derived cells (Jiang et al., 2002). During the past few decades, certain cells of ectodermal, neural crest or mesodermal origin, when isolated postnatally from orofacial tissues, have been shown to exhibit stem/progenitor cell properties such as self-renewal, clonogenicity, multi-lineage differentiation, and the ability to induce tissue formation in vivo. However, how orofacial stem/progenitor cells contribute to patterning in prenatal development, pathogenesis or tissue regeneration remains largely obscure at this time.
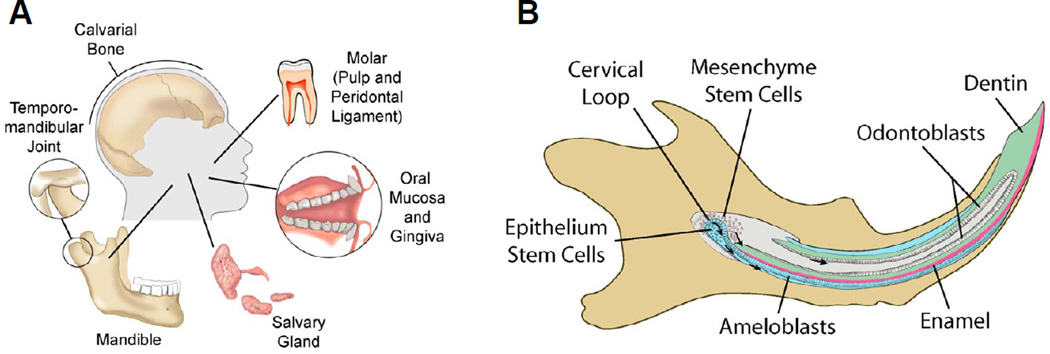
This review discusses two types of orofacial stem/progenitor cells: 1) stem/progenitor cells that are present in orofacial connective tissues including dental pulp, jaw bone, periodontal ligament, and lamina propria of oral mucosa, and 2) epithelial stem cells in oral epithelium, salivary glands and the developing tooth organ Fig. 1A, 1B). Rather than an exhaustive review, we choose to identify, in broad strokes, what is known and what needs to be known about orofacial stem/progenitor cells, and translational pathways for the development of putative regenerative therapeutics.
Figure 1.
Diagrams of human and mouse orofacial tissues from which stem/progenitor cells have been studied. A: Putative epithelial stem cells reside in the developing tooth germ, oral epithelium and salivary gland. Connective tissue stem/progenitor cells (of mesenchyme/mesoderm origin) have been isolated from calvarial bone, tooth pulp, dental papilla, the periodontal ligament and marrow of alveolar bone. B: The developing rodent incisors have been the most prevalent model for studying orofacial epithelium stem cells. Rodent incisors undergo continuous growth and eruption in life. The cervical loop of the developing incisor harbors both epithelial and mesenchyme stem cells. Epithelial stem cells are known to give rise to transient amplifying cells that propagate and migrate anteriorly and differentiate intoameloblasts that produce enamel matrix. Strikingly, enamel is produced only on the labial side in rodents. In contrast, mesenchyme stem cells migrate anteriorly to differentiateinto odontoblasts that produce dentin, in addition to likely giving rise to interstitial fibroblast-like cells in dental pulp, among which very few cells are stem/progenitor cells that are typically quiescent and serve to replenish pulp cells, including upon injury or pathological insult.
Connective tissue stem/progenitor cells in orofacial structures
Defining orofacial connective tissue stem cells
Bone marrow stromal cells frequently serve as a reference for the characterization of stem/progenitor cells that reside in orofacial connective tissues, given that both are of mesenchymal and/or mesodermal origins. Hematopoietic stem cells reside in bone marrow niches that are formed by stromal cells and osteoblasts (Sacchetti et al., 2007; Méndez-Ferrer et al., 2010; Song et al., 2010; Bianco, 2011). Colony-forming unit fibroblasts (CFU-F) were first identified as non-hematopoietic bone marrow cells that readily adhere to tissue culture polystyrene and, importantly, generate bone with marrow sinusoids upon in vivo heterotopic transplantation (Friedenstein et al., 1974; Owen and Friedenstein, 1988; Bianco et al., 2004). They were named as bone marrow stromal cells (BMSCs) to indicate their residence in bone marrow stroma, their primary function to support hematopoiesis and their ability to generate heterotopic bone (Friedenstein et al., 1974; Prockop, 1997; Robey, 2000; Bianco et al., 2004; Sacchetti et al., 2007). The term ‘mesenchymal stem cells’ (MSCs) was later coined to suggest their potency to generate or regenerate multiple connective tissues (Caplan, 1991; Caplan and Correa, 2011). However, evidence is lacking at this time to support the concept that progenies of a single MSC can generate an entire connective tissue (Bianco et al., 2008; Robey et al., 2011; Keating, 2012). Regardless of the name, one must recognize that commonly studied MSCs isolated from bone marrow, adipose or orofacial tissues as mono-nucleated and adherent cells are each highly heterogeneous cell populations (Gronthos et al., 2002; Guilak et al., 2004; Marion and Mao, 2006; Lee et al., 2010a; Keating, 2012). Given that mesenchyme only exists prenatally, we use “connective tissue stem/progenitor (CTS) cells” to refer to stem/progenitor cells in postnatal orofacial connective tissues. CTS cells therefore include all putative stem/progenitor cells that have been studied in orofacial connective tissues including dental pulp, jaw bone, periodontal ligament, and lamina propria of oral mucosa. Developmentally, orofacial CTS cells arise from 1) neural crest derived mesenchyme and/or 2) orofacial mesoderm.
Currently, mono-nucleated cells that are isolated from orofacial connective tissues and adhere to tissue culture polystyrene are deemed to be stem/progenitor cells (Table 1). Ex vivo differentiation of mononucleated and adherent cells into osteoblasts, chondrocytes and/or adipocytes is considered as evidence that they are stem cells (Table 1). However, mono-nucleated and adherent cells isolated from orofacial connective tissues, even if they differentiate into multiple lineages ex vivo, are far from pure stem cells. Additional rigor is essential to characterize orofacial CTS cells, including colony formation and clonogenecity, in vivo cell lineage tracing and orthotopic cell infusion (Table 1).
Table 1.
Existing and rigorous approaches for characterization of orofacial CTS cells.
| Existing approaches | Additional rigor |
|---|---|
| Colony formation1 | Cologenecity and clonal analysis2 |
| Multi-lineage differentiation in vitro3 |
In vivo cell tracing, lineage tracing, label retention, and functional assays4 |
| In vivo ectopic tissue formation5 | In vivo orthotopic tissue regeneration6 |
Sparsely seeded cells each forming a colony;
A single cell, when plated, yields a progeny;
Multi-lineage differentiation ex vivo: frequently into odontoblasts/osteoblasts, adipocytes and chondrocytes;
Transplanted cells are tagged with fluorescent marker or nanoparticles, and traced in vivo;
Frequently heterotopic implantation such as the dorsum or omentum.
Determine the fate of in vivo transplanted cells.
Dental pulp CTS cells
The bulk of the tooth in humans and many other mammalian species is formed by highly mineralized dentin. Dentin is covered by the enamel in the crown of the tooth and cementum in the root. Dental pulp is the only soft tissue in the tooth, and functions primarily to maintain its own homeostasis and that of dentin. Dental pulp is a heterogeneous cell reservoir, and consists of odontoblasts that reside on mineralized dentin surface, in addition to abundant interstitial fibroblasts that are located among a web of blood vessels and nerve endings. Dental pulp is highly cellular in the young, but its cellularity decreases with age (Smith et al., 1995; Nanci, 2007). Cranial neural crest cells are multipotent stem cells and give rise to dental mesenchyme in a structure known as the dental papilla (Chai et al., 2000). Dental papilla is the recognized origin of postnatal dental pulp stem/progenitor cells (Smith et al., 1995; Nanci, 2007; Chai et al., 2000). Mesenchymal cells in the developing E13.5 mouse tooth germ are multipotent and readily differentiate into non-dental lineages including chondrocytes and osteoblasts, in addition to odontoblasts (Yamazaki et al., 2007). Some, but far from all, of the mononucleated and adherent cells isolated from postnatal dental pulp demonstrate stem/progenitor cell properties including colonogenecity and differentiation into a limited number of cell lineages ex vivo (Gronthos et al., 2000; Batouli et al., 2003). At a clonal level, about 2/3 of dental pulp CTS cells generate ectopic dentin when transplanted heterotopically in vivo, but not the remaining 1/3 (Gronthos et al., 2002). The spatial distribution of dental pulp CTS cells has been recently demonstrated by in vivo cell tracing, showing that odontoblasts in dental pulp may originate from two different sources: perivascular and non-perivascular cells, both of which are capable of migrating to and potentially replenishing odontoblasts upon pulp injury (Feng et al., 2011). Importantly, few cells in dental pulp undergo migration in postnatal homeostasis (Feng et al., 2011). To date, few studies have focused on molecular signaling of orofacial CTS cells. Notably, Notch signaling has been shown to maintain the stemness of dental pulp CTS cells and attenuate their differentiation (Zhang et al., 2008). However, little else is known about the contribution of other molecular signaling pathways to the stemness of orofacial CTS cells.
Jaw bone CTS cells
Tissues in dental pulp are connected via the root apex with both the periodontal ligament and bone marrow in either the maxilla or mandible. Given that bone marrow MSCs were initially isolated from the marrow of appendicular bones such as the iliac crest, one would assume that the marrow of jaw bone also harbors stem/progenitor cells. Indeed, CTS cells have been isolated from jaw bones of both humans and rodents (Matsubara et al., 2005; Akintoye et al., 2006; Yamaza et al., 2011). Like iliac crest cells, stem/progenitor cells from the jaw bone are clonogenic and have potent osteogenic potential in vitro and in vivo (Matsubara et al., 2005). However, a number of differences exist between these two cell types. Compared to iliac crest cells, mandibular CTS cells proliferate more rapidly, exhibit delayed senescence, express alkaline phosphatase more robustly and accumulate more calcium when cultured in vitro (Akintoye et al., 2006). When transplanted heterotopically in vivo, MSCs from long bones yield greater bone marrow area than mandibular CTS cells (Yamaza et al., 2011), while mandibular bone marrow CTS cells yield greater bone volume than appendicular marrow MSCs (Akintoye et al., 2006; Yamaza et al., 2011). Interestingly, jaw bone CTS cells are far less chondrogenic and adipogenic than MSCs from the iliac crest (Matsubara et al., 2005). The underlying mechanisms for the observed differences between orofacial CTS cells and appendicular bone marrow MSCs are elusive at this time. Interestingly, MSCs isolated from the iliac crest and vertebral body are also known to differ (McLain et al., 2005). A meaningful reference is perhaps whether the differences between orofacial CTS cells and appendicular bone marrow MSCs are more pronounced than differences of MSCs isolated from the iliac crest and vertebral body.
Periodontal ligament CTS cells
The periodontal ligament (PDL) connects tooth roots to the surrounding alveolar bone, and primarily functions to maintain its own homeostasis and that of the cementum, in addition to transmitting mechanical stresses. Dental follicle cells, which originate from neural crest derived mesenchyme, differentiate into cells that form the periodontal ligament and are present in the developing tooth germ prior to root formation (Yao et al., 2008). Postnatal cells isolated from the periodontal ligament of extracted teeth differentiate into cementoblast-like cells, adipocytes, and collagen-forming cells under permissive conditions in vitro, and express Stro1, CD146 and scleraxis (Seo et al., 2004). When transplanted into immunocompromised rodents, human periodontal mesenchymal stem/progenitor cells yield cementum/PDL-like structures in porous calcium hydroxyapatite (Seo et al., 2004). However, in comparison to tendinopathy in which adipose tissue accumulates in tendons, there is no report of adipose tissue accumulation in the periodontal ligament, suggesting that native periodontal ligament CTS cells are perhaps incapable of adipogenesis.
Oral mucosa CTS cells
Oral mucosa consists of oral epithelium and the underlying lamina propria. Mononucleated and adherent cells isolated from postnatal lamina propria of gingival and alveolar mucosa are highly proliferative and contain putative stem/progenitor cells (Marynka-Kalmani et al., 2010). Oral mucosa CTS cells differ from dental pulp and periodontal ligament CTS cells by their high expression of CD49d (Integrin α2 or VLA-4) and weak expression of osteogenic transcriptional factors such as Runx2 (Lindroos et al., 2008). Compared to our marginal understanding of lamina propria CTS cells in oral mucosa, next to nothing is known about oral epithelial stem cells (e.g. Izumi et al., 2007).
Despite the original tenet that MSCs participate in tissue regeneration as tissue builders, recent data show that MSCs interact with inflammatory cells and immune cells that infiltrate in the wound. Similarly, gingival CTS cells prompt macrophages to acquire an anti-inflammatory M2 phenotype when co-cultured in vitro (Zhang et al., 2010). In vivo, systemically-infused gingival CTS cells improve wound repair by homing to skin wound sites and promoting macrophage polarization towards an M2 phenotype (Zhang et al., 2010). The M2 polarized macrophages play important roles in resolving inflammation by releasing trophic factors and suppressing the secretion of pro-inflammatory cytokines (Sica and Mantovani, 2012). Periodontal ligament CTS cells also suppress inflammatory cells such as peripheral blood monocytes, independent of cell contact (Wada et al., 2009), similar to bone marrow MSCs (Lee et al., 2008). These findings endorse the general concept that transplanted orofacial CTS cells, similar to appendicular MSCs, primarily serve as signaling cells in wound healing, rather than as tissue replacement cells (Wagner and Ho, 2007; Lee et al., 2008; Prockop, 2010b).
Orofacial CTS cells and appendicular bone marrow MSCs: are they different
Table 2 provides such a comparison, with the caveat that few studies have been performed with donor-matched samples. Additionally, molecular markers expressed by either orofacial CTS cells or appendicular bone marrow MSCs are sensitive to perturbation by a multitude of factors such as passaging, incubation medium, medium lot selection, plating density, and freezing and thawing (Sekiya et al., 2002; Smith et al., 2004; Lee et al., 2009). Bearing these caveats in mind, orofacial CTS cells and appendicular bone marrow MSCs indeed overlap in many molecular markers but nonetheless have several important differences. For example, CTS cells from either deciduous or adult dental pulp undergo more rapid proliferation ex vivo than appendicular bone marrow MSCs for reasons that are not well understood (Gronthos et al., 2000; Miura et al., 2003). When transplanted heterotopically in vivo, dental pulp CTS cells from both deciduous and permanent teeth, yield dentin nodules on the surface of dentin substrate or porous calcium phosphate (Gronthos et al., 2000; Batouli et al., 2003; Casagrande et al., 2010; Yu et al., 2007). A subset of bone marrow MSCs have the ability to generate orthotopic bone in vivo (Mankani et al., 2006). Importantly, dental pulp CTS cells lack the capacity of appendicular marrow MSCs to regenerate heterotopic bone (Robey, 2011).
Table 2.
Comparison of orofacial CTS cells with appendicular bone marrow MSCs.
| Orofacial CTS cells1 | Appendicular bone marrow MSCs2 |
|
|---|---|---|
| Tissue origin | Dental pulp, periodontal ligament, marrow of jaw bones, lamina propria of oral mucosa3 |
Marrow of appendicular bones or vertebrae |
|
Negative markers (non-exclusive)4 |
CD14, CD31, CD34, CD45 | CD14, CD31, CD34, CD45 |
|
Positive markers (non-exclusive)5 |
CD29, CD44, CD49d, CD73, CD90, CD105, CD106, CD146, Stro1, Oct4 and Nanog6, hTERT, endostatin, Stro1, nestin, scleroxis, etc. |
CD29, CD44, CD73, CD90, CD105, CD106, CD146, Oct4 and Nanog6, Stro1, nestin, etc. |
|
Heterotopic transplantation7 |
Dental pulp CTS cells yield dentin-like tissues; periodontal ligament CTS cells yield fibrous tissue and bone |
Heterotopic bone with marrow sinosuids6 |
|
Orthotopic transplantation8 |
Yields mineralized tissue in tooth root scaffolds |
Promote bone fracture healing although cell fate is uncertain |
Non-epithelium orofacial CTS cells.
Non-hematopoietic stem cells of bone marrow or bone marrow stromal cells.
These orofacial CTS cells express different molecular markers. See Huang et al., 2009 for detailed catalog of markers of orofacial CTS cells.
These markers are typically less than 1–2%.
These markers may vary from overwhelming expression (e.g. >90%) to definitive presence but not dominant (e.g. 10% or less).
Oct4 and Nanog expression in orofacial CTS cells or appendicular bone marrow MSCs is present but are thousands fold less than those in embryonic stem cells.
Heterotopic transplantation of orofacial CTS cells is exemplified by subcutaneous implantation in the dorsum or omentum.
Orthotopic transplantation refers to delivery of cells to the very location of their origin, such as bone marrow MSCs to fracture site.
Can orofacial CTS cells participate in the regeneration of non-orofacial tissues?
Dental pulp CTS cells have been differentiated, mostly in vitro, into putative hair follicle cells, hepatocyte-like cells, neuron-like cells, myocyte-like cells, islet-like cells and cardiomyocyte-like cells (Reynolds and Jahoda, 2004; Iohara et al., 2006; Ishkitiev et al., 2010; Yang et al., 2010; Sugiyama et al., 2011; Govindasamy et al., 2011), thus raising the possibility that they could participate in the regeneration of non-orofacial tissues. However, ex vivo differentiation, especially of heterogeneous orofacial CTS cells, is of limited value. In vivo functional and lineage tracing studies are necessary, as in Table 1, to appreciate whether wild type and/or selected fractions of orofacial CTS cells indeed trans-differentiate into non-orofacial lineages. In one study, only two out of dozens of clonal progenies of deciduous dental pulp CTS cells spontaneously fused into multinucleated myocyte-like cells that produce myosin heavy chain ex vivo (Yang et al., 2010), underscoring the rarity of cells in dental pulp with the ability to transform into natively unintended lineages. Nonetheless, when transplanted into injured skeletal muscle, myocyte-prone dental pulp clonal progenies successfully engraft and express human dystrophin, a protein that is missing in muscular dystrophy (Yang et al., 2010). Injection of GFP+ human dental pulp stem/progenitor cells into acute cardiac infarct sites in nude rats improves cardiac function with efficacy similar to appendicular marrow MSCs (Gandia et al., 2008). Interestingly, GFP+ dental pulp CTS cells fail to differentiate into cardiomyocytes, suggesting that dental pulp CTS cells promote cardiac infarct healing likely due to their ability to secrete proangiogenic and anti-apoptotic factors (Gandia et al., 2008). Implanted adult human dental pulp CTS cells from wisdom teeth promote the migration and sprouting of avian trigeminal ganglion via CXCL12/SDF1 and its receptor, CXCR4, in vivo (Arthur et al., 2009). Similarly, untreated rhesus dental pulp CTS cells delivered into the hippocampus of immunesuppressed mice recruit endogenous nestin+ cells and β-3-tubulin+ neurons to the site of the graft (Huang et al., 2009). Trans-differentiation of orofacial CTS cells, as outlined above, has been pursued as isolated examples and needs to be considered in the context of in vivo functional assays and perhaps also cellular programming/reprogramming in order to convincingly demonstrate a direct role in regeneration of non-orofacial tissues .
What we already know about orofacial CTS cells
Orofacial CTS cells have been intensely studied in the past decade or so. However most studies have relied on in vitro cultures of mononucleated and plastic-adherent cells that have been isolated from various orofacial structures. At best, these studies have extended our understanding of cells of orofacial tissues, including stem/progenitor cells that are rarely separately studied among heterogeneous cell populations.
Postnatal orofacial CTS cells are rare cells that remain quiescent or slow cycling in vivo at most times. It is virtually impossible to identify true stem cells without in vivo label retention, lineage tracing and/or serial transplantation experiments.
Typical cultures of isolated orofacial CTS cells as mononuclear and adherent cells from dental pulp (regardless of deciduous or permanent teeth), lamina propria of oral mucosa, periodontal ligament and mandibular bone marrow are each heterogeneous, and far from uniform “stem cell” cultures.
Orofacial CTS cells express a broad array of molecular markers that are also ascribed to as yet incompletely defined bone marrow MSCs, but nonetheless express little CD14 (innate immune marker), CD31 (PECAM-1), the hematopoeietic markers CD34 and CD45. Thus far, no single, or combination of, cell-surface markers has been identified to mark stemness in CTS populations or to differentiate between different CTS cell types.
Orofacial CTS cells from dental pulp, lamina propria of oral mucosa, periodontal ligament, and mandibular bone marrow, each as heterogeneously mixed cell populations, appear to undergo more rapid proliferation than bone marrow MSCs. Rapid proliferation does not necessarily guarantee that orofacial CTS cells can be propagated in greater numbers for therapeutic purposes.
Outstanding questions about orofacial CTS cells
Despite a well justified motivation to harness the presumed therapeutic potential of orofacial CTS cells, fundamental biology studies must be pursued and will fuel translational effort towards orofacial regeneration. Virtually untapped are the putative mechanisms by which stem/progenitor cells contribute to the pathogenesis of orofacial diseases.
In vivo lineage tracing studies that tag and track various orofacial CTS cells using transgenic and/or interventional models. In vitro multi-lineage differentiation of heterogeneous orofacial CTS cell populations is of little value. Clonal differentiation is valuable but in itself still does not fully establish stemness.
Focus on the understanding of how stem cells give rise to specialized orofacial cells that are not found elsewhere in the body including odontoblasts (and how they differ from osteoblasts), ameloblasts or enamel-forming cells (e.g. what equips them with outstanding mineralization), cementoblasts, salivary gland cells and oral mucosa cells.
Benchmark studies that compare orofacial CTS cells with appendicular marrow MSCs in humans and other species, including the use of donor-matched samples.
Immunoepitope panels and molecular assays that serve as hallmarks for each of the orofacial CTS cell populations at critical stages of differentiation and self-renewal.
Develop and validate heterotopic and orthotopic animal models that reproducibly test the behavior of transplanted and tagged orofacial CTS cells in vivo.
Signaling pathways that regulate stemness, differentiation and trophic effects of orofacial CTS cells have received little attention and need to be better understood.
Study how orofacial CTS cells may be involved in the pathogenesis of congenital anomalies and acquired diseases, exemplified as birth defects and periodontal disease or jaw joint disorders.
A critical question that needs to be answered is whether orofacial cells, including stem/progenitor cells, offer higher efficiency and safety for reprogramming, including direct transformation into cells that safely propagate into sufficient numbers and regenerate orofacial or non-orofacial tissues.
Epithelial stem cells in orofacial tissues: the tooth as a model
Tooth development is a classic model of epithelial-mesenchymal interactions. Rodent incisors continue to grow and erupt throughout life, providing a unique and powerful model for studying stem cells of the epithelium and mesenchyme. Epithelial stem cells in the developing rodent incisor reside in the cervical loop (Fig. 1B) and are surrounded by dental mesenchyme, somewhat similarly to the hair follicle bulge and the intestinal crypt (Turksen et al., 2004; Moore and Lemischka, 2006; Hsu et al., 2001; Thesleff, 2006). Mineralization of enamel and dentin, in comparison to the unmineralized dental pulp, affords a unique opportunity for studying the contrasting fate of a given population of stem cells, dental papilla in this case, that differentiate into mineralized dentin, and unmineralized dental pulp. Whereas the hair follicle bulge and the intestinal crypt are subjects of robust investigations towards understanding of stem cell behavior, less is known about lineage commitment, migration and differentiation of dental epithelium and mesenchymal stem cells of the developing tooth organ. There are also few studies on putative stem cells in oral epithelium and salivary gland epithelium. A notable exception is a recent report of an epithelial stem cell axis in the salivary gland, showing that acetylcholine signaling increased epithelial morphogenesis and proliferation of the keratin 5-positive progenitor cells, whereas parasympathetic innervation maintains the stemness of epithelial progenitor cell population (Knox et al., 2010).
During tooth development, DiI labeling and BrdU pulse chase/label retention shows that dental epithelial stem cells undergo continuous self renewal (Harada et al., 1999; Kawano et al., 2004). Dental epithelial stem cells further undergo asymmetric division, with some daughters retaining their stemness, while others depart from the niche, migrate and differentiate into ameloblasts, which are enamel-forming cells that synthesize enamel (Smith, 1980; Harada et al., 1999, 2002; Wang et al., 2007). Continuous self-renewal and asymmetric division of dental epithelial stem cells are directly responsible not only for the replenishment of functional ameloblasts, but also continuing eruption of rodent incisors (Harada et al., 1999, 2002; Wang et al., 2007).
The action of dental epithelium stem cells is only a part of the story in tooth organogenesis. Dental mesenchymal stem cells surround dental epithelium stem cells in the cervical loop (Fig. 1B) (Rothová et al., 2011). During epithelium-orchestrated amelogenesis, dental mesenchymal stem cells line up opposite the row of enameling-forming ameloblasts initially with nothing but a basement membrane in between (Harada et al., 1999, 2002; Thesleff, 2006; Wang et al., 2007). Ameloblasts, while laying down enamel matrix, generate an indispensable induction signal for mesenchymally derived odontoblasts to lay down dentin matrix (Kawano et al., 2004; Yoshida et al., 2008; Fujimori et al., 2010). By the time the developing tooth organ reaches the bud stage, dental mesenchyme takes over as signal generator for the developing ameloblasts to undergo maturation (Kollar and Fisher, 1980; Tucker and Sharpe, 2004; Thesleff, 2006). This mutual induction of dental epithelium and mesenchyme has contributed a great deal to the understanding of epithelial-mesenchymal interactions, along with observations in other organ systems such as the skin and hair follicle (Moore and Lemischka, 2006; Fuchs, 2008; Hsu et al., 2001). However, little is known about what governs the differentiation of dental mesenchyme stem cells into not only mineralized dentin and cementum, but also unmineralized dental pulp.
An additional striking feature of dental epithelium stem cells in rodent incisors is that enamel is only formed on the labial surface, but not the lingual surface (Fig. 1B), providing a rare model for studying the polarity of stem cell distribution and function (Harada et al., 1999, 2002; Thesleff, 2006; Wang et al., 2007). In the cervical loop, epithelial stem cells proliferate and migrate along the labial surface, differentiating into enamel-forming ameloblasts (Fig. 1B) (Wang et al., 2007). In contrast, the lingual cervical loop has few proliferating epithelial stem cells or ameloblasts, and hence is devoid of enamel formation (Fig. 1B) (Thesleff et al., 2007).
Considerable insight on signaling in tooth development has enriched our understanding of epithelial and mesenchymal stem cells. TGFβ, Wnt, FGF, Lrp4, and Hedgehog are among some of the highly conserved signaling pathways that regulate many aspects of dental stem cells in development (Thesleff, 2003; Järvinen et al., 2006; Yokohama-Tamaki et al., 2006; Klein et al., 2008; Lin et al., 2009). FGF signaling in dental mesenchyme regulates Notch signaling in dental epithelium (Karada et al., 1999; Kawano et al., 2004; Mitsiadis et al., 2010). Notch signaling, in turn, is required for regulating the survival of epithelial stem cells in the continuously growing mouse incisor (Felszeghy et al., 2010). Sonic hedgehog produced by the differentiating progeny of rodent incisor stem cells, though not necessary for survival, is essential for ameloblastic differentiation (Seidel et al., 2010). Activin signaling regulates the proliferation and differentiation of dental epithelial stem cells (Wang et al., 2004). Stimulation of Wnt or Wnt/BMP pathways in dental epithelium in transgenic mice not only mediates continuous growth of mouse incisors, but also leads to multiple newly formed teeth (Järvinen et al., 2006; Wang et al., 2009; O’Connell et al., 2012). However, signaling pathways in tooth development are only partially understood, and are virtually not studied at all in the context of tooth regeneration.
Regeneration of orofacial tissues
The face, including the oral cavity and the teeth, is of tremendous therapeutic interest for tissue regeneration (Mao et al., 2006). In addition to functional reconstruction, patients who suffer from tooth loss, cleft lip or facial trauma have a strong desire for restoring esthetics. Mammalian teeth do not spontaneously regenerate upon trauma or pathological insult. Sharks and certain lizards, however, continuously generate new sets of teeth, albeit root-less, throughout life in ways that are only peripherally understood (Boyne, 1970; Samuel et al., 1983; Handrigan et al., 2010). This section uses tooth regeneration as a model to exemplify challenges and strategies for orofacial regeneration.
The classic experiment of Kollar and Fisher (1980) shows that grafting of 5-day chick epithelium from the first/second pharyngeal arch combined with E16–18 mouse molar mesenchyme produced tooth crowns with enamel and dentin in the ocular chamber, suggesting that a) inductive signals for tooth organogenesis may derive from non-dental epithelium such as the tooth-less chick epithelium, and b) the oral cavity is not privileged for tooth formation. When embryonic dental epithelium is reconstituted with either dental or non-dental mesenchyme, odontogenesis genes are up-regulated and multiple tooth organs are formed upon transplantation in the adult renal capsule or jaw bone (Ohazama et al., 2004; Modino and Sharpe 2005; Mantesso and Sharpe, 2009). Similarly, E14.5 oral epithelium and dental mesenchyme can be reconstituted in collagen gel and, when cultured ex vivo, yield multiple dental tissues analogs (Nakao et al., 2007). When similarly reconstituted mouse E14.5 tooth germ cells were transplanted into tooth extraction sockets of 5-wk-old mice, a complete tooth organ was formed with both the crown and root, followed by eruption into the oral cavity (Ikeda et al., 2009). Recently, reconstituted E14.5 mouse tooth germ cells further yielded complex tooth organ structures with mechanical stiffness approaching that of native tooth structures and a putative periodontal ligament after eruption (Oshima et al., 2011). These studies underscore the capacity of embryonic dental epithelium and mesenchyme cells, even following disassociation and reconstitution, to form a complete tooth organ.
The developing tooth germ continues to grow in postnatal life, including in human wisdom teeth that are frequently extracted to alleviate or prevent peri-dental infections. However, whether these postnatal stem/progenitor cells, without reprogramming, are able to regenerate an entire tooth organ is not feasible at this time. Disassociated cells of postnatal porcine or rat tooth buds, when seeded in biomaterials and implanted in the abdominal cavity, yielded multiple dentin and enamel organs (Young et al., 2002; Duailibi et al., 2004). Transplantation of postnatal autologous tooth germ cells from un-erupted molar tooth yielded dentin/pulp-like structures with odontoblast-like cells and cementum-like structures (Kuo et al., 2008). Multipotent cells of the tooth apical papilla, a transient structural derivative of dental papilla, generated mineralized tissues with a putative periodontal ligament structure when transplanted in porous tricalcium phosphate in the extraction socket of an incisor in a miniature pig (Sonoyama et al., 2006). Seeding dental follicle cells from surgically extracted wisdom teeth in dentin matrix sheets activates expression of multiple odontogenesis/osteogenesis genes (Yang et al., 2012). In contrast to mouse E14.5 tooth germ cells, reconstituted postnatal tooth germ cells have only generated fragmented dental structures upon in vivo transplantation, rather than an anatomically correct tooth organ.
Given the presence of stem/progenitor cells in many dental tissues, the idea of promoting tooth regeneration through manipulating stem/progenitor cells is a clinically translatable but nonetheless under-explored possibility. A first attempt has recently been made to deliver two growth factors, SDF1 and BMP7, in the microchannels of anatomically correct biomaterial tooth scaffolds that were implanted orthotopically in tooth extraction sockets in vivo (Kim et al., 2010a). 9 weeks following implantation, co-delivery of SDF1 and BMP7 induced the regeneration of mineralized tissue in biomaterial root scaffolds with de novo formation of a putative periodontal ligament and newly formed alveolar bone by the recruitment of endogenous host cells (Kim et al., 2010a; Yildirim et al. 2010). Whether other factors, including other members of bone morphogenetic proteins, contribute to tooth regeneration warrants additional investigations (Nakashima and Reddi, 2003). However, amelogenesis was not observed (Kim et al., 2010a; Yildirim et al. 2010), similar to the lack of enamel formation upon transplantation of postnatal tooth germ cells or apical papilla cells (Sonoyama et al., 2006; Kuo et al., 2008). Tooth regeneration by recruitment of host endogenous stem/progenitor cells is consistent with tissue regeneration by cell homing in several other structures such cartilage, skeletal muscle and pancreatic tissues (Lee et al., 2006; Karp et al., 2009; Baird et al., 2009; Lee et al., 2010b), and appears to offer an alternative to cell transplantation. General difficulties associated with cell therapy also apply to cell sources that could potentially be used in tooth regeneration, including teratoma formation and inappropriate lineage differentiation for embryonic stem cells (ESCs) or induced pluripotent stem cells (iPS). Regardless of cell source, cell transplantation for tooth regeneration encounters additional translational barriers including excessive costs associated with ex vivo cell culture and manipulation, potential contamination, complexities of sterilization, shipping, storage and handling, and potential oncogenic mutation associated with ex vivo cell manipulation. Tumorigenecity becomes a real concern upon prolonged ex vivo culture or immortalization. Cell sources and biomaterial selections for tooth regeneration are topics of intense interest (for reviews see: Yelick and Vacanti, 2006; Thesleff and Tummers, 2008; Volponi et al., 2010; Yildirim et al. 2010; Keller et al., 2011; Yuan et al., 2001).
Cell sources for tooth regeneration
Developmentally, the tooth originates from the epithelium that forms the enamel, and the mesenchyme that differentiates into the dentin, cementum and dental pulp. Indeed, epithelium stem cells and mesenchyme stem cells from the embryonic tooth germ have formed tooth organs that erupt into the oral cavity in a rat model. However, embryonic tooth germ cells are difficult, if not impossible, to be applied clinically,
Autologous human embryonic tooth germ cells are inaccessible for regeneration in the adult. Allogeneic human embryonic tooth germ cells are ethically unacceptable, and also may cause immunorejection and pathogen transmission.
Xenogenic, non-human embryonic tooth germ cells suffer from immune rejection and tooth dysmorphogenesis resulting from genetically patterned crown and root shape, and altered numbers and dimensions of non-human species.
Postnatal autologous tooth germ cells (e.g. third molars) or autologous dental stem/progenitor cells are of limited availability, and appear to lack the potency to regenerate a complete tooth organ.
Clinical trials embedded with intrinsic risks and high cost may be justified for potentially life-threatening diseases that current medicine deems incurable, such as Parkinson’s disease, diabetes or spinal cord injuries, but likely not for tooth regeneration.
Tooth loss is the most common organ failure. By 2030, ~30 million individuals in the United States, where dental care is among the most advanced worldwide, will be completely edentulous (CDC). Can adult stem/progenitor cells, regardless of sources, regenerate a complete tooth? The short answer for now is no, as ameloblasts or enamel-forming cells are no longer present following crown formation and tooth eruption. However, the paucity of tissue progenitor cells for enamel regeneration is hardly a unique problem, as this challenge exists for regeneration of other tissues.
Projected strategies for tooth regeneration
Tooth regeneration needs to have multiple milestones with the eventual endpoint as regenerated entire tooth organs in patients. First, translational approaches are called for to regenerate singular or multiple dental tissues such as dental pulp and/or dentin (e.g. Cordeiro et al., 2008; Kim et al., 2010b; Iohara et al., 2011; Galler et al., 2012). In parallel, it is meritorious to produce scalable enamel and dentin crystals that serve as native replacement fillers (Du et al., 2005; Huang et al., 2010; Aida et al., 2012). Furthermore, there is a clinical need to regenerate a biological tooth root that is connected to the supporting alveolar bone with a periodontal ligament. A prosthetic tooth crown can readily be attached to a biologically regenerated tooth root and may serve as a first generation regenerative tooth therapy. The ultimate goal is to regenerate, entire. tooth organs with the enamel, dentin, cementum and dental pulp, as well as the periodontal ligament using clinically compatible cell types and approaches.
Life ends in numerous wild life species upon complete tooth loss, suggesting that spontaneous tooth regeneration is not phylogenically embedded in postnatal orofacial stem/progenitor cells. However, cellular reprogramming prompts the imagination of whether bioengineered embryonic-like cells, or reprogrammed tooth germ cells, can regenerate an entire tooth organ. After all, inductive signals that trigger dental mesenchyme for tooth organogenesis can originate from tooth-less species, or conversely, dental epithelium can direct non-dental epithelium towards tooth formation. Thus, it is perhaps not too farfetched to conceptualize that inductive signals with the same potency as embryonic dental epithelium and mesenchyme may be teased out by high-throughput screening approaches. Novel tools are necessary for advancing our understanding of fundamental biology and translation towards the development of therapeutics.
Concluding remarks
Diversity of the face not only among humans, but also among myriad vertebrate and invertebrate species intrigues many investigations about the amazing ability of stem cells of the embryonic epithelium, mesoderm and neural crest derived mesenchyme in patterning highly individualized structures. On the other hand, there is clearly a need for viable pathways to develop regenerative therapies for patients with congenital anomalies and acquired orofacial defects. Translational studies may well take place without the obligation to wait for full understanding of every thread of fundamental biology of orofacial stem/progenitor cells. However, basic understanding of the potency and limitations of orofacial stem/progenitor cells will serve as instructive cues for better translation. Despite recent exponential growth in the volume of studies on orofacial stem/progenitor cells, we only understand bits and pieces of their functions in development, pathogenesis and regeneration. At a minimum, orofacial structures including the tooth are among some of the powerful and under-explored models for studying how stem cells work in development, wound healing as well as genetic and acquired diseases. Is postnatal tissue regeneration a faithful recapitulation of embryonic development? Orofacial tissues appear to be well poised to address questions such as this. A photo of a child with cleft lip and palate stimulates unlimited imagination of how human face can possibly be reconstructed by innovative therapies based on the knowledge of stem/progenitor cells.
Acknowledgements
We thank Dr. N. Lumelsky from NIH/NIDCR for her critical comments and Dr. M. Embree for interactions with medical graphics for illustration. The work for composition of this manuscript is supported by NIH grants R01DE018248, R01EB009663 and RC2DE020767 (to J.J.M.), New York Stem Cell Foundation Grant (to J.J.M.); and NIH grants R01HL073755, P01RR17447, and R21EY020962 (to D.J.P.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contribution
All three authors conceived the general framework of the manuscript. J.J.M. composed the first draft, and revised the manuscript. P.G.R. and D.J.P. provided insightful and critical suggestions and participated in the revision of the manuscript.
References
- 1.Aida T, Meijer EW, Stupp SI. Functional supramolecular polymers. Science. 2012;335(6070):813–817. doi: 10.1126/science.1205962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akintoye SO, Lam T, Shi S, Brahim J, Collins MT, Robey PG. Skeletal site-specific characterization of orofacial and iliac crest human bone marrow stromal cells in same individuals. Bone. 2006;38:758–768. doi: 10.1016/j.bone.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 3.Arakaki M, Ishikawa M, Nakamura T, Iwamoto T, Yamada A, Fukumoto E, Saito M, Otsu K, Harada H, Yamada Y, Fukumoto S. Role of epithelial-stem cell interactions during dental cell differentiation. J Biol Chem. 2012 doi: 10.1074/jbc.M111.285874. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arthur A, Shi S, Zannettino AC, Fujii N, Gronthos S, Koblar SA. Implanted adult human dental pulp stem cells induce endogenous axon guidance. Stem Cells. 2009;27(9):2229–2237. doi: 10.1002/stem.138. [DOI] [PubMed] [Google Scholar]
- 5.Bi Y, Ehirchiou D, Kilts TM, Inkson CA, Embree MC, Sonoyama W, Li L, Leet AI, Seo BM, Zhang L, Shi S, Young MF. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007;13(10):1219–1227. doi: 10.1038/nm1630. [DOI] [PubMed] [Google Scholar]
- 6.Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2:313–319. doi: 10.1016/j.stem.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bianco P, Robey PG, Saggio I, Riminucci M. “Mesenchymal” stem cells in human bone marrow (skeletal stem cells): a critical discussion of their nature, identity, and significance in incurable skeletal disease. Hum Gene Ther. 2010;21:1057–1066. doi: 10.1089/hum.2010.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bianco P. Bone and the hematopoietic niche: a tale of two stem cells. Blood. 2011;117(20):5281–5288. doi: 10.1182/blood-2011-01-315069. [DOI] [PubMed] [Google Scholar]
- 9.Boyne PJ. Study of the chronologic development and eruption of teeth in elasmobranchs. J Dent Res. 1970;49(3):556–560. doi: 10.1177/00220345700490031501. [DOI] [PubMed] [Google Scholar]
- 10.Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9(5):641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 11.Caplan AI, Correa D. The MSC: an injury drugstore. Cell Stem Cell. 2011;8(1):11–15. doi: 10.1016/j.stem.2011.06.008. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chai Y, Jiang X, Ito Y, Bringas P, Jr, Han J, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development. 2000;127(8):1671–1679. doi: 10.1242/dev.127.8.1671. [DOI] [PubMed] [Google Scholar]
- 13.Cordero DR, Brugmann S, Chu Y, Bajpai R, Jame M, Helms JA. Cranial neural crest cells on the move: their roles in craniofacial development. Am J Med Genet A. 2011;155A(2):270–279. doi: 10.1002/ajmg.a.33702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cordeiro MM, Dong Z, Kaneko T, Zhang Z, Miyazawa M, Shi S, Smith AJ, Nör JE. Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. J Endod. 2008;34(8):962–969. doi: 10.1016/j.joen.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 15.Du C, Falini G, Fermani S, Abbott C, Moradian-Oldak J. Supramolecular assembly of amelogenin nanospheres into birefringent microribbons. Science. 2005;307(5714):1450–1454. doi: 10.1126/science.1105675. [DOI] [PubMed] [Google Scholar]
- 16.Felszeghy S, Suomalainen M, Thesleff I. Notch signalling is required for the survival of epithelial stem cells in the continuously growing mouse incisor. Differentiation. 2010;80(4–5):241–248. doi: 10.1016/j.diff.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Friedenstein AJ, Chailakhyan RK, Latsinik NV, Panasyuk AF, Keiliss-Borok IV. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues Cloning in vitro and retransplantation in vivo . Transplantation. 1974;17(4):331–340. doi: 10.1097/00007890-197404000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Fuchs E. The tortoise and the hair: slow-cycling cells in the stem cell race. Cell. 2009;137(5):811–819. doi: 10.1016/j.cell.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujimori S, Novak H, Weissenböck M, Jussila M, Gonçalves A, Zeller R, Galloway J, Thesleff I, Hartmann C. Wnt/β-catenin signaling in the dental mesenchyme regulates incisor development by regulating Bmp4. Dev Biol. 2010;348(1):97–106. doi: 10.1016/j.ydbio.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galler KM, Hartgerink JD, Cavender AC, Schmalz G, D’Souza RN. A customized self-assembling peptide hydrogel for dental pulp tissue engineering. Tissue Eng Part A. 2012;18(1–2):176–84. doi: 10.1089/ten.tea.2011.0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gandia C, Armiñan A, García-Verdugo JM, Lledó E, Ruiz A, Miñana MD, Sanchez-Torrijos J, Payá R, Mirabet V, Carbonell-Uberos F, Llop M, Montero JA, Sepúlveda P. Human dental pulp stem cells improve left ventricular function, induce angiogenesis, and reduce infarct size in rats with acute myocardial infarction. Stem Cells. 2008;26(3):638–645. doi: 10.1634/stemcells.2007-0484. [DOI] [PubMed] [Google Scholar]
- 22.Guilak F, et al. Clonal analysis of the differentiation potential of human adipose-derived adult stem cells. J Cell Physiol. 2006;206:229–237. doi: 10.1002/jcp.20463. [DOI] [PubMed] [Google Scholar]
- 23.Handrigan GR, Leung KJ, Richman JM. Identification of putative dental epithelial stem cells in a lizard with life-long tooth replacement. Development. 2010 Nov;137(21):3545–3549. doi: 10.1242/dev.052415. [DOI] [PubMed] [Google Scholar]
- 24.Harada H, Kettunen P, Jung HS, Mustonen T, Wang YA, Thesleff I. Localization of putative stem cells in dental epithelium and their association with Notch and FGF signaling. J Cell Biol. 1999;147(1):105–120. doi: 10.1083/jcb.147.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harada H, Toyono T, Toyoshima K, Yamasaki M, Itoh N, Kato S, Sekine K, Ohuchi H. FGF10 maintains stem cell compartment in developing mouse incisors. Development. 2002;129(6):1533–1541. doi: 10.1242/dev.129.6.1533. [DOI] [PubMed] [Google Scholar]
- 26.Hsu YC, Pasolli HA, Fuchs E. Dynamics between stem cells, niche, and progeny in the hair follicle. Cell. 2011;144(1):92–105. doi: 10.1016/j.cell.2010.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu B, Unda F, Bopp-Kuchler S, Jimenez L, Wang XJ, Haïkel Y, Wang SL, Lesot H. Bone marrow cells can give rise to ameloblast-like cells. J Dent Res. 2006;85(5):416–421. doi: 10.1177/154405910608500504. [DOI] [PubMed] [Google Scholar]
- 28.Huang GT, Gronthos S, Shi S. Mesenchymal stem cells derived from dental tissues vs those from other sources: their biology and role in regenerative medicine. J Dent Res. 2009;88(9):792–806. doi: 10.1177/0022034509340867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang Z, Newcomb CJ, Bringas P, Jr, Stupp SI, Snead ML. Biological synthesis of tooth enamel instructed by an artificial matrix. Biomaterials. 2010;31(35):9202–9211. doi: 10.1016/j.biomaterials.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ikeda E, Morita R, Nakao K, Ishida K, Nakamura T, Takano-Yamamoto T, et al. Fully functional bioengineered tooth replacement as an organ replacement therapy. Proc Natl Acad Sci U S A. 2009;106:13475–13480. doi: 10.1073/pnas.0902944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iohara K, Imabayashi K, Ishizaka R, Watanabe A, Nabekura J, Ito M, Matsushita K, Nakamura H, Nakashima M. Complete pulp regeneration after pulpectomy by transplantation of CD105+stem cells with stromal cell-derived factor-1. Tissue Eng Part A. 2011;17(15–16):1911–1920. doi: 10.1089/ten.TEA.2010.0615. [DOI] [PubMed] [Google Scholar]
- 32.Izumi K, Tobita T, Feinberg SE. Isolation of human oral keratinocyte progenitor/stem cells. J Dent Res. 2007;86(4):341–346. doi: 10.1177/154405910708600408. [DOI] [PubMed] [Google Scholar]
- 33.Järvinen E, Salazar-Ciudad I, Birchmeier W, Taketo MM, Jernvall J, Thesleff I. Continuous tooth generation in mouse is induced by activated epithelial Wnt/beta-catenin signaling. Proc Natl Acad Sci U S A. 2006;103(49):18627–18632. doi: 10.1073/pnas.0607289103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang X, Iseki S, Maxson RE, Sucov HM, Morriss-Kay GM. Tissue origins and interactions in the mammalian skull vault. Dev Biol. 2002;241(1):106–116. doi: 10.1006/dbio.2001.0487. [DOI] [PubMed] [Google Scholar]
- 35.Jo YY, Lee HJ, Kook SY, Choung HW, Park JY, Chung JH, Choung YH, Kim ES, Yang HC, Choung PH. Isolation and characterization of postnatal stem cells from human dental tissues. Tissue Eng. 2007;13(4):767–773. doi: 10.1089/ten.2006.0192. [DOI] [PubMed] [Google Scholar]
- 36.Karp JM, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. 2009;4(3):206–216. doi: 10.1016/j.stem.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 37.Keating A. Mesenchymal stromal cells: new directions. Cell Stem Cell. 2012;10(6):709–716. doi: 10.1016/j.stem.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 38.Keller L, Kuchler-Bopp S, Mendoza SA, Poliard A, Lesot H. Tooth engineering: searching for dental mesenchymal cells sources. Front Physiol. 2011;2 doi: 10.3389/fphys.2011.00007. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kawano S, Saito M, Handa K, Morotomi T, Toyono T, Seta Y, Nakamura N, Uchida T, Toyoshima K, Ohishi M, Harada H. Characterization of dental epithelial progenitor cells derived from cervical-loop epithelium in a rat lower incisor. J Dent Res. 2004;83(2):129–133. doi: 10.1177/154405910408300209. [DOI] [PubMed] [Google Scholar]
- 40.Kim JY, Xin X, Moioli EK, Chung J, Lee CH, Chen M, Fu SY, Koch PD, Mao JJ. Regeneration of dental-pulp-like tissue by chemotaxis-induced cell homing. Tissue Eng Part A. 2010a;16(10):3023–3031. doi: 10.1089/ten.tea.2010.0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim K, Lee CH, Kim BK, Mao JJ. Anatomically shaped tooth and periodontal regeneration by cell homing. J Dent Res. 2010b;89(8):842–847. doi: 10.1177/0022034510370803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klein OD, Lyons DB, Balooch G, Marshall GW, Basson MA, Peterka M, Boran T, Peterkova R, Martin GR. An FGF signaling loop sustains the generation of differentiated progeny from stem cells in mouse incisors. Development. 2008;135(2):377–385. doi: 10.1242/dev.015081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Knox SM, Lombaert IM, Reed X, Vitale-Cross L, Gutkind JS, Hoffman MP. Parasympathetic innervation maintains epithelial progenitor cells during salivary organogenesis. Science. 2010;329(5999):1645–1647. doi: 10.1126/science.1192046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kollar EJ, Fisher C. Tooth induction in chick epithelium: expression of quiescent genes for enamel synthesis. Science. 1980;207(4434):993–995. doi: 10.1126/science.7352302. [DOI] [PubMed] [Google Scholar]
- 45.Larson BL, Ylöstalo J, Prockop DJ. Human multipotent stromal cells undergo sharp transition from division to development in culture. Stem Cells. 2008 Jan;26(1):193–201. doi: 10.1634/stemcells.2007-0524. [DOI] [PubMed] [Google Scholar]
- 46.Lee CH, Shah B, Moioli EK, Mao JJ. CTGF directs fibroblast differentiation from human mesenchymal stem/stromal cells and defines connective tissue healing in a rodent injury model. J Clin Invest. 2010a;120(9):3340–3349. doi: 10.1172/JCI43230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee CH, Cook JL, Mendelson A, Moioli EK, Yao H, Mao JJ. Regeneration of the articular surface of the rabbit synovial joint by cell homing: a proof of concept study. Lancet. 2010b;376(9739):440–448. doi: 10.1016/S0140-6736(10)60668-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee RH, Seo MJ, Reger RL, Spees JL, Pulin AA, Olson SD, Prockop DJ. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc Natl Acad Sci U S A. 2006;103(46):17438–17443. doi: 10.1073/pnas.0608249103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, Semprun-Prieto L, Delafontaine P, Prockop DJ. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5(1):54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee RH, Seo MJ, Pulin AA, Gregory CA, Ylostalo J, Prockop DJ. The CD34-like protein PODXL and alpha6-integrin (CD49f) identify early progenitor MSCs with increased clonogenicity and migration to infarcted heart in mice. Blood. 2009;113(4):816–826. doi: 10.1182/blood-2007-12-128702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin Y, Cheng YS, Qin C, Lin C, D’Souza R, Wang F. FGFR2 in the dental epithelium is essential for development and maintenance of the maxillary cervical loop, a stem cell niche in mouse incisors. Dev Dyn. 2009;238(2):324–330. doi: 10.1002/dvdy.21778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Laird DJ, von Andrian UH, Wagers AJ. Stem cell trafficking in tissue development, growth, and disease. Cell. 2008;132:612–630. doi: 10.1016/j.cell.2008.01.041. [DOI] [PubMed] [Google Scholar]
- 53.Mao JJ, Giannobile WV, Helms JA, Hollister SJ, Krebsbach PH, Longaker MT, Shi S. Craniofacial tissue engineering by stem cells. J Dent Res. 2006;85(11):966–979. doi: 10.1177/154405910608501101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marion NW, Mao JJ. Mesenchymal stem cells and tissue engineering. Methods Enzymol. 2006;420:339–361. doi: 10.1016/S0076-6879(06)20016-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marynka-Kalmani K, Treves S, Yafee M, Rachima H, Gafni Y, Cohen MA, Pitaru S. The lamina propria of adult human oral mucosa harbors a novel stem cell population. Stem Cells. 2010;28(5):984–995. doi: 10.1002/stem.425. [DOI] [PubMed] [Google Scholar]
- 56.Matsubara T, Suardita K, Ishii M, Sugiyama M, Igarashi A, Oda R, Nishimura M, Saito M, Nakagawa K, Yamanaka K, Miyazaki K, Shimizu M, Bhawal UK, Tsuji K, Nakamura K, Kato Y. Alveolar bone marrow as a cell source for regenerative medicine: differences between alveolar and iliac bone marrow stromal cells. J Bone Miner Res. 2005;20(3):399–409. doi: 10.1359/JBMR.041117. [DOI] [PubMed] [Google Scholar]
- 57.McLain RF, Fleming JE, Boehm CA, Muschler GF. Aspiration of osteoprogenitor cells for augmenting spinal fusion: comparison of progenitor cell concentrations from the vertebral body and iliac crest. J Bone Joint Surg Am. 2005;87(12):2655–2661. doi: 10.2106/JBJS.E.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Medici D, Shore EM, Lounev VY, Kaplan FS, Kalluri R, Olsen BR. Conversion of vascular endothelial cells into multipotent stem-like cells. Nat Med. 2010 Dec;16(12):1400–1406. doi: 10.1038/nm.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Méndez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma’ayan A, Enikolopov GN, Frenette PS. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466(7308):829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mitsiadis TA, Graf D, Luder H, Gridley T, Bluteau G. BMPs and FGFs target Notch signalling via jagged 2 to regulate tooth morphogenesis and cytodifferentiation. Development. 2010;137(18):3025–3035. doi: 10.1242/dev.049528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moore KA, Lemischka IR. Stem cells and their niches. Science. 2006;311(5769):1880–1885. doi: 10.1126/science.1110542. [DOI] [PubMed] [Google Scholar]
- 62.Munne PM, Tummers M, Järvinen E, Thesleff I, Jernvall J. Tinkering with the inductive mesenchyme: Sostdc1 uncovers the role of dental mesenchyme in limiting tooth induction. Development. 2009;136(3):393–402. doi: 10.1242/dev.025064. [DOI] [PubMed] [Google Scholar]
- 63.Nakao K, Morita R, Saji Y, Ishida K, Tomita Y, Ogawa M, Saitoh M, Tomooka Y, Tsuji T. The development of a bioengineered organ germ method. Nat Methods. 2007;4(3):227–230. doi: 10.1038/nmeth1012. [DOI] [PubMed] [Google Scholar]
- 64.Nanci A. Ten Cate’s Oral Histology: Development, Structure, and Function. Mosby. 2007:46–52. [Google Scholar]
- 65.O’Connell DJ, Ho JW, Mammoto T, Turbe-Doan A, O’Connell JT, Haseley PS, Koo S, Kamiya N, Ingber DE, Park PJ, Maas RL. A Wnt-bmp feedback circuit controls intertissue signaling dynamics in tooth organogenesis. Sci Signal. 2012;5(206) doi: 10.1126/scisignal.2002414. 10; In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ohazama A, Johnson EB, Ota MS, Choi HY, Porntaveetus T, Oommen S, Itoh N, Eto K, Gritli-Linde A, Herz J, Sharpe PT. Lrp4 modulates extracellular integration of cell signaling pathways in development. PLoS One. 2008;3(12):e4092. doi: 10.1371/journal.pone.0004092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oshima M, Mizuno M, Imamura A, Ogawa M, Yasukawa M, Yamazaki H, Morita R, Ikeda E, Nakao K, Takano-Yamamoto T, Kasugai S, Saito M, Tsuji T. Functional tooth regeneration using a bioengineered tooth unit as a mature organ replacement regenerative therapy. PLoS One. 2011;6(7):e21531. doi: 10.1371/journal.pone.0021531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Owen M, Friedenstein AJ. Stromal stem cells: marrow-derived osteogenic precursors. Ciba Found Symp. 1988;136:42–60. doi: 10.1002/9780470513637.ch4. [DOI] [PubMed] [Google Scholar]
- 69.Park CH, Rios HF, Jin Q, Sugai JV, Padial-Molina M, Taut AD, Flanagan CL, Hollister SJ, Giannobile WV. Tissue engineering bone-ligament complexes using fiber-guiding scaffolds. Biomaterials. 2012;33(1):137–145. doi: 10.1016/j.biomaterials.2011.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276(5309):71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 71.Prockop DJ, Brenner M, Fibbe WE, Horwitz E, Le Blanc K, Phinney DG, Simmons PJ, Sensebe L, Keating A. Defining the risks of mesenchymal stromal cell therapy. Cytotherapy. 2010 Sep;12(5):576–578. doi: 10.3109/14653249.2010.507330. [DOI] [PubMed] [Google Scholar]
- 72.Prockop DJ, Kota DJ, Bazhanov N, Reger RL. Evolving paradigms for repair of tissues by adult stem/progenitor cells (MSCs) J Cell Mol Med. 2010;14(9):2190–2199. doi: 10.1111/j.1582-4934.2010.01151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Reynolds AJ, Jahoda CA. Cultured human and rat tooth papilla cells induce hair follicle regeneration and fiber growth. Differentiation. 2004;72(9–10):566–575. doi: 10.1111/j.1432-0436.2004.07209010.x. [DOI] [PubMed] [Google Scholar]
- 74.Robey PG. Stem cells near the century mark. J Clin Invest. 2000;105(11):1489–91. doi: 10.1172/JCI10256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Robey PG. Cell sources for bone regeneration: the good, the bad, and the ugly (but promising) Tissue Eng Part B Rev. 2011;17(6):423–430. doi: 10.1089/ten.teb.2011.0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rothová M, Feng J, Sharpe PT, Peterková R, Tucker AS. Contribution of mesoderm to the developing dental papilla. Int J Dev Biol. 2011;55(1):59–64. doi: 10.1387/ijdb.103083mr. [DOI] [PubMed] [Google Scholar]
- 77.Rothova M, Thompson H, Lickert H, Tucker AS. Lineage tracing of the endoderm during oral development. Dev Dyn. 2012 doi: 10.1002/dvdy.23804. In press. [DOI] [PubMed] [Google Scholar]
- 78.Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, Tagliafico E, Ferrari S, Robey PG, Riminucci M, Bianco P. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131(2):324–336. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 79.Samuel N, Bringas P, Jr, Santos V, Nanci A, Slavkin HC. Selachian tooth development: I Histogenesis, morphogenesis, and anatomical features in Squalus acanthias. J Craniofac Genet Dev Biol. 1983;3(1):29–41. [PubMed] [Google Scholar]
- 80.Schop D, Janssen FW, van Rijn LD, Fernandes H, Bloem RM, de Bruijn JD, van Dijkhuizen-Radersma R. Growth, metabolism, and growth inhibitors of mesenchymal stem cells. Tissue Eng Part A. 2009 Aug;15(8):1877–1886. doi: 10.1089/ten.tea.2008.0345. [DOI] [PubMed] [Google Scholar]
- 81.Seidel K, Ahn CP, Lyons D, Nee A, Ting K, Brownell I, Cao T, Carano RA, Curran T, Schober M, Fuchs E, Joyner A, Martin GR, de Sauvage FJ, Klein OD. Hedgehog signaling regulates the generation of ameloblast progenitors in the continuously growing mouse incisor. Development. 2010;137(22):3753–3761. doi: 10.1242/dev.056358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sekiya I, Larson BL, Smith JR, Pochampally R, Cui JG, Prockop DJ. Expansion of human adult stem cells from bone marrow stroma: conditions that maximize the yields of early progenitors and evaluate their quality. Stem Cells. 2002;20(6):530–541. doi: 10.1634/stemcells.20-6-530. [DOI] [PubMed] [Google Scholar]
- 83.Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, Young M, Robey PG, Wang CY, Shi S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364(9429):149–155. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- 84.Shinohara K, et al. Stromal Cell-Derived Factor-1 and Monocyte Chemotactic Protein-3 Improve Recruitment of Osteogenic Cells into Sites of Musculoskeletal Repair. Journal of Orthopaedic Research. 2011 doi: 10.1002/jor.21374. [DOI] [PubMed] [Google Scholar]
- 85.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122(3):787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Smith AJ, et al. Reactionary dentinogenesis. Int. J. Dev. Biol. 1995;39:273–280. [PubMed] [Google Scholar]
- 87.Smith CE. Cell turnover in the odontogenic organ of the rat incisor as visualized by graphic reconstructions following a single injection of 3H-thymidine. Am J Anat. 1980;158(3):321–343. doi: 10.1002/aja.1001580307. [DOI] [PubMed] [Google Scholar]
- 88.Smith JR, Pochampally R, Perry A, Hsu SC, Prockop DJ. Isolation of a highly clonogenic and multipotential subfraction of adult stem cells from bone marrow stroma. Stem Cells. 2004;22(5):823–831. doi: 10.1634/stemcells.22-5-823. [DOI] [PubMed] [Google Scholar]
- 89.Helms JA, Schneider RA. Cranial skeletal biology. Nature. 2003;423(6937):326–331. doi: 10.1038/nature01656. [DOI] [PubMed] [Google Scholar]
- 90.Song J, Kiel MJ, Wang Z, Wang J, Taichman RS, Morrison SJ, Krebsbach PH. An in vivo model to study and manipulate the hematopoietic stem cell niche. Blood. 2010;115(13):2592–2600. doi: 10.1182/blood-2009-01-200071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Suomalainen M, Thesleff I. Patterns of Wnt pathway activity in the mouse incisor indicate absence of Wnt/beta-catenin signaling in the epithelial stem cells. Dev Dyn. 2010;239(1):364–372. doi: 10.1002/dvdy.22106. [DOI] [PubMed] [Google Scholar]
- 92.Thesleff I. The genetic basis of tooth development and dental defects. Am J Med Genet A. 2006;140(23):2530–2535. doi: 10.1002/ajmg.a.31360. [DOI] [PubMed] [Google Scholar]
- 93.Thesleff I, Wang XP, Suomalainen M. Regulation of epithelial stem cells in tooth regeneration. C R Biol. 2007;330(6–7):561–564. doi: 10.1016/j.crvi.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 94.Thesleff I, Tummers M. StemBook [Internet] Cambridge MA: Harvard Stem Cell Institute; 2008-2009. Jan 31, Tooth organogenesis and regeneration. [PubMed] [Google Scholar]
- 95.Tolar J, Nauta AJ, Osborn MJ, Panoskaltsis Mortari A, McElmurry RT, Bell S, Xia L, Zhou N, Riddle M, Schroeder TM, Westendorf JJ, McIvor RS, Hogendoorn PC, Szuhai K, Oseth L, Hirsch B, Yant SR, Kay MA, Peister A, Prockop DJ, Fibbe WE, Blazar BR. Sarcoma derived from cultured mesenchymal stem cells. Stem Cells. 2007;25:371–379. doi: 10.1634/stemcells.2005-0620. [DOI] [PubMed] [Google Scholar]
- 96.Trainor PA. Craniofacial birth defects: The role of neural crest cells in the etiology and pathogenesis of Treacher Collins syndrome and the potential for prevention. Am J Med Genet A. 2010;152A(12):2984–2994. doi: 10.1002/ajmg.a.33454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tucker AS. Salivary gland development. Semin Cell Dev Biol. 2007;18(2):237–244. doi: 10.1016/j.semcdb.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 98.Tucker A, Sharpe P. The cutting-edge of mammalian development; how the embryo makes teeth. Nat Rev Genet. 2004;5(7):499–508. doi: 10.1038/nrg1380. [DOI] [PubMed] [Google Scholar]
- 99.Turksen K. Revisiting the bulge. Dev Cell. 2004;6(4):454–456. doi: 10.1016/s1534-5807(04)00105-4. [DOI] [PubMed] [Google Scholar]
- 100.Volponi AA, Pang Y, Sharpe PT. Stem cell-based biological tooth repair and regeneration. Trends Cell Biol. 2010;20(12):715–722. doi: 10.1016/j.tcb.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wagner W, Ho AD. Mesenchymal stem cell preparations--comparing apples and oranges. Stem Cell Rev. 2007;3:239–248. doi: 10.1007/s12015-007-9001-1. [DOI] [PubMed] [Google Scholar]
- 102.Wang XP, O’Connell DJ, Lund JJ, Saadi I, Kuraguchi M, Turbe-Doan A, Cavallesco R, Kim H, Park PJ, Harada H, Kucherlapati R, Maas RL. Apc inhibition of Wnt signaling regulates supernumerary tooth formation during embryogenesis and throughout adulthood. Development. 2009;136(11):1939–1949. doi: 10.1242/dev.033803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang XP, Suomalainen M, Jorgez CJ, Matzuk MM, Wankell M, Werner S, Thesleff I. Modulation of activin/bone morphogenetic protein signaling by follistatin is required for the morphogenesis of mouse molar teeth. Dev Dyn. 2004;231(1):98–108. doi: 10.1002/dvdy.20118. [DOI] [PubMed] [Google Scholar]
- 104.Wang XP, Suomalainen M, Felszeghy S, Zelarayan LC, Alonso MT, Plikus MV, Maas RL, Chuong CM, Schimmang T, Thesleff I. An integrated gene regulatory network controls stem cell proliferation in teeth. PLoS Biol. 2007;5(6):e159. doi: 10.1371/journal.pbio.0050159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yang B, Chen G, Li J, Zou Q, Xie D, Chen Y, Wang H, Zheng X, Long J, Tang W, Guo W, Tian W. Tooth root regeneration using dental follicle cell sheets in combination with a dentin matrix - based scaffold. Biomaterials. 2012;33(8):2449–2461. doi: 10.1016/j.biomaterials.2011.11.074. [DOI] [PubMed] [Google Scholar]
- 106.Yang R, Chen M, Lee CH, Yoon R, Lal S, Mao JJ. Clones of ectopic stem cells in the regeneration of muscle defects in vivo . PLoS One. 2010;5(10):e13547. doi: 10.1371/journal.pone.0013547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yao S, Pan F, Prpic V, Wise GE. Differentiation of stem cells in the dental follicle. J Dent Res. 2008;87(8):767–771. doi: 10.1177/154405910808700801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yuan Z, Nie H, Wang S, Lee CH, Li A, Fu SY, Zhou H, Chen L, Mao JJ. Biomaterial selection for tooth regeneration. Tissue Eng Part B Rev. 2011;17(5):373–388. doi: 10.1089/ten.teb.2011.0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yelick PC, Vacanti JP. Bioengineered teeth from tooth bud cells. Dent Clin North Am. 2006;50(2):191–203. doi: 10.1016/j.cden.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 110.Yokohama-Tamaki T, Ohshima H, Fujiwara N, Takada Y, Ichimori Y, Wakisaka S, Ohuchi H, Harada H. Cessation of Fgf10 signaling, resulting in a defective dental epithelial stem cell compartment, leads to the transition from crown to root formation. Development. 2006;133(7):1359–1366. doi: 10.1242/dev.02307. [DOI] [PubMed] [Google Scholar]
- 111.Yoshida T, Vivatbutsiri P, Morriss-Kay G, Saga Y, Iseki S. Cell lineage in mammalian craniofacial mesenchyme. Mech Dev. 2008;125(9–10):797–808. doi: 10.1016/j.mod.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 112.Yu J, Wang Y, Deng Z, Tang L, Li Y, Shi J, Jin Y. Odontogenic capability: bone marrow stromal stem cells versus dental pulp stem cells. Biol Cell. 2007;99(8):465–474. doi: 10.1042/BC20070013. [DOI] [PubMed] [Google Scholar]
- 113.Zhang C, Chang J, Sonoyama W, Shi S, Wang CY. Inhibition of human dental pulp stem cell differentiation by Notch signaling. J Dent Res. 2008;87(3):250–255. doi: 10.1177/154405910808700312. [DOI] [PubMed] [Google Scholar]
- 114.Zhang QZ, Su WR, Shi SH, Wilder-Smith P, Xiang AP, Wong A, Nguyen AL, Kwon CW, Le AD. Human gingiva-derived mesenchymal stem cells elicit polarization of m2 macrophages and enhance cutaneous wound healing. Stem Cells. 2010;28(10):1856–1868. doi: 10.1002/stem.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zheng Y, Wang XY, Wang YM, Liu XY, Zhang CM, Hou BX, Wang SL. Dentin regeneration using deciduous pulp stem/progenitor cells. J Dent Res. 2012;91(7):676–682. doi: 10.1177/0022034512449834. [DOI] [PubMed] [Google Scholar]